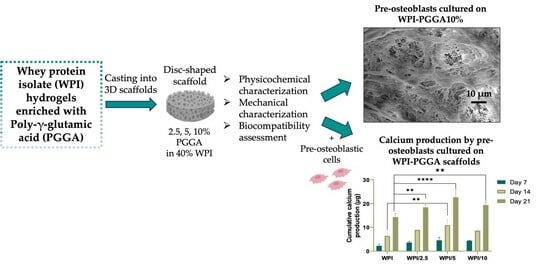
The Enrichment of Whey Protein Isolate Hydrogels with Poly-γ-Glutamic Acid Promotes the Proliferation and Osteogenic Differentiation of Preosteoblasts
Abstract
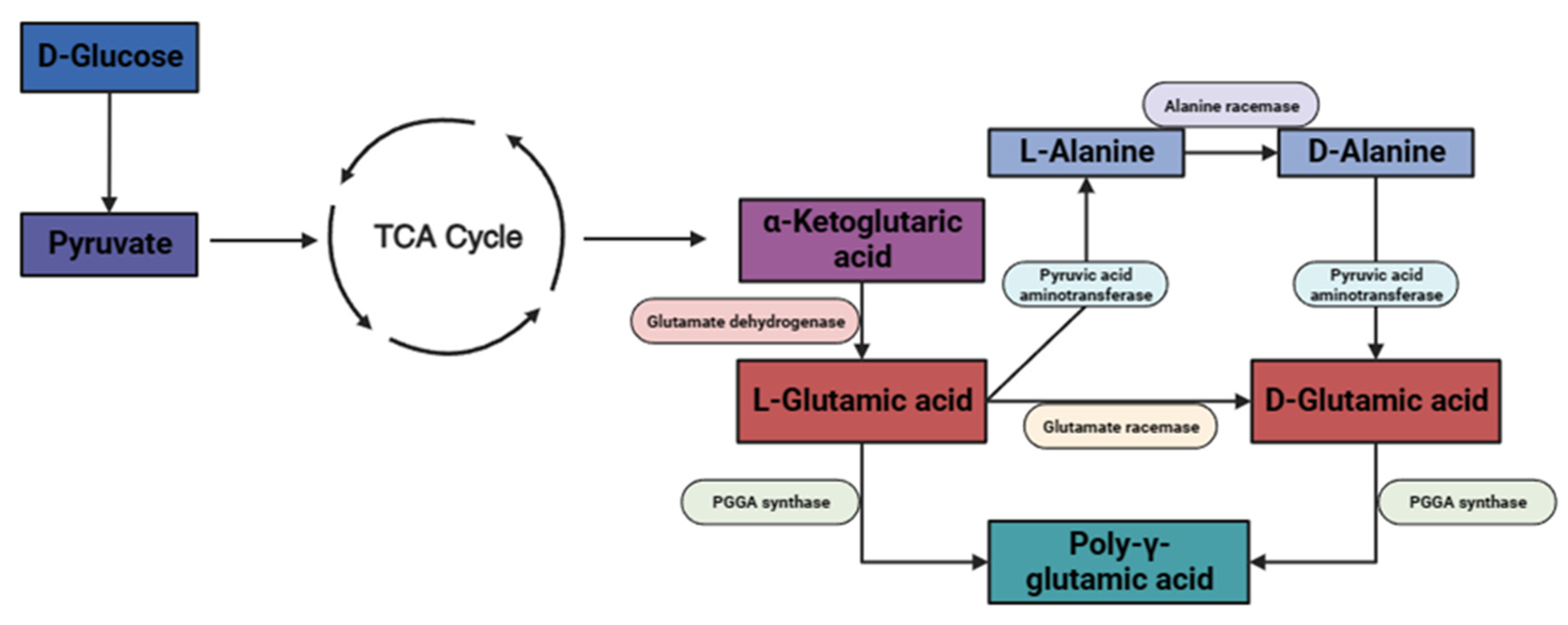
:1. Introduction
2. Results and Discussion
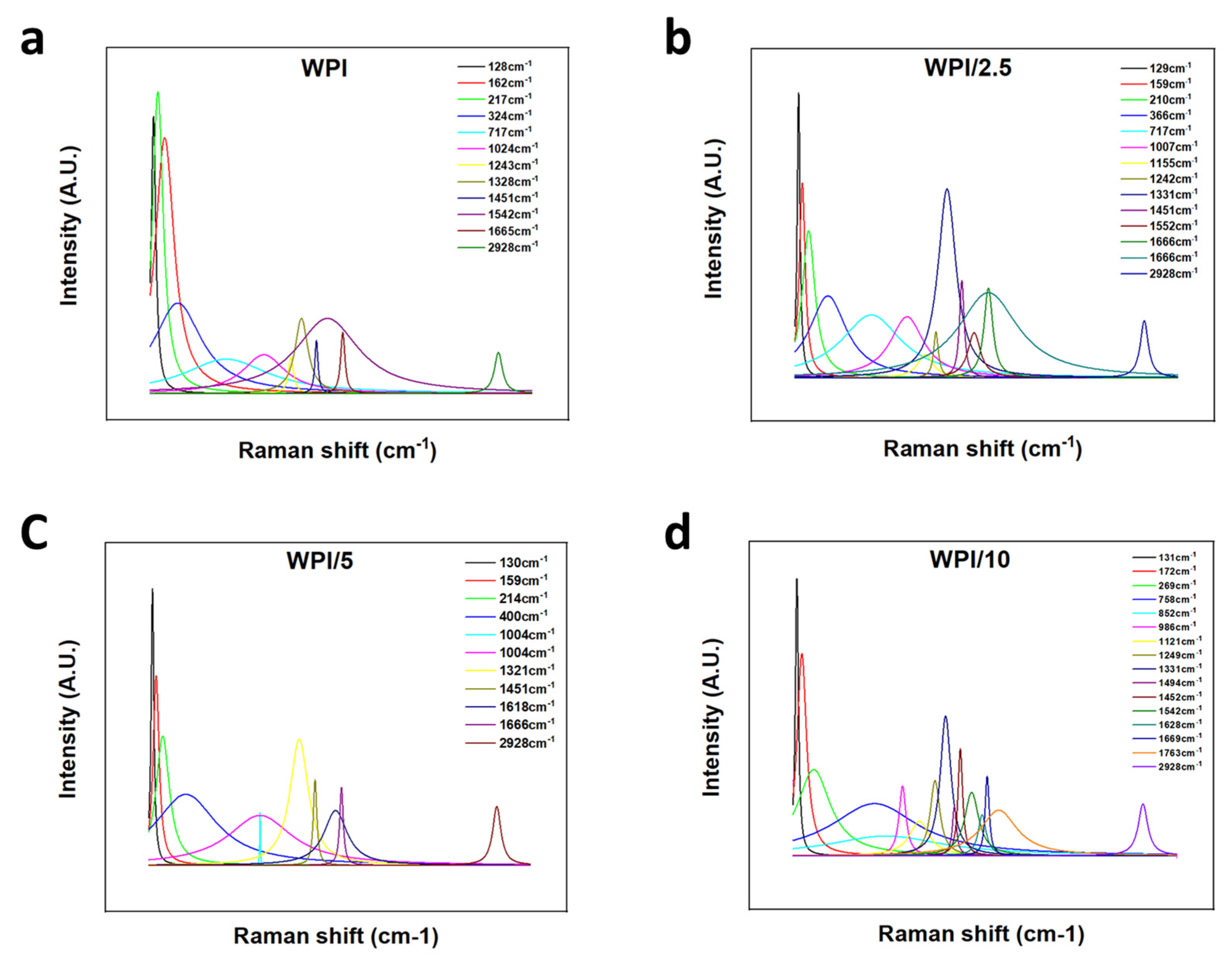
2.1. Raman Spectroscopy
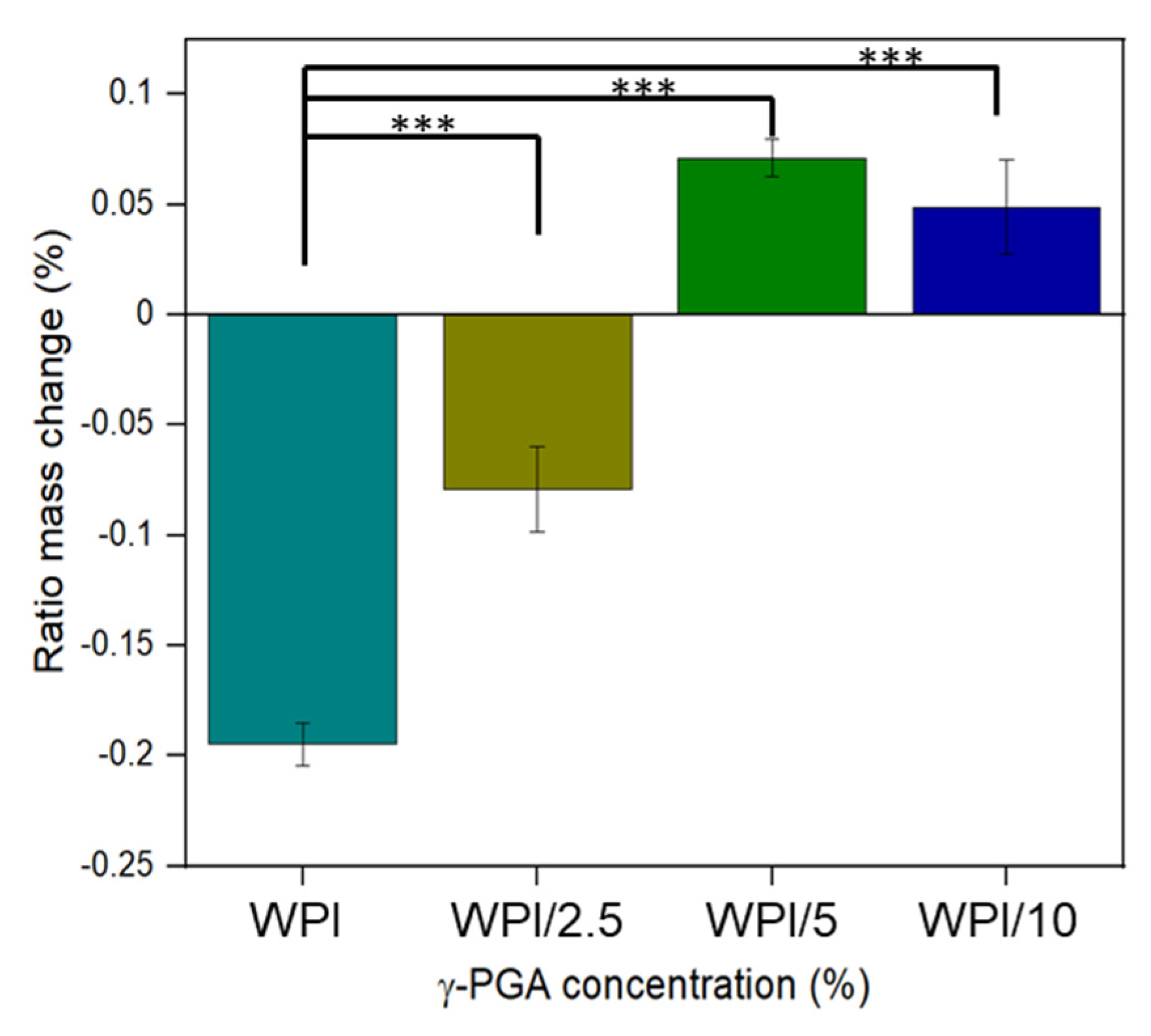
2.2. Swelling Analysis
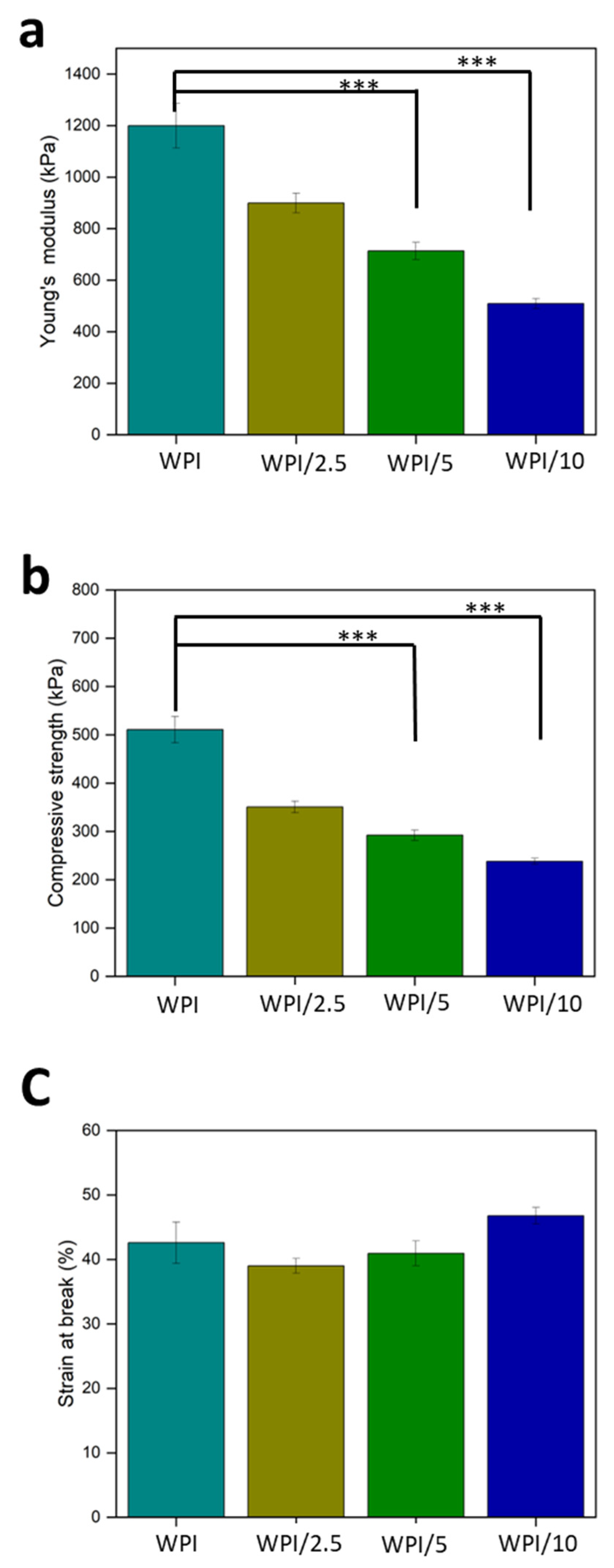
2.3. Compression Analysis
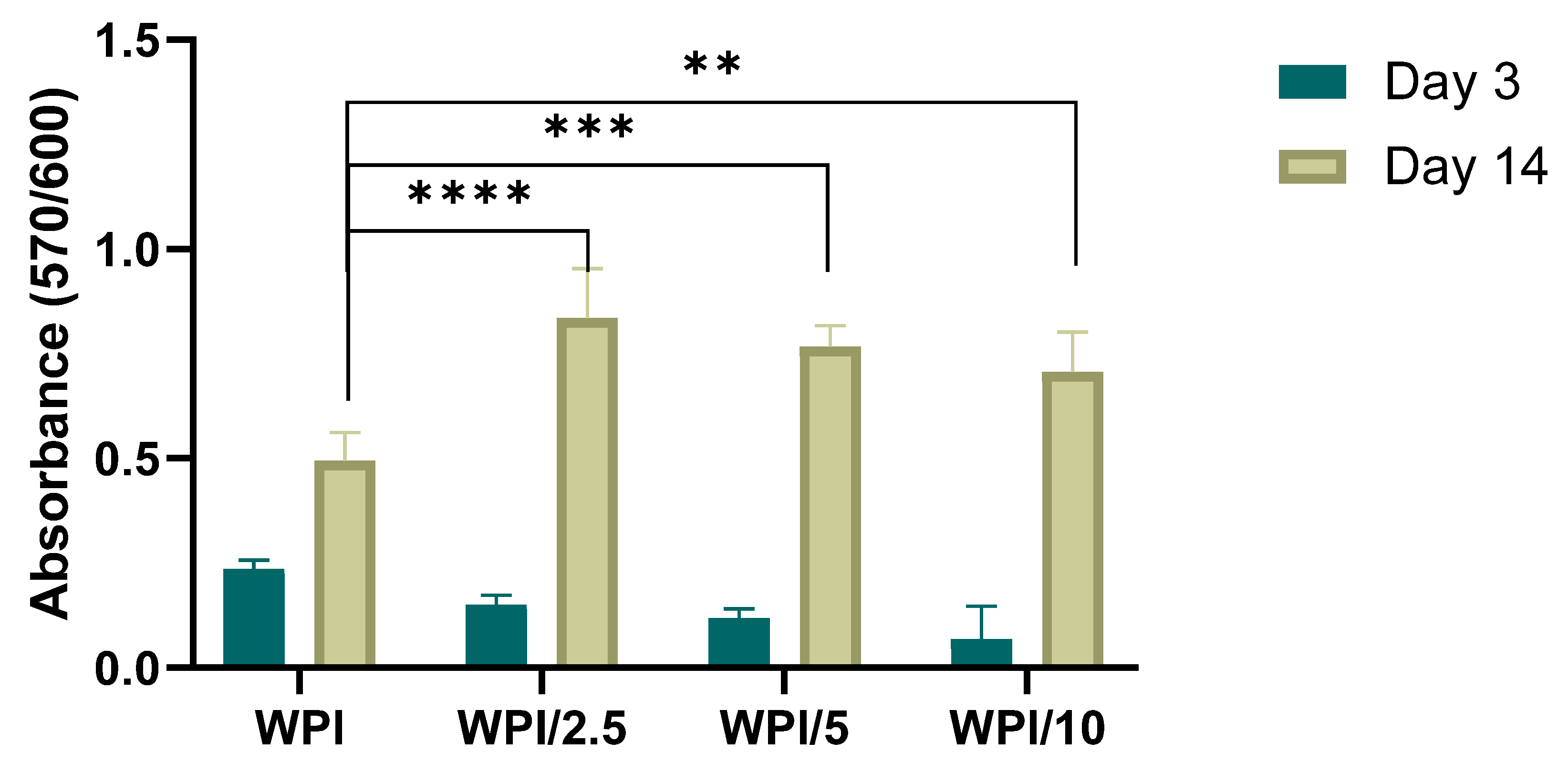
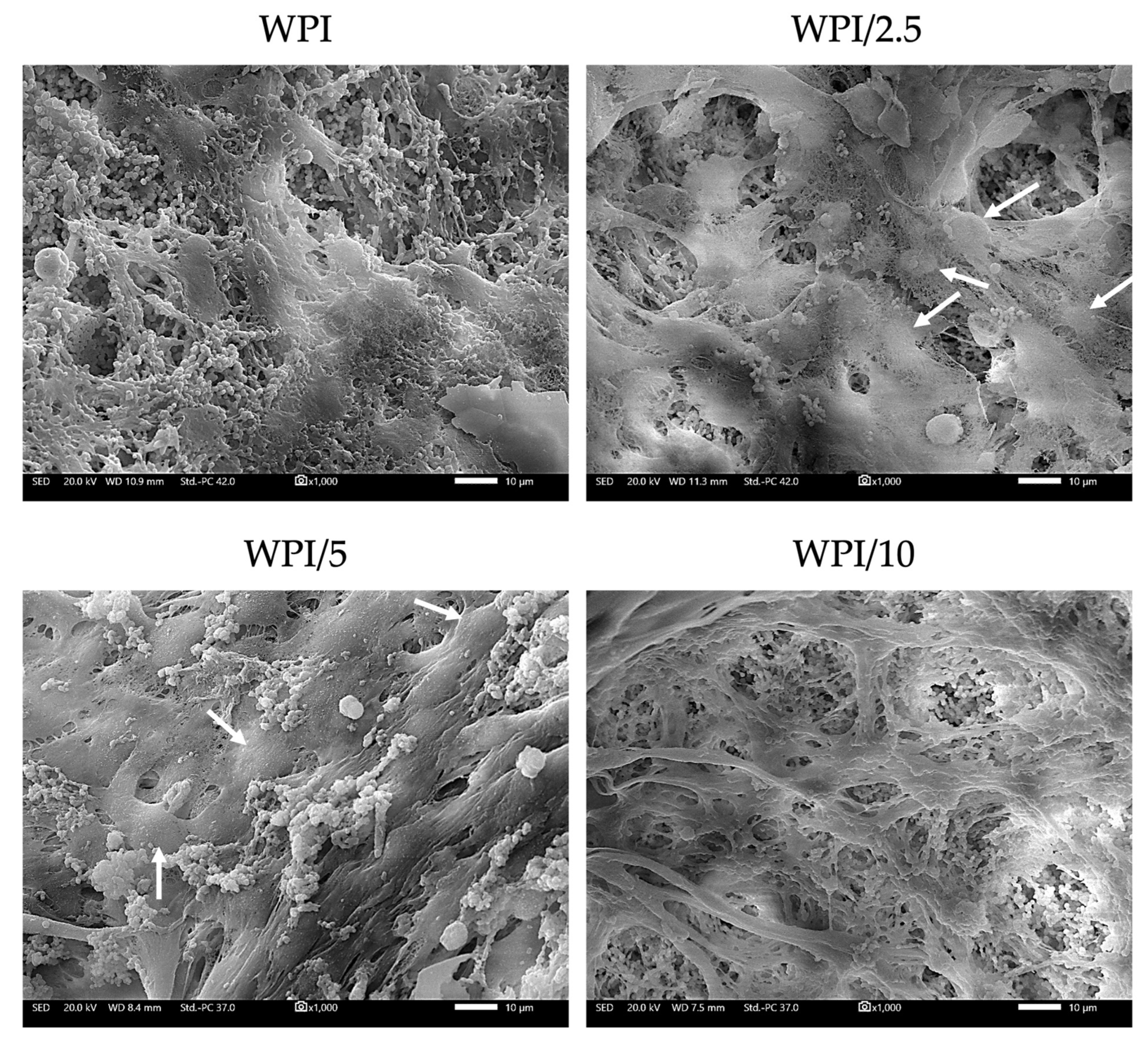
2.4. Biocompatibility and Osteogenic Capacity of the Scaffolds
3. Conclusions
4. Materials and Methods
4.1. Whey Protein Isolate–Poly-Gamma-Glutamic Acid Hydrogel Formation
4.2. Raman Spectroscopy Analysis
4.3. Swelling Analysis
4.4. Mechanical Testing
4.5. Cell Culture and Viability
4.6. Cell Adhesion and Morphology Evaluation using Scanning Electron Microscopy
4.7. Alkaline Phosphatase (ALP) Activity
4.8. Determination of the Produced Extracellular Collagen
4.9. Measurement of the Concentration of Calcium
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reginster, J.Y.; Nansa, B. Osteoporosis: A still increasing prevalence. Bone 2006, 38, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Barnsley, J.; Buckland, G.; Chan, P.E.; Ong, A.; Ramos, A.S.; Baxter, M.; Laskou, F.; Dennison, E.M.; Cooper, C.; Patel, H.P. Pathophysiology and treatment of osteoporosis: Challenges for clinical practice in older people. Aging Clin. Exp. Res. 2021, 33, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, T.; Zhang, Q.; Piao, Y.; Pan Bei, H.; Zhao, X. Biomimetic, Stiff, and