Interfacial Rheological Study of β-Casein/Pectin Mixtures at the Air/Water Interface
Abstract
1. Introduction
2. Results and Discussion
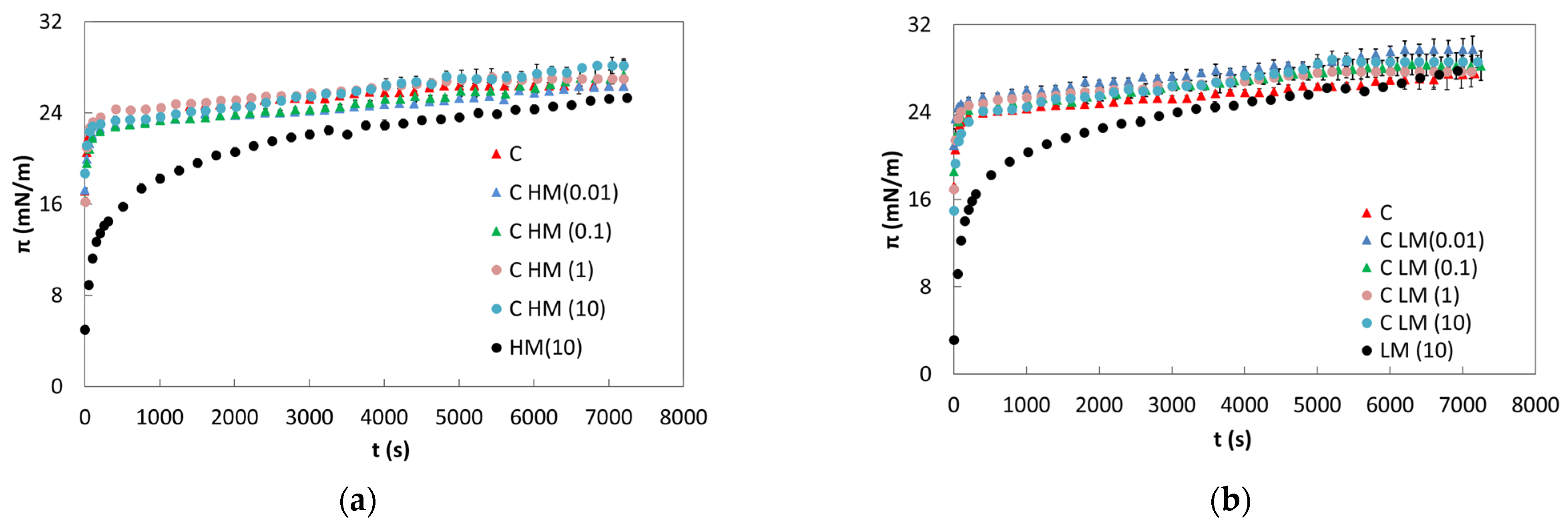
2.1. Static Measurements
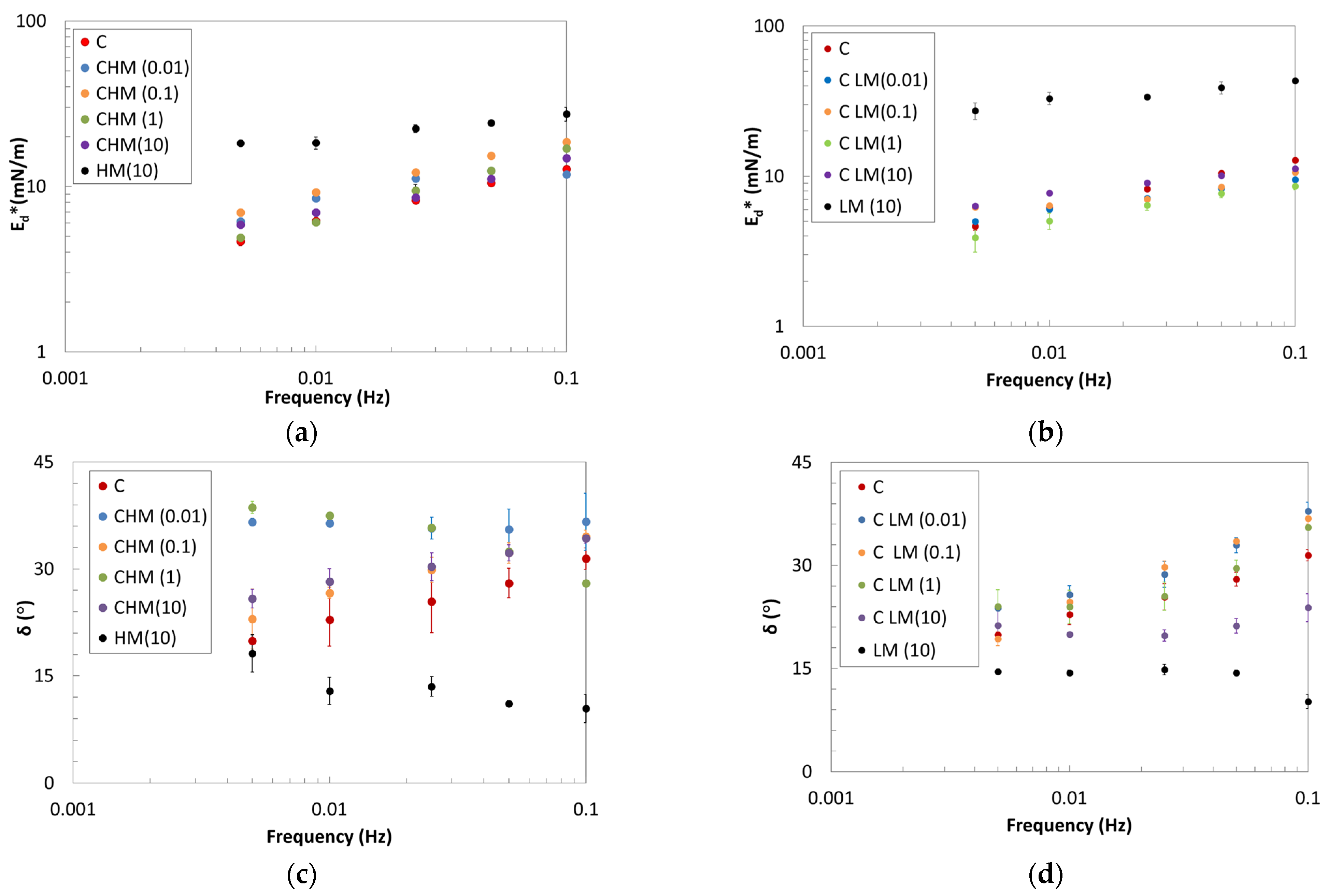
2.2. Oscillating Dilatational Rheology
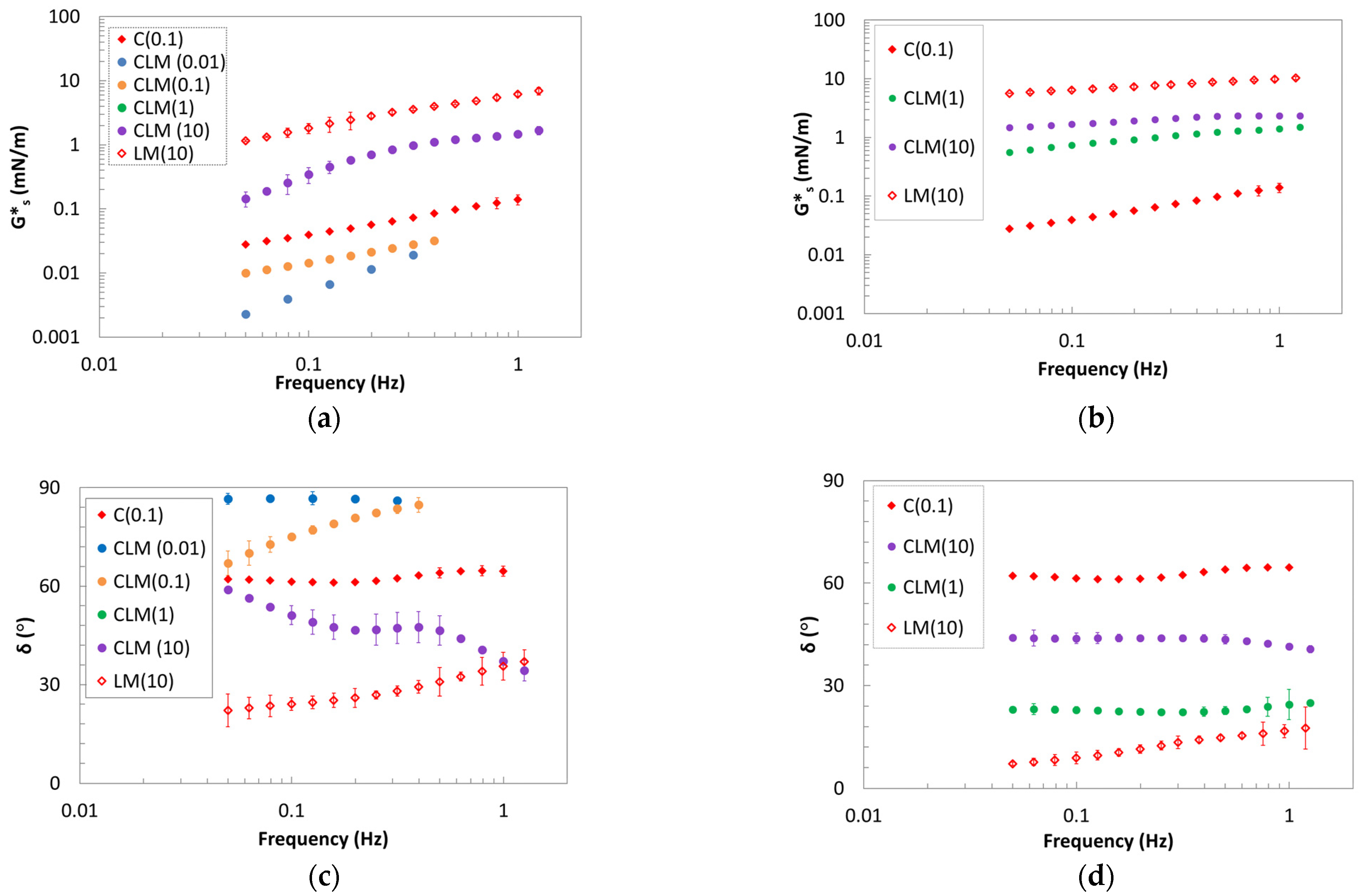
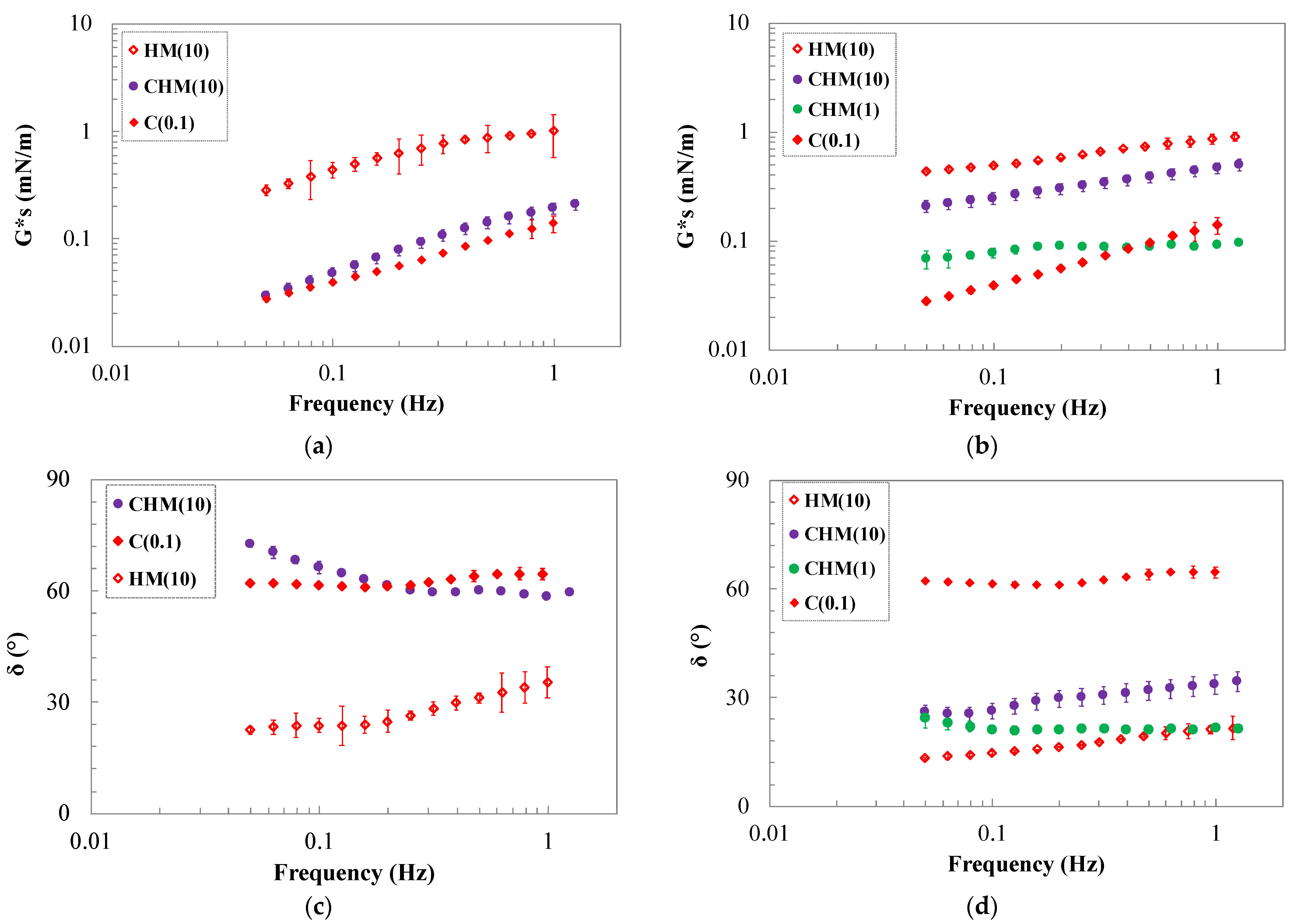
2.3. Oscillating Shear Rheology
3. Conclusions
4. Materials and Methods
4.1. Materials and Sample Preparation
4.2. Static Measurements and Adsorption Kinetics
4.3. Dilatational Rheological Analysis
4.4. Shear Rheological Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bos, M.A.; Van Vliet, T. Interfacial Rheological Properties of Adsorbed Protein Layers and Surfactants: A Review. Adv. Colloid Interface Sci. 2001, 91, 437–471. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Mixed Biopolymers at Interfaces: Competitive Adsorption and Multilayer Structures. Food Hydrocoll. 2011, 25, 1966–1983. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Ganzevles, R.A.; Cohen Stuart, M.A.; van Vliet, T.; de Jongh, H.H.J. Use of Polysaccharides to Control Protein Adsorption to the Air-Water Interface. Food Hydrocoll. 2006, 20, 872–878. [Google Scholar] [CrossRef]
- Dickinson, E. Adsorbed Protein Layers at Fluid Interfaces: Interactions, Structure and Surface Rheology. Colloids Surf. B Biointerfaces 1999, 15, 161–176. [Google Scholar] [CrossRef]
