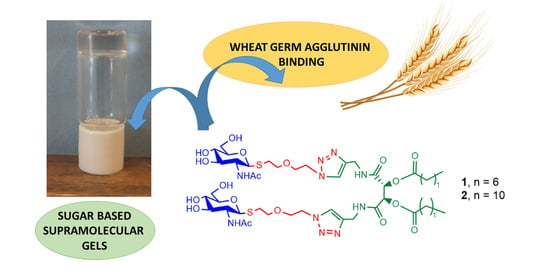
Amphiphilic Low-Molecular-Weight Gelators Bearing β-S-N-Acetylglucosamine Linked to a Tartaric Acid Scaffold: Synthesis, Self-Assembly and Wheat Germ Agglutinin Binding
Abstract
:1. Introduction
2. Results and Discussion
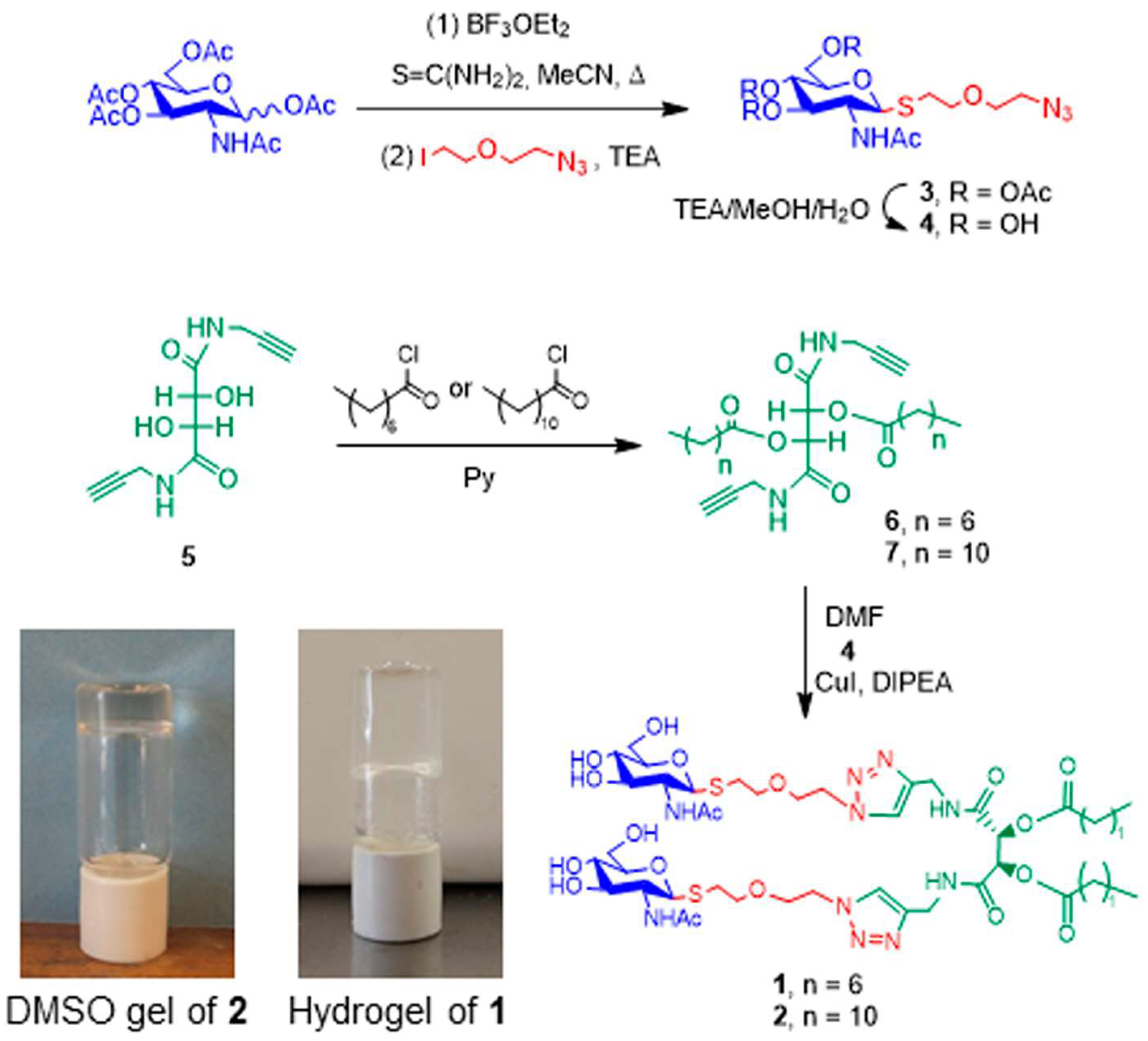
2.1. Synthesis of Amphiphiles
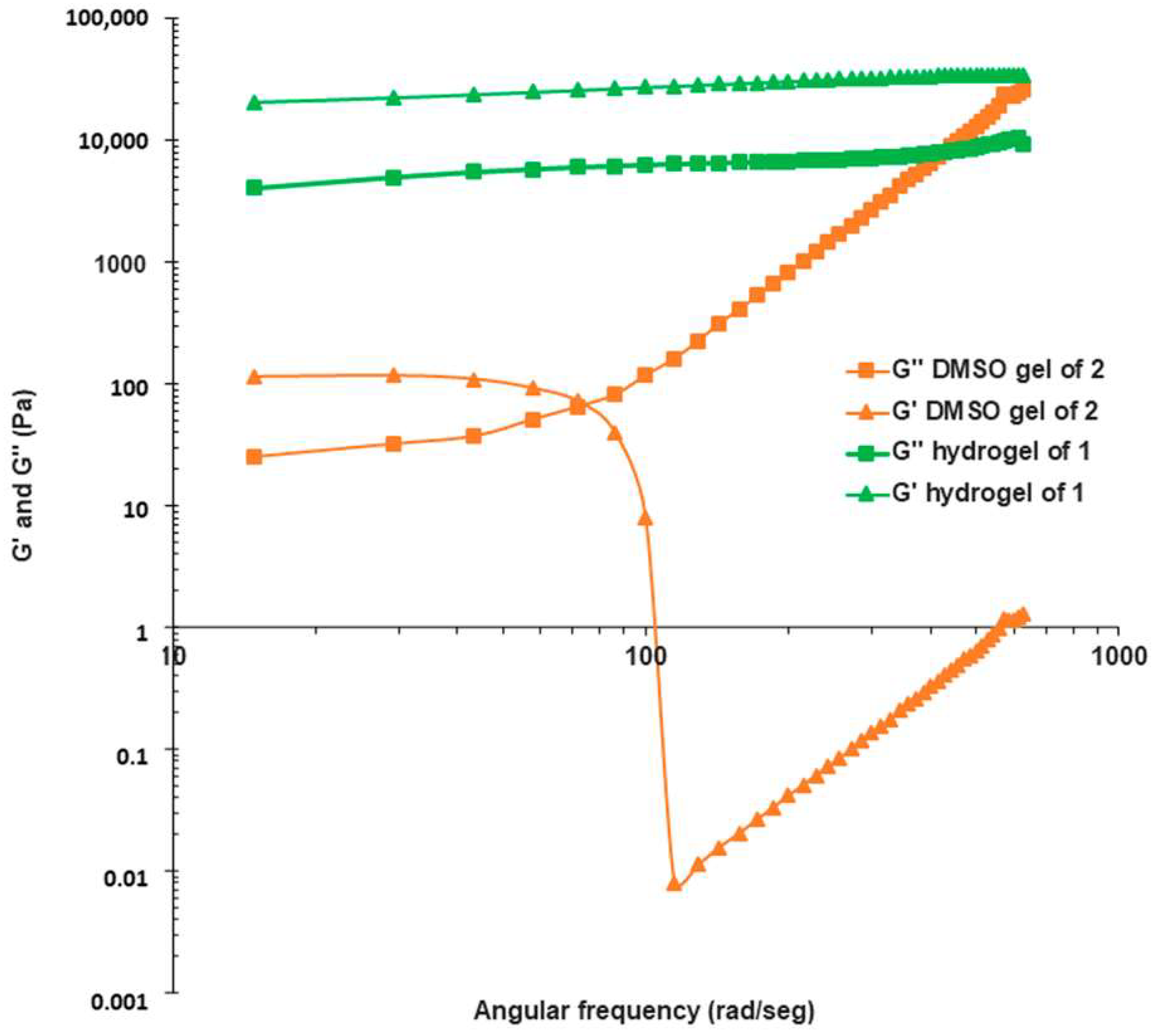
2.2. Gelation Scope and Characterization of Supramolecular Gels
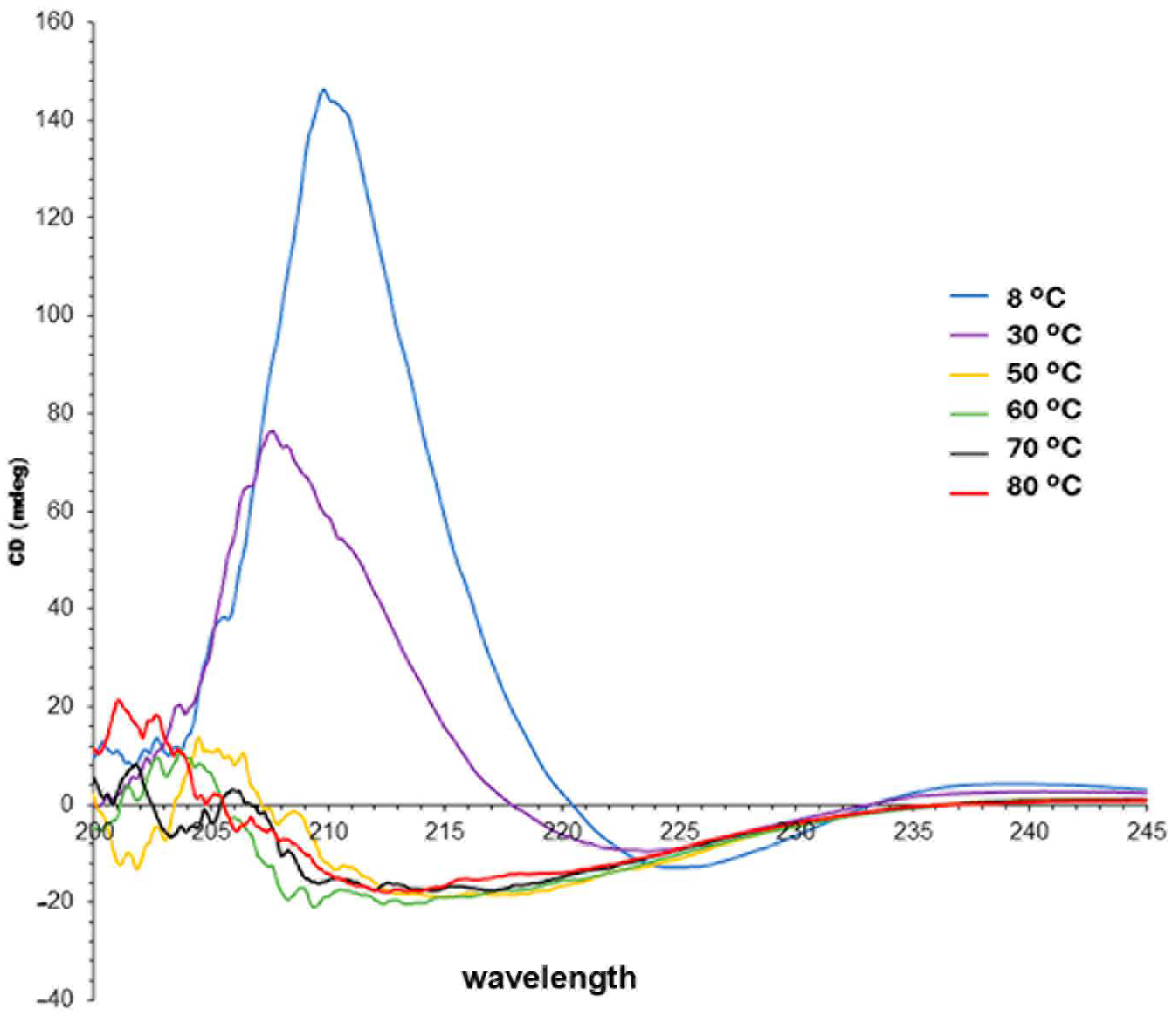
2.3. Circular Dichroism and Chirality of the Assemblies
2.4. Wheat Germ Lectin-Binding Assay
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. General Methods
4.3. Synthetic Methodologies
4.3.1. 2-(2-Azidoethoxy)ethanol
4.3.2. 1-Azido-2-(2-iodoethoxy)ethane
4.3.3. 2-(2-Azidoethoxy)ethyl 2-acetamido-3,4,5-tri-O-acetyl-2-deoxy-1-thio-β-d-glucopyranoside 3
4.3.4. 2-(2-Azidoethoxy)ethyl 2-acetamido-2-deoxy-1-thio-β-D-glucopyranoside 4
4.3.5. General Procedure for the Click Reaction
4.4. Characterization
4.4.1. Gelation Assays and Minimal Concentration for Gelation (MCG)
4.4.2. Scanning Electron Microscopy (SEM)
4.4.3. Turbidity Assay with Wheat Germ Agglutinin (WGA)
4.4.4. Circular Dichroism
4.4.5. Transmission Electron Microscopy (TEM)
4.4.6. X-ray Powder Diffraction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorrenti, A.; Illa, O.; Ortuño, R.M. Amphiphiles in Aqueous Solution: Well beyond a Soap Bubble. Chem. Soc. Rev. 2013, 42, 8200–8219. [Google Scholar] [CrossRef] [PubMed]