Adhesive Periosteum with Osteogenic-Angiogenic Coupling Effect for Bone Regeneration. Small 2021, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2018, 84, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Giannitelli, S.; Basoli, F.; Mozetic, P.; Piva, P.; Bartuli, F.; Luciani, F.; Arcuri, C.; Trombetta, M.; Rainer, A.; Licoccia, S. Graded porous polyurethane foam: A potential scaffold for oro-maxillary bone regeneration. Mater. Sci. Eng. C 2015, 51, 329–335. [Google Scholar] [CrossRef]
- Chandra, P.K.; Soker, S.S.; Atala, A.A. Tissue engineering: Current status and future perspectives. Princ. Tissue Eng. 2020, 5, 1–35. [Google Scholar]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Luck, P.; Vardhanabhuti, B. Milk Protein Products|Whey Protein Products. In Encyclopedia of Dairy Sciences, 2nd ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 873–878. [Google Scholar]
- Wu, J.; Chen, H.; Zhou, L.; Liu, W.; Zhong, J.; Liu, C. An insight into heat-induced gelation of whey protein isolate–lactose mixed and conjugate solutions: Rheological behaviour, microstructure, and molecular forces. Eur. Food Res. Technol. 2021, 247, 1711–1724. [Google Scholar] [CrossRef]
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017, 70, 69–81. [Google Scholar] [CrossRef]
- Dziadek, M.; Charuza, K.; Kudlackova, R.; Aveyard, J.; D’Sa, R.; Serafim, A.; Stancu, I.-C.; Iovu, H.; Kerns, J.G.; Allinson, S.; et al. Modification of heat-induced whey protein isolate hydrogel with highly bioactive glass particles results in promising biomaterial for bone tissue engineering. Mater. Des. 2021, 205, 109749. [Google Scholar] [CrossRef]
