Comprehensive Insights and Advancements in Gel Catalysts for Electrochemical Energy Conversion
Abstract
1. Introduction
2. Gel Material Synthesis Process
2.1. Radical Polymerization
2.2. Hydrothermal/Solvothermal Method
2.3. Sol–Gel Method
2.4. Ligand Substitution
3. Gel Classifications
3.1. Hydrogels
3.2. Aerogels
4. Gel as an Advanced Catalyst: Challenges and Solutions
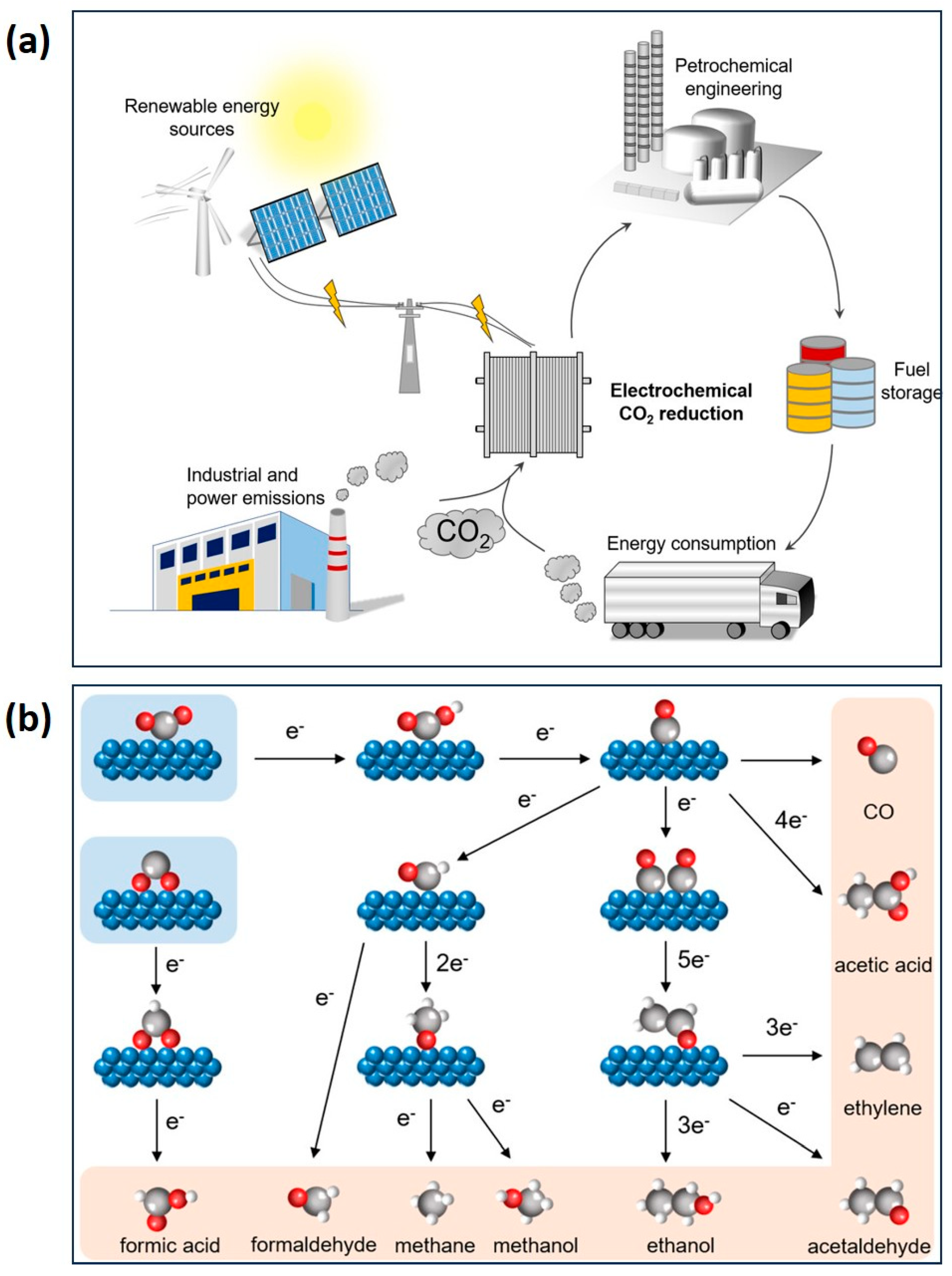
4.1. Gel Electrocatalysts for CO2RR
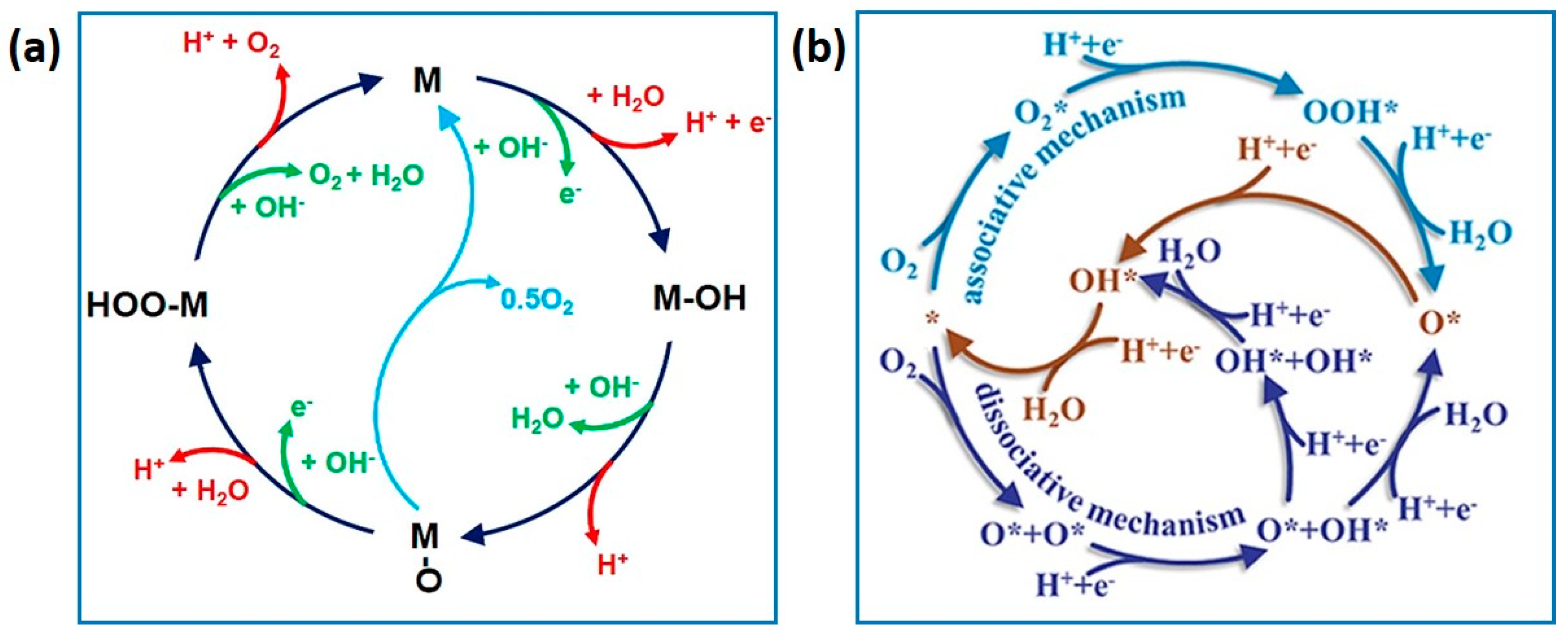
4.2. Gel Electrocatalysts for the ORR and OER
4.3. Gel Electrocatalysts for H2 Production
5. Summary and Outlook for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, T.-G.; Kang, H.-J.; Bari, G.A.R.; Park, J.-W.; Seo, H.-W.; An, B.-H.; Hwang, H.J.; Jun, Y.-S. Macroscopic graphitic carbon nitride monolith for efficient hydrogen production by photocatalytic reforming of glucose under sunlight. Chemosphere 2021, 283, 131174. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Lee, T.-G.; Bari, G.A.K.M.R.; Seo, H.-W.; Park, J.-W.; Hwang, H.J.; An, B.-H.; Suzuki, N.; Fujishima, A.; Kim, J.-H.; et al. Sulfuric acid treated G-CN as a precursor to generate high-efficient G-CN for hydrogen evolution from water under visible light irradiation. Catalysts 2021, 11, 37. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, L.; Yang, Z.; Li, X.; Wen, Z.; Chen, L. Porous Organic Polymer Gel Derived Electrocatalysts for Efficient Oxygen Reduction. ChemElectroChem 2019, 6, 485–492. [Google Scholar] [CrossRef]
- Pei, Z.; Tan, H.; Gu, J.; Lu, L.; Zeng, X.; Zhang, T.; Wang, C.; Ding, L.; Cullen, P.J.; Chen, Z.; et al. A polymeric hydrogel electrocatalyst for direct water oxidation. Nat. Commun. 2023, 14, 818. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Li, J.; Liu, J.-P.; Wang, H.; Peng, Y.; Liu, C.-S. Conferring supramolecular guanosine gel nanofiber with ZIF-67 for high-performance oxygen reduction catalysis in rechargeable zinc–air batteries. Appl. Catal. B Environ. 2021, 286, 119888. [Google Scholar] [CrossRef]
- Feng, X.; Xiao, Y.; Huang, H.; Wang, Q.; Wu, J.; Ke, Z.; Tong, Y.; Zhang, J. Phytic Acid-Based FeCo Bimetallic Metal-Organic Gels for Electrocatalytic Oxygen Evolution Reaction. Chem. Asian J. 2021, 16, 3213–3220. [Google Scholar] [CrossRef] [PubMed]
- Jena, B.K.; Raj, C.R. Electrocatalytic applications of nanosized pt particles self-assembled on sol-gel-derived three-dimensional silicate network. J. Phys. Chem. C 2008, 112, 3496–3502. [Google Scholar] [CrossRef]
- Yang, H.; Hu, H.; Xia, C.; You, F.; Yao, J.; Jiang, X.; Xia, B.Y. Progress on nanostructured gel catalysts for oxygen electrocatalysis. Nano Res. 2022, 15, 10343–10356. [Google Scholar] [CrossRef]
- Lu, J.; Hao, W.; Wu, X.; Shen, X.; Cui, S.; Shi, W. Electronic Modulation of the 3D Architectured Ni/Fe Oxyhydroxide Anchored N-Doped Carbon Aerogel with Much Improved OER Activity. Gels 2023, 9, 190. [Google Scholar] [CrossRef]
- Wang, H.; Chen, B.; Liu, D. Metal–Organic Frameworks and Metal–Organic Gels for Oxygen Electrocatalysis: Structural and Compositional Considerations. Adv. Mater. 2021, 33, 2008023. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, Y.; Zou, X.; Hu, B.; Qiang, Y.; Yu, D.; Yin, W.; Chen, C. Hydrothermal Synthesis of a New Kind of N-Doped Graphene Gel-like Hybrid As an Enhanced ORR Electrocatalyst. ACS Appl. Mater. Interfaces 2018, 10, 10842–10850. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Eychmüller, A. Promoting Electrocatalysis upon Aerogels. Adv. Mater. 2019, 31, e1804881. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, W.; Xue, Q.; Wang, C.; Liu, K. Solvent dispersion triggered the formation of NiFe-gel as an efficient electrocatalyst for enhancing the oxygen evolution reaction. Chem. Commun. 2020, 56, 7781–7784. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wu, P.; Qian, Y.; Yu, G. Gel-Derived Amorphous Bismuth–Nickel Alloy Promotes Electrocatalytic Nitrogen Fixation via Optimizing Nitrogen Adsorption and Activation. Angew. Chem. Int. Ed. 2020, 60, 4275–4281. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhang, A.; Wu, P.; Yu, G. Inorganic Cyanogels and Their Derivatives for Electrochemical Energy Storage and Conversion. ACS Mater. Lett. 2019, 1, 158–170. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, W.; Zhang, R.; Zeng, J.; Du, Y.; Qi, S.; Cong, C.; Ding, C.; Huang, X.; Wen, G.; et al. Mass Production of Large-Sized Nonlayered 2D Nanosheets Their Directed Synthesis by a Rapid ‘Gel-Blowing’ Strategy, and Applications in Li/Na Storage and Catalysis. Adv. Mater. 2018, 30, e1803569. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, Q. Controlled synthesis and energy applications of one-dimensional conducting polymer nanostructures: An overview. Adv. Energy Mater. 2012, 2, 179–218. [Google Scholar] [CrossRef]
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, B.C.K.; Shi, Y.; Cui, Y.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef]
- Shanmugam, S.; Xu, S.; Adnan, N.N.M.; Boyer, C. Heterogeneous Photocatalysis as a Means for Improving Recyclability of Organocatalyst in ‘living’ Radical Polymerization. Macromolecules 2018, 51, 779–790. [Google Scholar] [CrossRef]
- Cuthbert, J.; Balazs, A.C.; Kowalewski, T.; Matyjaszewski, K. STEM Gels by Controlled Radical Polymerization. Trends Chem. 2020, 2, 341–353. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Pan, L.; Ding, Y.; Zhao, Y.; Li, Y.; Shi, Y.; Yu, G. Dopant-Enabled Supramolecular Approach for Controlled Synthesis of Nanostructured Conductive Polymer Hydrogels. Nano Lett. 2015, 15, 7736–7741. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.; Hoffmann, A.; Janssen, L. Reducing the gel effect in free radical polymerization. Chem. Eng. Sci. 2001, 56, 911–915. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, G.-B.; Zhou, L.-L.; Li, W.-Y.; Dong, Y.-B. Nanoscale covalent organic frameworks as theranostic platforms for oncotherapy: Synthesis, functionalization, and applications. Nanoscale Adv. 2020, 2, 3656–3733. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Ploychompoo, S.; Chen, J.; Zhou, T.; Luo, H. Simultaneous Cr(VI) reduction and bisphenol A degradation by a 3D Z-scheme Bi2S3-BiVO4 graphene aerogel under visible light. Chem. Eng. J. 2020, 384, 123256. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Q.; Shi, G. Supercapacitors based on self-assembled graphene organogel. Phys. Chem. Chem. Phys. 2011, 13, 17249–17254. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Feinle, A.; Elsaesser, M.S.; Hüsing, N. Sol-gel synthesis of monolithic materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45, 3377–3399. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Kim, H. High-refractive-index and high-barrier-capable epoxy-phenoxy-based barrier film for organic electronics. Polym. Adv. Technol. 2020, 31, 1752–1764. [Google Scholar] [CrossRef]