- Argudo, P.G.; Spitzer, L.; Jerome, F.; Cramail, H.; Camacho, L.; Lecommandoux, S. Design and Self-Assembly of Sugar-Based Amphiphiles: Spherical to Cylindrical Micelles. Langmuir 2022, 38, 7535–7544. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S. Aqueous Sugar-Based Amphiphile Systems: Recent Advances in Phase Behavior and Nanoarchitectonics. J. Oleo Sci. 2023, 72, ess22391. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Mohnen, D.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. (Eds.) Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017. [Google Scholar]
- Ambrosi, M.; Cameron, N.R.; Davis, B.G. Lectins: Tools for the Molecular Understanding of the Glycocode. Org. Biomol. Chem. 2005, 3, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gupta, G.S. Applications of Mannose-Binding Lectins and Mannan Glycoconjugates in Nanomedicine. J. Nanopart. Res. 2022, 24, 228. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhang, Q.; Wang, S.; Bharate, P.; Varela-Aramburu, S.; Lu, M.; Seeberger, P.H.; Yin, J. Tumour-Targeted Drug Delivery with Mannose-Functionalized Nanoparticles Self-Assembled from Amphiphilic Β-Cyclodextrins. Chem. Eur. J. 2016, 22, 15216–15221. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, M.; Chandra, N. Lectins. Curr. Opin. Struct. Biol. 1999, 9, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Katoch, R.; Tripathi, A. Research Advances and Prospects of Legume Lectins. J. Biosci. 2021, 46, 104. [Google Scholar] [CrossRef]
- Barre, A.; Van Damme, E.J.M.; Klonjkowski, B.; Simplicien, M.; Sudor, J.; Benoist, H.; Rougé, P. Legume Lectins with Different Specificities as Potential Glycan Probes for Pathogenic Enveloped Viruses. Cells 2022, 11, 339. [Google Scholar] [CrossRef]
