Bone Spheroid Development Under Flow Conditions with Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells in a 3D Porous Hydrogel Supplemented with Hydroxyapatite
Abstract
1. Introduction
2. Results and Discussion
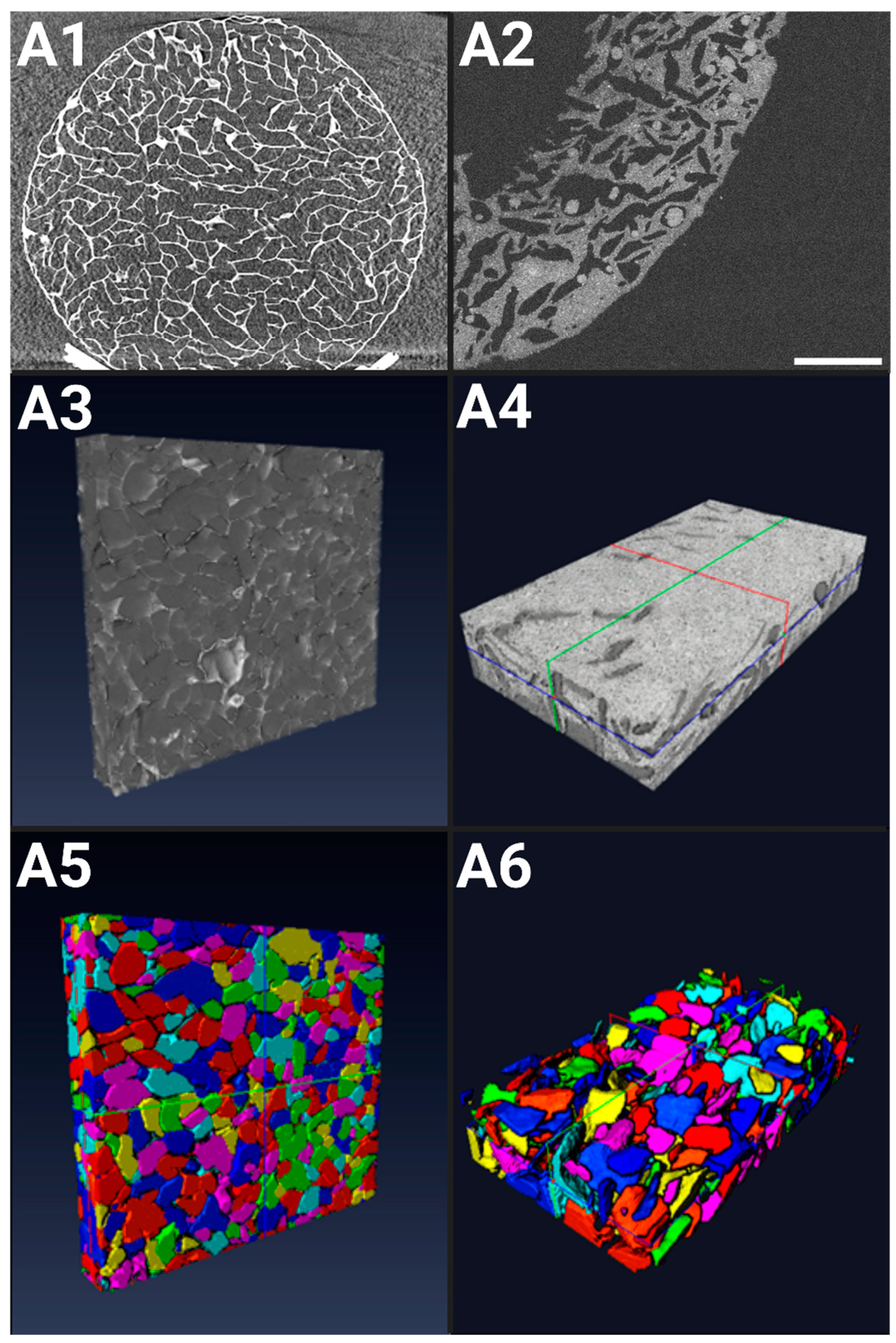
2.1. Structural Characterizations of 3D Scaffolds
2.1.1. X-ray Microtomography of Hydrogels in a Dry State
2.1.2. OCT of Hydrogels in a Hydrated State
2.2. Characterizations of MSC/HUVEC Spheroids Within Hydrogels
2.2.1. Spheroid Mapping
2.2.2. Spheroid Diameter
2.2.3. Spheroid Volume
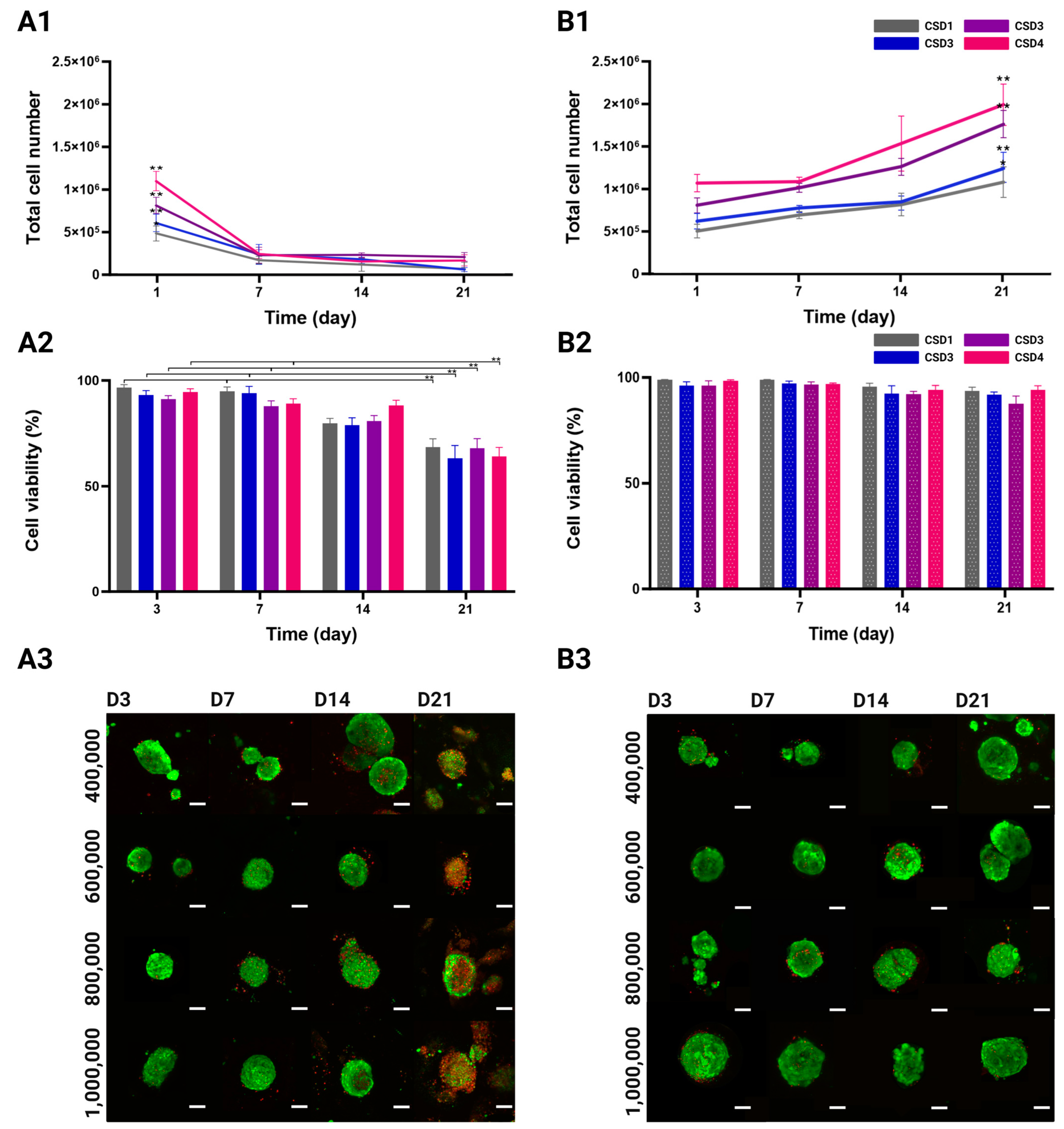
2.3. Proliferation and Viability of the MSC/HUVEC Spheroids
2.3.1. Cell Proliferation Quantification
2.3.2. Viability Quantification and Mapping
2.4. 3D Spatial Reorganization of the MSCs and the HUVECs
2.4.1. Reorganization of Cells
2.4.2. The Detection of Clustering Patterns Across the Entire Diameter of the Spheroid
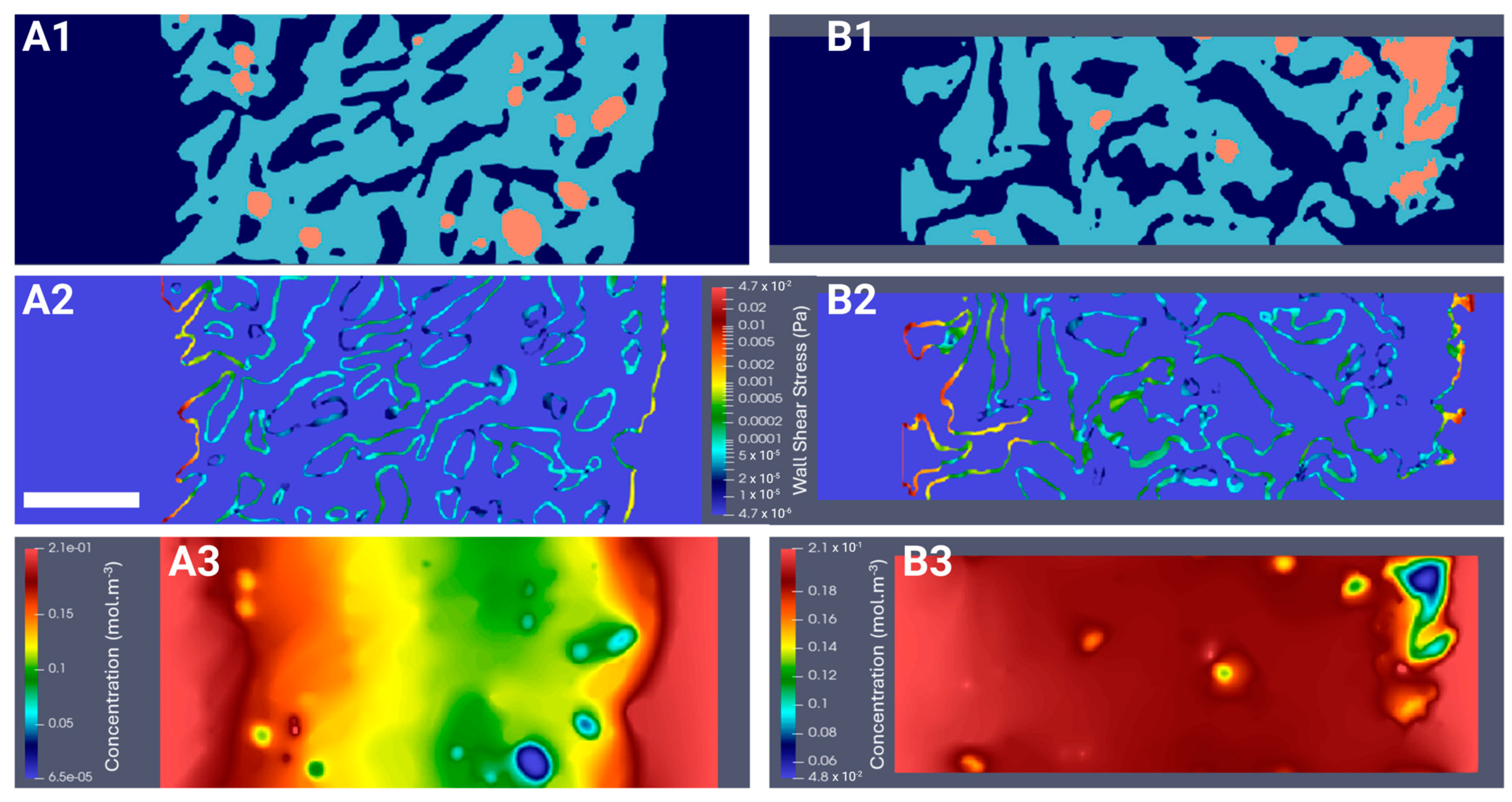
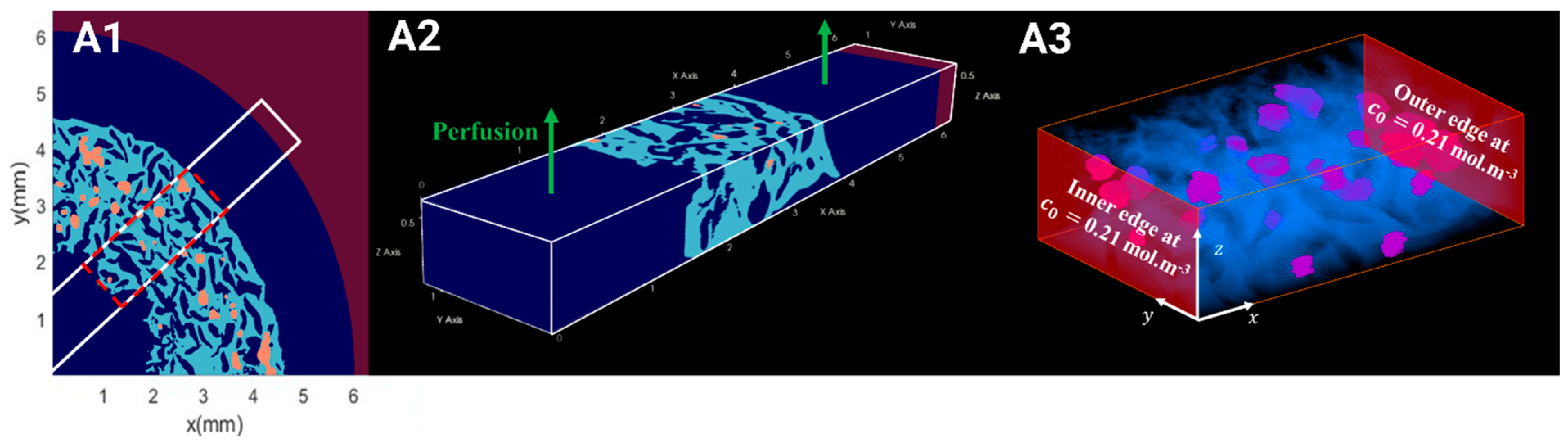
2.5. Computation of the WSS Within the Bioreactor and Oxygen Concentration Mapping in the Hydrogel
2.5.1. Hydrodynamics
2.5.2. Oxygen Diffusion
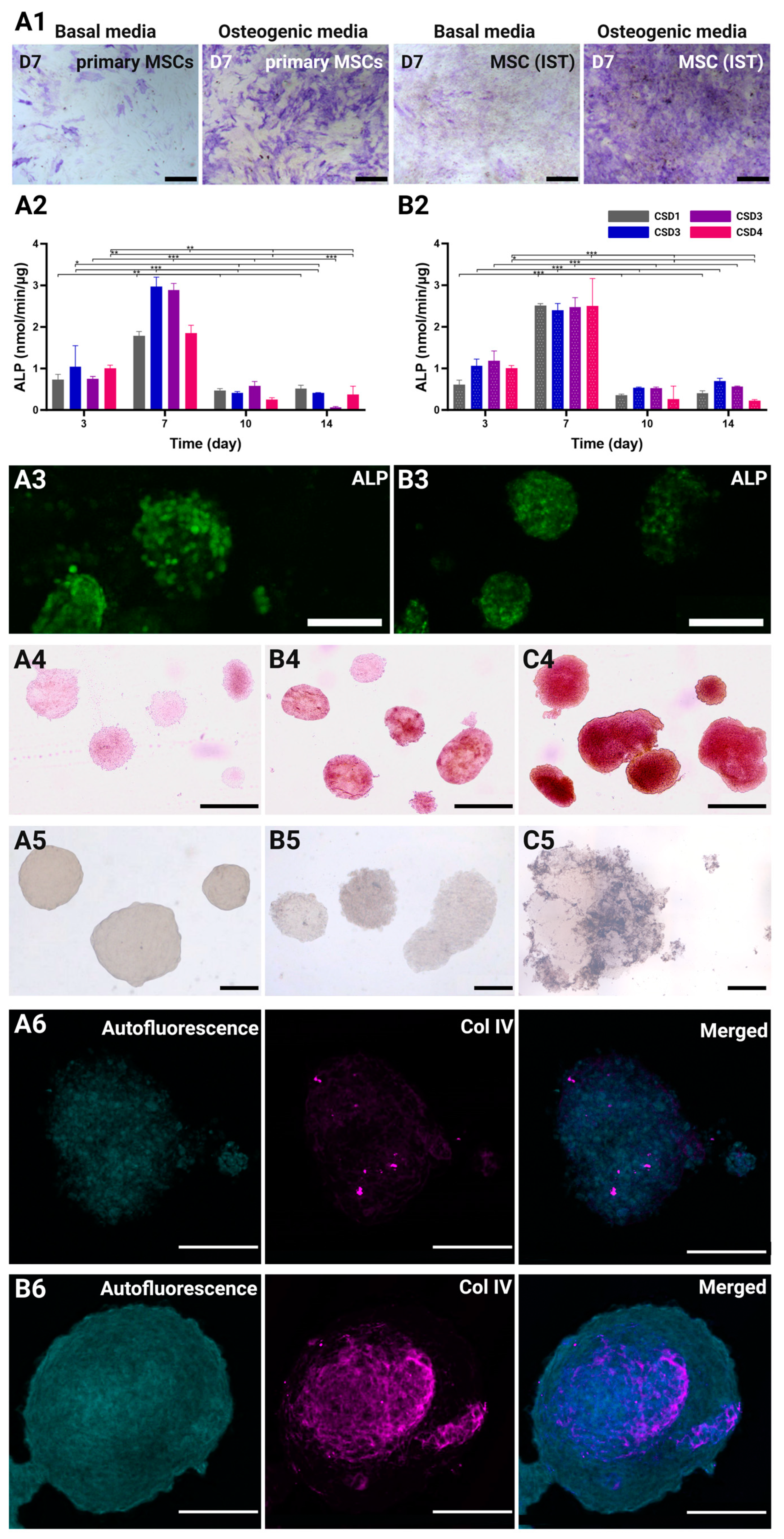
2.6. Osteogenic and Angiogenic Capacities of the MSC/the HUVEC Spheroids
2.6.1. Osteogenic Differentiation of Immortalized MSCs
2.6.2. Alkaline Phosphatase (ALP) Quantification
2.6.3. Mineralization of ECM Through Alizarin Red and Von Kossa Staining
2.6.4. Vasculogenic Capacity of the HUVECs in Co-Culture Spheroids
3. Conclusions
4. Materials and Methods
4.1. Preparation of Porous Polysaccharide-Based Hydrogel Scaffolds
4.2. X-ray Computed Microtomography Hydrogel Characterization
4.2.1. Image Acquisition
4.2.2. X-ray Computed Microtomography Image Reconstruction, Visualization, and Analysis
4.3. Cell Lines
SV40+hTERT-Immortalized Bone Marrow-Derived Mesenchymal Stem Cells Differentiation Capacity
4.4. Cell Seeding Within 3-D Macroporous Scaffold
4.5. Optical Coherence Tomography Hydrogel Characterization
4.5.1. Image Acquisition
4.5.2. Image Reconstruction, Visualization, and Analysis
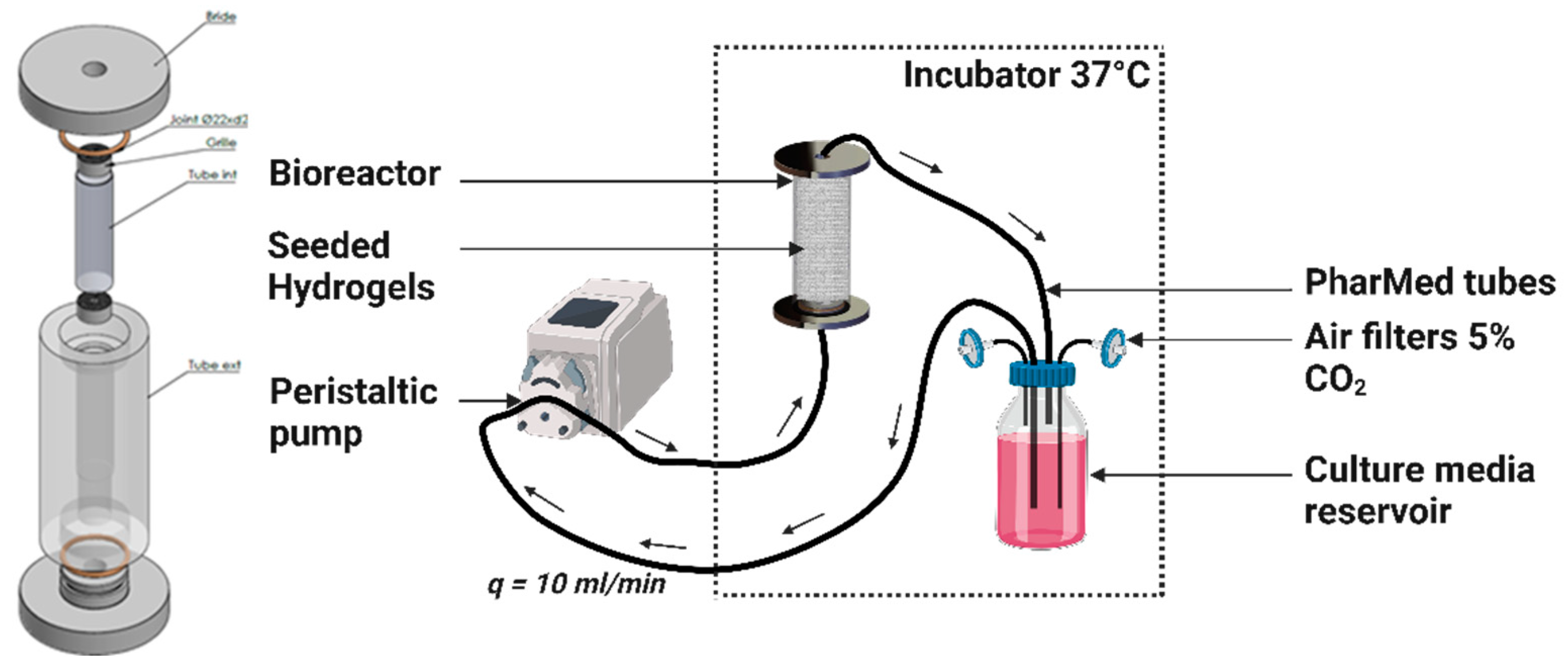
4.6. Dynamic Culture in a Perfusion Bioreactor
4.7. The MSC and HUVEC Coculture
4.7.1. Cell Proliferation Assay
4.7.2. Cell Viability Assessment
4.7.3. Alkaline Phosphatase (ALP) Activity Assay
4.7.4. ALP Live Stain
4.7.5. Mineralization
- 1.
- Von Kossa staining
- 2.
- Alizarin red staining
- 3.
- Collagen type IV immunocytochemistry
4.8. Confocal Image Reconstruction, Visualization, and Spatial Analysis
4.8.1. 3D Plots
4.8.2. Ripley’s K Function
4.9. Computational Fluid Dynamics (CFD) Simulation of Porous Scaffold Perfusion
4.9.1. Modeling and Lattice Boltzmann Method (LBM) Implementation
4.9.2. Computational Configuration
4.10. Simulation of Oxygen Transport and Consumption Within a Porous Scaffold
4.10.1. Modeling and LBM Implementation
4.10.2. Computational Configuration
4.11. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Farjam, P.; Hekman, E.E.G.; Rouwkema, J.; Verkerke, G.J. Bone Fixation Techniques for Managing Joint Disorders and Injuries: A Review Study. J. Mech. Behav. Biomed. Mater. 2022, 126, 104982. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Ofotokun, I. Physiological and Pathophysiological Bone Turnover—Role of the Immune System. Nat. Rev. Endocrinol. 2016, 12, 518–532. [Google Scholar] [CrossRef]