- Bari, G.A.R.; Kim, H. High-barrier polymeric multilayer film with organic and interactive materials. Prog. Org. Coat. 2020, 147, 105814. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Poggetto, G.D.; Pasquali, M.; Dell’era, A.; Ciprioti, S.V. Influence of the heat treatment on the particles size and on the crystalline phase of TiO2 synthesized by the sol-gel method. Materials 2018, 11, 2364. [Google Scholar] [CrossRef] [PubMed]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol-gel’ chemistry as a technique for materials synthesis. Mater. Horizons 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Esposito, S. ‘Traditional’ sol-gel chemistry as a powerful tool for the preparation of supported metal and metal oxide catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Bari, G.A.R.; Park, S.; Parveen, A.S.; Lee, S.; Kim, H. High barrier performance of the multilayer film based on epoxy and montmorillonite. Prog. Org. Coat. 2019, 126, 1–7. [Google Scholar] [CrossRef]
- Fang, Z.; Wu, P.; Yu, K.; Li, Y.; Zhu, Y.; Ferreira, P.J.; Liu, Y.; Yu, G. Hybrid Organic-Inorganic Gel Electrocatalyst for Stable Acidic Water Oxidation. ACS Nano 2019, 13, 14368–14376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, X.; Zhang, C.; Yu, Z.; Zhou, Y.; Tang, Y.; Wu, P.; Guo, S. 3D Space-Confined Pyrolysis of Double-Network Aerogels Containing In–Fe Cyanogel and Polyaniline: A New Approach to Hierarchically Porous Carbon with Exclusive Fe–Nx Active Sites for Oxygen Reduction Catalysis. Small Methods 2017, 1, 1700167. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; García-Melchor, M.; Han, L.; Xu, J.; Liu, M.; Zheng, L.; et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 2016, 352, 333–337. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.; Wang, Z.; Xi, G.; Mao, R.; Ma, Y.; Wang, D.; Tang, M.; Xu, Z.; Luan, H. Ultrastretchable, Self-Healing Conductive Hydrogel-Based Triboelectric Nanogenerators for Human-Computer Interaction. ACS Appl. Mater. Interfaces 2023, 15, 5128–5138. [Google Scholar] [CrossRef]
- Wu, S.; Wang, B.; Chen, D.; Liu, X.; Wang, H.; Song, Z.; Yu, D.; Li, G.; Ge, S.; Liu, W. Highly sensitive and self-healing conductive hydrogels fabricated from cationic cellulose nanofiber-dispersed liquid metal for strain sensors. Sci. China Mater. 2023, 66, 1923–1933. [Google Scholar] [CrossRef]
- Wang, X.; Weng, L.; Zhang, X.; Guan, L.; Li, X. Constructing conductive and mechanical strength self-healing hydrogel for flexible sensor. J. Sci. Adv. Mater. Devices 2023, 8, 100563. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Hedenqvist, M.S.; Chen, C.; Cai, C.; Li, H.; Liu, H.; Fu, J. Multifunctional conductive hydrogels and their applications as smart wearable devices. J. Mater. Chem. B 2021, 9, 2561–2583. [Google Scholar] [CrossRef] [PubMed]
- Green, R.A.; Hassarati, R.T.; Goding, J.A.; Baek, S.; Lovell, N.H.; Martens, P.J.; Poole-Warren, L.A. Conductive Hydrogels: Mechanically Robust Hybrids for Use as Biomaterials. Macromol. Biosci. 2012, 12, 494–501. [Google Scholar] [CrossRef]
- Zheng, C.; Lu, K.; Lu, Y.; Zhu, S.; Yue, Y.; Xu, X.; Mei, C.; Xiao, H.; Wu, Q.; Han, J. A stretchable, self-healing conductive hydrogels based on nanocellulose supported graphene towards wearable monitoring of human motion. Carbohydr. Polym. 2020, 250, 116905. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, Q.; Wang, H.; Wu, Z.; Gui, X.; Li, C.; Hu, N.; Tao, K.; Wu, J. Engineering Smart Composite Hydrogels for Wearable Disease Monitoring. Nano-Micro Lett. 2023, 15, 105. [Google Scholar] [CrossRef]
- Cong, J.; Fan, Z.; Pan, S.; Tian, J.; Lian, W.; Li, S.; Wang, S.; Zheng, D.; Miao, C.; Ding, W.; et al. Polyacrylamide/Chitosan-Based Conductive Double Network Hydrogels with Outstanding Electrical and Mechanical Performance at Low Temperatures. ACS Appl. Mater. Interfaces 2021, 13, 34942–34953. [Google Scholar] [CrossRef]
- Song, B.; Ren, Z.; Gu, H. Totally dynamically cross-linked dual-network conductive hydrogel with superb and rapid self-healing ability for motion detection sensors. Mater. Today Commun. 2023, 35, 105919. [Google Scholar] [CrossRef]
- Shi, Z.; Gao, X.; Ullah, M.W.; Li, S.; Wang, Q.; Yang, G. Electroconductive natural polymer-based hydrogels. Biomaterials 2016, 111, 40–54. [Google Scholar] [CrossRef]
- Deng, Z.; Yu, R.; Guo, B. Stimuli-responsive conductive hydrogels: Design, properties, and applications. Mater. Chem. Front. 2021, 5, 2092–2123. [Google Scholar] [CrossRef]
- Rai, N.; Chauhan, I. Multifunctional Aerogels: A comprehensive review on types, synthesis and applications of aerogels. J. Sol-Gel Sci. Technol. 2023, 105, 324–336. [Google Scholar] [CrossRef]
- Thapliyal, P.C.; Singh, K. Aerogels as Promising Thermal Insulating Materials: An Overview. J. Mater. 2014, 2014, 127049. [Google Scholar] [CrossRef]
- Błaszczyński, T.; Ślosarczyk, A.; Morawski, M. Synthesis of silica aerogel by supercritical drying method. Procedia Eng. 2013, 57, 200–206. [Google Scholar] [CrossRef]
- Obrey, K.A.; Wilson, K.V.; Loy, D.A. Enhancing mechanical properties of silica aerogels. J. Non-Cryst. Solids 2011, 357, 3435–3441. [Google Scholar] [CrossRef]
- Lee, S.; Cha, Y.C.; Hwang, H.J.; Moon, J.-W.; Han, I.S. The effect of pH on the physicochemical properties of silica aerogels prepared by an ambient pressure drying method. Mater. Lett. 2007, 61, 3130–3133. [Google Scholar] [CrossRef]