- Norris, K.; Kocot, M.; Tryba, A.M.; Chai, F.; Talari, A.; Aston, L.; Parakhonskiy, B.V.; Samal, S.K.; Blanchemain, N.; Pamula, E.; et al. Marine-Inspired Enzymatic Mineralization of Dairy-Derived Whey Protein Isolate (WPI) Hydrogels for Bone Tissue Regeneration. Mar Drugs 2020, 18, 294. [Google Scholar] [CrossRef] [PubMed]
- Słota, D.; Głąb, M.; Tyliszczak, B.; Douglas, T.E.L.; Rudnicka, K.; Miernik, K.; Urbaniak, M.M.; Rusek-Wala, P.; Sobczak-Kupiec, A. Composites Based on Hydroxyapatite and Whey Protein Isolate for Applications in Bone Regeneration. Materials 2021, 14, 2317. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Kocot, M.; Tryba, A.M.; Serafim, A.; Stancu, I.C.; Jaegermann, Z.; Pamuła, E.; Reilly, G.C.; Douglas, T.E. Novel naturally derived whey protein isolate and aragonite biocomposite hydrogels have potential for bone regeneration. Mater. Des. 2020, 188, 108408. [Google Scholar] [CrossRef]
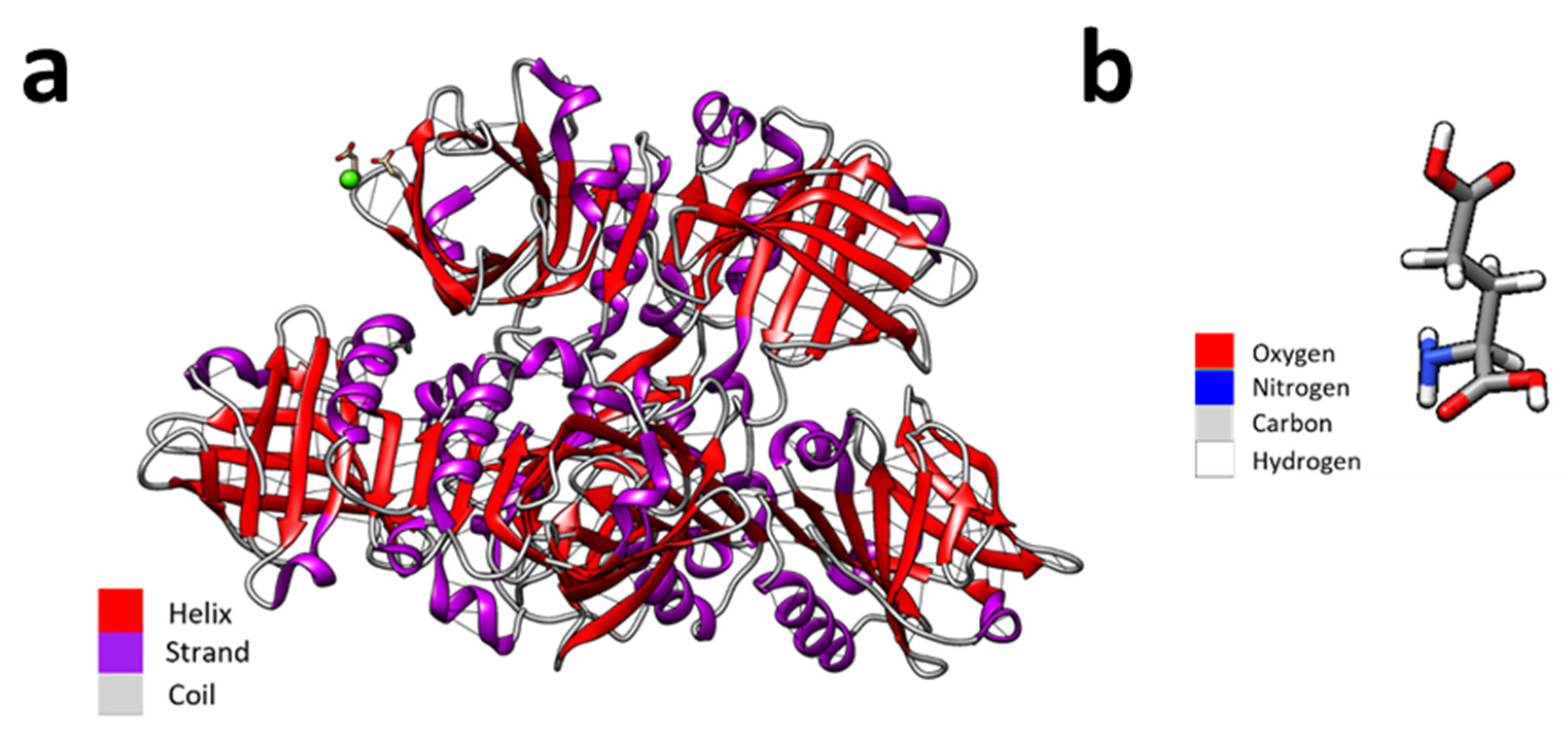
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera?A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Bajaj, L.; Singhai, R. Sequential optimisation approach for enhanced production of poly-gamma-glutamic acid from newly isolated Bacillus subtilis. Food Technol. Biotechnol. 2009, 47, 313–322. [Google Scholar]
- Ajayeoba, T.A.; Dula, S.; Ijabadeniyi, O.A. Properties of Poly-γ-Glutamic Acid Producing-Bacillus Species Isolated From Ogi Liquor and Lemon-Ogi Liquor. Front. Microbiol. 2019, 10, 771. [Google Scholar] [CrossRef]
- Nair, P.; Navale, G.R.; Dharne, M.S. Poly-gamma-glutamic acid biopolymer: A sleeping giant with diverse applications and unique opportunities for commercialization. Biomass- Convers. Biorefin. 2023, 13, 4555–4573. [Google Scholar] [CrossRef]
