Development of Personalised Immediate-Release Gel-Based Formulations Using Semi-Solid Extrusion
Abstract
1. Introduction
2. Results and Discussion
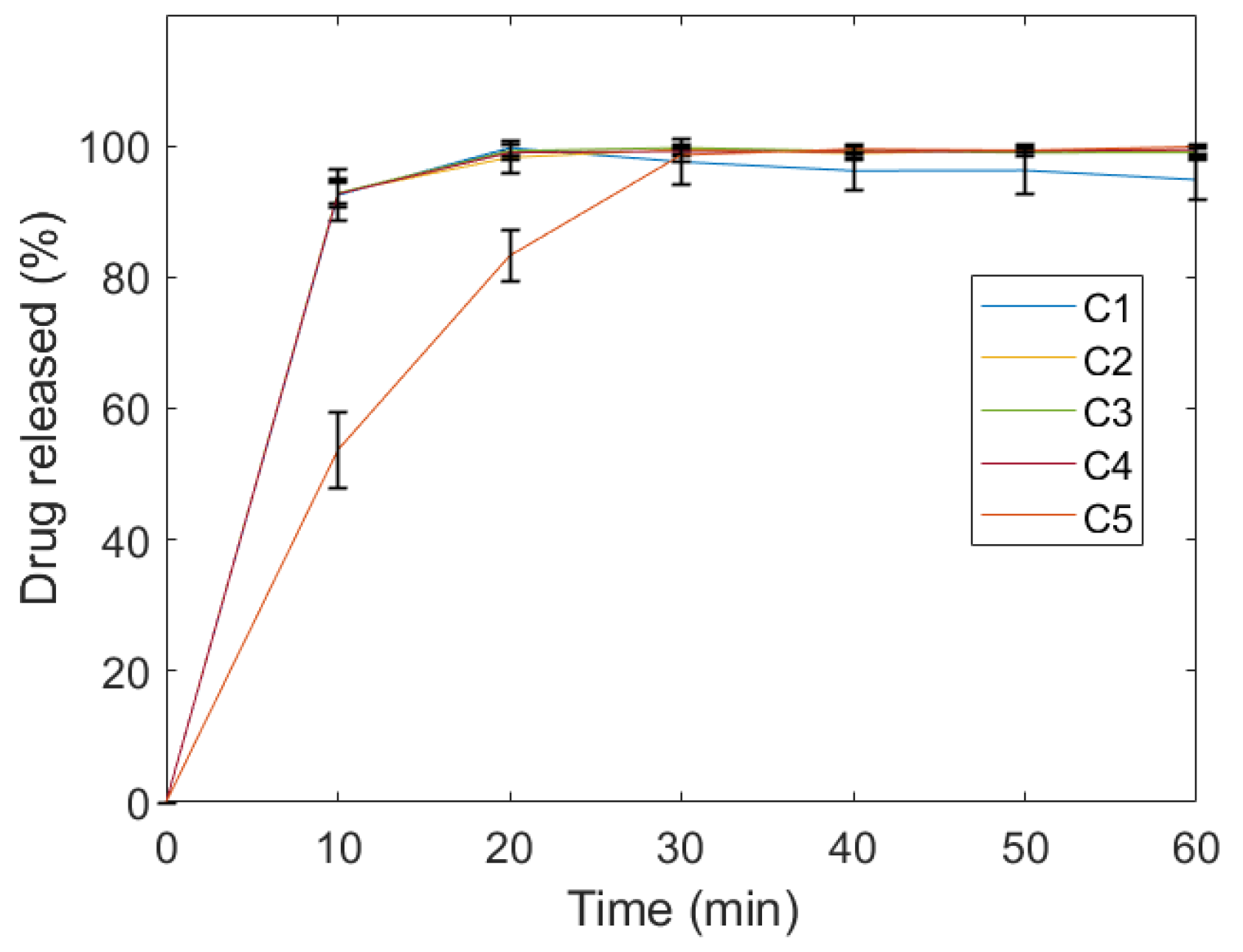
2.1. Evaluation of Quality Control Attributes
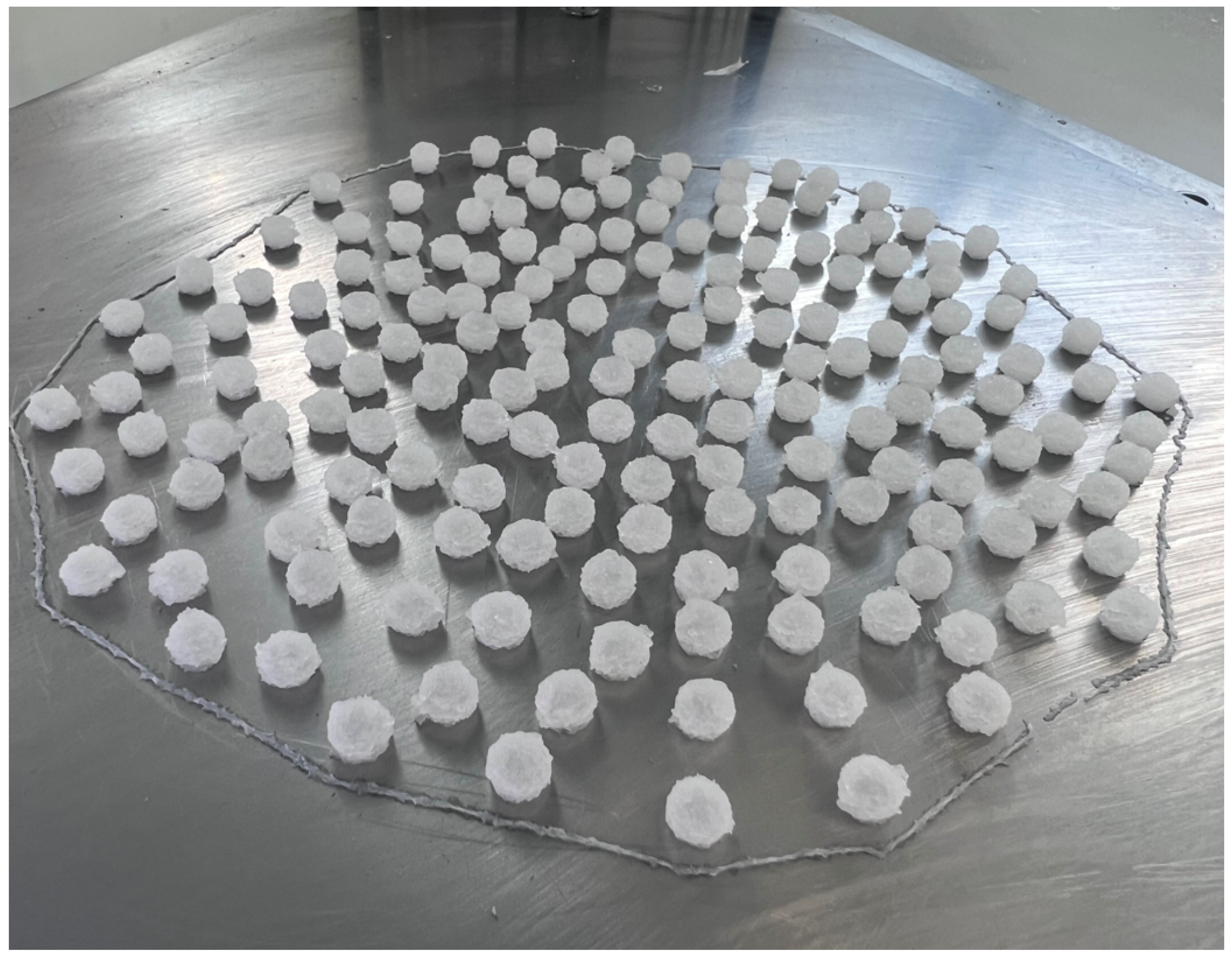
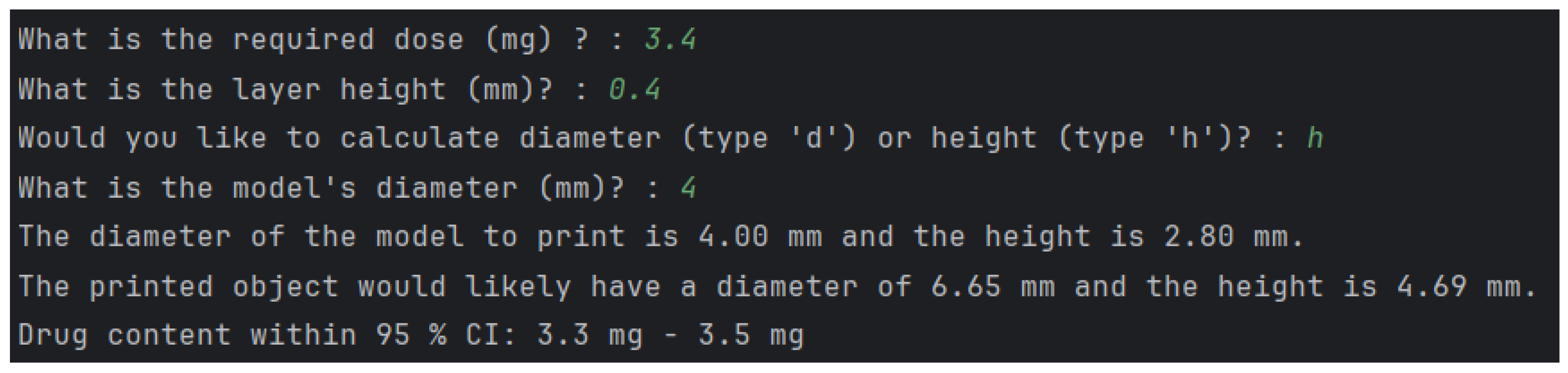
2.2. Personalisation of Dosage Form
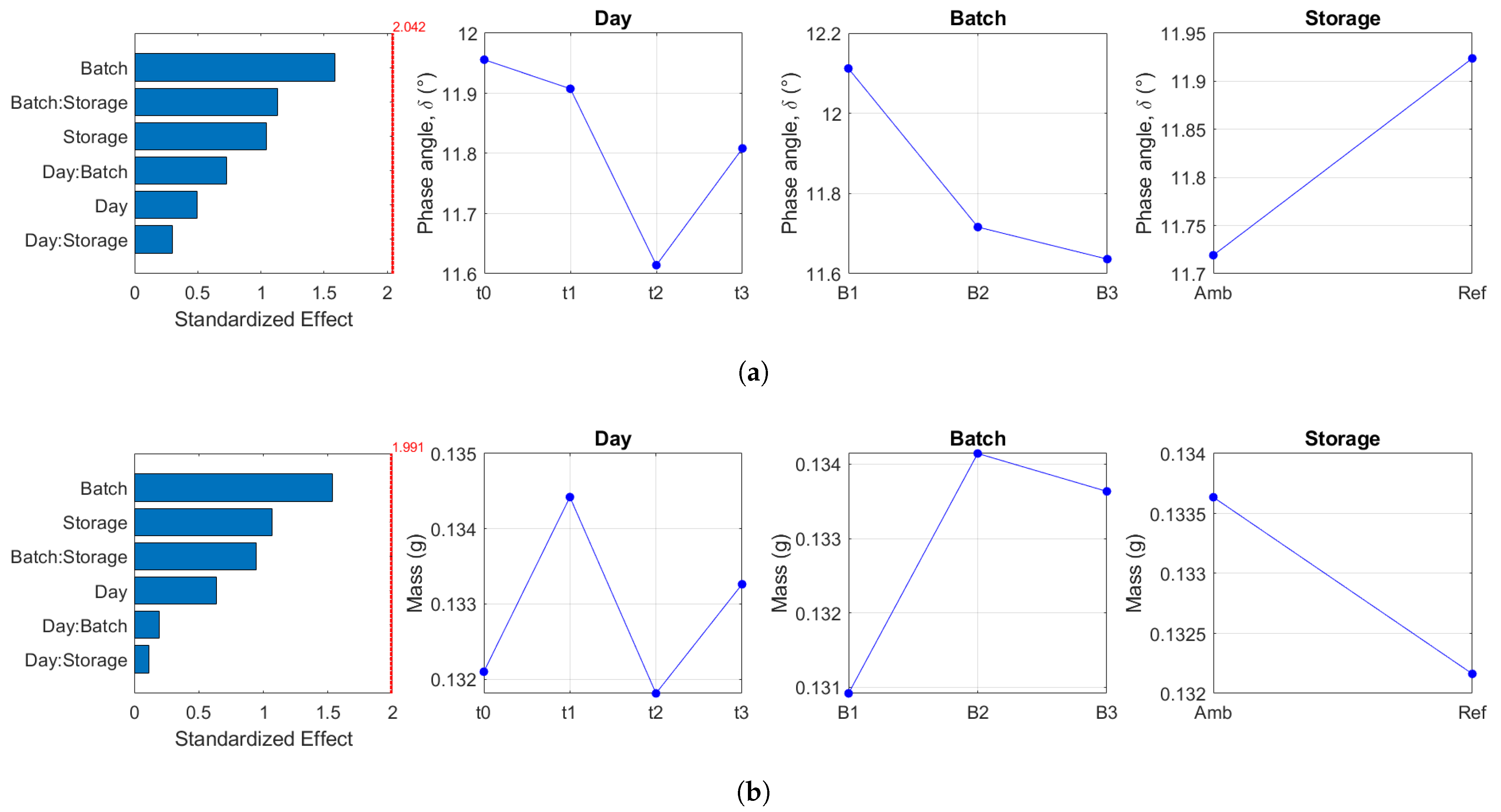
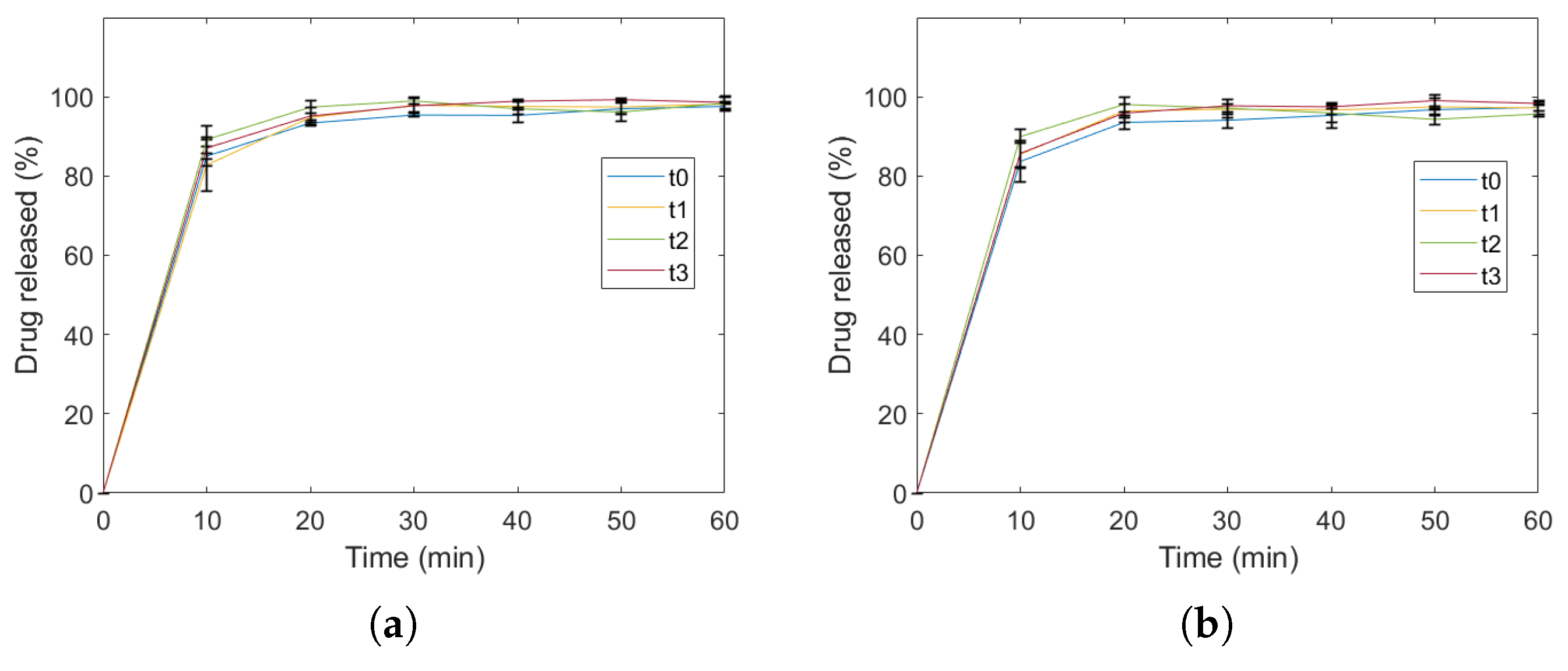
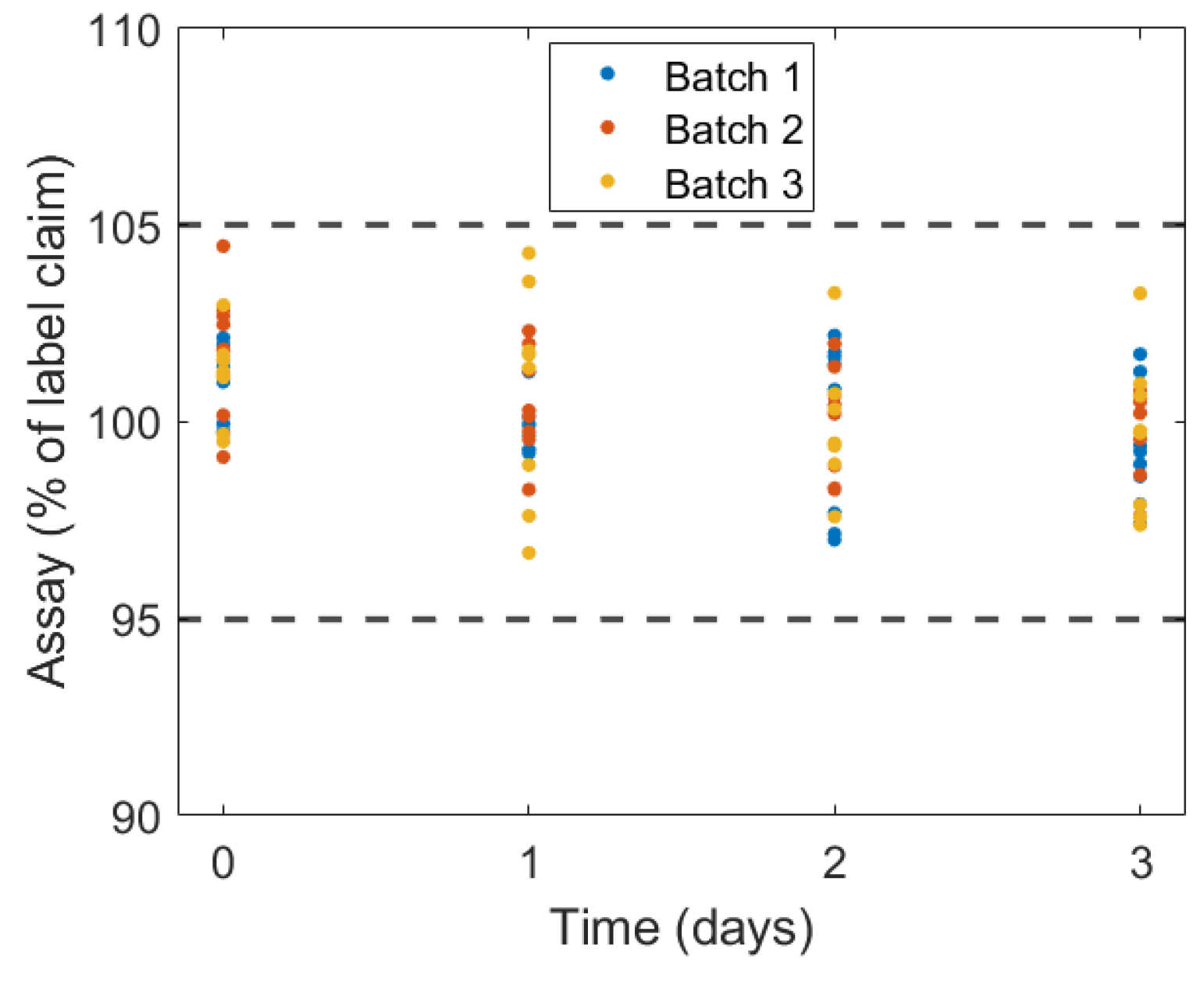
2.3. Identification of Variability
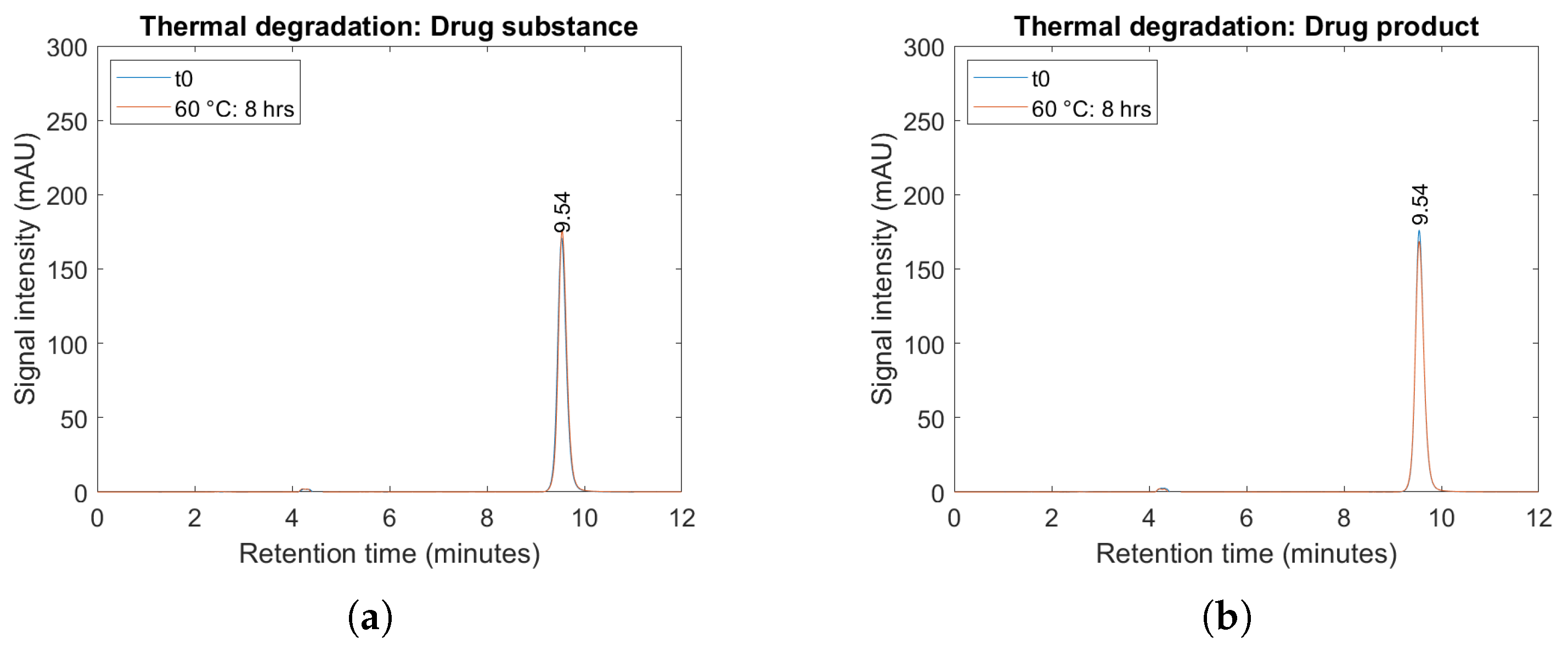
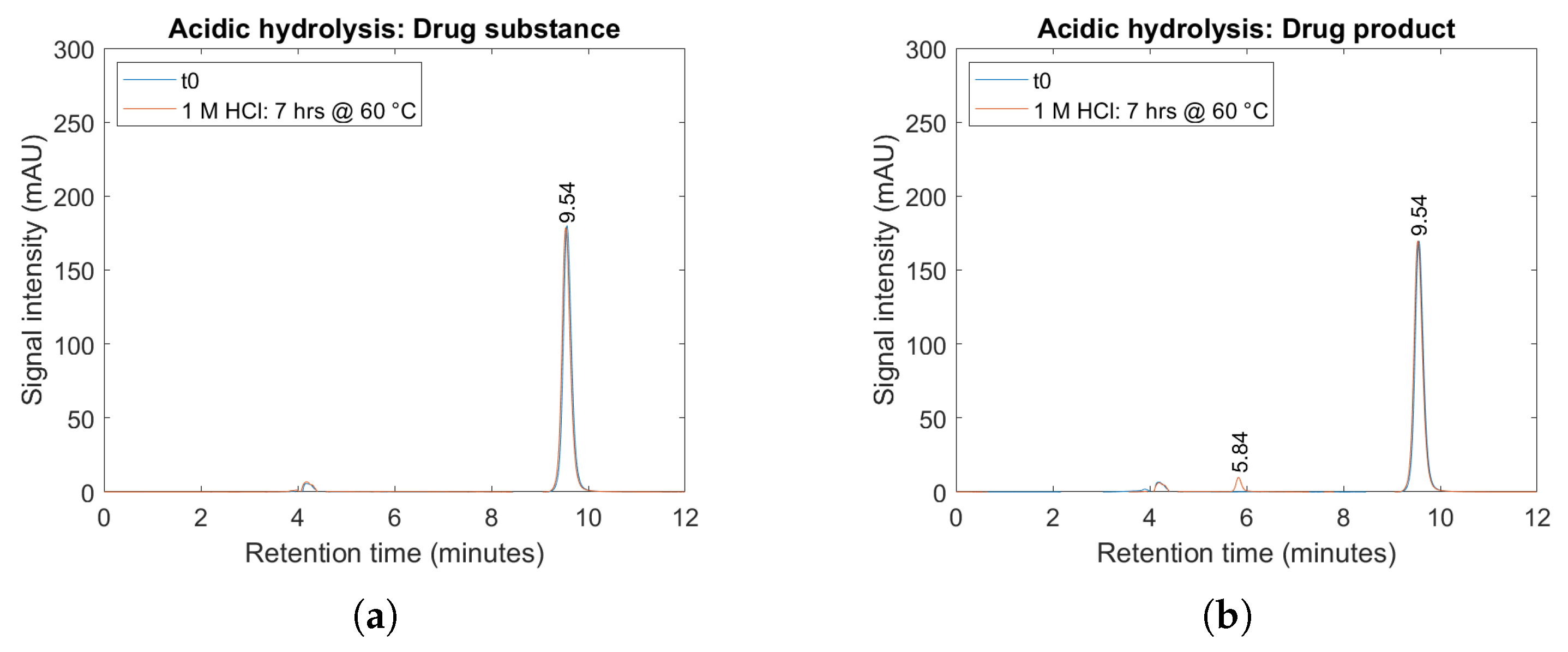
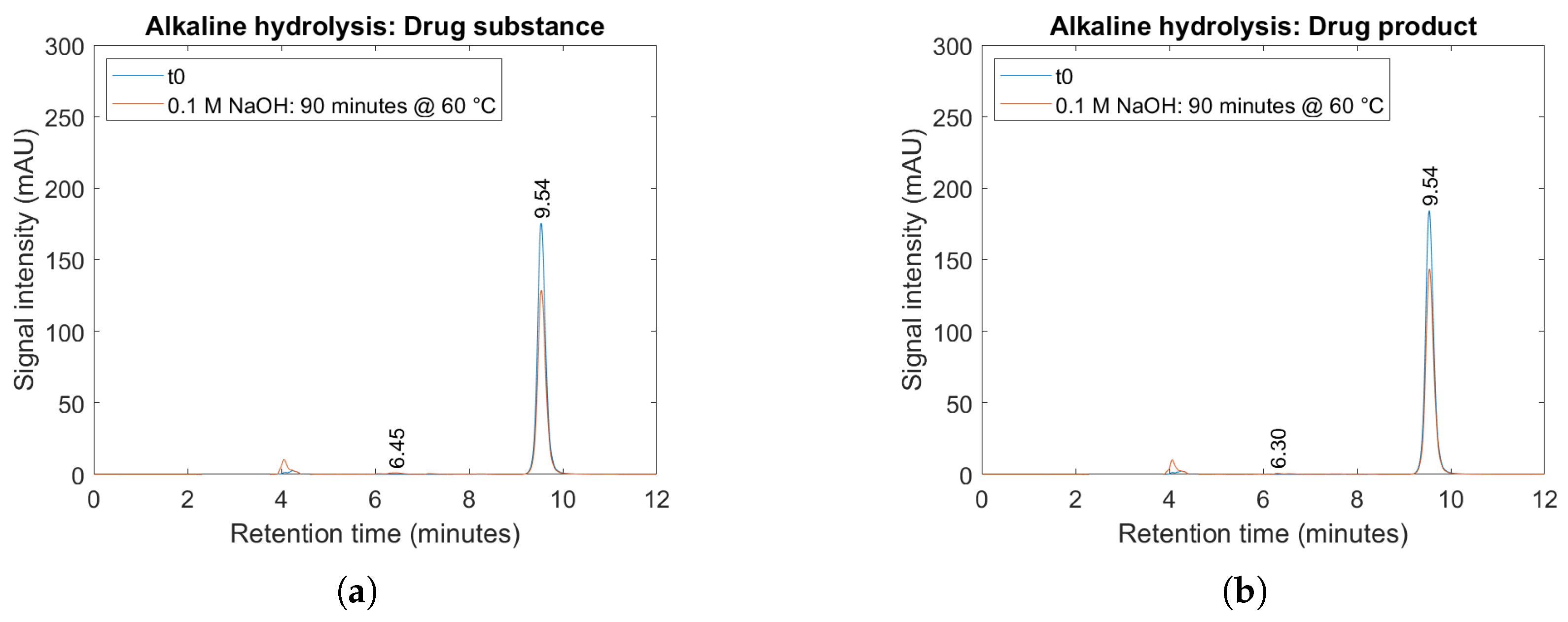
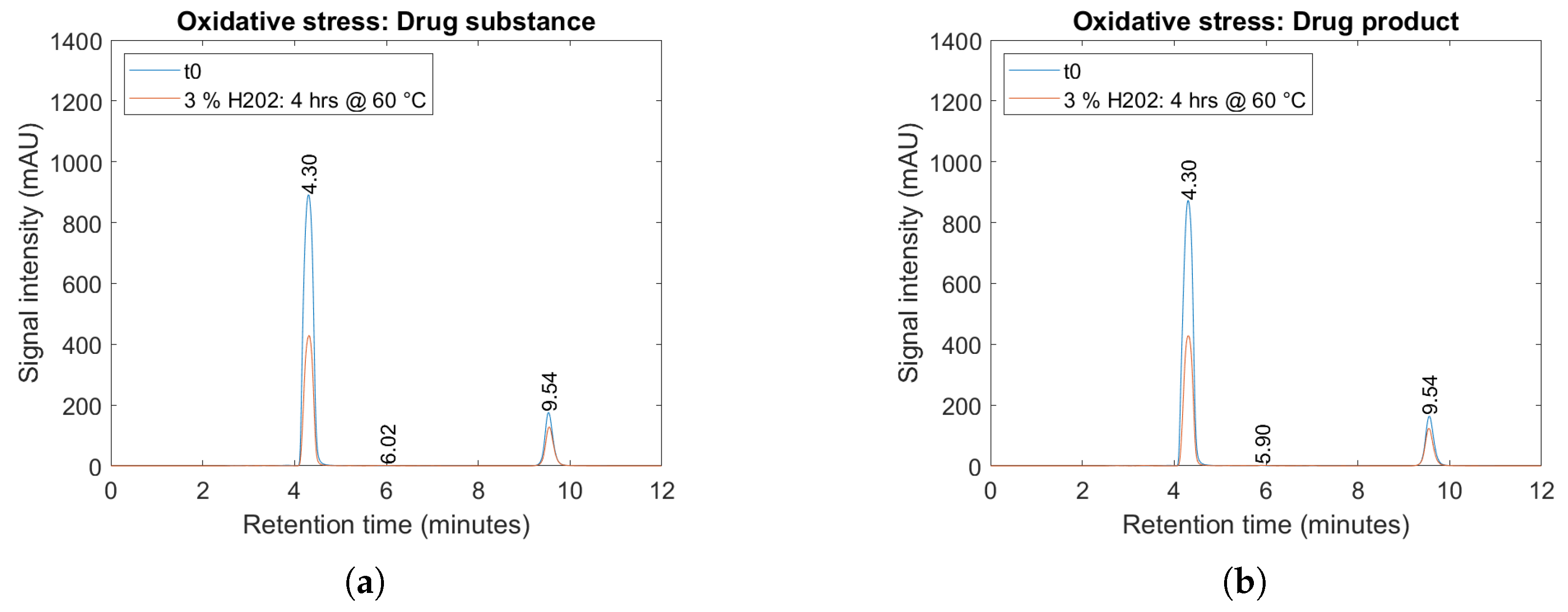
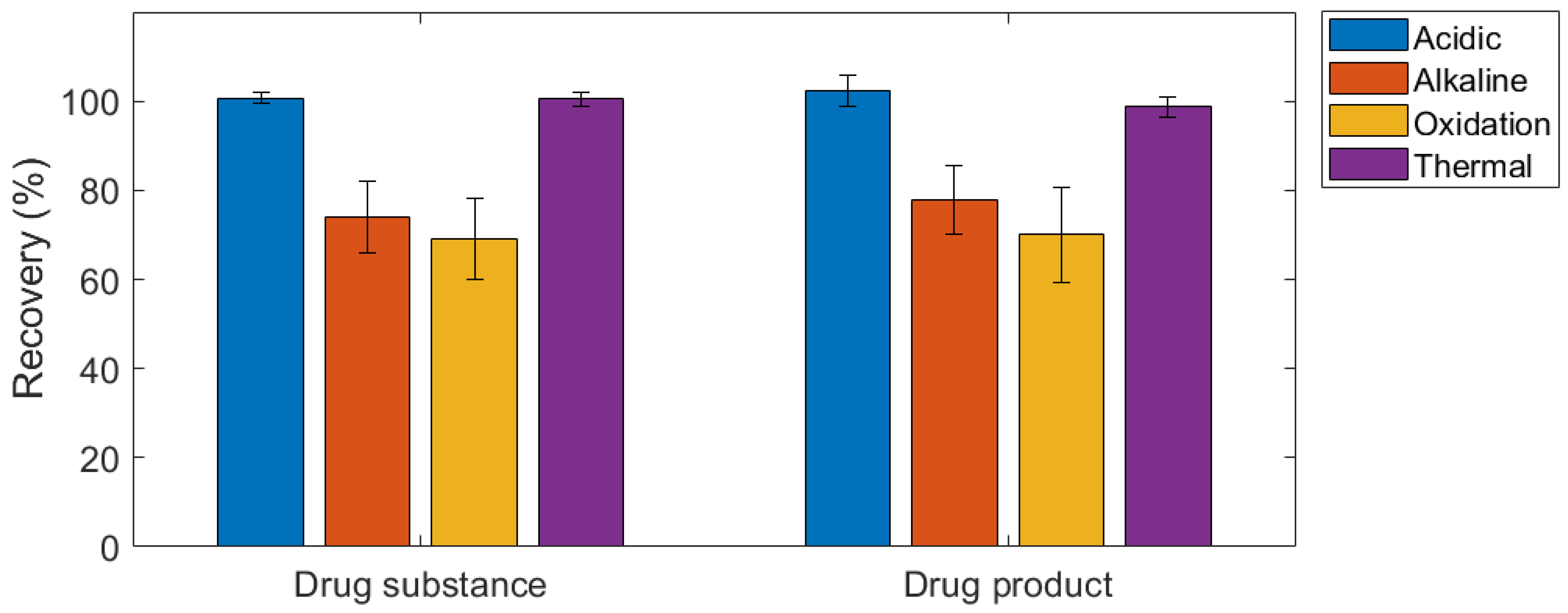
2.4. Forced Degradation Studies
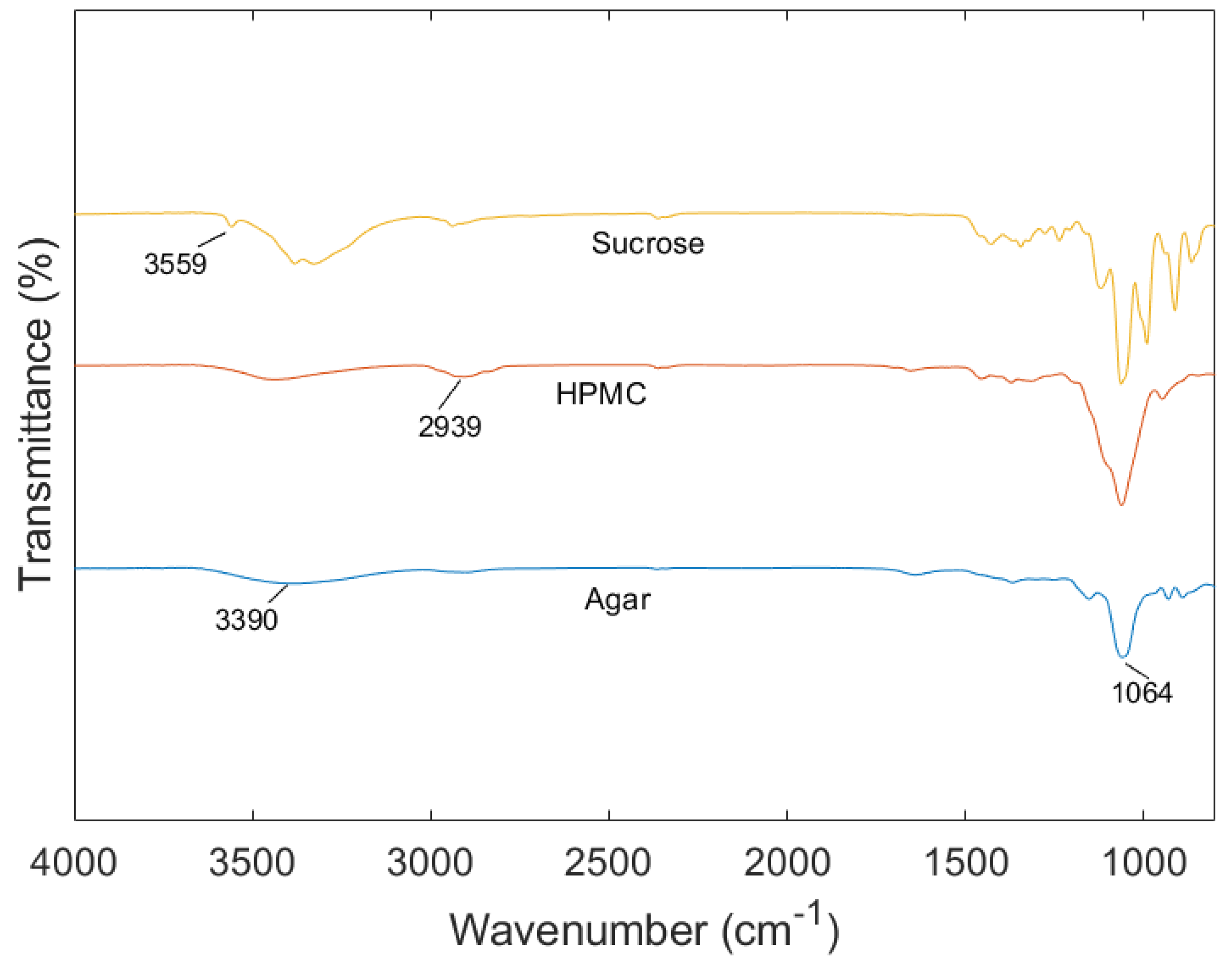
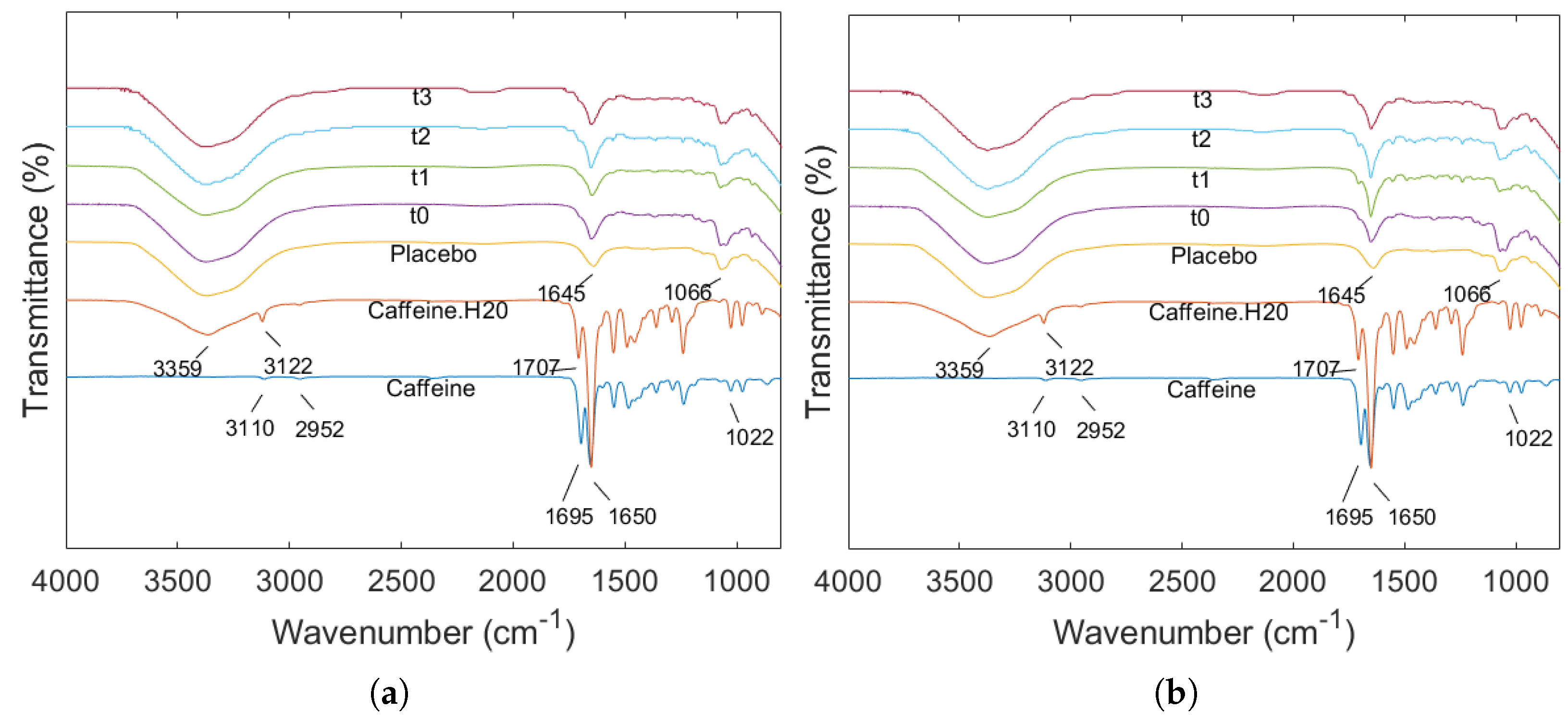
2.5. ATR-FTIR
3. Conclusions
4. Materials & Methods
4.1. Materials
4.2. Preparation of Hydrogels
4.3. Printing Process
4.4. Method of Assay
4.5. Evaluation of Quality Control Attributes
4.5.1. Calculation of Volumes and Dimensional Measurement of Printed Objects
4.5.2. Uniformity of Mass
4.5.3. Uniformity of Dosage Units
4.5.4. Disintegration Time
4.5.5. Dissolution Study
4.6. Identification of Variability
4.7. Forced Degradation Study
4.8. ATR-FTIR
4.9. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A



References
- Willson, C. The Clinical Toxicology of Caffeine: A Review and Case Study. Toxicol. Rep. 2018, 5, 1140–1152. [Google Scholar] [CrossRef]
- Nonappa, N.; Kolehmainen, E. Caffeine as a Gelator. Gels 2016, 2, 9. [Google Scholar] [CrossRef]
- Bright, M.; Raman, V.; Laupland, K.B. Use of Therapeutic Caffeine in Acute Care Postoperative and Critical Care Settings: A Scoping Review. BMC Anesthesiol. 2021, 21, 100. [Google Scholar] [CrossRef]
- Dobson, N.R.; Hunt, C.E. Pharmacology Review: Caffeine Use in Neonates: Indications, Pharmacokinetics, Clinical Effects, Outcomes. NeoReviews 2013, 14, e540–e550. [Google Scholar] [CrossRef]
- Abdel-Hady, H.; Nasef, N.; Shabaan, A.E.; Nour, I. Caffeine Therapy in Preterm Infants. World J. Clin. Pediatr. 2015, 4, 81–93. [Google Scholar] [CrossRef]
- Shrestha, B.; Jawa, G. Caffeine Citrate—Is It a Silver Bullet in Neonatology? Pediatr. Neonatol. 2017, 58, 391–397. [Google Scholar] [CrossRef]
- McCloskey, A.P.; Bracken, L.; Vasey, N.; Ehtezazi, T. 3D Printing—An Alternative Strategy for Pediatric Medicines. Expert Rev. Clin. Pharmacol. 2023, 16, 613–616. [Google Scholar] [CrossRef]