- Cavada, B.S.; de Oliveira, M.V.; Osterne, V.J.S.; Pinto-Junior, V.R.; Martins, F.W.V.; Correia-Neto, C.; Pinheiro, R.F.; Leal, R.B.; Nascimento, K.S. Recent Advances in the Use of Legume Lectins for the Diagnosis and Treatment of Breast Cancer. Biochimie 2023, 208, 100–116. [Google Scholar] [CrossRef]
- Balčiūnaitė-Murzienė, G.; Dzikaras, M. Wheat Germ Agglutinin—From Toxicity to Biomedical Applications. Appl. Sci. 2021, 11, 884. [Google Scholar] [CrossRef]
- Goyard, D.; Ortiz, A.M.-S.; Boturyn, D.; Renaudet, O. Multivalent Glycocyclopeptides: Conjugation Methods and Biological Applications. Chem. Soc. Rev. 2022, 51, 8756–8783. [Google Scholar] [CrossRef] [PubMed]
- Schwefel, D.; Maierhofer, C.; Beck, J.G.; Seeberger, S.; Diederichs, K.; Möller, H.M.; Welte, W.; Wittmann, V. Structural Basis of Multivalent Binding to Wheat Germ Agglutinin. J. Am. Chem. Soc. 2010, 132, 8704–8719. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N.; Lis, H. Lectins; Springer: Dordrecht, The Netherlands, 2007; ISBN 978-1-4020-6605-4. [Google Scholar]
- Auth, J.; Fröba, M.; Große, M.; Rauch, P.; Ruetalo, N.; Schindler, M.; Morokutti-Kurz, M.; Graf, P.; Dolischka, A.; Prieschl-Grassauer, E.; et al. Lectin from Triticum Vulgaris (WGA) Inhibits Infection with SARS-CoV-2 and Its Variants of Concern Alpha and Beta. Int. J. Mol. Sci. 2021, 22, 10205. [Google Scholar] [CrossRef] [PubMed]
- Pellegrina, C.D.; Perbellini, O.; Scupoli, M.T.; Tomelleri, C.; Zanetti, C.; Zoccatelli, G.; Fusi, M.; Peruffo, A.; Rizzi, C.; Chignola, R. Effects of Wheat Germ Agglutinin on Human Gastrointestinal Epithelium: Insights from an Experimental Model of Immune/Epithelial Cell Interaction. Toxicol. Appl. Pharmacol. 2009, 237, 146–153. [Google Scholar] [CrossRef]
- Van Buul, V.J.; Brouns, F.J.P.H. Health Effects of Wheat Lectins: A Review. J. Cereal Sci. 2014, 59, 112–117. [Google Scholar] [CrossRef]