- Poologasundarampillai, G.; Yu, B.; Tsigkou, O.; Valliant, E.; Yue, S.; Lee, P.D.; Hamilton, R.W.; Stevens, M.M.; Kasuga, T.; Jones, J.R. Bioactive silica–poly(γ-glutamic acid) hybrids for bone regeneration: Effect of covalent coupling on dissolution and mechanical properties and fabrication of porous scaffolds. Soft Matter 2012, 8, 4822–4832. [Google Scholar] [CrossRef]
- Parati, M.; Clarke, L.; Anderson, P.; Hill, R.; Khalil, I.; Tchuenbou-Magaia, F.; Stanley, M.S.; McGee, D.; Mendrek, B.; Kowalczuk, M.; et al. Microbial Poly-γ-Glutamic Acid (γ-PGA) as an Effective Tooth Enamel Protectant. Polymers 2022, 14, 2937. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wei, Y.; Fu, C.; Sablani, S.S.; Huang, Z.; Han, C.; Li, D.; Sun, Z.; Qin, H. Antimicrobial activity of gam-ma-poly (glutamic acid), a preservative coating for cherries. Colloids Surf B Biointerfaces 2023, 225, 113272. [Google Scholar] [CrossRef] [PubMed]
- Gamarra-Montes, A.; Missagia, B.; Morató, J.; Muñoz-Guerra, S. Antibacterial Films Made of Ionic Complexes of Poly(γ-glutamic acid) and Ethyl Lauroyl Arginate. Polymers 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Freire, P.; Moreira, B.; Alves de Lima, F., Jr.; José, F.; Melo, F.; Filho, J. Raman Spectroscopy of Amino Acid Crystals. Raman Spectrosc. Appl. 2017, 10, 65480. [Google Scholar]
- Barth, A. The infrared absorption of amino acid side chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Guangyong, Z.; Xian, Z.; Qi, F.; Xueliang, W. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 78, 1187–1195. [Google Scholar]
