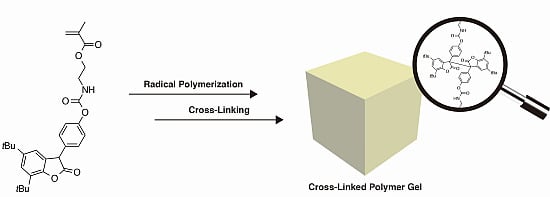
Diarylbibenzofuranone-Based Dynamic Covalent Polymer Gels Prepared via Radical Polymerization and Subsequent Polymer Reaction
Abstract
:1. Introduction

2. Results and Discussion
2.1. Radical Copolymerization of DABBF-Containing Bifunctional Monomer and MMA
2.2. Radical Copolymerization of ABF-Containing Monomer and MMA
| Entry | Initiator a | [3]/[MMA] b | Temp. (°C) | Time (h) | Conv. (%) c | Yield (%) | 3/MMA d | Mn e | MW/Mn e |
|---|---|---|---|---|---|---|---|---|---|
| 1 | AIBN | 1:9 | 60 | 24 | 37 | 29 | 1:9.0 | 20,700 | 1.83 |
| 2 | AIBN | 1:4 | 60 | 24 | 11 | 5 | 1:4.1 | 24,400 | 1.44 |
| 3 | AIBN | 1:1 | 60 | 72 | 7 | 7 | 1:0.5 | 15,900 | 1.27 |
| 4 | AIBN | 1:0 | 60 | 120 | – | 3 | 1:0 | 14,400 | 1.19 |
| 5 | V-70 | 1:9 | 40 | 61 | 54 | 40 | 1:8.8 | 22,300 | 1.81 |
| 6 | V-70 | 1:4 | 40 | 61 | 33 | 23 | 1:3.6 | 19,000 | 1.61 |
| 7 | V-70 | 1:1 | 40 | 61 | 13 | – | – | – | – |
| 8 | V-70 | 1:0 | 40 | 120 | 5 | 4 | 1:0 | 12,300 | 1.39 |
| 9 | redox | 1:9 | 0 | 72 | 22 | 14 | 1:16.0 | 47,700 | 2.13 |
| 10 | redox | 1:4 | 0 | 72 | 10 | 3 | 1:5.3 | 34,300 | 1.75 |
| 11 | redox | 1:1 | 0 | 72 | 12 | 1 | 1:0.6 | 39,700 | 1.97 |
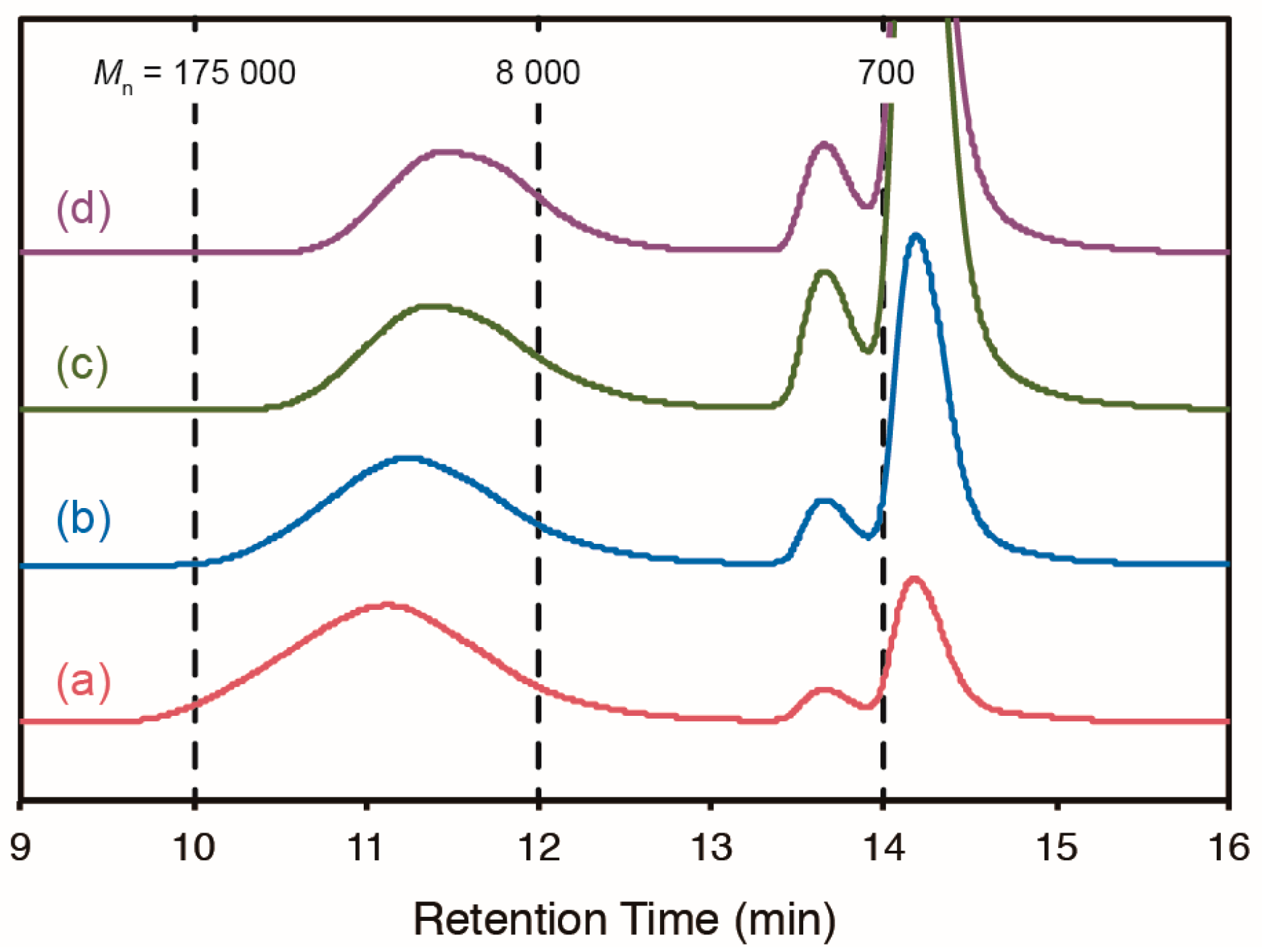
2.3. Cross-Linking of ABF-Containing Linear Polymers by Oxidative Coupling
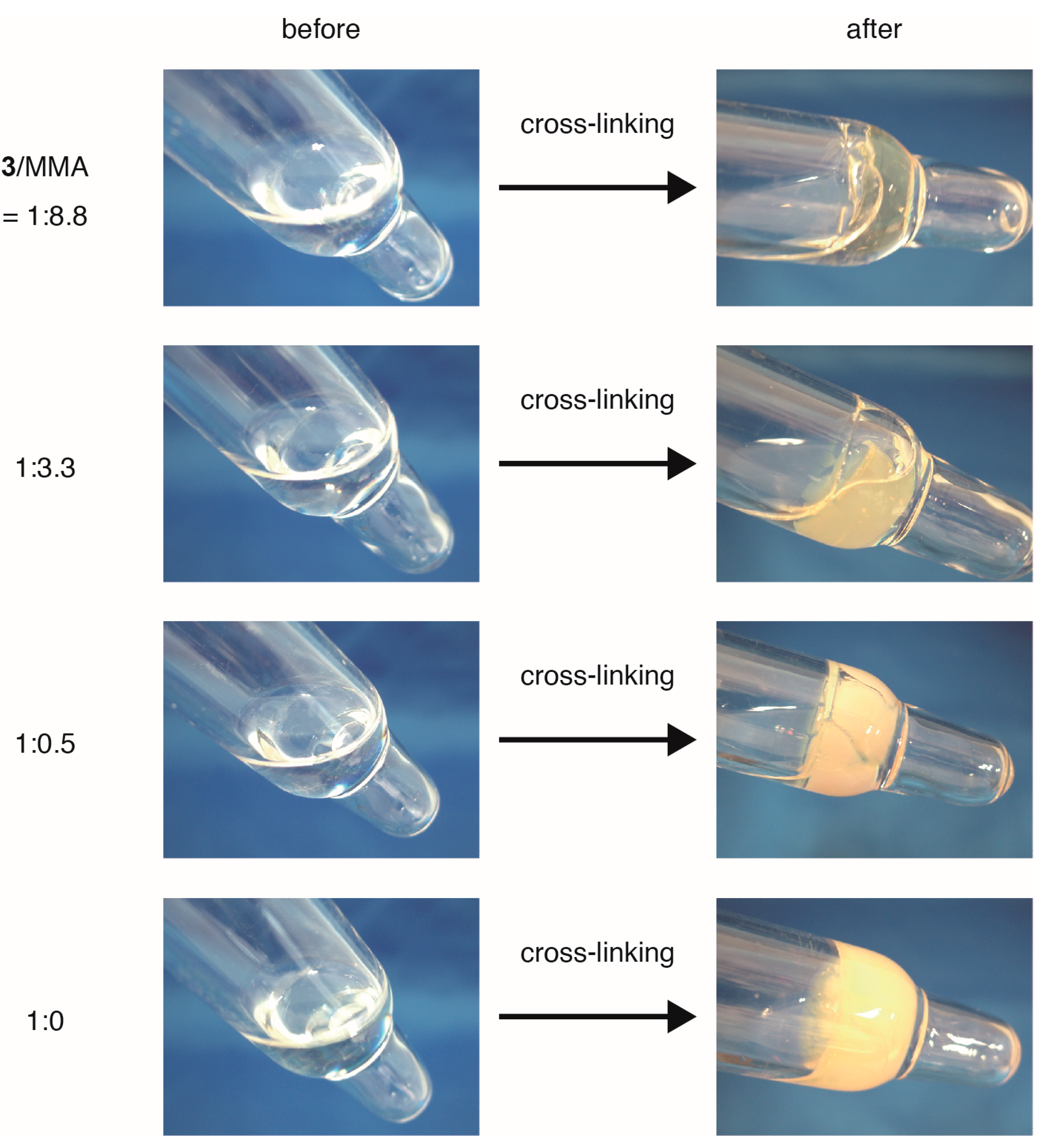
| 3/MMA a | Mn b | Tg before Cross-Linking (°C) c | Tg after Cross-Linking (°C) c |
|---|---|---|---|
| 1:8.8 | 22,300 | 108 | 120 |
| 1:3.6 | 19,000 | 111 | 126 |
| 1:0.5 | 15,900 | 113 | 131 |
| 1:0 | 12,300 | 105 | 130 |
2.4. De-Cross-Linking of Polymers with DABBF Cross-Linkages