- Vidal, L.; Kampleitner, C.; Brennan, M.Á.; Hoornaert, A.; Layrolle, P. Reconstruction of Large Skeletal Defects: Current Clinical Therapeutic Strategies and Future Directions Using 3D Printing. Front. Bioeng. Biotechnol. 2020, 8, 61. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous Bone Graft: Is It Still the Gold Standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Shegarfi, H.; Reikeras, O. Review Article: Bone Transplantation and Immune Response. J. Orthop. Surg. 2009, 17, 206–211. [Google Scholar] [CrossRef]
- Mercado-Pagán, Á.E.; Stahl, A.M.; Shanjani, Y.; Yang, Y. Vascularization in Bone Tissue Engineering Constructs. Ann. Biomed. Eng. 2015, 43, 718–729. [Google Scholar] [CrossRef]
- Kocherova, I.; Bryja, A.; Mozdziak, P.; Angelova Volponi, A.; Dyszkiewicz-Konwińska, M.; Piotrowska-Kempisty, H.; Antosik, P.; Bukowska, D.; Bruska, M.; Iżycki, D.; et al. Human Umbilical Vein Endothelial Cells (HUVECs) Co-Culture with Osteogenic Cells: From Molecular Communication to Engineering Prevascularised Bone Grafts. J. Clin. Med. 2019, 8, 1602. [Google Scholar] [CrossRef]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in Tissue Engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef]
- Sweeney, M.; Foldes, G. It Takes Two: Endothelial-Perivascular Cell Cross-Talk in Vascular Development and Disease. Front. Cardiovasc. Med. 2018, 5, 154. [Google Scholar] [CrossRef]
- Au, P.; Tam, J.; Fukumura, D.; Jain, R.K. Bone Marrow–Derived Mesenchymal Stem Cells Facilitate Engineering of Long-Lasting Functional Vasculature. Blood 2008, 111, 4551–4558. [Google Scholar] [CrossRef]
- Mendes, L.F.; Pirraco, R.P.; Szymczyk, W.; Frias, A.M.; Santos, T.C.; Reis, R.L.; Marques, A.P. Perivascular-Like Cells Contribute to the Stability of the Vascular Network of Osteogenic Tissue Formed from Cell Sheet-Based Constructs. PLoS ONE 2012, 7, e41051. [Google Scholar] [CrossRef]
- Aguirre, A.; Planell, J.A.; Engel, E. Dynamics of Bone Marrow-Derived Endothelial Progenitor Cell/Mesenchymal Stem Cell Interaction in Co-Culture and Its Implications in Angiogenesis. Biochem. Biophys. Res. Commun. 2010, 400, 284–291. [Google Scholar] [CrossRef]
- Anada, T.; Pan, C.-C.; Stahl, A.M.; Mori, S.; Fukuda, J.; Suzuki, O.; Yang, Y. Vascularized Bone-Mimetic Hydrogel Constructs by 3D Bioprinting to Promote Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2019, 20, 1096. [Google Scholar] [CrossRef]
- Ayan, B.; Heo, D.N.; Zhang, Z.; Dey, M.; Povilianskas, A.; Drapaca, C.; Ozbolat, I.T. Aspiration-Assisted Bioprinting for Precise Positioning of Biologics. Sci. Adv. 2020, 6, eaaw5111. [Google Scholar] [CrossRef]
- Chen, L.; Wu, J.; Wu, C.; Xing, F.; Li, L.; He, Z.; Peng, K.; Xiang, Z. Three-Dimensional Co-Culture of Peripheral Blood-Derived Mesenchymal Stem Cells and Endothelial Progenitor Cells for Bone Regeneration. J. Biomed. Nanotechnol. 2019, 15, 248–260. [Google Scholar] [CrossRef]
- Dahlin, R.L.; Gershovich, J.G.; Kasper, F.K.; Mikos, A.G. Flow Perfusion Co-Culture of Human Mesenchymal Stem Cells and Endothelial Cells on Biodegradable Polymer Scaffolds. Ann. Biomed. Eng. 2014, 42, 1381–1390. [Google Scholar] [CrossRef]