- Ivory-Cousins, T.; Nurzynska, A.; Klimek, K.; Baines, D.K.; Truszkiewicz, W.; Pałka, K.; Douglas, T.E.L. Whey Protein Isolate/Calcium Silicate Hydrogels for Bone Tissue Engineering Applications-Preliminary In Vitro Evaluation. Materials 2023, 16, 6484. [Google Scholar] [CrossRef]
- Platania, V.; Douglas, T.E.; Zubko, M.K.; Ward, D.; Pietryga, K.; Chatzinikolaidou, M. Phloroglucinol-enhanced whey protein isolate hydrogels with antimicrobial activity for tissue engineering. Mater. Sci. Eng. C 2021, 129, 112412. [Google Scholar] [CrossRef]
- Douglas, T.E.; Vandrovcová, M.; Kročilová, N.; Keppler, J.K.; Zárubová, J.; Skirtach, A.G.; Bačáková, L. Application of whey protein isolate in bone regeneration: Effects on growth and osteogenic differentiation of bone-forming cells. J. Dairy Sci. 2018, 101, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Chen, Y.; Liu, Y.; Tong, L.; Chu, J.; Xiao, K.; Zhou, Z.; Dong, W.; Chu, X. Preparation, Characterization and Properties of Alginate/Poly (γ-glutamic acid) Composite Microparticles. Mar. Drugs 2017, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chiu, J.Y. Glycerol-modified γ-PGA and gellan composite hydrogel materials with tunable physicochemical and thermal properties for soft tissue engineering application. Polymer 2021, 230, 124049. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, J.; Chen, J.; Zhu, P.; Gao, C.C. An injectable bioactive poly (γ-glutamic acid) modified magnesium phosphate bone cement for bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 112, e35316. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Chung, C.