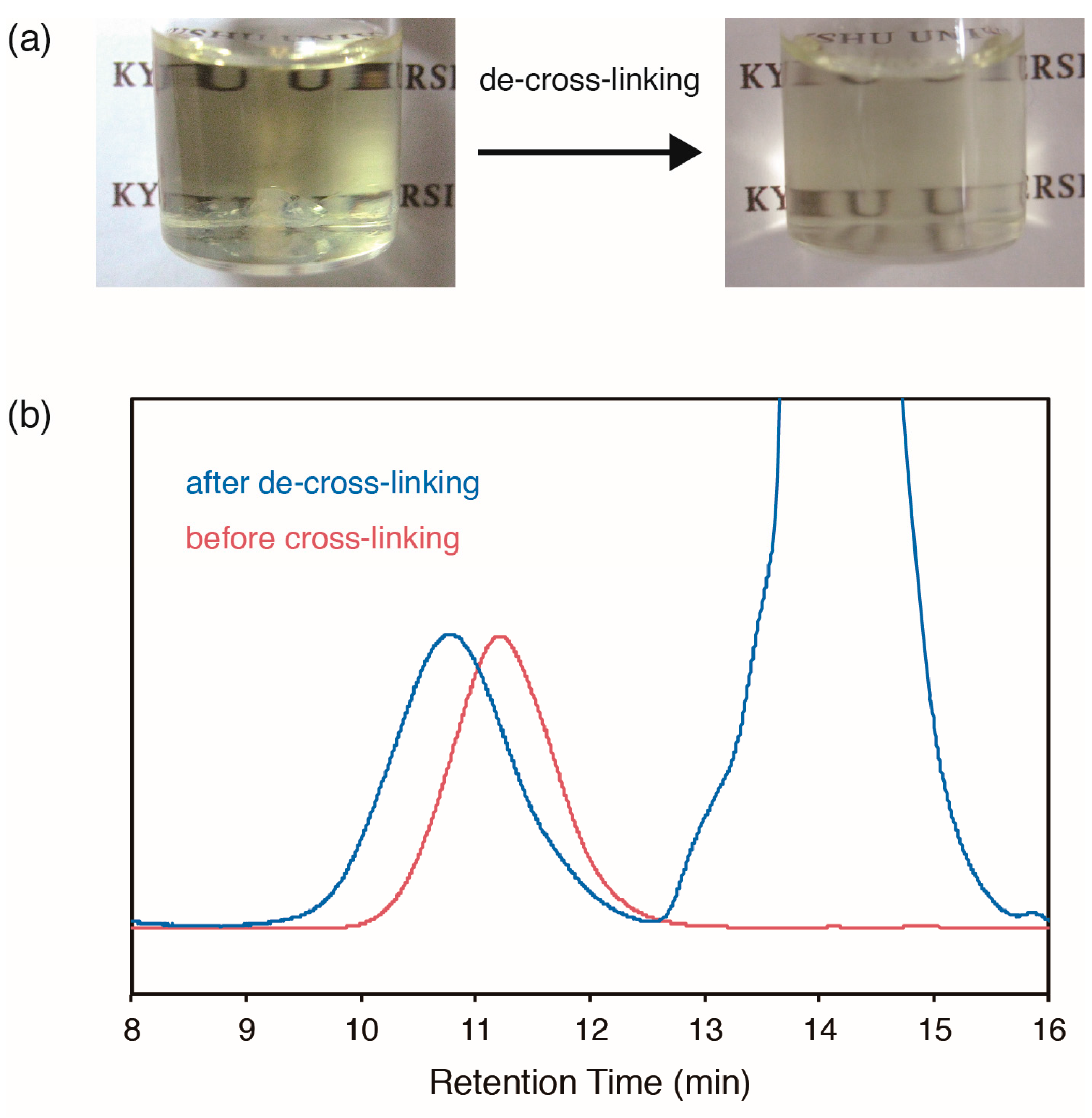
| 3/MMA a | Cross-linked DABBF (%) b |
|---|---|
| 1:8.8 | 53 |
| 1:3.6 | 63 |
| 1:0.5 | 56 |
| 1:0 | 60 |
3. Conclusions
4. Experimental Section
4.1. Radical Copolymerization of DABBF-Containing Bifunctional Monomer and MMA
4.2. Radical Copolymerization of ABF-Containing Monomer and MMA
4.3. Cross-Linking of ABF-Containing Linear Polymers
4.4. De-Cross-Linking of Gels with DABBF Cross-Linkages
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burattini, S.; Greenland, B.W.; Chappell, D.; Colquhoun, H.M.; Hayes, W. Healable polymeric materials: A tutorial review. Chem. Soc. Rev. 2010, 39, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Wojtecki, R.J.; Meador, M.A.; Rowan, S.J. Using the dynamic bond to access macroscopically responsive structurally dynamic polymers. Nat. Mater. 2011, 10, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular polymers. Chem. Rev. 2001, 101, 4071–4098. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.J.; Cantrill, S.J.; Cousins, G.R.L.; Sanders, J.K.M.; Stoddart, J.F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 2002, 41, 898–952. [Google Scholar] [CrossRef]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Burattini, S.; Colquhoun, H.M.; Fox, J.D.; Friedmann, D.; Greenland, B.W.; Harris, P.J.F.; Hayes, W.; Mackay, M.E.; Rowan, S.J. A Self-Repairing, Supramolecular polymer system: Healability as a consequence of donor–acceptor π–π stacking interactions. Chem. Commun. 2009, 6717–6719. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host-guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef] [PubMed]
