Lippia origanoides Essential Oil or Thymol in Combination with Fluconazole Produces Damage to Cells and Reverses the Azole-Resistant Phenotype of a Candida tropicalis Strain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Minimum Inhibitory Concentration
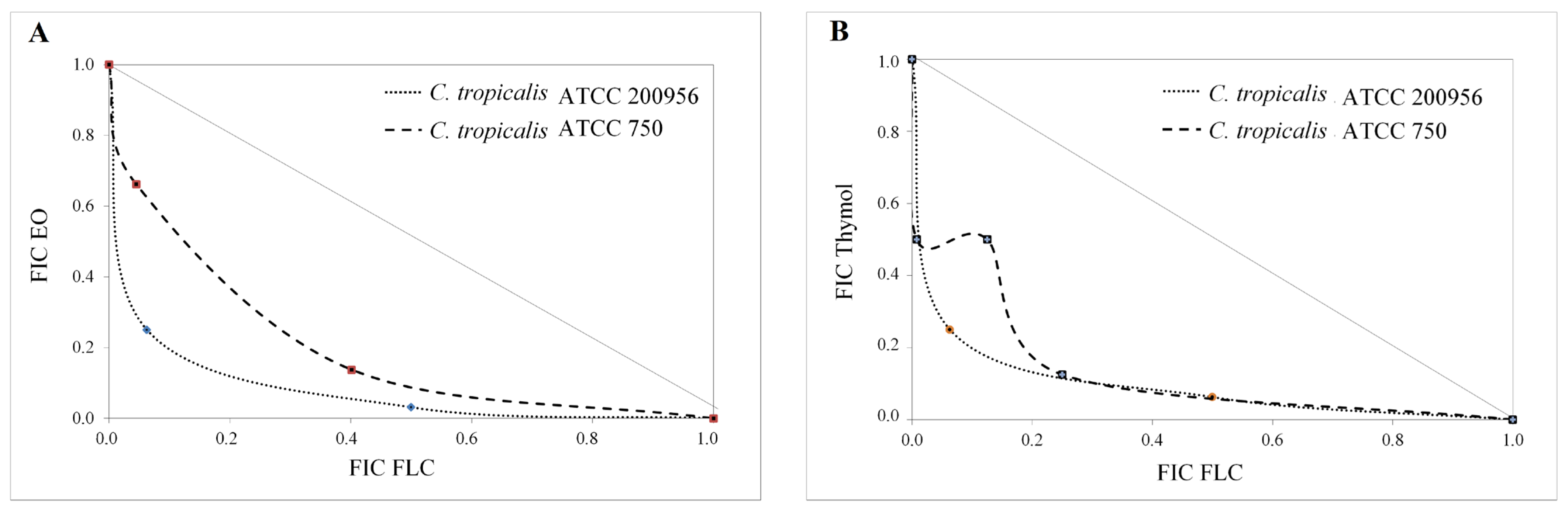