- Montenegro, H.O.; Di Chenna, P.H.; Spagnuolo, C.C.; Uhrig, M.L. Multivalent Assembly of a Pyrene Functionalized Thio-N-Acetylglucosamine: Synthesis, Spectroscopic and WGA Binding Studies. Carbohydr. Res. 2019, 479, 6–12. [Google Scholar] [CrossRef]
- Morris, J.; Bietsch, J.; Bashaw, K.; Wang, G. Recently Developed Carbohydrate Based Gelators and Their Applications. Gels 2021, 7, 24. [Google Scholar] [CrossRef]
- Rosa Nunes, D.; Reche-Tamayo, M.; Ressouche, E.; Raynal, M.; Isare, B.; Foury-Leylekian, P.; Albouy, P.-A.; Brocorens, P.; Lazzaroni, R.; Bouteiller, L. Organogel Formation Rationalized by Hansen Solubility Parameters: Shift of the Gelation Sphere with the Gelator Structure. Langmuir 2019, 35, 7970–7977. [Google Scholar] [CrossRef]
- Liu, M.; Ouyang, G.; Niu, D.; Sang, Y. Supramolecular Gelatons: Towards the Design of Molecular Gels. Org. Chem. Front. 2018, 5, 2885–2900. [Google Scholar] [CrossRef]
- Dastidar, P. Designing Supramolecular Gelators: Challenges, Frustrations, and Hopes. Gels 2019, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Prathap, A.; Sureshan, K.M. Sugar-Based Organogelators for Various Applications. Langmuir 2019, 35, 6005–6014. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, P.; Roy, R.; Parveen, R.; Sarkar, K. Supramolecular Synthon Approach in Designing Molecular Gels for Advanced Therapeutics. Adv. Ther. 2019, 2, 1800061. [Google Scholar] [CrossRef]
- Mukherjee, A. Building upon Supramolecular Synthons: Some Aspects of Crystal Engineering. Cryst. Growth Des. 2015, 15, 3076–3085. [Google Scholar] [CrossRef]