- Perez, A.A.; Sánchez, C.C.; Patino, J.M.R.; Rubiolo, A.C.; Santiago, L.G. Milk Whey Proteins and Xanthan Gum Interactions in Solution and at the Air–Water Interface: A Rheokinetic Study. Colloids Surf. B Biointerfaces 2010, 81, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.L.; Agnely, F. Proteins, Polysaccharides, and Their Complexes Used as Stabilizers for Emulsions: Alternatives to Synthetic Surfactants in the Pharmaceutical Field? Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Camino, N.A.; Pérez, O.E.; Sanchez, C.C.; Rodriguez Patino, J.M.; Pilosof, A.M.R. Hydroxypropylmethylcellulose Surface Activity at Equilibrium and Adsorption Dynamics at the Air-Water and Oil-Water Interfaces. Food Hydrocoll. 2009, 23, 2359–2368. [Google Scholar] [CrossRef]
- Pérez, O.E.; Sánchez, C.C.; Pilosof, A.M.R.; Rodríguez Patino, J.M. Kinetics of Adsorption of Whey Proteins and Hydroxypropyl-Methyl-Cellulose Mixtures at the Air–Water Interface. J. Colloid Interface Sci. 2009, 336, 485–496. [Google Scholar] [CrossRef]
- Fischer, P. Rheology of Interfacial Protein-Polysaccharide Composites. Eur. Phys. J. Spec. Top. 2013, 222, 73–81. [Google Scholar] [CrossRef][Green Version]
- Rodríguez Patino, J.M.; Pilosof, A.M.R. Protein–Polysaccharide Interactions at Fluid Interfaces. Food Hydrocoll. 2011, 25, 1925–1937. [Google Scholar] [CrossRef]
- Wusigale; Liang, L.; Luo, Y. Casein and Pectin: Structures, Interactions, and Applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, D.; Zeng, M.; Chen, J.; Mei, X.; Cao, X.; Liu, J. Milk β-Casein as Delivery Systems for Luteolin: Multi-Spectroscopic, Computer Simulations, and Biological Studies. J. Food Biochem. 2022, 46, e14133. [Google Scholar] [CrossRef]
- Singh, H. Aspects of Milk-Protein-Stabilised Emulsions. Food Hydrocoll. 2011, 25, 1938–1944. [Google Scholar] [CrossRef]