- Burnworth, M.; Tang, L.; Kumpfer, J.R.; Duncan, A.J.; Beyer, F.L.; Fiore, G.L.; Rowan, S.J.; Weder, C. Optically healable supramolecular polymers. Nature 2011, 472, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, K.; Uyama, K.; Tanimoto, H. Self-healing in nanocomposite hydrogels. Macromol. Rapid Commun. 2011, 32, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kushner, A.M.; Williams, G.A.; Guan, Z. Multiphase design of autonomic self-healing thermoplastic elastomers. Nat. Chem. 2012, 4, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Bin Ihsan, A.; Akasaki, T.; Sato, K.; Haque, M.A.; Nakajima, T.; Gong, J.P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A Thermally Re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Reutenauer, P.; Buhler, E.; Boul, P.J.; Candau, S.J.; Lehn, J.M. Room temperature dynamic polymers based on diels-alder chemistry. Chem. Eur. J. 2009, 15, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Tang, C.; Li, F.; Jiang, H.; Chen, Y. Covalent Cross-linked polymer gels with reversible sol-gel transition and self-healing properties. Macromolecules 2010, 43, 1191–1194. [Google Scholar] [CrossRef]
- Yoshie, N.; Watanabe, M.; Araki, H.; Ishida, K. Thermo-Responsive Mending of polymers crosslinked by thermally reversible covalent bond: polymers from bisfuranic terminated poly(ethylene adipate) and tris-maleimide. Polym. Degrad. Stab. 2010, 95, 826–829. [Google Scholar] [CrossRef]
- He, L.; Fullenkamp, D.E.; Rivera, J.G.; Messersmith, P.B. pH responsive self-healing hydrogels formed by boronate–catechol complexation. Chem. Commun. 2011, 47, 7497–7499. [Google Scholar] [CrossRef] [PubMed]