- Mittal, A.; Krishna; Aarti; Prasad, S.; Mishra, P.K.; Sharma, S.K.; Parshad, B. Self-Assembly of Carbohydrate-Based Small Amphiphiles and Their Applications in Pathogen Inhibition and Drug Delivery: A Review. Mater. Adv. 2021, 2, 3459–3473. [Google Scholar] [CrossRef]
- Faig, A.; Arthur, T.D.; Fitzgerald, P.O.; Chikindas, M.; Mintzer, E.; Uhrich, K.E. Biscationic Tartaric Acid-Based Amphiphiles: Charge Location Impacts Antimicrobial Activity. Langmuir 2015, 31, 11875–11885. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.E.; Di Chenna, P.H.; Lesur, D.; Wolosiuk, A.; Kovensky, J.; Uhrig, M.L. Chirality Inversion, Supramolecular Hydrogelation and Lectin Binding of Two Thiolactose Amphiphiles Constructed on a Di-Lauroyl-L-Tartaric Acid Scaffold. New J. Chem. 2017, 41, 14754–14765. [Google Scholar] [CrossRef]
- Almeida, M.M.; Perez, K.R.; Faig, A.; Uhrich, K.E.; Riske, K.A. Location of the Positive Charges in Cationic Amphiphiles Modulates Their Mechanism of Action against Model Membranes. Langmuir 2019, 35, 14117–14123. [Google Scholar] [CrossRef]
- Shankar, B.V.; Patnaik, A. A New PH and Thermo-Responsive Chiral Hydrogel for Stimulated Release. J. Phys. Chem. B 2007, 111, 9294–9300. [Google Scholar] [CrossRef]
- Novotný, M.; Hrabálek, A.; Janůšová, B.; Novotný, J.; Vávrová, K. Dicarboxylic Acid Esters as Transdermal Permeation Enhancers: Effects of Chain Number and Geometric Isomers. Bioorg. Med. Chem. Lett. 2009, 19, 344–347. [Google Scholar] [CrossRef]
- Raghavan, V.; Polavarapu, P.L. Specific Optical Rotation Is a Versatile Tool for the Identification of Critical Micelle Concentration and Micellar Growth of Tartaric Acid–Based Diastereomeric Amphiphiles. Chirality 2017, 29, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Kosma, P.; Wrodnigg, T.; Stütz, A. (Eds.) Carbohydrate Chemistry; Series: Proven Synthetic Methods; CRC Press: Boca Raton, FL, USA, 2021; Volume 5, ISBN 9781351256087. [Google Scholar]