- Bantchev, G.B.; Schwartz, D.K. Surface Shear Rheology of β-Casein Layers at the Air/Solution Interface: Formation of a Two-Dimensional Physical Gel. Langmuir 2003, 19, 2673–2682. [Google Scholar] [CrossRef]
- de Kruif, C.G.; Huppertz, T.; Urban, V.S.; Petukhov, A.V. Casein Micelles and Their Internal Structure. Adv. Colloid Interface Sci. 2012, 171–172, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Głąb, T.K.; Boratyński, J. Potential of Casein as a Carrier for Biologically Active Agents. Top. Curr. Chem. 2017, 375, 71. [Google Scholar] [CrossRef] [PubMed]
- Dauphas, S.; Mouhous-Riou, N.; Metro, B.; MacKie, A.R.; Wilde, P.J.; Anton, M.; Riaublanc, A. The Supramolecular Organisation of β-Casein: Effect on Interfacial Properties. Food Hydrocoll. 2005, 19, 387–393. [Google Scholar] [CrossRef]
- Bhat, M.Y.; Dar, T.A.; Singh, L.R. Casein Proteins: Structural and Functional Aspects. In Milk Proteins; Dar, T.A., Ed.; IntechOpen: Rijeka, Croatia, 2016; Ch. 1; ISBN 978-953-51-2537-2. [Google Scholar]
- Eskin, N.A.M.; Goff, H.D. Chapter 4—Milk. In Biochemistry of Foods, 3rd ed.; Eskin, N.A.M., Shahidi, F., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 187–214. ISBN 978-0-12-242352-9. [Google Scholar]
- Beverung, C.J.; Radke, C.J.; Blanch, H.W. Protein Adsorption at the Oil/Water Interface: Characterization of Adsorption Kinetics by Dynamic Interfacial Tension Measurements. Biophys. Chem. 1999, 81, 59–80. [Google Scholar] [CrossRef]
- Perez, A.A.; Sánchez, C.C.; Rodríguez Patino, J.M.; Rubiolo, A.C.; Santiago, L.G. Surface Adsorption Behaviour of Milk Whey Protein and Pectin Mixtures under Conditions of Air-Water Interface Saturation. Colloids Surf. B Biointerfaces 2011, 85, 306–315. [Google Scholar] [CrossRef]