- Ullah, M.; Wahab, A.; Khan, S.U.; Naeem, M.; ur Rehman, K.; Ali, H.; Ullah, A.; Khan, A.; Khan, N.R.; Rizg, W.Y.; et al. 3D Printing Technology: A New Approach for the Fabrication of Personalized and Customized Pharmaceuticals. Eur. Polym. J. 2023, 195, 112240. [Google Scholar] [CrossRef]
- Lafeber, I.; Ruijgrok, E.J.; Guchelaar, H.J.; Schimmel, K.J.M. 3D Printing of Pediatric Medication: The End of Bad Tasting Oral Liquids?—A Scoping Review. Pharmaceutics 2022, 14, 416. [Google Scholar] [CrossRef]
- Xue, A.; Li, W.; Tian, W.; Zheng, M.; Shen, L.; Hong, Y. A Bibliometric Analysis of 3D Printing in Personalized Medicine Research from 2012 to 2022. Pharmaceuticals 2023, 16, 1521. [Google Scholar] [CrossRef]
- Funk, N.L.; Leão, J.; de Oliveira, T.V.; Beck, R.C.R. Semi-Solid Extrusion (SSE) in Pharmaceuticals. In Additive Manufacturing in Pharmaceuticals; Banerjee, S., Ed.; Springer Nature: Singapore, 2023; pp. 171–200. [Google Scholar] [CrossRef]
- Zhang, B.; Belton, P.; Yi Teoh, X.; Gleadall, A.; Bibb, R.; Qi, S. An Investigation into the Effects of Ink Formulations of Semi-Solid Extrusion 3D Printing on the Performance of Printed Solid Dosage Forms. J. Mater. Chem. B 2024, 12, 131–144. [Google Scholar] [CrossRef]
- Wang, X.m.; Li, B.; Zhang, T.; Li, X.y. Performance of Nanofiltration Membrane in Rejecting Trace Organic Compounds: Experiment and Model Prediction. Desalination 2015, 370, 7–16. [Google Scholar] [CrossRef]
- Kulkarni, U.D.; Mahalingam, R.; Li, X.; Pather, I.; Jasti, B. Effect of Experimental Temperature on the Permeation of Model Diffusants Across Porcine Buccal Mucosa. AAPS PharmSciTech 2011, 12, 579–586. [Google Scholar] [CrossRef]
- Johannesson, J.; Wu, M.; Johansson, M.; Bergström, C.A.S. Quality Attributes for Printable Emulsion Gels and 3D-printed Tablets: Towards Production of Personalized Dosage Forms. Int. J. Pharm. 2023, 646, 123413. [Google Scholar] [CrossRef]
- Sjöholm, E.; Mathiyalagan, R.; Lindfors, L.; Wang, X.; Ojala, S.; Sandler, N. Semi-Solid Extrusion 3D Printing of Tailored ChewTs for Veterinary Use—A Focus on Spectrophotometric Quantification of Gabapentin. Eur. J. Pharm. Sci. 2022, 174, 106190. [Google Scholar] [CrossRef]
- Zheng, Z.; Lv, J.; Yang, W.; Pi, X.; Lin, W.; Lin, Z.; Zhang, W.; Pang, J.; Zeng, Y.; Lv, Z.; et al. Preparation and Application of Subdivided Tablets Using 3D Printing for Precise Hospital Dispensing. Eur. J. Pharm. Sci. 2020, 149, 105293. [Google Scholar] [CrossRef]
- Roche, A.; Sanchez-Ballester, N.M.; Aubert, A.; Rossi, J.C.; Begu, S.; Soulairol, I. Preliminary Study on the Development of Caffeine Oral Solid Form 3D Printed by Semi-Solid Extrusion for Application in Neonates. AAPS PharmSciTech 2023, 24. [Google Scholar] [CrossRef]