- Weiss, R.G. Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Weiss, R.G., Terech, P., Eds.; Springer: Dordrecht, The Netherlands, 2006; ISBN 978-1-4020-3352-0. [Google Scholar]
- Dawn, A.; Kumari, H. Low Molecular Weight Supramolecular Gels Under Shear: Rheology as the Tool for Elucidating Structure-Function Correlation. Chem. Eur. J. 2018, 24, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, J.J.; Toone, E.J. The Cluster Glycoside Effect. Chem. Rev. 2002, 102, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-R.; An, H.-W.; Wang, Y.-Q.; Zhang, J.-C.; Li, X.-L. Multivalent Glycoclusters Constructed by Chiral Self-Assembly of Mannose Functionalized Perylene Bisimide. Org. Biomol. Chem. 2013, 11, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Cairo, C.W.; Gestwicki, J.E.; Kanai, M.; Kiessling, L.L. Control of Multivalent Interactions by Binding Epitope Density. J. Am. Chem. Soc. 2002, 124, 1615–1619. [Google Scholar] [CrossRef]
- Gan, W.; Cao, X.; Shi, Y.; Gao, H. Chain-growth Polymerization of Azide–Alkyne Difunctional Monomer: Synthesis of Star Polymer with Linear Polytriazole Arms from a Core. J. Polym. Sci. 2020, 58, 84–90. [Google Scholar] [CrossRef]
- Cristófalo, A.E.; Cagnoni, A.J.; Uhrig, M.L. Synthesis of N-Acetylglucosamine and N-Acetylallosamine Resorcinarene-Based Multivalent β-Thio-Glycoclusters: Unexpected Affinity of N-Acetylallosamine Ligands towards Wheat Germ Agglutinin. Org. Biomol. Chem. 2020, 18, 6853–6865. [Google Scholar] [CrossRef]