- Baldino, N.; Mileti, O.; Lupi, F.; Gabriele, D. Chapter 1—Interfacial Rheology of Food: Protein as a Model Food. In Advances in Food Rheology and Its Applications, 2nd ed.; Ahmed, J., Basu, S., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 3–26. ISBN 978-0-12-823983-4. [Google Scholar]
- Baldino, N.; Mileti, O.; Lupi, F.R.; Gabriele, D. Rheological Surface Properties of Commercial Citrus Pectins at Different PH and Concentration. LWT 2018, 93, 124–130. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and Uses of Pectin—A Review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Alba, K.; Kontogiorgos, V. Pectin at the Oil-Water Interface: Relationship of Molecular Composition and Structure to Functionality. Food Hydrocoll. 2017, 68, 211–218. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Belshaw, N.J.; Waldron, K.W.; Morris, V.J. Pectin—An Emerging New Bioactive Food Polysaccharide. Trends Food Sci. Technol. 2012, 24, 64–73. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New Insights into an Old Polymer Are Starting to Gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Ngouémazong, E.; Christiaens, S.; Shpigelman, A.; van Loey, A.; Hendrickx, M. The Emulsifying and Emulsion-Stabilizing Properties of Pectin: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Siew, C.K.; Williams, P.A. Role of Protein and Ferulic Acid in the Emulsification Properties of Sugar Beet Pectin. J. Agric. Food Chem. 2008, 56, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Lupi, F.R.; Gabriele, D.; Seta, L.; Baldino, N.; de Cindio, B.; Marino, R. Rheological Investigation of Pectin-Based Emulsion Gels for Pharmaceutical and Cosmetic Uses. Rheol. Acta 2015, 54, 41–52. [Google Scholar] [CrossRef]