- Amamoto, Y.; Kamada, J.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Repeatable photoinduced self-healing of covalently cross-linked polymers through reshuffling of trithiocarbonate units. Angew. Chem. Int. Ed. 2011, 50, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Canadell, J.; Goossens, H.; Klumperman, B. Self-healing materials based on disulfide links. Macromolecules 2011, 44, 2536–2541. [Google Scholar] [CrossRef]
- Yuan, C.; Rong, M.Z.; Zhang, M.Q.; Zhang, Z.P.; Yuan, Y.C. Self-healing of polymers via synchronous covalent bond fission/radical recombination. Chem. Mater. 2011, 23, 5076–5081. [Google Scholar] [CrossRef]
- Zheng, P.; McCarthy, T.J. A Surprise from 1954: Siloxane equilibration is a simple, robust, and obvious polymer self-healing mechanism. J. Am. Chem. Soc. 2012, 134, 2024–2027. [Google Scholar] [CrossRef] [PubMed]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-catalyzed transesterification for healing and assembling of thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef] [PubMed]
- Amamoto, Y.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Self-healing of covalently cross-linked polymers by reshuffling thiuram disulfide moieties in air under visible light. Adv. Mater. 2012, 24, 3975–3980. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-X.; Guan, Z. Olefin metathesis for effective polymer healing via dynamic exchange of strong carbon–carbon double bonds. J. Am. Chem. Soc. 2012, 134, 14226–14231. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Zhang, Y.; Cheng, J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat. Commun. 