- Ghanaati, S.; Fuchs, S.; Webber, M.J.; Orth, C.; Barbeck, M.; Gomes, M.E.; Reis, R.L.; James Kirkpatrick, C. Rapid Vascularization of Starch–Poly(Caprolactone) in Vivo by Outgrowth Endothelial Cells in Co-Culture with Primary Osteoblasts. J. Tissue Eng. Regen. Med. 2011, 5, e136–e143. [Google Scholar] [CrossRef]
- Gurel Pekozer, G.; Torun Kose, G.; Hasirci, V. Influence of Co-Culture on Osteogenesis and Angiogenesis of Bone Marrow Mesenchymal Stem Cells and Aortic Endothelial Cells. Microvasc. Res. 2016, 108, 1–9. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.N.; Lim, K.-T.; Kim, Y.; Pandey, S.; Garg, P.; Choung, Y.-H.; Choung, P.-H.; Suh, K.-Y.; Chung, J.H. Synergistic Effects of Nanotopography and Co-Culture with Endothelial Cells on Osteogenesis of Mesenchymal Stem Cells. Biomaterials 2013, 34, 7257–7268. [Google Scholar] [CrossRef]
- Moor, L.D.; Smet, J.; Plovyt, M.; Bekaert, B.; Vercruysse, C.; Asadian, M.; Geyter, N.D.; Vlierberghe, S.V.; Dubruel, P.; Declercq, H. Engineering Microvasculature by 3D Bioprinting of Prevascularized Spheroids in Photo-Crosslinkable Gelatin. Biofabrication 2021, 13, 045021. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, M.; Ye, Z.; Zhou, Y.; Tan, W.-S. Fabrication of Viable and Functional Pre-Vascularized Modular Bone Tissues by Coculturing MSCs and HUVECs on Microcarriers in Spinner Flasks. Biotechnol. J. 2017, 12, 1700008. [Google Scholar] [CrossRef]
- Böhrnsen, F.; Schliephake, H. Supportive Angiogenic and Osteogenic Differentiation of Mesenchymal Stromal Cells and Endothelial Cells in Monolayer and Co-Cultures. Int. J. Oral Sci. 2016, 8, 223–230. [Google Scholar] [CrossRef]
- Grellier, M.; Bordenave, L.; Amédée, J. Cell-to-Cell Communication between Osteogenic and Endothelial Lineages: Implications for Tissue Engineering. Trends Biotechnol. 2009, 27, 562–571. [Google Scholar] [CrossRef]
- Stahl, A.; Wenger, A.; Weber, H.; Stark, G.B.; Augustin, H.G.; Finkenzeller, G. Bi-Directional Cell Contact-Dependent Regulation of Gene Expression between Endothelial Cells and Osteoblasts in a Three-Dimensional Spheroidal Coculture Model. Biochem. Biophys. Res. Commun. 2004, 322, 684–692. [Google Scholar] [CrossRef]
- Harold, F.M. Molecules into Cells: Specifying Spatial Architecture. Microbiol. Mol. Biol. Rev. 2005, 69, 544–564. [Google Scholar] [CrossRef]
- Xavier da Silveira dos Santos, A.; Liberali, P. From Single Cells to Tissue Self-organization. Febs J. 2019, 286, 1495–1513. [Google Scholar] [CrossRef]
- Grenier, J.; David, B.; Journé, C.; Cicha, I.; Letourneur, D.; Duval, H. Perfusion of MC3T3E1 Preosteoblast Spheroids within Polysaccharide-Based Hydrogel Scaffolds: An Experimental and Numerical Study at the Bioreactor Scale. Bioengineering 2023, 10, 849. [Google Scholar] [CrossRef]
- Fricain, J.C.; Schlaubitz, S.; Le Visage, C.; Arnault, I.; Derkaoui, S.M.; Siadous, R.; Catros, S.; Lalande, C.; Bareille, R.; Renard, M.; et al. A Nano-Hydroxyapatite—Pullulan/Dextran Polysaccharide Composite Macroporous Material for Bone Tissue Engineering. Biomaterials 2013, 34, 2947–2959. [Google Scholar] [CrossRef]