- O’Brien, F.; Clapham, D.; Krysiak, K.; Batchelor, H.; Field, P.; Caivano, G.; Pertile, M.; Nunn, A.; Tuleu, C. Making Medicines Baby Size: The Challenges in Bridging the Formulation Gap in Neonatal Medicine. Int. J. Mol. Sci. 2019, 20, 2688. [Google Scholar] [CrossRef]
- Krueger, L.; Cao, Y.; Zheng, Z.; Ward, J.; Miles, J.A.; Popat, A. 3D Printing Tablets for High-Precision Dose Titration of Caffeine. Int. J. Pharm. 2023, 642, 123132. [Google Scholar] [CrossRef]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef]
- Kaliampakou, C.; Lagopati, N.; Pavlatou, E.A.; Charitidis, C.A. Alginate–Gelatin Hydrogel Scaffolds; An Optimization of Post-Printing Treatment for Enhanced Degradation and Swelling Behavior. Gels 2023, 9, 857. [Google Scholar] [CrossRef]
- Dabbaghi, A.; Ramazani, A.; Farshchi, N.; Rezaei, A.; Bodaghi, A.; Rezayati, S. Synthesis, Physical and Mechanical Properties of Amphiphilic Hydrogels Based on Polycaprolactone and Polyethylene Glycol for Bioapplications: A Review. J. Ind. Eng. Chem. 2021, 101, 307–323. [Google Scholar] [CrossRef]
- Saqib, M.N.; Khaled, B.M.; Liu, F.; Zhong, F. Hydrogel Beads for Designing Future Foods: Structures, Mechanisms, Applications, and Challenges. Food Hydrocoll. Health 2022, 2, 100073. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhang, X.; Rahman, S.E.; Su, S.; Wei, J.; Ning, F.; Hu, Z.; Martínez-Zaguilán, R.; Sennoune, S.R.; et al. 3D Printed Agar/ Calcium Alginate Hydrogels with High Shape Fidelity and Tailorable Mechanical Properties. Polymer 2021, 214, 123238. [Google Scholar] [CrossRef]
- Ramli, N.A.; Adam, F.; Mohd Amin, K.N.; Nor, A.M.; Ries, M.E. Evaluation of Mechanical and Thermal Properties of Carrageenan/Hydroxypropyl Methyl Cellulose Hard Capsule. Can. J. Chem. Eng. 2023, 101, 1219–1234. [Google Scholar] [CrossRef]
- Kittipongpatana, O.S.; Trisopon, K.; Wattanaarsakit, P.; Kittipongpatana, N. Fabrication and Characterization of Orodispersible Composite Film from Hydroxypropylmethyl Cellulose-Crosslinked Carboxymethyl Rice Starch. Membranes 2022, 12, 594. [Google Scholar] [CrossRef]
- Cavelier, M.; Gondé, H.; Costa, D.; Lamoureux, F.; Pereira, T.; Buchbinder, N.; Varin, R.; Hervouët, C. Development of an Oral Liquid Formulation of Nicardipine Hydrochloride Compounded with Simple Excipients for the Treatment of Pediatric Hypertension. Pharmaceutics 2023, 15, 446. [Google Scholar] [CrossRef]
- Su, H.; Yang, S.; Chen, S.; Chen, X.; Guo, M.; Zhu, L.; Xu, W.; Liu, H. What Happens in the Gut during the Formation of Neonatal Jaundice—Underhand Manipulation of Gut Microbiota? Int. J. Mol. Sci. 2024, 25, 8582. [Google Scholar] [CrossRef]