- Fissore, E.N.; Rojas, A.M.; Gerschenson, L.N.; Williams, P.A. Butternut and Beetroot Pectins: Characterization and Functional Properties. Food Hydrocoll. 2013, 31, 172–182. [Google Scholar] [CrossRef]
- Funami, T.; Zhang, G.; Hiroe, M.; Noda, S.; Nakauma, M.; Asai, I.; Cowman, M.K.; Al-Assaf, S.; Phillips, G.O. Effects of the Proteinaceous Moiety on the Emulsifying Properties of Sugar Beet Pectin. Food Hydrocoll. 2007, 21, 1319–1329. [Google Scholar] [CrossRef]
- Yapo, B.M.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of Extraction Conditions on the Yield, Purity and Surface Properties of Sugar Beet Pulp Pectin Extracts. Food Chem. 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- Baeza, R.; Pilosof, A.M.R.; Sanchez, C.C.; Rodríguez Patino, J.M. Adsorption and Rheological Properties of Biopolymers at the Air-Water Interface. AIChE J. 2006, 52, 2627–2638. [Google Scholar] [CrossRef]
- Ganzevles, R.A.; Fokkink, R.; van Vliet, T.; Cohen Stuart, M.A.; de Jongh, H.H.J. Structure of Mixed β-Lactoglobulin/Pectin Adsorbed Layers at Air/Water Interfaces; a Spectroscopy Study. J. Colloid Interface Sci. 2008, 317, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Seta, L.; Baldino, N.; Gabriele, D.; Lupi, F.R.; de Cindio, B. Rheology and Adsorption Behaviour of β-Casein and β-Lactoglobulin Mixed Layers at the Sunflower Oil/Water Interface. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 669–677. [Google Scholar] [CrossRef]