- Tajiri, K.; Igarashi, K.; Nishio, T. Effects of supercritical drying media on structure and properties of silica aerogel. J. Non-Cryst. Solids 1995, 186, 83–87. [Google Scholar] [CrossRef]
- García-González, C.; Camino-Rey, M.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, H.; Li, C.; Li, S.; Liu, K.; Wang, L. Facile coordination driven synthesis of metal-organic gels toward efficiently electrocatalytic overall water splitting. Appl. Catal. B Environ. 2021, 299, 120641. [Google Scholar] [CrossRef]
- Nandi, A.K.; Chatterjee, D.P. Chatterjee, Hybrid polymer gels for energy applications. J. Mater. Chem. A 2023, 11, 12593–12642. [Google Scholar] [CrossRef]
- Kuzina, M.A.; Kartsev, D.D.; Stratonovich, A.V.; Levkin, P.A. Organogels versus Hydrogels: Advantages, Challenges, and Applications. Adv. Funct. Mater. 2023, 33, 2301421. [Google Scholar] [CrossRef]
- Fang, Z.; Li, P.; Yu, G. Gel Electrocatalysts: An Emerging Material Platform for Electrochemical Energy Conversion. Adv. Mater. 2020, 32, e2003191. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Y. Preparation and electrocatalysis application of pure metallic aerogel: A review. Catalysts 2020, 10, 1376. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Z.; Chandrasekaran, S.; Liu, Y.; Wu, M. Self-healing, antibacterial, and conductive double network hydrogel for strain sensors. Carbohydr. Polym. 2023, 303, 120468. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, H.; Gong, X.; Hu, L.; Tong, Y.-X.; Zhang, J. Electrochemical Activation of Heterometallic Nanofibers for Hydrogen Evolution. ACS Appl. Nano Mater. 2020, 3, 2393–2401. [Google Scholar] [CrossRef]
- Kim, B.-S.; Im, J.-S.; Baek, S.-T.; Lee, J.-O.; Sigeta, M.; Yoshinaga, K. Synthesis of polyglycidol hydrogel films crosslinked with carboxyl-terminated poly(ethylene glycol). Polym. J. 2006, 38, 335–342. [Google Scholar] [CrossRef][Green Version]
- Zheng, O.; Sun, Q.; Dong, A.; Han, Z.; Wang, Z.; Wei, S.; Xia, Q.; Liu, Y.; Ji, H.; Liu, S. Gelation Process Optimization of Shrimp Surimi Induced by Dense Phase Carbon Dioxide and Quality Evaluation of Gel. Foods 2022, 11, 3807. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Bronic, J. Mechanism of zeolite formation: Seed-gel interaction. Zeolites 1994, 14, 250–255. [Google Scholar] [CrossRef]
- Milivojević, M.; Popović, A.; Pajić-Lijaković, I.; Šoštarić, I.; Kolašinac, S.; Stevanović, Z.D. Alginate Gel-Based Carriers for Encapsulation of Carotenoids: On Challenges and Applications. Gels 2023, 9, 620. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Jeong, J.-H. Porous Carbon for CO2 Capture Technology: Unveiling Fundamentals and Innovations. Surfaces 2023, 6, 316–340. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Kang, H.-J.; Lee, T.-G.; Hwang, H.J.; An, B.-H.; Seo, H.-W.; Ko, C.H.; Hong, W.H.; Jun, Y.-S. Dual-templating-derived porous carbons for low-pressure CO2 capture. Carbon Lett. 2023, 33, 811–822. [Google Scholar] [CrossRef]
- Quan, Y.; Zhu, J.; Zheng, G. Electrocatalytic Reactions for Converting CO2 to Value-Added Products. Small Sci. 2021, 1, 2100043. [Google Scholar] [CrossRef]
- Masoumi, Z.; Tayebi, M.; Tayebi, M.; Lari, S.A.M.; Sewwandi, N.; Seo, B.; Lim, C.-S.; Kim, H.-G.; Kyung, D. Electrocatalytic Reactions for Converting CO2 to Value-Added Products: Recent Progress and Emerging Trends. Int. J. Mol. Sci. 2023, 24, 9952. [Google Scholar] [CrossRef]
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5, 486–519. [Google Scholar] [CrossRef]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.; Wagner, P.; Gambhir, S.; Jalili, R.; Byun, S.; Sayyar, S.; Lee, Y.M.; MacFarlane, D.R.; Wallace, G.G.; et al. Energy efficient electrochemical reduction of CO2 to CO using a three-dimensional porphyrin/graphene hydrogel. Energy Environ. Sci. 2019, 12, 747–755. [Google Scholar] [CrossRef]
- Todorova, T.K.; Schreiber, M.W.; Fontecave, M. Mechanistic Understanding of CO2 Reduction Reaction (CO2RR) Toward Multicarbon Products by Heterogeneous Copper-Based Catalysts. ACS Catal. 2020, 10, 1754–1768. [Google Scholar] [CrossRef]
- Ren, D.; Fong, J.; Yeo, B.S. The effects of currents and potentials on the selectivities of copper toward carbon dioxide electroreduction. Nat. Commun. 2018, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [PubMed]
- Bertheussen, E.; Hogg, T.V.; Abghoui, Y.; Engstfeld, A.K.; Chorkendorff, I.; Stephens, I.E.L. Electroreduction of CO on Polycrystalline Copper at Low Overpotentials. ACS Energy Lett. 2018, 3, 634–640. [Google Scholar] [CrossRef]
- Phattharasupakun, N.; Wutthiprom, J.; Duangdangchote, S.; Sawangphruk, M. A 3D free-standing lithiophilic silver nanowire aerogel for lithium metal batteries without lithium dendrites and volume expansion: In operando X-ray diffraction. Chem. Commun. 2019, 55, 5689–5692. [Google Scholar] [CrossRef]
- Wu, Y.; Jiao, L.; Xu, W.; Gu, W.; Zhu, C.; Du, D.; Lin, Y. Polydopamine-Capped Bimetallic AuPt Hydrogels Enable Robust Biosensor for Organophosphorus Pesticide Detection. Small 2019, 15, e1900632. [Google Scholar] [CrossRef]
- Jiang, X.; Du, R.; Hübner, R.; Hu, Y.; Eychmüller, A. A Roadmap for 3D Metal Aerogels: Materials Design and Application Attempts. Matter 2021, 4, 54–94. [Google Scholar] [CrossRef]
- Wen, D.; Liu, W.; Haubold, D.; Zhu, C.; Oschatz, M.; Holzschuh, M.; Wolf, A.; Simon, F.; Kaskel, S.; Eychmueller, A. Gold Aerogels: Three-Dimensional Assembly of Nanoparticles and Their Use as Electrocatalytic Interfaces. ACS Nano 2016, 10, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Jin, W.; Wu, H.; Hübner, R.; Zhou, L.; Xue, G.; Hu, Y.; Eychmueller, A. Rapid synthesis of gold-palladium core-shell aerogels for selective and robust electrochemical CO2 reduction. J. Mater. Chem. A 2021, 9, 17189–17197. [Google Scholar] [CrossRef]
- Luc, W.; Collins, C.; Wang, S.; Xin, H.; He, K.; Kang, Y.; Jiao, F. Ag-sn bimetallic catalyst with a core-shell structure for CO2 reduction. J. Am. Chem. Soc. 2017, 139, 1885–1893. [Google Scholar] [CrossRef]