- Radwan, I.M.; Sakr, M.M.A.; Mohamed, S.A. Is Oral Agar Combined with Phototherapy Superior than Phototherapy in Treatment of Neonatal Indirect Hyperbilirubinemia. Sci. J. Med Sch. 2023, 2, 25–28. [Google Scholar] [CrossRef]
- Blessy, M.; Patel, R.D.; Prajapati, P.N.; Agrawal, Y.K. Development of Forced Degradation and Stability Indicating Studies of Drugs—A Review. J. Pharm. Anal. 2014, 4, 159–165. [Google Scholar] [CrossRef]
- Shah, U.; Kavad, M.; Raval, M. Development and Validation of Stability-indicating RP-HPLC Method for Estimation of Pamabrom in Tablets. Indian J. Pharm. Sci. 2014, 76, 198–202. [Google Scholar]
- Sonawane, S.; Jadhav, S.; Rahade, P.; Chhajed, S.; Kshirsagar, S. Development and Validation of Stability-Indicating Method for Estimation of Chlorthalidone in Bulk and Tablets with the Use of Experimental Design in Forced Degradation Experiments. Scientifica 2016, 2016, 4286482. [Google Scholar] [CrossRef]
- Baertschi, S.W. Forced Degradation and Its Relation to Real Time Drug Product Stability. In Pharmaceutical Stability Testing to Support Global Markets; Huynh-Ba, K., Ed.; Springer: New York, NY, USA, 2010; pp. 107–116. [Google Scholar] [CrossRef]
- Bom, S.; Ribeiro, R.; Ribeiro, H.M.; Santos, C.; Marto, J. On the Progress of Hydrogel-Based 3D Printing: Correlating Rheological Properties with Printing Behaviour. Int. J. Pharm. 2022, 615, 121506. [Google Scholar] [CrossRef]
- Sastri, T.K.; Gupta, V.N.; Chakraborty, S.; Madhusudhan, S.; Kumar, H.; Chand, P.; Jain, V.; Veeranna, B.; Gowda, D.V. Novel Gels: An Emerging Approach for Delivering of Therapeutic Molecules and Recent Trends. Gels 2022, 8, 316. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, A.; Puri, V.; Aggarwal, G.; Maman, P.; Huanbutta, K.; Nagpal, M.; Sangnim, T. Chitosan-Based Polymer Blends for Drug Delivery Systems. Polymers 2023, 15, 2028. [Google Scholar] [CrossRef]
- Jia, D.; Muthukumar, M. Theory of Charged Gels: Swelling, Elasticity, and Dynamics. Gels 2021, 7, 49. [Google Scholar] [CrossRef]
- Es Sayed, J.; Khoonkari, M.; Oggioni, M.; Perrin, P.; Sanson, N.; Kamperman, M.; Włodarczyk-Biegun, M.K. Multi-Responsive Jammed Micro-Gels Ink: Toward Control over the Resolution and the Stability of 3D Printed Scaffolds. Adv. Funct. Mater. 2022, 32, 2207816. [Google Scholar] [CrossRef]
- Laffleur, F.; Keckeis, V. Advances in Drug Delivery Systems: Work in Progress Still Needed? Int. J. Pharm. 2020, 590, 119912. [Google Scholar] [CrossRef]
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of Geometry on Drug Release from 3D Printed Tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef]