| Compound 1 | Compound 2 | |||
|---|---|---|---|---|
| Solvent | Sol/Gel 1 | MCG (wt%) | Sol/Gel 1 | MCG (wt%) |
| Water | G | 0.3 | P | - |
| Chloroform | I | - | I | - |
| Dichloromethane | I | - | I | - |
| Acetone | I | - | I | - |
| Ethanol | P | - | P | - |
| Ethyl acetate | I | - | I | - |
| n-Hexane | I | - | I | - |
| Ethanol:water 2:1 | S | - | P | - |
| Methanol:water 2:1 | S | - | P | - |
| DMSO | S | - | G | 0.8 |
| Methanol | P | - | S | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña García, V.L.; Di Chenna, P.H.; Uhrig, M.L. Amphiphilic Low-Molecular-Weight Gelators Bearing β-S-N-Acetylglucosamine Linked to a Tartaric Acid Scaffold: Synthesis, Self-Assembly and Wheat Germ Agglutinin Binding. Gels 2024, 10, 5. https://doi.org/10.3390/gels10010005
Peña García VL, Di Chenna PH, Uhrig ML. Amphiphilic Low-Molecular-Weight Gelators Bearing β-S-N-Acetylglucosamine Linked to a Tartaric Acid Scaffold: Synthesis, Self-Assembly and Wheat Germ Agglutinin Binding. Gels. 2024; 10(1):5. https://doi.org/10.3390/gels10010005
Chicago/Turabian StylePeña García, Vicente Leafar, Pablo Héctor Di Chenna, and María Laura Uhrig. 2024. "Amphiphilic Low-Molecular-Weight Gelators Bearing β-S-N-Acetylglucosamine Linked to a Tartaric Acid Scaffold: Synthesis, Self-Assembly and Wheat Germ Agglutinin Binding" Gels 10, no. 1: 5. https://doi.org/10.3390/gels10010005
APA StylePeña García, V. L., Di Chenna, P. H., & Uhrig, M. L. (2024). Amphiphilic Low-Molecular-Weight Gelators Bearing β-S-N-Acetylglucosamine Linked to a Tartaric Acid Scaffold: Synthesis, Self-Assembly and Wheat Germ Agglutinin Binding. Gels, 10(1), 5. https://doi.org/10.3390/gels10010005