- Liu, M.; Pang, Y.; Zhang, B.; De Luna, P.; Voznyy, O.; Xu, J.; Zheng, X.; Dinh, C.T.; Fan, F.; Cao, C.; et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 2016, 537, 382–386. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Z.; Zhang, X.; Li, L.; Li, Y.; Xu, H.; Li, X.; Yu, X.; Zhang, Z.; Liang, Y.; et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures. Nat. Commun. 2017, 8, 14675. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.K.; Singh, S.K.; Kumar, V.; Rana, S.; Kurungot, S.; Ballav, N. High-Level Supercapacitive Performance of Chemically Reduced Graphene Oxide. Chem 2017, 3, 846–860. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, J.; Pan, L.; Shi, Y.; Yu, G. Energy gels: A bio-inspired material platform for advanced energy applications. Nano Today 2016, 11, 738–762. [Google Scholar] [CrossRef]
- Lu, L.; Sun, X.; Ma, J.; Yang, D.; Wu, H.; Zhang, B.; Zhang, J.; Han, B. Highly Efficient Electroreduction of CO2 to Methanol on Palladium–Copper Bimetallic Aerogels. Angew. Chem. 2018, 130, 14345–14349. [Google Scholar] [CrossRef]
- Rosser, T.E.; Windle, C.D.; Reisner, E. Electrocatalytic and Solar-Driven CO2 Reduction to CO with aMole cular Manganese Catalyst Immobilized on Mesoporous TiO2. Angew. Chem. Int. Ed. 2016, 55, 7388–7392. [Google Scholar] [CrossRef]
- Reuillard, B.; Ly, K.H.; Rosser, T.E.; Kuehnel, M.F.; Zebger, I.; Reisner, E. Tuning Product Selectivity for Aqueous CO2 Reduction with a Mn(bipyridine)-pyrene Catalyst Immobilized on a Carbon Nanotube Electrode. J. Am. Chem. Soc. 2017, 139, 14425–14435. [Google Scholar] [CrossRef]
- Varela, A.S.; Sahraie, N.R.; Steinberg, J.; Ju, W.; Oh, H.; Strasser, P. Metal-Doped Nitrogenated Carbon as an Efficient Catalyst for Direct CO2 Electroreduction to CO and Hydrocarbons. Angew. Chem. Int. Ed. 2015, 54, 10758–10762. [Google Scholar] [CrossRef] [PubMed]
- Bourrez, M.; Orio, M.; Molton, F.; Vezin, H.; Duboc, C.; Deronzier, A.; Chardon-Noblat, S. Pulsed-EPR Evidence of a Manganese(II) Hydroxycarbonyl Intermediate in the Electrocatalytic Reduction of Carbon Dioxide by a Manganese Bipyridyl Derivative. Angew. Chem. Int. Ed. 2013, 53, 240–243. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.; Ding, J.; Xu, N.; Bernards, M.T.; He, Y.; Shi, Y. Three-Dimensional Nitrogen-Doped Graphene Aerogel-Supported MnO Nanoparticles as Efficient Electrocatalysts for CO2 Reduction to CO. ACS Sustain. Chem. Eng. 2020, 8, 4983–4994. [Google Scholar] [CrossRef]
- Gu, J.; Hsu, C.-S.; Bai, L.; Chen, H.M.; Hu, X. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 2019, 364, 1091–1094. [Google Scholar] [CrossRef]
- Jiao, L.; Yang, W.; Wan, G.; Zhang, R.; Zheng, X.; Zhou, H.; Yu, S.; Jiang, H. Single-Atom Electrocatalysts from Multivariate Metal–Organic Frameworks for Highly Selective Reduction of CO2 at Low Pressures. Angew. Chem. Int. Ed. 2020, 59, 20589–20595. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, S.; Yue, T.; Zhu, Y.; Fang, M.; Li, X.; Xiao, G.; Dai, L. Universal domino reaction strategy for mass production of single-atom metal-nitrogen catalysts for boosting CO2 electroreduction. Nano Energy 2020, 82, 105689. [Google Scholar] [CrossRef]
- Möller, T.; Ju, W.; Bagger, A.; Wang, X.; Luo, F.; Thanh, T.N.; Varela, A.S.; Rossmeisl, J.; Strasser, P. Efficient CO2 to CO electrolysis on solid Ni-N-C catalysts at industrial current densities. Energy Environ. Sci. 2019, 12, 640–647. [Google Scholar] [CrossRef]
- Wen, C.F.; Mao, F.; Liu, Y.; Zhang, X.Y.; Fu, H.Q.; Zheng, L.R.; Liu, P.F.; Yang, H.G. Nitrogen-Stabilized Low-Valent Ni Motifs for Efficient CO2 Electrocatalysis. ACS Catal. 2020, 10, 1086–1093. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zheng, S.; Yang, B.; Li, Z.; Lu, J.; Zhang, Q.; Adli, N.M.; Lei, L.; Wu, G.; et al. Hierarchical Cross-Linked Carbon Aerogels with Transition Metal-Nitrogen Sites for Highly Efficient Industrial-Level CO2 Electroreduction. Adv. Funct. Mater. 2021, 31, 2104377. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, H.; Cai, W.; Wen, Z.; Jia, B.; Wang, L.; Jin, W.; Ma, T. Engineering Bismuth–Tin Interface in Bimetallic Aerogel with a 3D Porous Structure for Highly Selective Electrocatalytic CO2 Reduction to HCOOH. Angew. Chem. Int. Ed. 2021, 60, 12554–12559. [Google Scholar] [CrossRef]
- Li, H.; Yue, X.; Che, J.; Xiao, Z.; Yu, X.; Sun, F.; Xue, C.; Xiang, J. High Performance 3D Self-Supporting Cu?Bi Aerogels for Electrocatalytic Reduction of CO2 to Formate Huaxin. ChemSusChem 2022, 15, e202200226. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Abdinejad, M.; Motlagh, M.K.; Noroozifar, M.; Kraatz, H.-B. Effect of peptide aerogel composite on silver nanoparticles as a catalyst for electrochemical CO2 reduction. J. Environ. Chem. Eng. 2023, 11, 110567. [Google Scholar] [CrossRef]
- Ren, B.; Wen, G.; Gao, R.; Luo, D.; Zhang, Z.; Qiu, W.; Ma, Q.; Wang, X.; Cui, Y.; Ricardez–Sandoval, L.; et al. Nano-crumples induced Sn-Bi bimetallic interface pattern with moderate electron bank for highly efficient CO2 electroreduction. Nat. Commun. 2022, 13, 2486. [Google Scholar] [CrossRef] [PubMed]
- Detweiler, Z.M.; White, J.L.; Bernasek, S.L.; Bocarsly, A.B. Anodized indium metal electrodes for enhanced carbon dioxide reduction in aqueous electrolyte. Langmuir 2014, 30, 7593–7600. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, S.; Guo, T.; Zhang, S.; Wang, J.; Wu, Y.; Chen, Y. Advances in Sn-Based Catalysts for Electrochemical CO2 Reduction. Nano-Micro Lett. 2019, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Pander, J.E.; Lum, J.W.J.; Yeo, B.S. The importance of morphology on the activity of lead cathodes for the reduction of carbon dioxide to formate. J. Mater. Chem. A 2019, 7, 4093–4101. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Abdinejad, M.; Mirza, Z.; Zhang, X.-A.; Kraatz, H.-B. Enhanced Electrocatalytic Activity of Primary Amines for CO2 Reduction Using Copper Electrodes in Aqueous Solution. ACS Sustain. Chem. Eng. 2020, 8, 1715–1720. [Google Scholar] [CrossRef]
- Dinh, C.-T.; Burdyny, T.; Kibria, M.G.; Seifitokaldani, A.; Gabardo, C.M.; de Arquer, F.P.G.; Kiani, A.; Edwards, J.P.; De Luna, P.; Bushuyev, O.S.; et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 2018, 360, 783–787. [Google Scholar] [CrossRef]