- Fricain, J.C.; Aid, R.; Lanouar, S.; Maurel, D.B.; Le Nihouannen, D.; Delmond, S.; Letourneur, D.; Amedee Vilamitjana, J.; Catros, S. In-Vitro and in-Vivo Design and Validation of an Injectable Polysaccharide-Hydroxyapatite Composite Material for Sinus Floor Augmentation. Dent. Mater. 2018, 34, 1024–1035. [Google Scholar] [CrossRef]
- Schlaubitz, S.; Derkaoui, S.M.; Marosa, L.; Miraux, S.; Renard, M.; Catros, S.; Visage, C.L.; Letourneur, D.; Amédée, J.; Fricain, J.-C. Pullulan/Dextran/nHA Macroporous Composite Beads for Bone Repair in a Femoral Condyle Defect in Rats. PLoS ONE 2014, 9, e110251. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of Ceramic Materials as Permanently Implantable Skeletal Prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the Effect of Mean Pore Size on Cell Activity in Collagen-Glycosaminoglycan Scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef]
- Ai, C.; Liu, L.; Goh, J.C.-H. Pore Size Modulates in Vitro Osteogenesis of Bone Marrow Mesenchymal Stem Cells in Fibronectin/Gelatin Coated Silk Fibroin Scaffolds. Mater. Sci. Eng. C 2021, 124, 112088. [Google Scholar] [CrossRef]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of Pore Formation in Hydrogel Scaffolds Textured by Freeze-Drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef]
- Grenier, J.; Duval, H.; Lv, P.; Barou, F.; Le Guilcher, C.; Aid, R.; David, B.; Letourneur, D. Interplay between Crosslinking and Ice Nucleation Controls the Porous Structure of Freeze-Dried Hydrogel Scaffolds. Biomater. Adv. 2022, 139, 212973. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Walthers, C.M.; Nazemi, A.; Patel, S.; Wu, B.; Dunn, J.C.Y. The Effect of Scaffold Macroporosity on Angiogenesis and Cell Survival in Tissue-Engineered Smooth Muscle. Biomaterials 2014, 35, 5129–5137. [Google Scholar] [CrossRef]
- Caprio, N.D.; Burdick, J.A. Engineered Biomaterials to Guide Spheroid Formation, Function, and Fabrication into 3D Tissue Constructs. Acta Biomater. 2023, 165, 4–18. [Google Scholar] [CrossRef]
- Morley, C.D.; Tordoff, J.; O’Bryan, C.S.; Weiss, R.; Angelini, T.E. 3D Aggregation of Cells in Packed Microgel Media. Soft Matter 2020, 16, 6572–6581. [Google Scholar] [CrossRef]
- Duttenhoefer, F.; Lara de Freitas, R.; Meury, T.; Loibl, M.; Benneker, L.M.; Hermann, M.; Richards, R.G.; Alini, M.; Verrier, S. 3D Scaffolds Co-Seeded with Human Endothelial Progenitor and Mesenchymal Stem Cells: Evidence of Prevascularisation within 7 Days. Eur. Cells Mater. ECM 2013, 26, 64–65. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhu, L.; Hargreaves, K.M.; Jin, L.; Zhang, C. Scaffold-Free Prevascularized Microtissue Spheroids for Pulp Regeneration. J. Dent. Res. 2014, 93, 1296–1303. [Google Scholar] [CrossRef]
- Saleh, F.; Whyte, M.; Genever, P. Effects of Endothelial Cells on Human Mesenchymal Stem Cell Activity in a Three-Dimensional in Vitro Model. Eur. Cell Mater. 2011, 22, 242–257. [Google Scholar] [CrossRef]
- Marshall, J.; Barnes, A.; Genever, P. Analysis of the Intrinsic Self-Organising Properties of Mesenchymal Stromal Cells in Three-Dimensional Co-Culture Models with Endothelial Cells. Bioengineering 2018, 5, 92. [Google Scholar] [CrossRef]