- Khizer, Z.; Akram, M.R.; Sarfraz, R.M.; Nirwan, J.S.; Farhaj, S.; Yousaf, M.; Hussain, T.; Lou, S.; Timmins, P.; Conway, B.R.; et al. Plasticiser-Free 3D Printed Hydrophilic Matrices: Quantitative 3D Surface Texture, Mechanical, Swelling, Erosion, Drug Release and Pharmacokinetic Studies. Polymers 2019, 11, 1095. [Google Scholar] [CrossRef]
- Domsta, V.; Hänsch, C.; Lenz, S.; Gao, Z.; Matin-Mann, F.; Scheper, V.; Lenarz, T.; Seidlitz, A. The Influence of Shape Parameters on Unidirectional Drug Release from 3D Printed Implants and Prediction of Release from Implants with Individualized Shapes. Pharmaceutics 2023, 15, 1276. [Google Scholar] [CrossRef]
- Funk, N.L.; Fantaus, S.; Beck, R.C.R. Immediate Release 3D Printed Oral Dosage Forms: How Different Polymers Have Been Explored to Reach Suitable Drug Release Behaviour. Int. J. Pharm. 2022, 625, 122066. [Google Scholar] [CrossRef]
- Fanous, M.; Gold, S.; Muller, S.; Hirsch, S.; Ogorka, J.; Imanidis, G. Simplification of Fused Deposition Modeling 3D-printing Paradigm: Feasibility of 1-Step Direct Powder Printing for Immediate Release Dosage Form Production. Int. J. Pharm. 2020, 578, 119124. [Google Scholar] [CrossRef]
- Onsawai, P.; Phetpan, K.; Khurnpoon, L.; Sirisomboon, P. Evaluation of Physiological Properties and Texture Traits of Durian Pulp Using Near-Infrared Spectra of the Pulp and Intact Fruit. Measurement 2021, 174, 108684. [Google Scholar] [CrossRef]
- Malik, A.N.; Khan, S.A.; Lazoglu, I. A Novel Hybrid Frost Detection and Defrosting System for Domestic Refrigerators. Int. J. Refrig. 2020, 117, 256–268. [Google Scholar] [CrossRef]
- Metze, F.K.; Sant, S.; Meng, Z.; Klok, H.A.; Kaur, K. Swelling-Activated, Soft Mechanochemistry in Polymer Materials. Langmuir 2023, 39, 3546–3557. [Google Scholar] [CrossRef]
- Krakovský, I.; Hanyková, L.; Štastná, J. Phase Transition in Polymer Hydrogels Investigated by Swelling, DSC, FTIR and NMR. J. Therm. Anal. Calorim. 2024, 422, 1–18. [Google Scholar] [CrossRef]
- Tillinghast, G.; Sánchez-Rivera, K.L.; Huber, G.W.; Winter, H.H.; Rothstein, J.P. Shear and Extensional Rheology of Polyethylenes Recycled Using a Solvent Dissolution Process. Rheol. Acta 2024, 63, 345–360. [Google Scholar] [CrossRef]
- Li, S.; Zhang, M.; Vogt, B.D. Delayed Swelling and Dissolution of Hydrophobically Associated Hydrogel Coatings by Dilute Aqueous Surfactants. ACS Appl. Polym. Mater. 2022, 4, 250–259. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Javadzadeh, Y. The Effect of Storage Conditions on the Physical Stability of Tablets. Pharm. Technol. Eur. 2007, 19. [Google Scholar]