-Y. TATVHL peptide-grafted alginate/poly(γ-glutamic acid) scaffolds with inverted colloidal crystal topology for neuronal differentiation of iPS cells. Biomaterials 2012, 33, 8955–8966. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Z.; Miszuk, J.M.; Zeng, E.; Sun, H. High Molecular Weight Poly(glutamic acid) to Improve BMP2-Induced Osteogenic Differentiation. Mol. Pharm. 2022, 19, 4565–4575. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.C.; Zheng, Z.Z.; Liu, X.X.; Li, H.H.; Zhang, M.M. Effect of γ-PGA on the formation of collagen fibrils in vitro. Connect. Tissue Res. 2016, 57, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.; Yang, H.; Shen, L.; Liu, W.; Li, G. Glutamic acid concentration dependent collagen mineralization in aqueous solution. Colloids Surf. B Biointerfaces 2020, 190, 110892. [Google Scholar] [CrossRef]
- Liu, X.; Dan, N.; Dan, W. Insight into the collagen assembly in the presence of lysine and glutamic acid: An in vitro study. Mater. Sci. Eng. C 2017, 70, 689–700. [Google Scholar] [CrossRef]
- Sugino, A.; Miyazaki, T.; Ohtsuki, C. Apatite-forming ability of polyglutamic acid hydrogels in a body-simulating environment. J. Mater. Sci. Mater. Med. 2008, 19, 2269–2274. [Google Scholar] [CrossRef]
- Miyazaki, T.T.; Kuramoto, A.; Hirakawa, A.; Shirosaki, Y.; Ohtsuki, C. Biomineralization on chemically synthesized collagen containing immobilized poly-γ-glutamic acid. Dent. Mater. J. 2013, 32, 544–549. [Google Scholar] [CrossRef]
- Karaman, O.; Kumar, A.; Moeinzadeh, S.; He, X.; Cui, T.; Jabbari, E. Effect of surface modification of nanofibres with glutamic acid peptide on calcium phosphate nucleation and osteogenic differentiation of marrow stromal cells. J. Tissue Eng. Regen. Med. 2016, 10, 132–146. [Google Scholar] [CrossRef]