- Jiang, Q.; Wu, C.; Wang, Z.; Wang, A.C.; He, J.-H.; Wang, Z.L.; Alshareef, H.N. MXene electrochemical microsupercapacitor integrated with triboelectric nanogenerator as a wearable self-charging power unit. Nano Energy 2018, 45, 266–272. [Google Scholar] [CrossRef]
- Lipton, J.; Weng, G.-M.; Alhabeb, M.; Maleski, K.; Antonio, F.; Kong, J.; Gogotsi, Y.; Taylor, A.D. Mechanically strong and electrically conductive multilayer MXene nanocomposites. Nanoscale 2019, 11, 20295–20300. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Li, X.; Bai, Z.; Lu, S. Recent Advances in Layered Ti3C2Tx MXene for Electrochemical Energy Storage. Small 2018, 14, e1703419. [Google Scholar] [CrossRef] [PubMed]
- VahidMohammadi, A.; Mojtabavi, M.; Caffrey, N.M.; Wanunu, M.; Beidaghi, M. Assembling 2D MXenes into Highly Stable Pseudocapacitive Electrodes with High Power and Energy Densities. Adv. Mater. 2019, 31, e1806931. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, H.; Xie, X.; Yang, R.; Liu, Z.; Liu, Y.; Yu, Z. MMultifunctional, Superelastic, and Lightweight MXene/Polyimide Aerogels. Small 2018, 14, e1802479. [Google Scholar] [CrossRef] [PubMed]
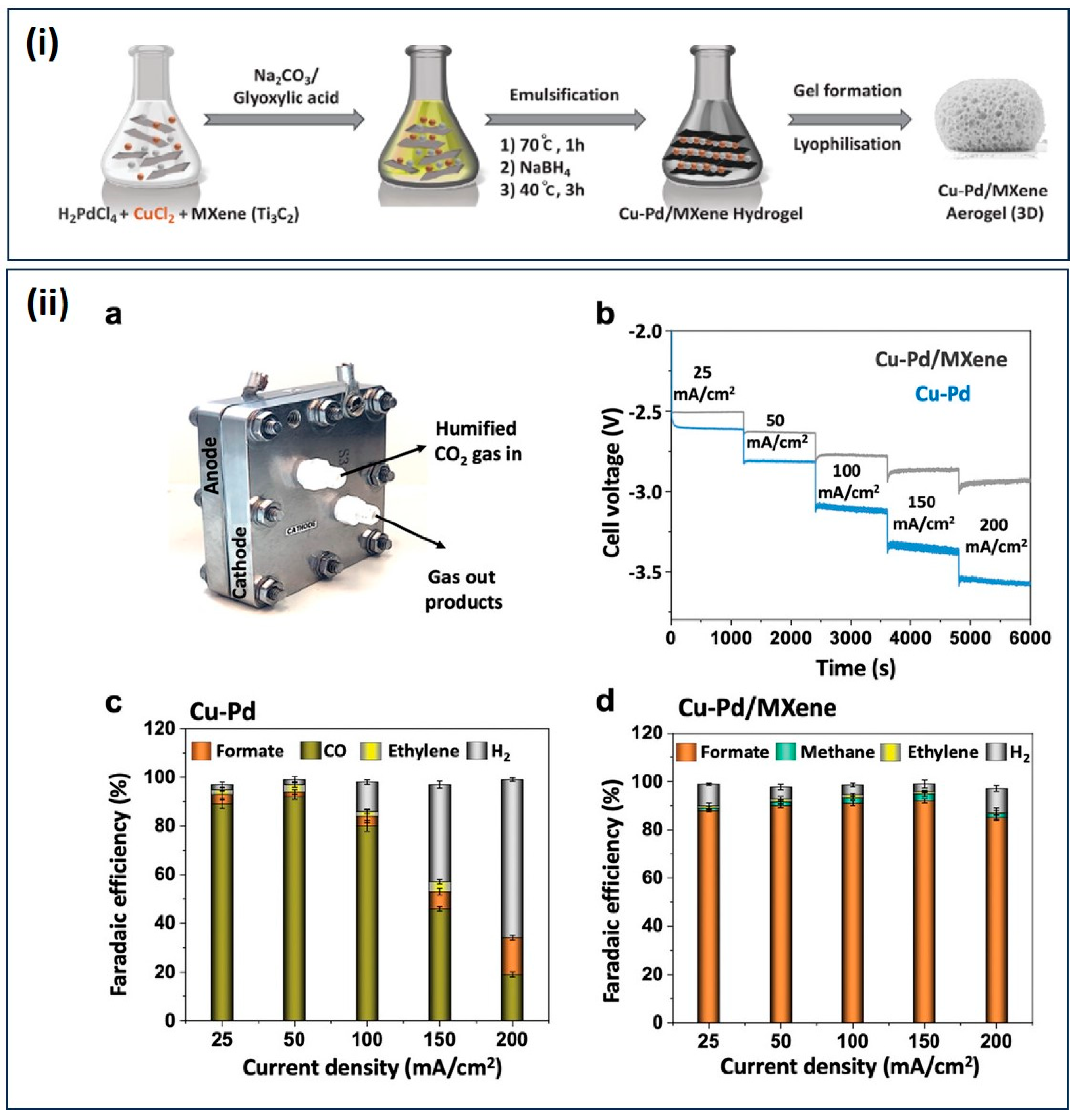
- Abdinejad, M.; Subramanian, S.; Motlagh, M.K.; Noroozifar, M.; Duangdangchote, S.; Neporozhnii, I.; Ripepi, D.; Pinto, D.; Li, M.; Tang, K.; et al. Insertion of MXene-Based Materials into Cu–Pd 3D Aerogels. Adv. Energy Mater. 2023, 13, 2300402. [Google Scholar] [CrossRef]
- Watanabe, E.; Ushiyama, H.; Yamashita, K. Theoretical studies on the mechanism of oxygen reduction reaction on clean and O-substituted Ta3N5(100) surfaces. Catal. Sci. Technol. 2015, 5, 2769–2776. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Z.; Wang, X. Understanding the Mechanism of the Oxygen Evolution Reaction with Consideration of Spin. Electrochem. Energy Rev. 2021, 4, 136–145. [Google Scholar] [CrossRef]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef]
- Ma, J.; Gong, L.; Shen, Y.; Sun, D.; Liu, B.; Zhang, J.; Liu, D.; Zhang, L.; Xia, Z. Detrimental Effects and Prevention of Acidic Electrolytes on Oxygen Reduction Reaction Catalytic Performance of Heteroatom-Doped Graphene Catalysts. Front. Mater. 2019, 6, 294. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Cheng, H.; Li, N.; Ma, T.Y.; Su, Y.-Z. ZnCo2O4 Quantum Dots Anchored on Nitrogen-Doped Carbon Nanotubes as Reversible Oxygen Reduction/Evolution Electrocatalysts. Adv. Mater. 2016, 28, 3777–3784. [Google Scholar] [CrossRef]
- Deng, D.; Yu, L.; Chen, X.; Wang, G.; Jin, L.; Pan, X.; Deng, J.; Sun, G.; Bao, X. Iron Encapsulated within Pod-like Carbon Nanotubes for Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yu, H.; Jiang, C.; Zhang, T.; Zhan, R.; Li, X.; Li, J.; Tian, J.; Yang, R. NiCo Alloy Nanoparticles Decorated on N-Doped Carbon Nanofibers as Highly Active and Durable Oxygen Electrocatalyst. Adv. Funct. Mater. 2017, 28, 1705094. [Google Scholar] [CrossRef]
- Fu, G.; Chen, Y.; Cui, Z.; Li, Y.; Zhou, W.; Xin, S.; Tang, Y.; Goodenough, J.B. Novel Hydrogel-Derived Bifunctional Oxygen Electrocatalyst for Rechargeable Air Cathodes. Nano Lett. 2016, 16, 6516–6522. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zeng, X.; Wang, D.; Cao, D. Biomass-derived FeNi alloy and nitrogen-codoped porous carbons as highly efficient oxygen reduction and evolution bifunctional electrocatalysts for rechargeable Zn-air battery. Energy Storage Mater. 2018, 12, 277–283. [Google Scholar] [CrossRef]
- Guo, X.; Liu, P.; Han, J.; Ito, Y.; Hirata, A.; Fujita, T.; Chen, M. 3D Nanoporous Nitrogen-Doped Graphene with Encapsulated RuO2 Nanoparticles for Li–O2 Batteries. Adv. Mater. 2015, 27, 6137–6143. [Google Scholar] [CrossRef]
- Yilmaz, E.; Yogi, C.; Yamanaka, K.; Ohta, T.; Byon, H.R. Promoting Formation of Noncrystalline Li2O2 in the Li-O2 Battery with RuO2 Nanoparticles. Nano Lett. 2013, 13, 4679–4684. [Google Scholar] [CrossRef]
- Fu, G.; Liu, Y.; Chen, Y.; Tang, Y.; Goodenough, J.B.; Lee, J.-M. Robust N-doped carbon aerogels strongly coupled with iron-cobalt particles as efficient bifunctional catalysts for rechargeable Zn-air batteries. Nanoscale 2018, 10, 19937–19944. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Dong, S.; Wang, E. Transition-Metal (Co, Ni and Fe)-Based Electrocatalysts for the Water Oxidation Reaction. Adv. Mater. 2016, 28, 9266–9291. [Google Scholar] [CrossRef]
- Ma, R.; Wang, J.; Tang, Y.; Wang, J. Design Strategies for Single-Atom Iron Electrocatalysts toward Efficient Oxygen Reduction. J. Phys. Chem. Lett. 2022, 13, 168–174. [Google Scholar] [CrossRef]
- Joo, S.H.; Lee, J.S. Metal carbides as alternative electrocatalysts for energy conversion reactions. J. Catal. 2021, 404, 911–924. [Google Scholar] [CrossRef]
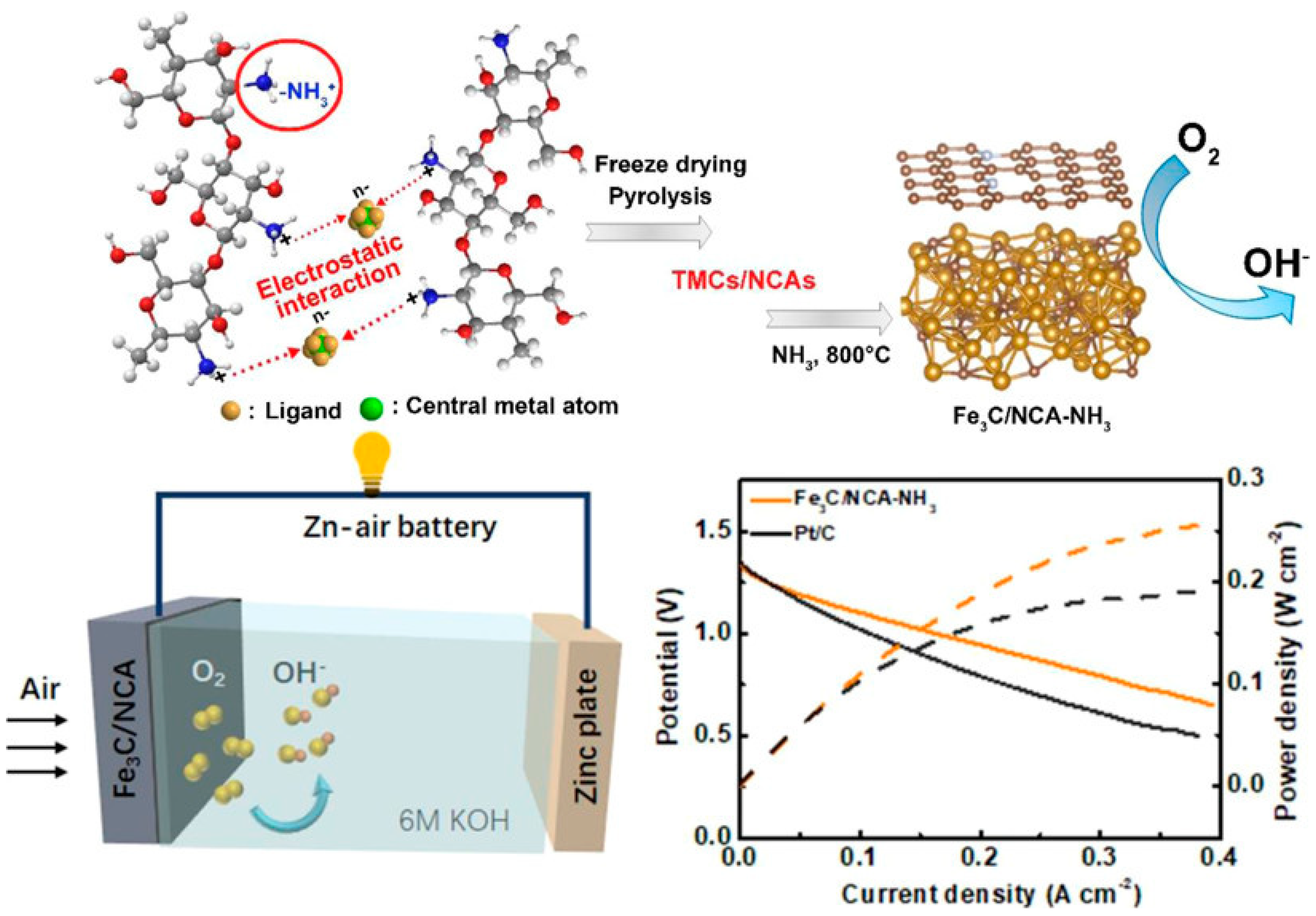
- Liu, C.; Liu, C.; Wang, X.; Wang, X.; Kong, Z.; Kong, Z.; Zhang, L.; Zhang, L.; Xin, Z.; Xin, Z.; et al. Electrostatic Interaction in Amino Protonated Chitosan-Metal Complex Anion Hydrogels: A Simple Approach to Porous Metal Carbides/N-Doped Carbon Aerogels for Energy Conversion. ACS Appl. Mater. Interfaces 2022, 14, 22151–22160. [Google Scholar] [CrossRef] [PubMed]
- Stoerzinger, K.A.; Diaz-Morales, O.; Kolb, M.; Rao, R.R.; Frydendal, R.; Qiao, L.; Wang, X.R.; Halck, N.B.; Rossmeisl, J.; Hansen, H.A.; et al. Orientation-Dependent Oxygen Evolution on RuO2 without Lattice Exchange. ACS Energy Lett. 2017, 2, 876–881. [Google Scholar] [CrossRef]
- Fu, S.; Song, J.; Zhu, C.; Xu, G.-L.; Amine, K.; Sun, C.; Li, X.; Engelhard, M.H.; Du, D.; Lin, Y. Ultrafine and highly disordered Ni2Fe1 nanofoams enabled highly efficient oxygen evolution reaction in alkaline electrolyte. Nano Energy 2017, 44, 319–326. [Google Scholar] [CrossRef]