- Graham, D.E.; Phillips, M.C. Proteins at Liquid Interfaces II: Adsorption Isotherms. J. Colloid Interface Sci. 1979, 70, 415–426. [Google Scholar] [CrossRef]
- Rafe, A.; Selahbarzin, S.; Kulozik, U.; Hesarinejad, M.A. Dilatational Rheology-Property Relationships of β-Lactoglobulin /High Methoxyl Pectin Mixtures in Aqueous Foams. Food Hydrocoll. 2022, 130, 107683. [Google Scholar] [CrossRef]
- Arboleya, J.-C.; Wilde, P.J. Competitive Adsorption of Proteins with Methylcellulose and Hydroxypropyl Methylcellulose. Food Hydrocoll. 2005, 19, 485–491. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Wang, W.-J.; Lin, L.-W.; Chen, L.-J. Systematic Effects of Bubble Volume on the Surface Tension Measured by Pendant Bubble Profiles. Colloids Surf. A Physicochem. Eng. Asp. 1996, 114, 31–39. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as Thickening and Gelling Agents in Food: A Critical Review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Arboleya, J.C.; García-Quiroga, M.; Lasa, D.; Oliva, O.; Luis-Aduriz, A. Effect of Highly Aerated Food on Expected Satiety. Int. J. Gastron. Food Sci. 2014, 2, 14–21. [Google Scholar] [CrossRef]
- Mileti, O.; Baldino, N.; Carmona, J.A.; Lupi, F.R.; Muñoz, J.; Gabriele, D. Shear and Dilatational Rheological Properties of Vegetable Proteins at the Air/Water Interface. Food Hydrocoll. 2022, 126, 107472. [Google Scholar] [CrossRef]
- Seta, L.; Baldino, N.; Gabriele, D.; Lupi, F.R.; De Cindio, B. The Effect of Surfactant Type on the Rheology of Ovalbumin Layers at the Air/Water and Oil/Water Interfaces. Food Hydrocoll. 2012, 29, 247–257. [Google Scholar] [CrossRef]