- Guerrero, J.; Catros, S.; Derkaoui, S.-M.; Lalande, C.; Siadous, R.; Bareille, R.; Thébaud, N.; Bordenave, L.; Chassande, O.; Le Visage, C.; et al. Cell Interactions between Human Progenitor-Derived Endothelial Cells and Human Mesenchymal Stem Cells in a Three-Dimensional Macroporous Polysaccharide-Based Scaffold Promote Osteogenesis. Acta Biomater. 2013, 9, 8200–8213. [Google Scholar] [CrossRef]
- Heo, D.N.; Hospodiuk, M.; Ozbolat, I.T. Synergistic Interplay between Human MSCs and HUVECs in 3D Spheroids Laden in Collagen/Fibrin Hydrogels for Bone Tissue Engineering. Acta Biomater. 2019, 95, 348–356. [Google Scholar] [CrossRef]
- Vorwald, C.E.; Joshee, S.; Leach, J.K. Spatial Localization of Endothelial Cells in Heterotypic Spheroids Influences Notch Signaling. J. Mol. Med. 2020, 98, 425–435. [Google Scholar] [CrossRef]
- Kuss, M.A.; Wu, S.; Wang, Y.; Untrauer, J.B.; Li, W.; Lim, J.Y.; Duan, B. Prevascularization of 3D Printed Bone Scaffolds by Bioactive Hydrogels and Cell Co-Culture. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1788–1798. [Google Scholar] [CrossRef]
- Merino-Casallo, F.; Gomez-Benito, M.J.; Hervas-Raluy, S.; Garcia-Aznar, J.M. Unravelling Cell Migration: Defining Movement from the Cell Surface. Cell Adhes. Migr. 2022, 16, 25–64. [Google Scholar] [CrossRef]
- Kim, S.; Byun, H.; Lee, S.; Kim, E.; Lee, G.M.; Huh, S.J.; Joo, J.; Shin, H. Spatially Arranged Encapsulation of Stem Cell Spheroids within Hydrogels for the Regulation of Spheroid Fusion and Cell Migration. Acta Biomater. 2022, 142, 60–72. [Google Scholar] [CrossRef]
- Bi, M.; Yang, K.; Yu, T.; Wu, G.; Li, Q. Cell-Based Mechanisms and Strategies of Co-Culture System Both in Vivo and Vitro for Bone Tissue Engineering. Biomed. Pharmacother. 2023, 169, 115907. [Google Scholar] [CrossRef]
- Rovere, M.; Reverberi, D.; Arnaldi, P.; Palamà, M.E.F.; Gentili, C. Spheroid Size Influences Cellular Senescence and Angiogenic Potential of Mesenchymal Stromal Cell-Derived Soluble Factors and Extracellular Vesicles. Front. Bioeng. Biotechnol. 2023, 11, 1297644. [Google Scholar] [CrossRef]
- Shemesh, J.; Jalilian, I.; Shi, A.; Yeoh, G.H.; Tate, M.L.K.; Warkiani, M.E. Flow-Induced Stress on Adherent Cells in Microfluidic Devices. Lab. Chip 2015, 15, 4114–4127. [Google Scholar] [CrossRef]
- Gonzalez, B.A.; Perez-Nevarez, M.; Mirza, A.; Perez, M.G.; Lin, Y.-M.; Hsu, C.-P.D.; Caobi, A.; Raymond, A.; Gomez Hernandez, M.E.; Fernandez-Lima, F.; et al. Physiologically Relevant Fluid-Induced Oscillatory Shear Stress Stimulation of Mesenchymal Stem Cells Enhances the Engineered Valve Matrix Phenotype. Front. Cardiovasc. Med. 2020, 7, 69. [Google Scholar] [CrossRef]
- Stavenschi, E.; Labour, M.-N.; Hoey, D.A. Oscillatory Fluid Flow Induces the Osteogenic Lineage Commitment of Mesenchymal Stem Cells: The Effect of Shear Stress Magnitude, Frequency, and Duration. J. Biomech. 2017, 55, 99–106. [Google Scholar] [CrossRef]
- Zheng, W.; Xie, Y.; Zhang, W.; Wang, D.; Ma, W.; Wang, Z.; Jiang, X. Fluid Flow Stress Induced Contraction and Re-Spread of Mesenchymal Stem Cells: A Microfluidic Study. Integr. Biol. 2012, 4, 1102–1111. [Google Scholar] [CrossRef]
- Shih, H.-C.; Lee, T.-A.; Wu, H.-M.; Ko, P.-L.; Liao, W.-H.; Tung, Y.-C. Microfluidic Collective Cell Migration Assay for Study of Endothelial Cell Proliferation and Migration under Combinations of Oxygen Gradients, Tensions, and Drug Treatments. Sci. Rep. 2019, 9, 8234. [Google Scholar] [CrossRef]