- Gao, M.; Sheng, W.; Zhuang, Z.; Fang, Q.; Gu, S.; Jiang, J.; Yan, Y. Efficient Water Oxidation Using Nanostructured α Nickel-Hydroxide as an Electrocatalyst. J. Am. Chem. Soc. 2014, 136, 7077–7084. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Meng, F.; Cabán-Acevedo, M.; Li, L.; Forticaux, A.; Xiu, L.; Wang, Z.; Jin, S. Hydrothermal continuous flow synthesis and exfoliation of NiCo layered double hydroxide nanosheets for enhanced oxygen evolution catalysis. Nano Lett. 2015, 15, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Zhang, L.; Yoon, Y.; Weber, P.K.; Wang, H.; Guo, J.; Dai, H. N-doping of graphene through electrothermal reactions with ammonia. Science 2009, 324, 768–771. [Google Scholar] [CrossRef]
- Gogoi, N.; Barooah, M.; Majumdar, G.; Chowdhury, D. Carbon Dots Rooted Agarose Hydrogel Hybrid Platform for Optical Detection and Separation of Heavy Metal Ions. ACS Appl. Mater. Interfaces 2015, 7, 3058–3067. [Google Scholar] [CrossRef]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.B.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J.; et al. Carbon dots rooted agarose hydrogel hybrid platform for optical detection and separation of heavy metal ions. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef]
- Liu, W.; Hu, X.; Li, H.; Yu, H. Pseudocapacitive Ni-Co-Fe Hydroxides/N-Doped Carbon Nanoplates-Based Electrocatalyst for Efficient Oxygen Evolution. Small 2018, 14, e1801878. [Google Scholar] [CrossRef]
- Li, F.; Shao, Q.; Huang, X.; Lang, J. Nanoscale Trimetallic Metal–Organic Frameworks Enable Efficient Oxygen Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2018, 57, 1888–1892. [Google Scholar] [CrossRef]
- Cao, Z.; Jiang, Z.; Li, Y.; Huang, C.; Li, Y.F. Metal–Organic Gel-Derived Multimetal Oxides as Effective Electrocatalysts for the Oxygen Evolution Reaction. ChemSusChem 2019, 12, 2480–2486. [Google Scholar] [CrossRef]
- Moi, R.; Nath, K.; Ghosh, D.; Biradha, K. Metal-Organic Gels of Tris-tetrazole-tri-amido Molecule with Co(II) and Ni(II) as Effective Electrocatalysts for Oxygen Evolution Reaction: Effect of Metal Ion, Porosity and Morphology on the Catalytic Activity of MOGs. ChemCatChem 2023, 15, e202300694. [Google Scholar] [CrossRef]
- Wei, S.; Wang, Y.; Chen, W.; Li, Z.; Cheong, W.-C.; Zhang, Q.; Gong, Y.; Gu, L.; Chen, C.; Wang, D.; et al. Atomically dispersed Fe atoms anchored on COF-derived N-doped carbon nanospheres as efficient multi-functional catalysts. Chem. Sci. 2020, 11, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.Q.; Hunt, A.; Greif, R. Mean free path and apparent thermal conductivity of a gas in a porous medium. J. Heat Transf. 1995, 117, 758–761. [Google Scholar] [CrossRef]
- Ren, G.; Chen, Q.; Zheng, J.; Huang, B.; Qian, Y. N-doped carbon nanofibers aerogels derived from aramid as efficient electrocatalysts for oxygen reduction reaction in alkaline and acidic media. J. Electroanal. Chem. 2018, 829, 177–183. [Google Scholar] [CrossRef]
- Mao, J.; Iocozzia, J.; Huang, J.; Meng, K.; Lai, Y.; Lin, Z. Graphene aerogels for efficient energy storage and conversion. Energy Environ. Sci. 2018, 11, 772–799. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, J.; Chen, Y.; Feng, J.; Wang, L.; Li, L.; Jiang, Y.; Lei, Y.; Feng, J. A stabilization synthesis strategy for atomically dispersed metal-N4 electrocatalysts via aerogel confinement and ammonia pyrolyzing. Nano Energy 2022, 104, 107869. [Google Scholar] [CrossRef]
- Panja, S.; Adams, D.J. Stimuli responsive dynamic transformations in supramolecular gels. Chem. Soc. Rev. 2021, 50, 5165–5200. [Google Scholar] [CrossRef]
- Schroeder, T.B.H.; Guha, A.; Lamoureux, A.; VanRenterghem, G.; Sept, D.; Shtein, M.; Yang, J.; Mayer, M. An electric-eel-inspired soft power source from stacked hydrogels. Nature 2017, 552, 214–218. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Kim, H. An Easy Fabricable Film for Organic Electronics Based on Phenoxy and Epoxy. Macromol. Chem. Phys. 2019, 220, 1900135. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Lu, H.; Wang, Y.; Xu, J.; Zhu, J.; Zhang, C.; Liu, T. Cryopolymerization enables anisotropic polyaniline hybrid hydrogels with superelasticity and highly deformation-tolerant electrochemical energy storage. Nat. Commun. 2020, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Bari, G.A.R.; Kim, H. Composite organic encapsulate film with epoxy and benzoxazine. Eur. Polym. J. 2019, 116, 453–462. [Google Scholar] [CrossRef]
- Gu, Y.; Zhao, J.; Johnson, J.A. Polymer Networks: From Plastics and Gels to Porous Frameworks. Angew. Chem. Int. Ed. 2020, 59, 5022–5049. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, H.; Mahmood, A.; Zhu, B.; Liang, Z.; Zhong, R.; Guo, S.; Zou, R. Recent advances in confining metal-based nanoparticles into carbon nanotubes for electrochemical energy conversion and storage devices. Energy Environ. Sci. 2019, 12, 2924–2956. [Google Scholar] [CrossRef]
- Deng, J.; Ren, P.; Deng, D.; Yu, L.; Yang, F.; Bao, X. Highly active and durable non-precious-metal catalysts encapsulated in carbon nanotubes for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 1919–1923. [Google Scholar] [CrossRef]
- Gu, D.; Li, W.; Wang, F.; Bongard, H.; Spliethoff, B.; Schmidt, W.; Weidenthaler, C.; Xia, Y.; Zhao, D.; Schüth, F. Controllable Synthesis of Mesoporous Peapod-like Co3O4@Carbon Nanotube Arrays for High-Performance Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2015, 54, 7060–7064. [Google Scholar] [CrossRef]
- Pan, F.; Li, B.; Sarnello, E.; Fei, Y.; Gang, Y.; Xiang, X.; Du, Z.; Zhang, P.; Wang, G.; Nguyen, H.T.; et al. Atomically Dispersed Iron-Nitrogen Sites on Hierarchically Mesoporous Carbon Nanotube and Graphene Nanoribbon Networks for CO2 Reduction. ACS Nano 2020, 14, 5506–5516. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, R.F.; Chen, X.M.; Tang, Y.H.; Qiao, S.Z. Fe–N Fe-N decorated hybrids of CNTs grown on hierarchically porous carbon for high-performance oxygen reduction. Adv. Mater. 2014, 26, 6074–6079. [Google Scholar] [CrossRef]
- Liu, J.; Gu, C.; Wang, M.; Cui, Y.; Liu, C.-S. Controllable fabrication of metal-confined carbon nanotubes from the self-templated conversion of supramolecular gel nanofibers. Mater. Today Chem. 2022, 24, 100798. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, C.; Jian, H.; Cheng, X.; Hu, T.; Wang, D.; Shang, L.; Chen, G.; Schaaf, P.; Wang, X.; et al. Substitutionally Dispersed High-Oxidation CoOx Clusters in the Lattice of Rutile TiO2 Triggering Efficient Co-Ti Cooperative Catalytic Centers for Oxygen Evolution Reactions. Adv. Funct. Mater. 2020, 31, 2009610. [Google Scholar] [CrossRef]