- Mileti, O.; Baldino, N.; Lupi, F.R.; Gabriele, D. Interfacial Behavior of Vegetable Protein Isolates at Sunflower Oil/Water Interface. Colloids Surf. B Biointerfaces 2023, 221, 113035. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.F.H.; Tordai, L. Time-Dependence of Boundary Tensions of Solutions I. The Role of Diffusion in Time-Effects. J. Chem. Phys. 1946, 14, 453–461. [Google Scholar] [CrossRef]
- Derkach, S.R.; Krägel, J.; Miller, R. Methods of Measuring Rheological Properties of Interfacial Layers (Experimental Methods of 2D Rheology). Colloid J. 2009, 71, 1–17. [Google Scholar] [CrossRef]
- Ravera, F.; Loglio, G.; Kovalchuk, V.I. Interfacial Dilational Rheology by Oscillating Bubble/Drop Methods. Curr. Opin. Colloid Interface Sci. 2010, 15, 217–228. [Google Scholar] [CrossRef]
- Dicharry, C.; Arla, D.; Sinquin, A.; Graciaa, A.; Bouriat, P. Stability of Water/Crude Oil Emulsions Based on Interfacial Dilatational Rheology. J. Colloid Interface Sci. 2006, 297, 785–791. [Google Scholar] [CrossRef]
- Brooks, C.F.; Fuller, G.G.; Frank, C.W.; Robertson, C.R. Interfacial Stress Rheometer to Study Rheological Transitions in Monolayers at the Air-Water Interface. Langmuir 1999, 15, 2450–2459. [Google Scholar] [CrossRef]

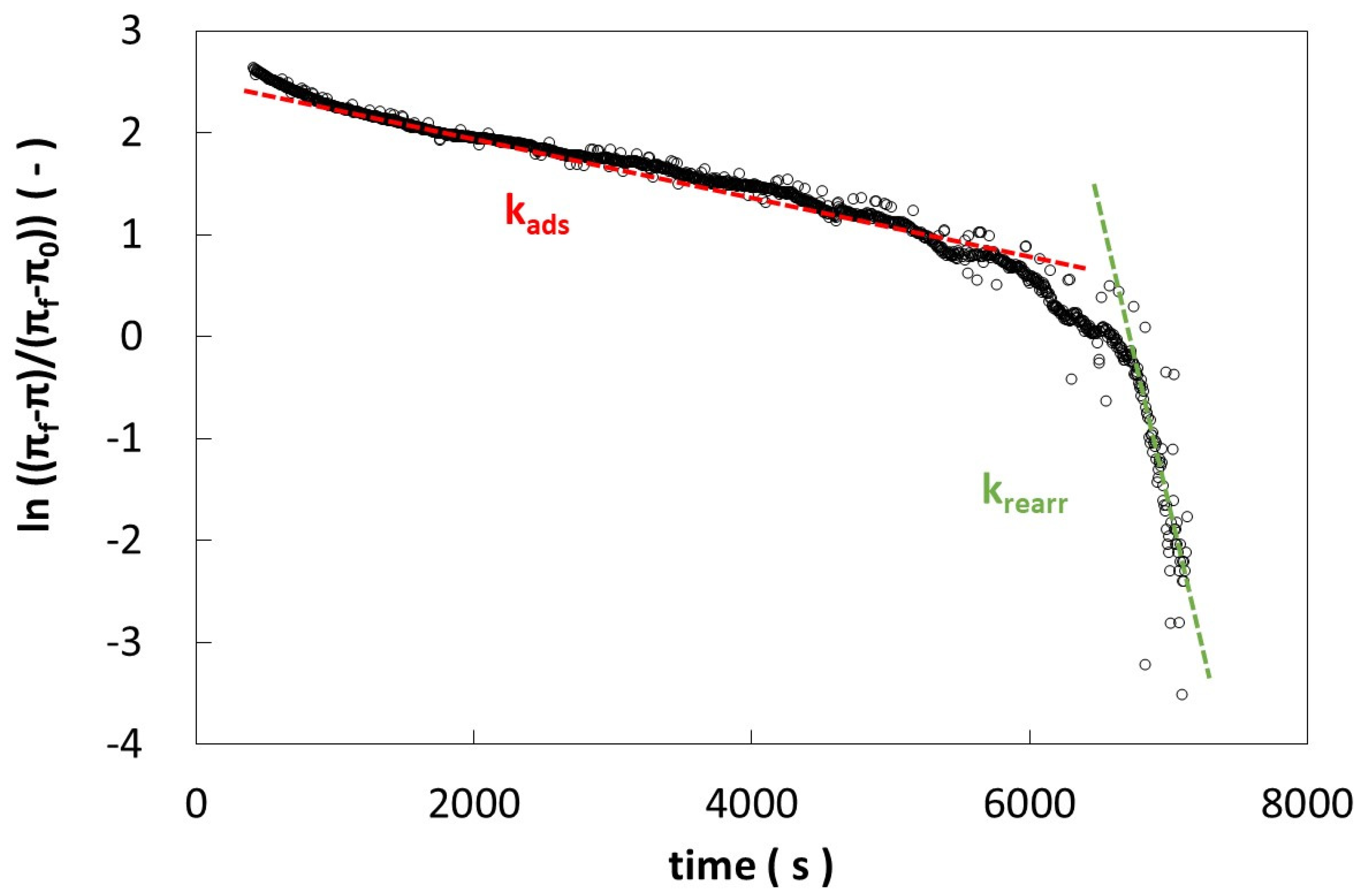
| Pectin (g/L) | β-Casein (g/L) | kads × 104 (s−1) | krearr × 104 (s−1) | ||
|---|---|---|---|---|---|
| CHM | CLM | CHM | CLM | ||
| 0 | 0.1 | 2.62 ± 0.01 | 2.62 ± 0.01 | 28.5 ± 0.6 | 28.5 ± 0.6 |
| 0.01 | 0.1 | 1.44 ± 0.03 | 2.12 ± 0.01 | 9.0 ± 0.2 | 9.3 ± 0.1 |
| 0.1 | 0.1 | 1.35 ± 0.01 | 1.56 ± 0.01 | 12.1 ± 0.1 | 12.1 ± 0.6 |