- Hsu, H.-H.; Ko, P.-L.; Wu, H.-M.; Lin, H.-C.; Wang, C.-K.; Tung, Y.-C. Study 3D Endothelial Cell Network Formation under Various Oxygen Microenvironment and Hydrogel Composition Combinations Using Upside-Down Microfluidic Devices. Small 2021, 17, 2006091. [Google Scholar] [CrossRef]
- Hsu, H.-H.; Ko, P.-L.; Peng, C.-C.; Cheng, Y.-J.; Wu, H.-M.; Tung, Y.-C. Studying Sprouting Angiogenesis under Combination of Oxygen Gradients and Co-Culture of Fibroblasts Using Microfluidic Cell Culture Model. Mater. Today Bio 2023, 21, 100703. [Google Scholar] [CrossRef]
- Lin, X.; Chen, Q.; Liu, W.; Zhang, J.; Wang, S.; Lin, Z.; Lin, J.-M. Oxygen-Induced Cell Migration and on-Line Monitoring Biomarkers Modulation of Cervical Cancers on a Microfluidic System. Sci. Rep. 2015, 5, 9643. [Google Scholar] [CrossRef]
- Buravkova, L.B.; Andreeva, E.R.; Gogvadze, V.; Zhivotovsky, B. Mesenchymal Stem Cells and Hypoxia: Where Are We? Mitochondrion 2014, 19, 105–112. [Google Scholar] [CrossRef]
- Iida, T.; Mine, S.; Fujimoto, H.; Suzuki, K.; Minami, Y.; Tanaka, Y. Hypoxia-Inducible Factor-1α Induces Cell Cycle Arrest of Endothelial Cells. Genes Cells 2002, 7, 143–149. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Siekierzycka, A.; Płoska, A.; Dobrucki, I.T.; Kalinowski, L. Endothelial Dysfunction Driven by Hypoxia—The Influence of Oxygen Deficiency on NO Bioavailability. Biomolecules 2021, 11, 982. [Google Scholar] [CrossRef]
- Stempien-Otero, A.; Karsan, A.; Cornejo, C.J.; Xiang, H.; Eunson, T.; Morrison, R.S.; Kay, M.; Winn, R.; Harlan, J. Mechanisms of Hypoxia-Induced Endothelial Cell Death: ROLE OF P53 IN APOPTOSIS*. J. Biol. Chem. 1999, 274, 8039–8045. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, J.; Cong, X.; Hu, S.; Chen, X. Hypoxia and Serum Deprivation-Induced Apoptosis in Mesenchymal Stem Cells. Stem Cells 2006, 24, 416–425. [Google Scholar] [CrossRef]
- Murphy, K.C.; Hung, B.P.; Browne-Bourne, S.; Zhou, D.; Yeung, J.; Genetos, D.C.; Leach, J.K. Measurement of Oxygen Tension within Mesenchymal Stem Cell Spheroids. J. R. Soc. Interface 2017, 14, 20160851. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Šromová, V.; Sobola, D.; Kaspar, P. A Brief Review of Bone Cell Function and Importance. Cells 2023, 12, 2576. [Google Scholar] [CrossRef]
- Bahramsoltani, M.; Slosarek, I.; De Spiegelaere, W.; Plendl, J. Angiogenesis and Collagen Type IV Expression in Different Endothelial Cell Culture Systems. Anat. Histol. Embryol. 2014, 43, 103–115. [Google Scholar] [CrossRef]
- Summer, S.; Rossmanith, E.; Pasztorek, M.; Fiedler, C.; Gröger, M.; Rauscher, S.; Weber, V.; Fischer, M.B. Mesenchymal Stem Cells Support Human Vascular Endothelial Cells to Form Vascular Sprouts in Human Platelet Lysate-Based Matrices. PLoS ONE 2022, 17, e0278895. [Google Scholar] [CrossRef]
- Bancroft, G.N.; Sikavitsas, V.I.; Mikos, A.G. Technical Note: Design of a Flow Perfusion Bioreactor System for Bone Tissue-Engineering Applications. Tissue Eng. 2003, 9, 549–554. [Google Scholar] [CrossRef]
- Fröhlich, M.; Grayson, W.L.; Marolt, D.; Gimble, J.M.; Kregar-Velikonja, N.; Vunjak-Novakovic, G. Bone Grafts Engineered from Human Adipose-Derived Stem Cells in Perfusion Bioreactor Culture. Tissue Eng. Part A 2010, 16, 179–189. [Google Scholar] [CrossRef]