- Weingarten, A.S.; Kazantsev, R.V.; Palmer, L.C.; McClendon, M.; Koltonow, A.R.; Samuel, A.P.S.; Kiebala, D.J.; Wasielewski, M.R.; Stupp, S.I. Self-assembling hydrogel scaffolds for photocatalytic hydrogen production. Nat. Chem. 2014, 6, 964–970. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Q.; Yuan, A.; Shi, Q.; Jiang, L.; Yang, W.; Yang, T.; Hou, X. A facile and green strategy for mass production of dispersive FeCo-rich phosphides@N,P-doped carbon electrocatalysts toward efficient and stable rechargeable Zn-air battery and water splitting. J. Mater. Sci. Technol. 2023, 182, 1–11. [Google Scholar] [CrossRef]
- Wu, X.; Ni, C.; Man, J.; Shen, X.; Cui, S.; Chen, X. A strategy to promote the ORR electrocatalytic activity by the novel engineering bunched three-dimensional Pd-Cu alloy aerogel. Chem. Eng. J. 2023, 454, 140293. [Google Scholar] [CrossRef]
- Álvarez-Manuel, L.; Alegre, C.; Sebastián, D.; Lázaro, M.J. Organic xerogels combined with iron and nitrogen as PGM-free catalysts for the oxygen reduction reaction. Int. J. Hydrogen Energy 2023, 52, 1076–1089. [Google Scholar] [CrossRef]
- Wu, X.; Liu, L.; Yuan, K.; Shao, Y.; Shen, X.; Cui, S.; Chen, X. Modulating Electronic Structure and Atomic Insights into the Novel Hierarchically Porous PdCuFe Trimetallic Alloy Aerogel for Efficient Oxygen Reduction. Small 2023, e2307243. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Xu, S.; Chen, Z.; Li, W.; Zhu, R.; Xi, S.; Xu, C.; Xiang, X.D. Developing an FexCoyLaz-based amorphous aerogel catalyst for the oxygen evolution reaction via high throughput synthesis. J. Mater. Chem. A Mater. 2023. [CrossRef]
- Zhang, T.; Dai, M.; Lang, X.; Huang, J.; Li, Q.; Chen, Y.; Lin, H. Self-oxidized amorphous FeOx@NiOy electrocatalyst with double-shell hollow nanoarchitecture for boosting oxygen evolution reaction. Ceram. Int. 2023, 50, 4415–4422. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Fei, J.; Yu, W.; Zhu, J.; Zhang, Y.; Shi, Y.; Tian, M.; Lai, J.; Wang, L. Sub-nanometric Pt clusters supported Co aerogel electrocatalyst with hierarchical micro/nano-porous structure for hydrogen evolution reaction. Appl. Catal. B Environ. 2023, 343, 123546. [Google Scholar] [CrossRef]
- Zhang, B.; Gong, S.; Wang, G.; Wu, C.; Zhao, G.; Lv, X. Cobalt-nitrogen-carbon sites on carbon aerogels for enhanced electrocatalytic CO2 activity. Appl. Surf. Sci. 2023, 630, 157437. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, M.; Ye, Z.; Tang, H.; Hong, Z.; Zhi, M. Cu-Sn Aerogels for Electrochemical CO2 Reduction with High CO Selectivity. Molecules 2023, 28, 1033. [Google Scholar] [CrossRef]
- Yu, W.; Liu, L.; Yang, Y.; Li, N.; Chen, Y.; Yin, X.; Niu, J.; Wang, J.; Ding, S. N, O-diatomic dopants activate catalytic activity of 3D self-standing graphene carbon aerogel for long-cycle and high-efficiency Li-CO2 batteries. Chem. Eng. J. 2023, 465, 142787. [Google Scholar] [CrossRef]
- Lam, C.V.; Vy, D.N.C.; Duyen, N.H.K.; Xuan, N.T.T.; Trinh, T.T.; Linh, N.H.G.; Ngan, N.X.; Tu, P.M.; Son, N.T.; Hieu, N.H. Zinc oxide-doped carbon aerogel derived from bagasse cellulose/sodium alginate/zinc nitrate composite for dye adsorption, storage energy and electrochemical sensing Cao. J. Chem. Technol. Biotechnol. 2023, 99, 279–293. [Google Scholar] [CrossRef]
- Yang, K.; Fan, Q.; Zhang, Y.; Ren, G.; Huang, X.; Fu, P. Hierarchical porous carbon aerogels as a versatile electrode material for high-stability supercapacitors. RSC Adv. 2024, 14, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
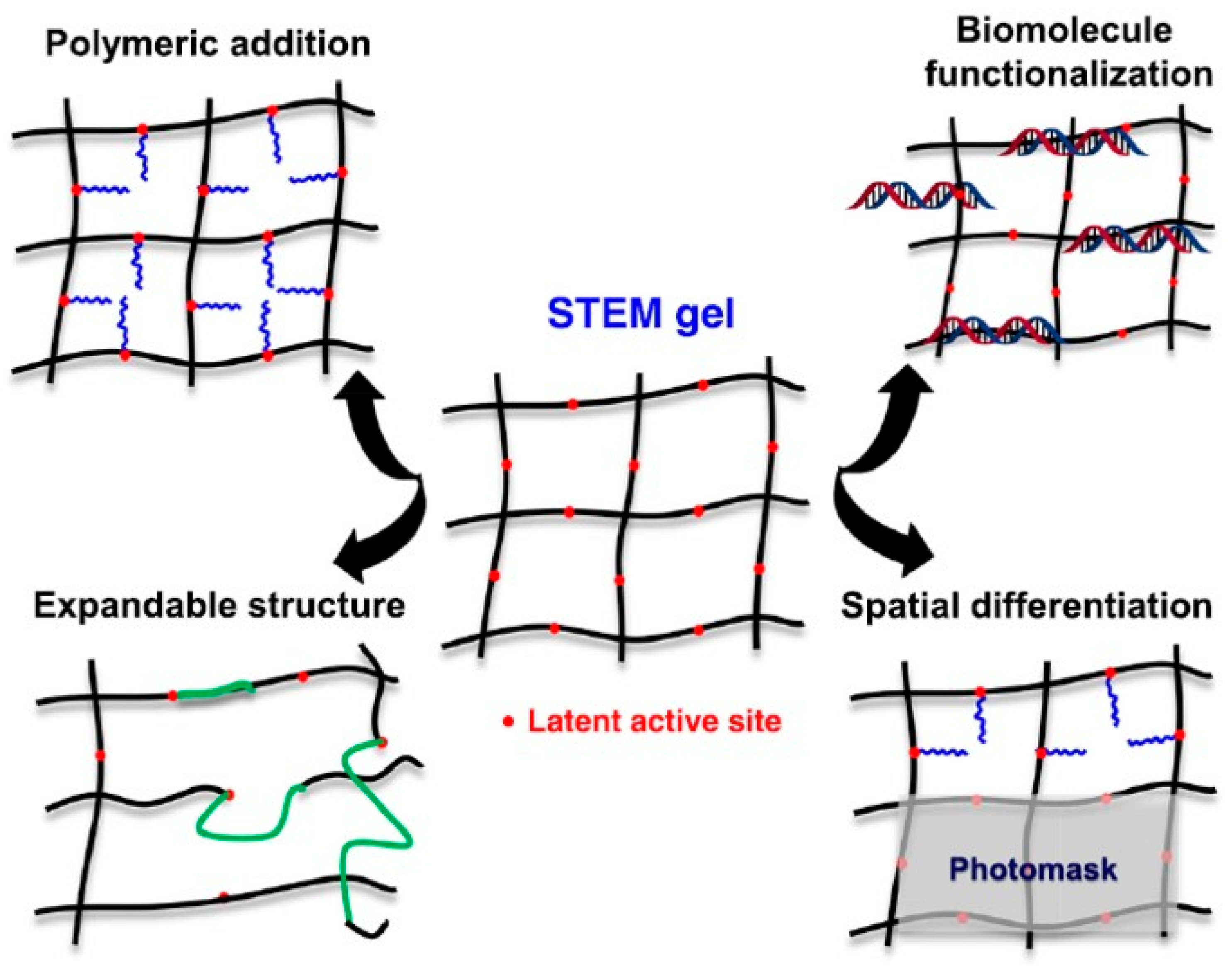

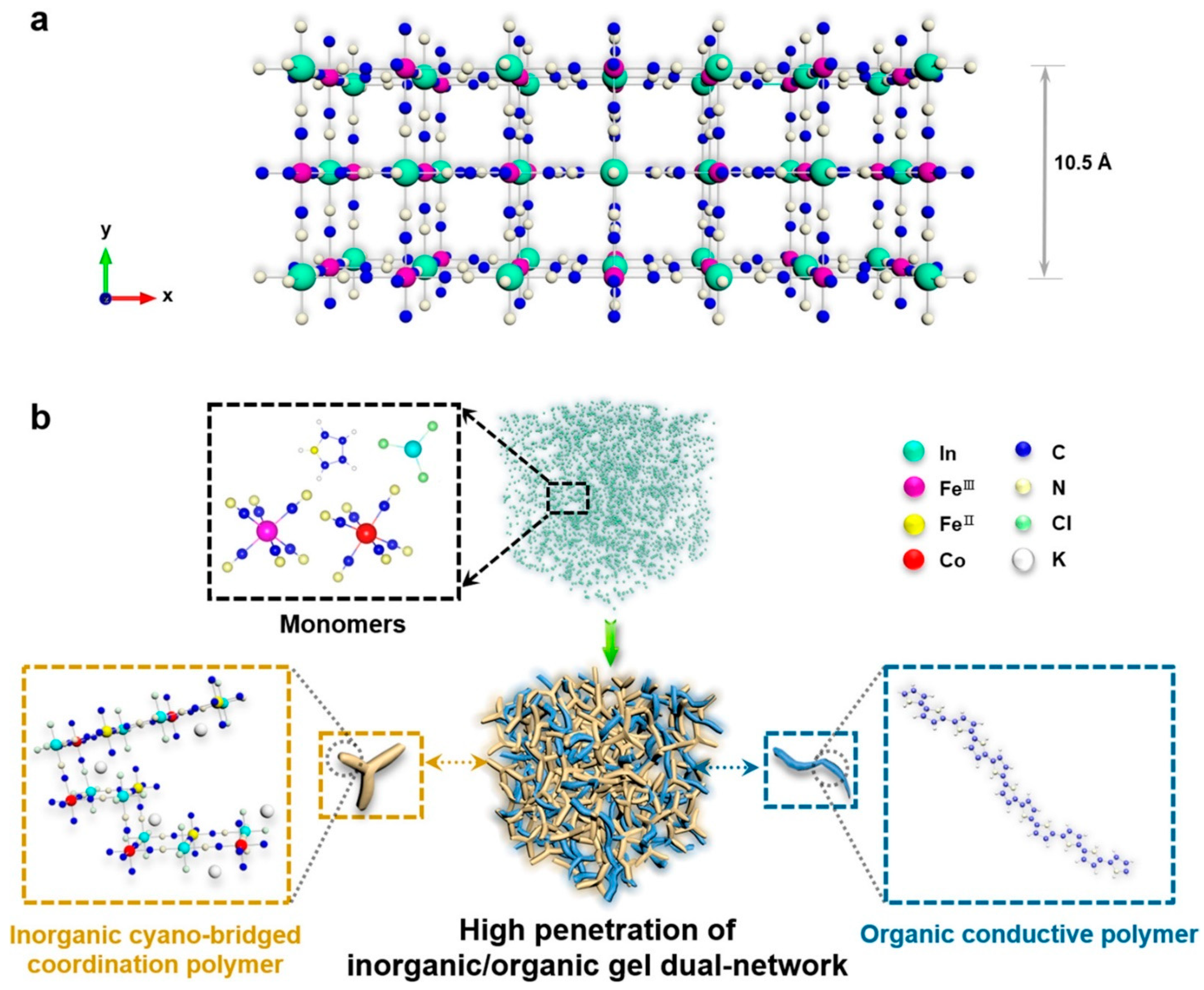
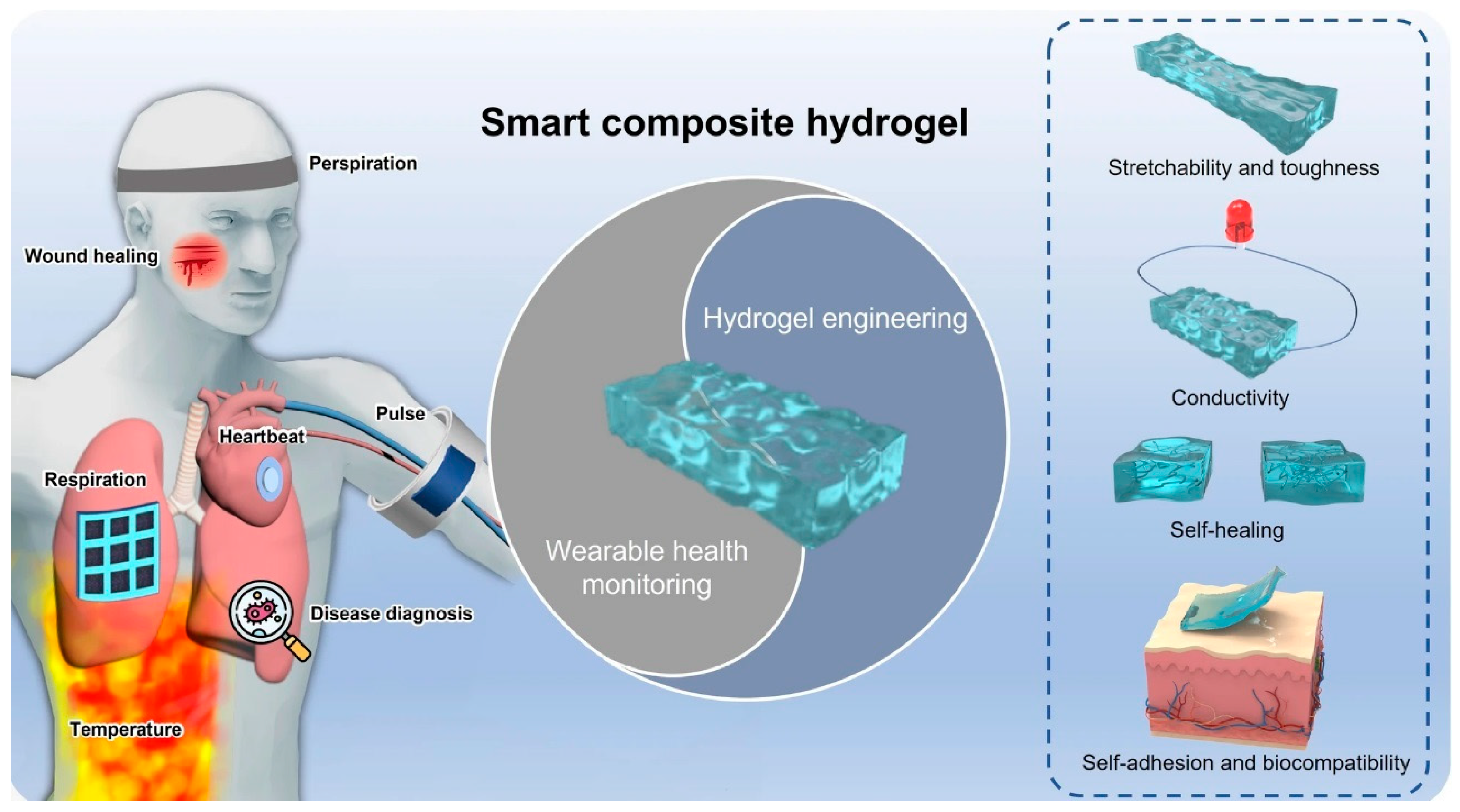

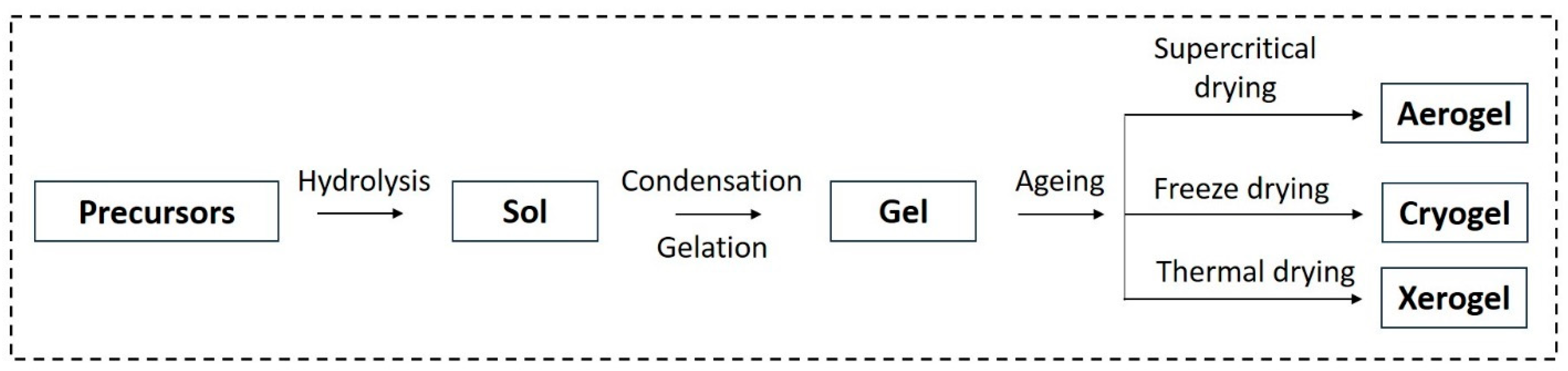




| Applications | Materials | Performance | Ref. |
|---|---|---|---|
| Zn–air battery (ORR) | Fe3C/N-doped C aerogels | P 253 mW cm−2 at current density 0.4 A cm−2, Eon 0.93 V, Tafel slope 76 mV dec−1, specific capacity 820 mA h g−1 | [132] |
| Zn–air battery (ORR) | 20% Pt/C | P 190 mW cm−2 at current density 0.4 A cm−2, Tafel slope 75 mV dec−1, specific capacity 742 mA h g−1, η 0.39 V at current density 10 mA cm−2 | [132] |
| Zn–air battery (ORR) | FeCo/N-DNC | P 115 mW cm−2 at current density 0.2 A cm−2, Eon 0.89 V, E1/2 0.81 V, specific capacity 804 mA h g−1, E 988 W h Kg−1 at 5 mA cm−2 | [128] |
| Zn–air battery (ORR) | Pt/C + RuO2 | P 109 mW cm−2 at current density 0.15 A cm−2, Eon 0.98 V, E1/2 0.84 V, specific capacity 699 mA h g−1, E 870 W h Kg−1 at 5 mA cm−2 | [128] |
| Zn–air battery (ORR) | Fe–N4 carbon aerogel | P 167 mW cm−2 at current density 0.22 A cm−2, E 956 Wh kg−1, E1/2 0.93 V, Tafel slope 53 mV dec−1, Cdl 9.3 mF cm−2 | [148] |
| Zn–air battery (ORR) | FeCo/Co2P/Fe2P-N-doped C aerogel | P 174 mW cm−2 at current density 0.3 A cm−2, E Eon 0.88 V, E1/2 0.79 V, Tafel slope 117 mV dec−1, specific capacity 730 mA h g−1, E 956 W h Kg−1 at 5 mA cm−2 | [163] |
| ORR | Pd3Cu aerogel | Limiting current density 5.8 mA cm−2, E1/2 0.9 V, Cdl 8 mF cm−2, TOF 500 s−1 at 0.85 V | [164] |
| ORR | Organic xerogel (Fe-N-C) | Eonset 0.73–0.76 V, E1/2 0.49–0.54 V, Jd–−2.88 to −3.88 mA cm−2, Tafel slope 70–93 mV dec−1 | [165] |
| ORR | Pd3CuFe0.5 aerogels | E1/2 0.92 V, limiting current density −7.6 mA cm−2, Cdl 21.9 mF cm−2, Tafel slope 96 mV dec−1 | [166] |
| Zn–CO2 battery | Ni-N-containing carbon aerogel | P 0.5 mW cm−2 at current density 3 mA cm−2, Tafel slope 86.6 mV dec−1, FECO 91% (300 mA cm−2) | [100] |
| OER | NiCoFe hydroxide nanoplates/N–doped C hydrogel hybrid | E 31.5 Wh Kg−1 at specific capacitance 1849 F g−1, Tafel slope 31 mV dec−1, η 250 mV (j = 10 mA cm−2), TOF 0.17 s−1, Cdl 34.9 mF cm−2 | [140] |
| OER | FeCo/Co2P/Fe2P-N-doped C aerogel | η 281 mV at current density 10 mA cm−2, Tafel slope 85 mV dec−1, ECSA 353.1 m2 g−1, Cdl 8.6 mF cm−2 | [163] |
| OER | Co–MOGs | η 312 mV at current density 10 mA cm−2, Tafel slope 84 mV dec−1 | [143] |