- Markl, D.; Maclean, N.; Mann, J.; Williams, H.; Abbott, A.; Mead, H.; Khadra, I. Tablet Disintegration Performance: Effect of Compression Pressure and Storage Conditions on Surface Liquid Absorption and Swelling Kinetics. Int. J. Pharm. 2021, 601, 120382. [Google Scholar] [CrossRef]
- Lenz, J.; Finke, J.H.; Bunjes, H.; Kwade, A.; Juhnke, M. Tablet Formulation Development Focusing on the Functional Behaviour of Water Uptake and Swelling. Int. J. Pharm. X 2021, 3, 100103. [Google Scholar] [CrossRef]
- Azevedo, J.V.C.; Hausnerova, B.; Möginger, B.; Sopik, T. Effect of Chain Extending Cross-Linkers on the Disintegration Behavior of Composted PBAT/PLA Blown Films. Int. J. Mol. Sci. 2023, 24, 4525. [Google Scholar] [CrossRef]
- Zheng, A.Y.; Heng, P.W.S.; Chan, L.W. Tablet Disintegratability: Sensitivity of Superdisintegrants to Temperature and Compaction Pressure. Pharmaceutics 2022, 14, 2725. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Lakerveld, R. Process Intensification for Pharmaceutical Crystallization. Chem. Eng. Process. Process. Intensif. 2018, 127, 111–126. [Google Scholar] [CrossRef]
- Andreeta, M.R.B. Crystallization: Science and Technology; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Javadzadeh, Y.; Hamedeyazdan, S.; Asnaashari, S. Recrystallization of Drugs: Significance on Pharmaceutical Processing. In Recrystallization; IntechOpen: Rijeka, Croatia, 2012; pp. 425–446. [Google Scholar] [CrossRef]
- Aranda, J.V.; Beharry, K.D. Pharmacokinetics, Pharmacodynamics and Metabolism of Caffeine in Newborns. Semin. Fetal Neonatal Med. 2020, 25, 101183. [Google Scholar] [CrossRef]
- Sugino, M.; Kuboi, T.; Noguchi, Y.; Nishioka, K.; Tadatomo, Y.; Kawaguchi, N.; Sadamura, T.; Nakano, A.; Konishi, Y.; Koyano, K.; et al. Serum Caffeine Concentrations in Preterm Infants: A Retrospective Study. Sci. Rep. 2023, 13, 10305. [Google Scholar] [CrossRef]
- Long, J.Y.; Guo, H.L.; He, X.; Hu, Y.H.; Xia, Y.; Cheng, R.; Ding, X.S.; Chen, F.; Xu, J. Caffeine for the Pharmacological Treatment of Apnea of Prematurity in the NICU: Dose Selection Conundrum, Therapeutic Drug Monitoring and Genetic Factors. Front. Pharmacol. 2021, 12, 681842. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Sankari, G.; Ponnusamy, S. Vibrational Spectral Investigation on Xanthine and Its Derivatives—Theophylline, Caffeine and Theobromine. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2005, 61, 117–127. [Google Scholar] [CrossRef]
- González-González, J.; Zuñiga, O.; Hernández-Galindo, M. Hydrated Solid Forms of Theophylline and Caffeine Obtained by Mechanochemistry. IOSR J. Pharm. 2017, 7, 28–30. [Google Scholar] [CrossRef]