- Yourek, G.; McCormick, S.M.; Mao, J.J.; Reilly, G.C. Shear Stress Induces Osteogenic Differentiation of Human Mesenchymal Stem Cells. Regen. Med. 2010, 5, 713–724. [Google Scholar] [CrossRef]
- Dellaquila, A.; Dujardin, C.; Le Bao, C.; Chaumeton, C.; Carré, A.; Le Guilcher, C.; Lam, F.; Simon-Yarza, T. Fibroblasts Mediate Endothelium Response to Angiogenic Cues in a Newly Developed 3D Stroma Engineered Model. Biomater. Adv. 2023, 154, 213636. [Google Scholar] [CrossRef]
- Le Guilcher, C.; Merlen, G.; Dellaquila, A.; Labour, M.-N.; Aid, R.; Tordjmann, T.; Letourneur, D.; Simon-Yarza, T. Engineered Human Liver Based on Pullulan-Dextran Hydrogel Promotes Mice Survival after Liver Failure. Mater. Today Bio 2023, 19, 100554. [Google Scholar] [CrossRef]
- Simon-Yarza, T.; Labour, M.-N.; Aid, R.; Letourneur, D. Channeled Polysaccharide-Based Hydrogel Reveals Influence of Curvature to Guide Endothelial Cell Arrangement in Vessel-like Structures. Mater. Sci. Eng. C 2021, 118, 111369. [Google Scholar] [CrossRef]
- Ahmed Omar, N.; Amédée, J.; Letourneur, D.; Fricain, J.-C.; Fenelon, M. Recent Advances of Pullulan and/or Dextran-Based Materials for Bone Tissue Engineering Strategies in Preclinical Studies: A Systematic Review. Front. Bioeng. Biotechnol. 2022, 10, 889481. [Google Scholar] [CrossRef]
- Ribot, E.J.; Tournier, C.; Aid-Launais, R.; Koonjoo, N.; Oliveira, H.; Trotier, A.J.; Rey, S.; Wecker, D.; Letourneur, D.; Amedee Vilamitjana, J.; et al. 3D Anatomical and Perfusion MRI for Longitudinal Evaluation of Biomaterials for Bone Regeneration of Femoral Bone Defect in Rats. Sci. Rep. 2017, 7, 6100. [Google Scholar] [CrossRef]
- Bidarra, S.J.; Barrias, C.C.; Barbosa, M.A.; Soares, R.; Amédée, J.; Granja, P.L. Phenotypic and Proliferative Modulation of Human Mesenchymal Stem Cells via Crosstalk with Endothelial Cells. Stem Cell Res. 2011, 7, 186–197. [Google Scholar] [CrossRef]
- Cacciamali, A.; Villa, R.; Dotti, S. 3D Cell Cultures: Evolution of an Ancient Tool for New Applications. Front. Physiol. 2022, 13, 836480. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Life Is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol. 2017, 35, 133–144. [Google Scholar] [CrossRef]
- Baddeley, A.; Rubak, E.; Turner, R. Spatial Point Patterns: Methodology and Applications with R; CRC Press: Boca Raton, FL, USA, 2015; ISBN 1-4822-1021-5. [Google Scholar]
- Lakhotia, S.; Papoutsakis, E.T. Agitation Induced Cell Injury in Microcarrier Cultures. Protective Effect of Viscosity Is Agitation Intensity Dependent: Experiments and Modeling. Biotechnol. Bioeng. 1992, 39, 95–107. [Google Scholar] [CrossRef]
- d’Humières, D. Multiple–Relaxation–Time Lattice Boltzmann Models in Three Dimensions. Philos. Trans. R. Soc. Lond. Ser. Math. Phys. Eng. Sci. 2002, 360, 437–451. [Google Scholar] [CrossRef]
- Duval, H.; Masson, D.; Guillot, J.-B.; Schmitz, P.; d’Humières, D. Two-Dimensional Lattice–Boltzmann Model of Hydrosol Depth Filtration. AIChE J. 2006, 52, 39–48. [Google Scholar] [CrossRef]
- Thibeaux, R.; Duval, H.; Smaniotto, B.; Vennat, E.; Néron, D.; David, B. Assessment of the Interplay between Scaffold Geometry, Induced Shear Stresses, and Cell Proliferation within a Packed Bed Perfusion Bioreactor. Biotechnol. Prog. 2019, 35, e2880. [Google Scholar] [CrossRef]