| OER | Ni–MOGs | η 418 mV at current density 10 mA cm−2, Tafel slope 107 mV dec−1 | [143] |
| OER | NiFe/B, N-CNT | η 355 mV at current density 10 mA cm−2, Tafel slope 116.2 mV dec−1, Cdl 11.9 mF cm−2 | [160] |
| OER | Co/TiO2 | η 400 mV at current density 10 mA cm−2, Tafel slope 65 mV dec−1, catalyst surface area (jBET) 2.04 mA cm−2 at 1.65 V, Cdl 0.26 mF cm−2, TOF 3.2 s−1 at 350 mV | [161] |
| OER | FexCoyLaz aerogel | ηonset 201 mV, η10 209 mV, η100 319 mV, Tafel slope 49.8 mV dec−1, mass activity 505.4 A gaerogel−1 at 1.63 V (vs. RHE) | [167] |
| OER | FeNi–O aerogel | η 280 mV at current density 50 mA cm−2, Tafel slope 3.25 mV dec−1, ECSA 148.5 cm2 g−1, Cdl 5.9 mF cm−2 | [168] |
| HER | Pd–Co@Pd NPs–NF (MOGs) | η 57 mV at current density 10 mA cm−2, Tafel slope 55 mV dec−1 | [62] |
| HER | Pt0.25Co aerogel | η 23 mV at current density 10 mA cm−2, Tafel slope 28 mV dec−1, Cdl 4.8 mF cm−2, ECSA 152.4 m2 g−1, TOF 63 s−1 at 0.1 V, Rac 2.13 Ω cm−2, Rd 1.0 cm−2 | [169] |
| CO2RR | Porphyrin-based graphene hydrogel (FePGH) | CO FE 96.2%, η 280 mV at current density 0.42 mA cm−2, TOF 0.8 s−1, Tafel slope 118 mV dec−1 | [74] |
| CO2RR | Pd–Cu aerogels | CH3OH FE 80%, at current density 31.8 mA cm−2, η 0.24 V, Tafel slope 124.4 mV dec−1 | [89] |
| CO2RR | Ni-N-containing carbon aerogel | CO FE 98%, at −0.8 V, current density 300 mA cm−2, Tafel slope 86.6 mV dec−1, Cdl 9.3 mF cm−2 | [100] |
| CO2RR | CoPc@-N-C aerogel | CO FE 92.4%, at −0.8 V, current density 21.7 mA cm−2, TOF 1.23 s−1, Tafel slope 188 mV dec−1, Cdl 8.4 mF cm−2, ECSA 211.5 cm2 | [170] |
| CO2RR | Bi–Sn aerogel | HCOOH FE 93.9%, at −1.0 V, current density 9.3 mA cm−2, Cdl 2.14 mF cm−2 | [101] |
| CO2RR | Au-Pd core–shell aerogel | CO FE 99.9%, at −0.5 V, η 390 mV, Tafel slope 182 mV dec−1 | [83] |
| CO2RR | Cu–Bi aerogel | CO FE 96.5%, at −0.9 V, Cdl 0.22 mF cm−2 | [102] |
| CO2RR | MA-FF-GoX-Ag | CO FE 88%, at −0.7 V | [103] |
| CO2RR | Cu–Pd/MXene aerogels | formate FE 93%, jformate: 150 mA cm−2, Tafel slope 182 mV dec−1, ECSA 0.18 cm2 | [116] |
| CO2RR | Cu95Sn5 aerogels | CO FE 93% with 6.58 mA cm−2 current density (−0.9 vs. RHE) | [171] |
| Li–CO2 battery (CO2RR) | N,O-diatomic dopants graphene C aerogels | Initial energy efficiency 78.4%, discharge areal capacity 18.6 mAh cm−2 at 20 A cm−2 | [172] |
| Sensing | ZnO-doped C aerogel | Adsorption capacity 39 mg g−1 (crystal violet pigment), capacitance 164.7 F g−1, glucose sensing | [173] |
| Supercapacitors | C aerogels | Specific capacitance 138 F g−1, E 10 W h Kg−1, P 181 W Kg−1 | [174] |
| Types | Example | Advantages | Challenges |
|---|---|---|---|
| Transition metal oxides/OH/complex gels | Fe porphyrin, MnO | Multiple oxidation states, pseudocapacitance, high theoretical activities, energy storage | Poor wettability, self-agglomeration, poisoning via intermediates |
| Noble metal gels | Au, Ru, Ir, Pd, Au-Pd | High conductivity, abundant electron/mass transfer channels, robust structure, plasmonic properties | Low gelation kinetics, difficulty in controlling microstructure, high cost |
| Bimetallic gels | Noble–noble metal alloys (Au–Pd) | Mass transport facilitation, moderate adsorption energies, optimized energy barrier, selective catalytic activities, synergetic effects, cost-effective with noble metals, higher intrinsic polarity | Metal interaction understanding, reproducibility concerns, stability and agglomeration, homogeneous alloying, precise control, scale-up considerations |
| Noble–transition metal alloys (Pd–Cu) | |||
| Transition–transition metal alloys (NiFe, NiCo, FeCoRu) | |||
| Non-transition metal alloys (Bi–Sn) | |||
| Transition–non-transition metal alloys (Cu–Bi) | |||
| Carbon/graphene with heteroatom-doped gels | FeCo-N-dual-network carbon, Ag-GO | High surface area, rational porosity, homogenized conductive pathways, cost efficiency, chemical stability, hierarchical structure, low onset potential, dual network Improved intimate contact between active and conducting components, high wettability, lowers local working function, single-atom active sites | Exposure to extreme conditions such as high current densities or extended operation times lead to structural degradations, competing reactions may affect selectivity |
| Single-atom-doped C gels | (Fe, Co, Ni, Cu)-doped N-containing carbon | Provides industrial level current density over 100 mA cm−2 | Optimization of different reaction conditions is challenging for designing a universal catalyst, scaling-up production, aggregation or leaching, catalyst poisoning |
| Transition metal carbide gels | Fe3C | Broadening d-orbitals induces high catalytic activity | Aggregation causes crystallization growth |
| Metal–organic gels | Co/Ni-containing organic gels | Efficient mass and charge transport, abundant active defect sites | Potential metal ion leaching within organic gel, temperature sensitivity |
| Nanostructured supramolecular gels | Guanosine-based supramolecular gels | Increased wettability, improved access to electrolytes, soft functional materials, tunable functionalization, composition, hydrophilic features, 3D network, hierarchical structure, efficient electron transfer chassis, effective gas diffusion | Structural stability under repeated cycle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bari, G.A.K.M.R.; Jeong, J.-H. Comprehensive Insights and Advancements in Gel Catalysts for Electrochemical Energy Conversion. Gels 2024, 10, 63. https://doi.org/10.3390/gels10010063
Bari GAKMR, Jeong J-H. Comprehensive Insights and Advancements in Gel Catalysts for Electrochemical Energy Conversion. Gels. 2024; 10(1):63. https://doi.org/10.3390/gels10010063
Chicago/Turabian StyleBari, Gazi A. K. M. Rafiqul, and Jae-Ho Jeong. 2024. "Comprehensive Insights and Advancements in Gel Catalysts for Electrochemical Energy Conversion" Gels 10, no. 1: 63. https://doi.org/10.3390/gels10010063
APA StyleBari, G. A. K. M. R., & Jeong, J.-H. (2024). Comprehensive Insights and Advancements in Gel Catalysts for Electrochemical Energy Conversion. Gels, 10(1), 63. https://doi.org/10.3390/gels10010063