- Švecová, M.; Palounek, D.; Volochanskyi, O.; Prokopec, V. Vibrational Spectroscopic Study of Selected Alkaloids with Therapeutic Effects. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2020, 229, 117969. [Google Scholar] [CrossRef]
- Paradkar, M.; Irudayaraj, J. A Rapid FTIR Spectroscopic Method for Estimation of Caffeine in Soft Drinks and Total Methylxanthines in Tea and Coffee. J. Food Sci. 2002, 67, 2507–2511. [Google Scholar] [CrossRef]
- Kwaśniewska-Sip, P.; Woźniak, M.; Jankowski, W.; Ratajczak, I.; Cofta, G. Chemical Changes of Wood Treated with Caffeine. Materials 2021, 14, 497. [Google Scholar] [CrossRef]
- Świderski, G.; Kalinowska, M.; Gołębiewska, E.; Świsłocka, R.; Lewandowski, W.; Kowalczyk, N.; Naumowicz, M.; Cudowski, A.; Pietryczuk, A.; Nalewajko-Sieliwoniuk, E.; et al. Structures, Antioxidant Properties, and Antimicrobial Properties of Eu(III), Gd(III), and Dy(III) Caffeinates and p-Coumarates. Molecules 2023, 28, 6506. [Google Scholar] [CrossRef]
- Wang, N.; Sun, H.; Dong, J.; Ouyang, D. PharmDE: A New Expert System for Drug-Excipient Compatibility Evaluation. Int. J. Pharm. 2021, 607, 120962. [Google Scholar] [CrossRef]
- Omari, D.M.; Akkam, Y.; Sallam, A. Drug-Excipient Interactions: An Overview on Mechanisms and Effects on Drug Stability and Bioavailability. Ann. Rom. Soc. Cell Biol. 2021, 25, 8402–8429. [Google Scholar]
- Aina, M.; Baillon, F.; Sescousse, R.; Sanchez-ballester, N.M.; Begu, S.; Soulairol, I.; Sauceau, M. Evaluation of the Printability of Agar and Hydroxypropyl Methylcellulose Gels as Gummy Formulations: Insights from Rheological Properties. Int. J. Pharm. 2024, 654, 123937. [Google Scholar] [CrossRef]
- Chew, S.W.T.; Shah, A.H.; Zheng, M.; Chang, H.; Wiraja, C.; Steele, T.W.J.; Xu, C. A Self-Adhesive Microneedle Patch with Drug Loading Capability through Swelling Effect. Bioeng. Transl. Med. 2020, 5, e10157. [Google Scholar] [CrossRef]
- Zahra, Q.; Minhas, M.U.; Khan, S.; Wu, P.C.; Suhail, M.; Iqbal, R.; Bashir, M. Fabrication of Polyethylene Glycol Hydrogels with Enhanced Swelling; Loading Capacity and Release Kinetics. Polym. Bull. 2022, 79, 5389–5415. [Google Scholar] [CrossRef]
- Ye, C.; Miao, C.; Yu, L.; Dong, Z.; Zhang, J.; Mao, Y.; Lu, X.; Lyu, Q. Factors Affecting the Efficacy and Safety of Aminophylline in Treatment of Apnea of Prematurity in Neonatal Intensive Care Unit. Pediatr. Neonatol. 2019, 60, 43–49. [Google Scholar] [CrossRef]
- Ahmad, W.; Hassan, Y.A.; Ahmad, A.; Suroor, M.; Sarafroz, M.; Alam, P.; Wahab, S.; Salam, S. A Simple Stability-Indicating UPLC Method for the Concurrent Assessment of Paracetamol and Caffeine in Pharmaceutical Formulations. Separations 2023, 10, 50. [Google Scholar] [CrossRef]