2014, 5, 3218. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Jones, G.O.; Virwani, K.; McCloskey, B.D.; Boday, D.J.; ter Huurne, G.M.; Horn, H.W.; Coady, D.J.; Bintaleb, A.M.; Alabdulrahman, A.M.S.; et al. Recyclable, strong thermosets and organogels via paraformaldehyde condensation with diamines. Science 2014, 344, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Luo, Q.; Qiao, S.; Wang, L.; Dong, Z.; Xu, J.; Liu, J. Enzymetically regulating the self-healing of protein hydrogels with high healing efficiency. Angew. Chem. Int. Ed. 2014, 53, 9343–9346. [Google Scholar] [CrossRef] [PubMed]
- Frenette, M.; Aliaga, C.; Font-Sanchis, E.; Scaiano, J.C. Bond dissociation energies for radical dimers derived from highly stabilized carbon-centered radicals. Org. Lett. 2004, 6, 2579–2582. [Google Scholar] [CrossRef] [PubMed]
- Frenette, M.; MacLean, P.D.; Barclay, L.R.C.; Scaiano, J.C. Radically different antioxidants: thermally generated carbon-centered radicals as chain-breaking antioxidants. J. Am. Chem. Soc. 2006, 128, 16432–16433. [Google Scholar] [CrossRef] [PubMed]
- Imato, K.; Nishihara, M.; Kanehara, T.; Amamoto, Y.; Takahara, A.; Otsuka, H. Self-healing of chemical gels cross-linked by diarylbibenzofuranone-based trigger-free dynamic covalent bonds at room temperature. Angew. Chem. Int. Ed. 2012, 51, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Imato, K.; Takahara, A.; Otsuka, H. Self-healing of a cross-linked polymer with dynamic covalent linkages at ambient temperature and evaluation at macroscopic and molecular levels. unpublished.