- Averianov, I.V.; Stepanova, M.A.; Gofman, I.V.; Lavrentieva, A.; Korzhikov-Vlakh, V.A.; Korzhikova-Vlakh, E.G. Osteoconductive biocompatible 3D-printed composites of poly-d,l-lactide filled with nanocrystalline cellulose modified by poly(glutamic acid). Mendeleev Commun. 2022, 32, 810–812. [Google Scholar] [CrossRef]
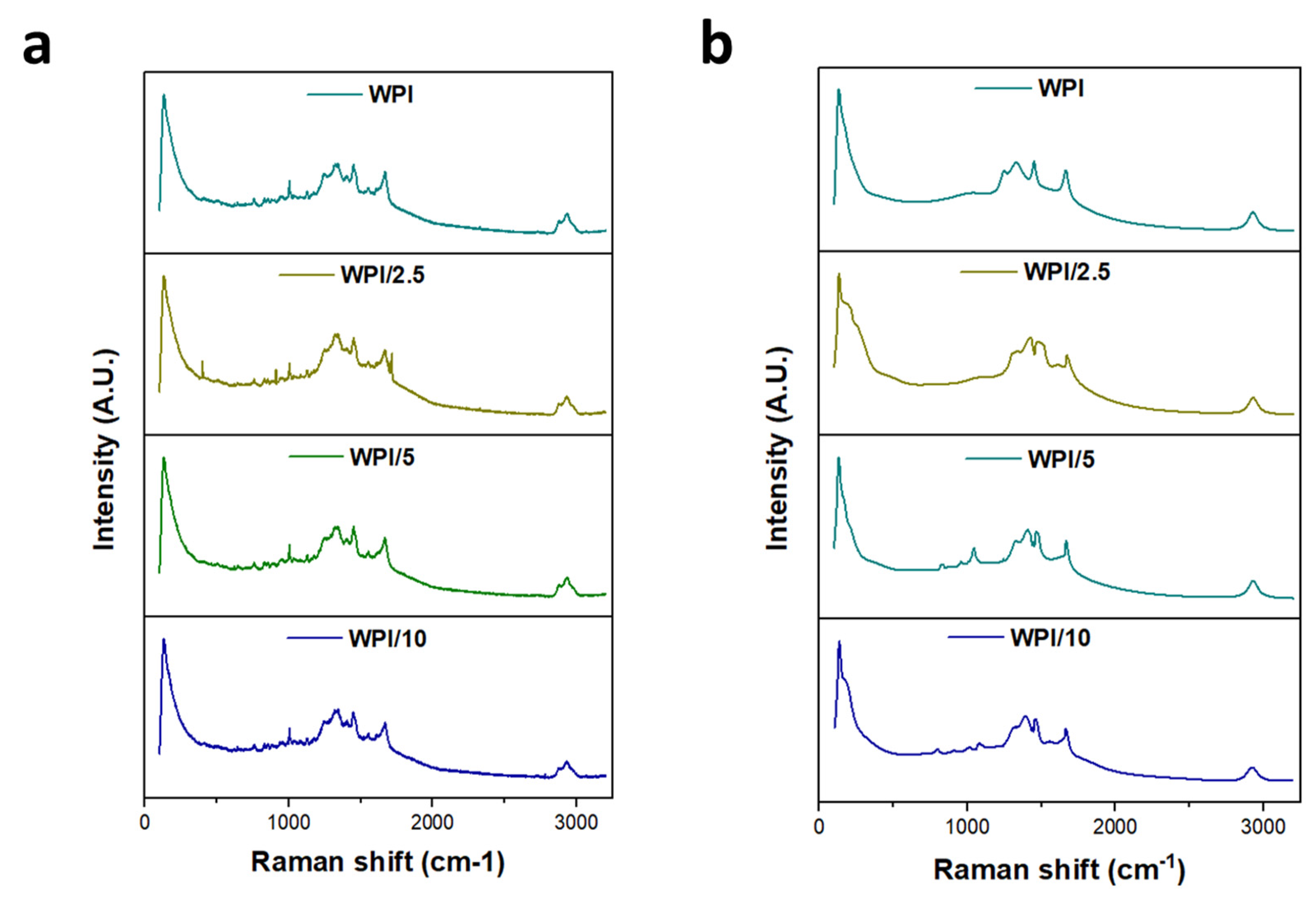



| WPI | WPI/2.5 | WPI/5 | WPI/10% | Interaction | Reference |
|---|---|---|---|---|---|
| 128 | 129 | 130 | 131 | Lattice rocking vibrations | [27] |
| 162 | 159 | 159 | 172 | CO2 torsion, lattice rocking vibrations | [27] |
| 217 | 210 | 214 | 269 | L-glutamic acid skeleton vibrations | [27] |
| 758–852 | CH2 rocking vibrations, COOH deformation vibrations | [28] | |||
| 1024 | 1007 | 1004 | 986 | CC stretching vibrations, C–C–N stretching vibrations | [27,29] |
| 1155 | 1121 | NH3+ rocking vibrations, C–O stretching vibrations, CH2 twisting vibrations | [27,28] | ||
| 1243 | 1242 | 1249 | C–O stretching vibrations, CH3 wagging vibrations, COH in plane bending vibrations, CH3COOH (H-bonded) | [28] | |
| 1328 | 1331 | 1321 | 1331 | COH in plane bending vibrations, CH3 wagging vibrations, CH in plane bending vibrations, COO– symmetric stretching vibrations | [28,29] |
| 1451 | 1451 | 1451 | 1494 | CH3 antisymmetric in plane bending vibrations, COO– symmetric stretching vibrations, COH in plane bending vibrations | [28,29] |
| 1542 | 1552 | 1542 | COO– anti-symmetric stretching vibrations | [28] | |
| 1665 | 1666 | 1666 | 1669/1763 | C=O stretching vibrations | [27] |