- Billowria, K.; Sandhu, N.K. Degradation Pathway of Caffeine in Bulk Drug and Pharmaceutical Dosage Form. Int. J. Creat. Res. Thoughts (IJCRT) 2023, 11, 440–475. [Google Scholar]
- Xie, F.; Ji, S.; Cheng, Z. In Vitro Dissolution Similarity Factor (F2) and in Vivo Bioequivalence Criteria, How and When Do They Match? Using a BCS Class II Drug as a Simulation Example. Eur. J. Pharm. Sci. 2015, 66, 163–172. [Google Scholar] [CrossRef]
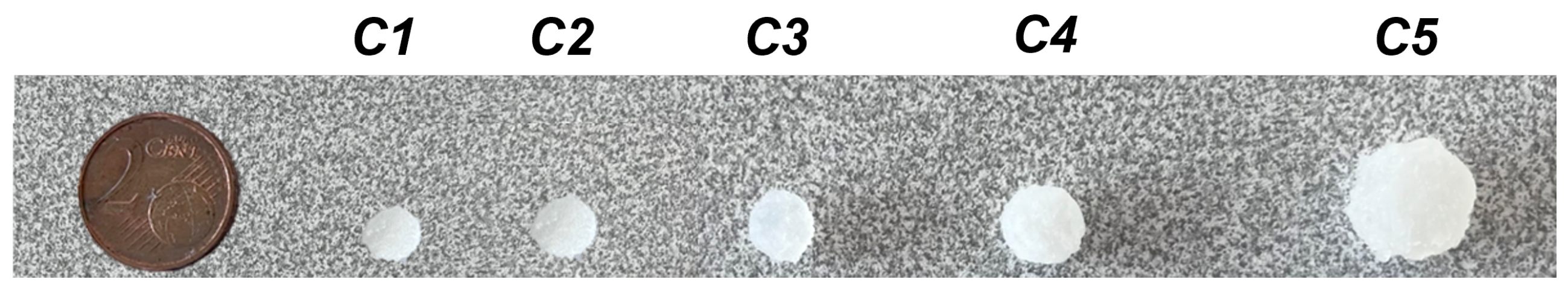


| Notation | Model | Printed Object | ||||
|---|---|---|---|---|---|---|
| Average | RSD | |||||
| Diameter (mm) | Height (mm) | Diameter (mm) | Height (mm) | Diameter (%) | Height (%) | |
| C1 | 4 | 2.4 | 5.9 | 4.0 | 1.1 | 3.0 |
| C2 | 5 | 2.4 | 8.5 | 4.7 | 2.5 | 4.1 |
| C3 | 6.9 | 3.2 | 11.0 | 5.8 | 1.9 | 1.7 |
| C4 | 7 | 3.6 | 11.5 | 6.0 | 1.5 | 3.2 |
| C5 | 8 | 5.2 | 14.1 | 8.2 | 2.4 | 4.0 |
| Notation | Mass | Disintegration Time | Drug Content | ||||
|---|---|---|---|---|---|---|---|
| SA/V (mm−1) | Average (g) | RSD (%) | Average (s) | SD (s) | Label Claim (mg of Caffeine) | Acceptance Value (AV) | |
| C1 | 1.18 | 0.125 | 2.4 | 65 | 11 | 2.8 | 0.1 |
| C2 | 0.89 | 0.241 | 2.5 | 67 | 4 | 4.7 | 0.3 |
| C3 | 0.71 | 0.563 | 1.9 | 82 | 3 | 11.2 | 0.7 |
| C4 | 0.68 | 0.704 | 2.2 | 83 | 6 | 13.6 | 0.6 |
| C5 | 0.53 | 1.219 | 2.0 | 345 | 9 | 23.8 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aina, M.; Baillon, F.; Sescousse, R.; Sanchez-Ballester, N.M.; Begu, S.; Soulairol, I.; Sauceau, M. Development of Personalised Immediate-Release Gel-Based Formulations Using Semi-Solid Extrusion. Gels 2024, 10, 665. https://doi.org/10.3390/gels10100665
Aina M, Baillon F, Sescousse R, Sanchez-Ballester NM, Begu S, Soulairol I, Sauceau M. Development of Personalised Immediate-Release Gel-Based Formulations Using Semi-Solid Extrusion. Gels. 2024; 10(10):665. https://doi.org/10.3390/gels10100665
Chicago/Turabian StyleAina, Morenikeji, Fabien Baillon, Romain Sescousse, Noelia M. Sanchez-Ballester, Sylvie Begu, Ian Soulairol, and Martial Sauceau. 2024. "Development of Personalised Immediate-Release Gel-Based Formulations Using Semi-Solid Extrusion" Gels 10, no. 10: 665. https://doi.org/10.3390/gels10100665
APA StyleAina, M., Baillon, F., Sescousse, R., Sanchez-Ballester, N. M., Begu, S., Soulairol, I., & Sauceau, M. (2024). Development of Personalised Immediate-Release Gel-Based Formulations Using Semi-Solid Extrusion. Gels, 10(10), 665. https://doi.org/10.3390/gels10100665