- Ginzburg, I.; d’Humières, D. Multireflection Boundary Conditions for Lattice Boltzmann Models. Phys. Rev. E 2003, 68, 066614. [Google Scholar] [CrossRef]
- Krüger, T.; Varnik, F.; Raabe, D. Shear Stress in Lattice Boltzmann Simulations. Phys. Rev. E 2009, 79, 046704. [Google Scholar] [CrossRef]
- Matyka, M.; Koza, Z.; Mirosław, Ł. Wall Orientation and Shear Stress in the Lattice Boltzmann Model. Comput. Fluids 2013, 73, 115–123. [Google Scholar] [CrossRef]
- Coyle, R.; Yao, J.; Richards, D.; Mei, Y. The Effects of Metabolic Substrate Availability on Human Adipose-Derived Stem Cell Spheroid Survival. Tissue Eng. Part A 2019, 25, 620–631. [Google Scholar] [CrossRef]
- Abaci, H.E.; Truitt, R.; Tan, S.; Gerecht, S. Unforeseen Decreases in Dissolved Oxygen Levels Affect Tube Formation Kinetics in Collagen Gels. Am. J. Physiol.-Cell Physiol. 2011, 301, C431–C440. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A.; Doetsch, J.; Mueller-Klieser, W.; Groebe, K. Proliferative Activity and Tumorigenic Conversion: Impact on Cellular Metabolism in 3-D Culture. Am. J. Physiol.-Cell Physiol. 2000, 278, C765–C780. [Google Scholar] [CrossRef]
- Ginzburg, I. Prediction of the Moments in Advection-Diffusion Lattice Boltzmann Method. I. Truncation Dispersion, Skewness, and Kurtosis. Phys. Rev. E 2017, 95, 013304. [Google Scholar] [CrossRef]


| CSD1 | CSD2 | CSD3 | CSD4 | |
|---|---|---|---|---|
| Cell Seeding Density (CSD)/scaffold | 400,000 | 600,000 | 800,000 | 1,000,000 |
| Average spheroid volume (µm3) × 105 | 6.0 ± 0.8 | 7.6 ± 0.1 | 6.3 ± 0.4 | 10.9 ± 0.1 |
| Spheroid number | 773 ± 252 | 805 ± 54 | 2384 ± 203 | 1368 ± 249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hajj, S.; Ntaté, M.B.; Breton, C.; Siadous, R.; Aid, R.; Dupuy, M.; Letourneur, D.; Amédée, J.; Duval, H.; David, B. Bone Spheroid Development Under Flow Conditions with Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells in a 3D Porous Hydrogel Supplemented with Hydroxyapatite. Gels 2024, 10, 666. https://doi.org/10.3390/gels10100666
El Hajj S, Ntaté MB, Breton C, Siadous R, Aid R, Dupuy M, Letourneur D, Amédée J, Duval H, David B. Bone Spheroid Development Under Flow Conditions with Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells in a 3D Porous Hydrogel Supplemented with Hydroxyapatite. Gels. 2024; 10(10):666. https://doi.org/10.3390/gels10100666
Chicago/Turabian StyleEl Hajj, Soukaina, Martial Bankoué Ntaté, Cyril Breton, Robin Siadous, Rachida Aid, Magali Dupuy, Didier Letourneur, Joëlle Amédée, Hervé Duval, and Bertrand David. 2024. "Bone Spheroid Development Under Flow Conditions with Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells in a 3D Porous Hydrogel Supplemented with Hydroxyapatite" Gels 10, no. 10: 666. https://doi.org/10.3390/gels10100666
APA StyleEl Hajj, S., Ntaté, M. B., Breton, C., Siadous, R., Aid, R., Dupuy, M., Letourneur, D., Amédée, J., Duval, H., & David, B. (2024). Bone Spheroid Development Under Flow Conditions with Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells in a 3D Porous Hydrogel Supplemented with Hydroxyapatite. Gels, 10(10), 666. https://doi.org/10.3390/gels10100666