- Nishihara, M.; Imato, K.; Irie, A.; Kanehara, T.; Kano, A.; Maruyama, A.; Takahara, A.; Otsuka, H. Reversibly crosslinked polymeric micelles formed by autonomously exchangeable dynamic covalent bonds. Chem. Lett. 2013, 42, 377–379. [Google Scholar] [CrossRef]
- Imato, K.; Ohishi, T.; Nishihara, M.; Takahara, A.; Otsuka, H. Network reorganization of dynamic covalent polymer gels with exchangeable diarylbibenzofuranone at ambient temperature. J. Am. Chem. Soc. 2014, 136, 11839–11845. [Google Scholar] [CrossRef] [PubMed]
- Imato, K.; Irie, A.; Kosuge, T.; Ohishi, T.; Nishihara, M.; Takahara, A.; Otsuka, H. Mechanophores with a reversible radical system and freezing-induced mechanochemistry in polymer solutions and gels. Angew. Chem. Int. Ed. 2015, 54, 6168–6172. [Google Scholar] [CrossRef] [PubMed]
- Imato, K.; Kanehara, T.; Ohishi, T.; Nishihara, M.; Yajima, H.; Ito, M.; Takahara, A.; Otsuka, H. Mechanochromic dynamic covalent elastomers: quantitative damage evaluation and autonomous recovery. unpublished.
- Filippenko, V.; Frenette, M.; Scaiano, J.C. Solvent-independent antioxidant activity from thermally generated carbon-centered radical antioxidants. Org. Lett. 2009, 11, 3634–3637. [Google Scholar] [CrossRef] [PubMed]
- Aliaga, C.; Stuart, D.R.; Aspée, A.; Scaiano, J.C. Solvent effects on hydrogen abstraction reactions from lactones with antioxidant properties. Org. Lett. 2005, 7, 3665–3668. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-Q.; Zhou, J.; Wang, C.-H.; Li, X.-T.; Jing, S. Actual structure, thermodynamic driving force, and mechanism of benzofuranone-typical compounds as antioxidants in solution. J. Phys. Chem. B 2011, 115, 3588–3603. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imato, K.; Nishihara, M.; Irie, A.; Takahara, A.; Otsuka, H. Diarylbibenzofuranone-Based Dynamic Covalent Polymer Gels Prepared via Radical Polymerization and Subsequent Polymer Reaction. Gels 2015, 1, 58-68. https://doi.org/10.3390/gels1010058
Imato K, Nishihara M, Irie A, Takahara A, Otsuka H. Diarylbibenzofuranone-Based Dynamic Covalent Polymer Gels Prepared via Radical Polymerization and Subsequent Polymer Reaction. Gels. 2015; 1(1):58-68. https://doi.org/10.3390/gels1010058
Chicago/Turabian StyleImato, Keiichi, Masamichi Nishihara, Atsushi Irie, Atsushi Takahara, and Hideyuki Otsuka. 2015. "Diarylbibenzofuranone-Based Dynamic Covalent Polymer Gels Prepared via Radical Polymerization and Subsequent Polymer Reaction" Gels 1, no. 1: 58-68. https://doi.org/10.3390/gels1010058
APA StyleImato, K., Nishihara, M., Irie, A., Takahara, A., & Otsuka, H. (2015). Diarylbibenzofuranone-Based Dynamic Covalent Polymer Gels Prepared via Radical Polymerization and Subsequent Polymer Reaction. Gels, 1(1), 58-68. https://doi.org/10.3390/gels1010058