| 1 | 0.1 | 1.26 ± 0.02 | 1.34 ± 0.01 | 17.5 ± 0.3 | 12.5 ± 0.1 |
| 10 | 0.1 | 1.23 ± 0.01 | 1.49 ± 0.02 | 13.3 ± 0.3 | 12.1 ± 0.1 |
| 10 | 0 | 3.31 ± 0.09 | 3.04 ± 0.01 | 11.2 ± 0.05 | 19.43 ± 0.01 |
| Pectin (g/L) | β-Casein (g/L) | kd (mN/m·s) | nd (-) | ||
|---|---|---|---|---|---|
| CHM | CLM | CHM | CLM | ||
| 0 | 0.1 | 27 ± 1 | 27 ± 1 | 0.32 ± 0.01 | 0.32 ± 0.01 |
| 0.01 | 0.1 | 17 ± 2 | 15.3 ± 0.4 | 0.15 ± 0.04 | 0.21 ± 0.01 |
| 0.1 | 0.1 | 40 ± 1 | 15 ± 1 | 0.32 ± 0.01 | 0.19 ± 0.02 |
| 1 | 0.1 | 44 ± 1 | 15 ± 1 | 0.42 ± 0.01 | 0.24 ± 0.02 |
| 10 | 0.1 | 30 ± 2 | 16.9 ± 0.5 | 0.32 ± 0.02 | 0.17 ± 0.01 |
| 10 | 0 | 32 ± 3 | 59 ± 4 | 0.15 ± 0.02 | 0.14 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mileti, O.; Baldino, N.; Luzzi, S.; Lupi, F.R.; Gabriele, D. Interfacial Rheological Study of β-Casein/Pectin Mixtures at the Air/Water Interface. Gels 2024, 10, 41. https://doi.org/10.3390/gels10010041
Mileti O, Baldino N, Luzzi S, Lupi FR, Gabriele D. Interfacial Rheological Study of β-Casein/Pectin Mixtures at the Air/Water Interface. Gels. 2024; 10(1):41. https://doi.org/10.3390/gels10010041
Chicago/Turabian StyleMileti, Olga, Noemi Baldino, Stefania Luzzi, Francesca R. Lupi, and Domenico Gabriele. 2024. "Interfacial Rheological Study of β-Casein/Pectin Mixtures at the Air/Water Interface" Gels 10, no. 1: 41. https://doi.org/10.3390/gels10010041
APA StyleMileti, O., Baldino, N., Luzzi, S., Lupi, F. R., & Gabriele, D. (2024). Interfacial Rheological Study of β-Casein/Pectin Mixtures at the Air/Water Interface. Gels, 10(1), 41. https://doi.org/10.3390/gels10010041