| 2928 | 2928 | 2928 | 2928 | CH2 stretching vibrations | [27] |
| Sample | % WPI | % γ-PGA |
|---|---|---|
| WPI | 40 | 0 |
| WPI/2.5 | 40 | 2.5 |
| WPI/5 | 40 | 5 |
| WPI/10 | 40 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baines, D.K.; Platania, V.; Tavernaraki, N.N.; Parati, M.; Wright, K.; Radecka, I.; Chatzinikolaidou, M.; Douglas, T.E.L. The Enrichment of Whey Protein Isolate Hydrogels with Poly-γ-Glutamic Acid Promotes the Proliferation and Osteogenic Differentiation of Preosteoblasts. Gels 2024, 10, 18. https://doi.org/10.3390/gels10010018
Baines DK, Platania V, Tavernaraki NN, Parati M, Wright K, Radecka I, Chatzinikolaidou M, Douglas TEL. The Enrichment of Whey Protein Isolate Hydrogels with Poly-γ-Glutamic Acid Promotes the Proliferation and Osteogenic Differentiation of Preosteoblasts. Gels. 2024; 10(1):18. https://doi.org/10.3390/gels10010018
Chicago/Turabian StyleBaines, Daniel K., Varvara Platania, Nikoleta N. Tavernaraki, Mattia Parati, Karen Wright, Iza Radecka, Maria Chatzinikolaidou, and Timothy E. L. Douglas. 2024. "The Enrichment of Whey Protein Isolate Hydrogels with Poly-γ-Glutamic Acid Promotes the Proliferation and Osteogenic Differentiation of Preosteoblasts" Gels 10, no. 1: 18. https://doi.org/10.3390/gels10010018
APA StyleBaines, D. K., Platania, V., Tavernaraki, N. N., Parati, M., Wright, K., Radecka, I., Chatzinikolaidou, M., & Douglas, T. E. L. (2024). The Enrichment of Whey Protein Isolate Hydrogels with Poly-γ-Glutamic Acid Promotes the Proliferation and Osteogenic Differentiation of Preosteoblasts. Gels, 10(1), 18. https://doi.org/10.3390/gels10010018