2.3. Interaction Assay
2.4. Assessment of Plasmatic Membrane Integrity
2.5. Evaluation of Mitochondrial Membrane Potential (∆ψm)
2.6. Mitochondrial Superoxide Indicator
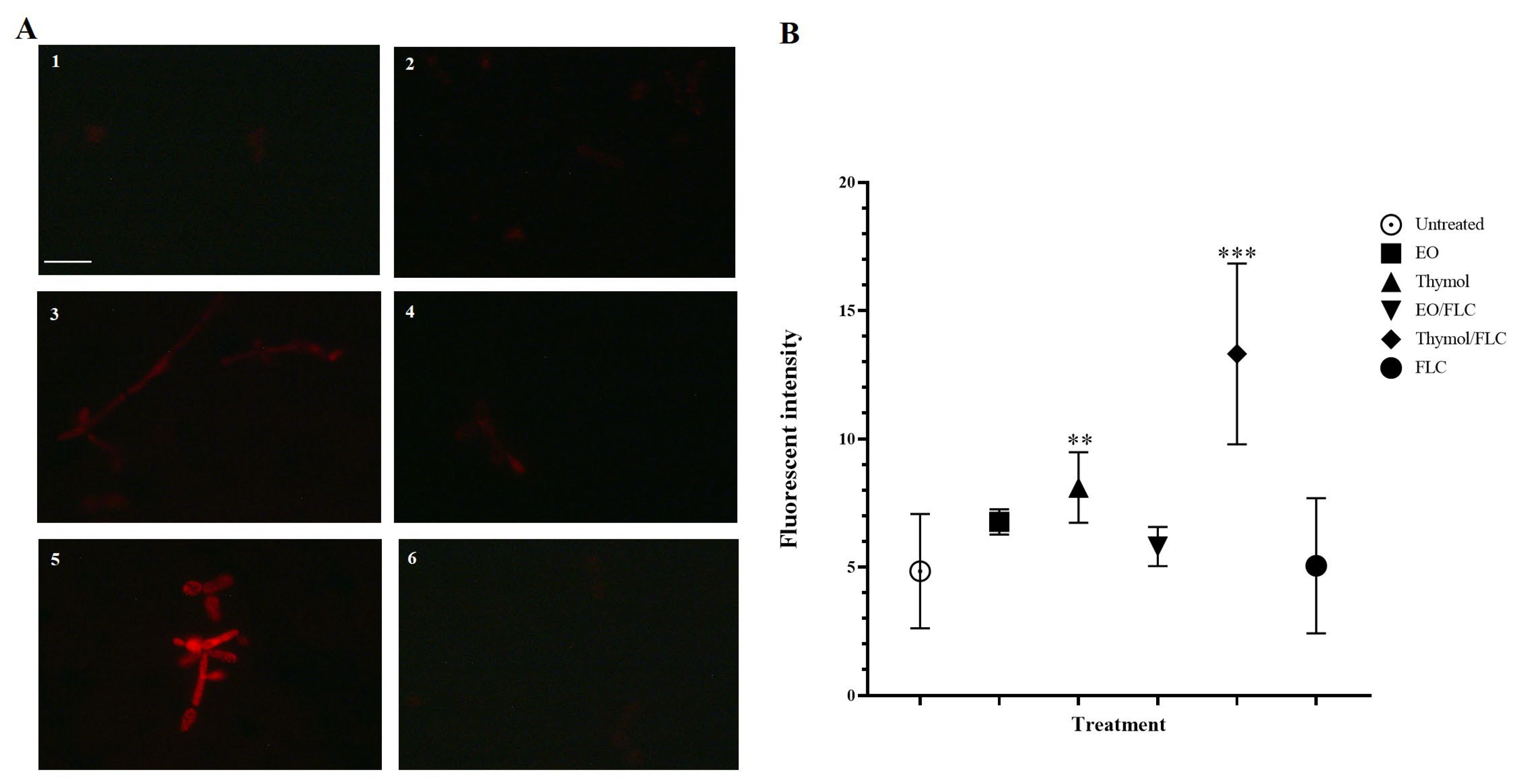
2.7. Nuclear Effect Assessment
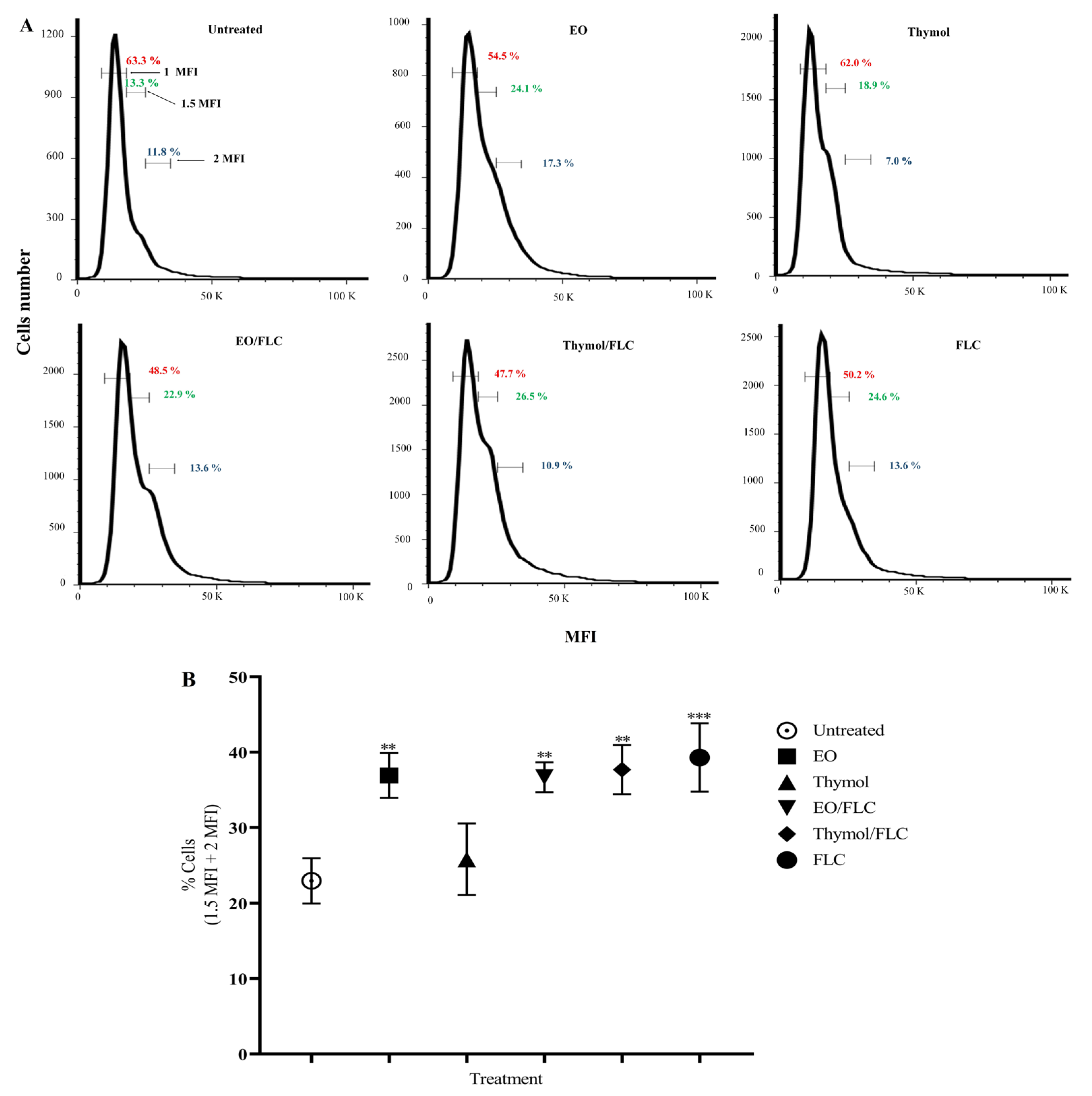
2.8. Cell Cycle Assessment
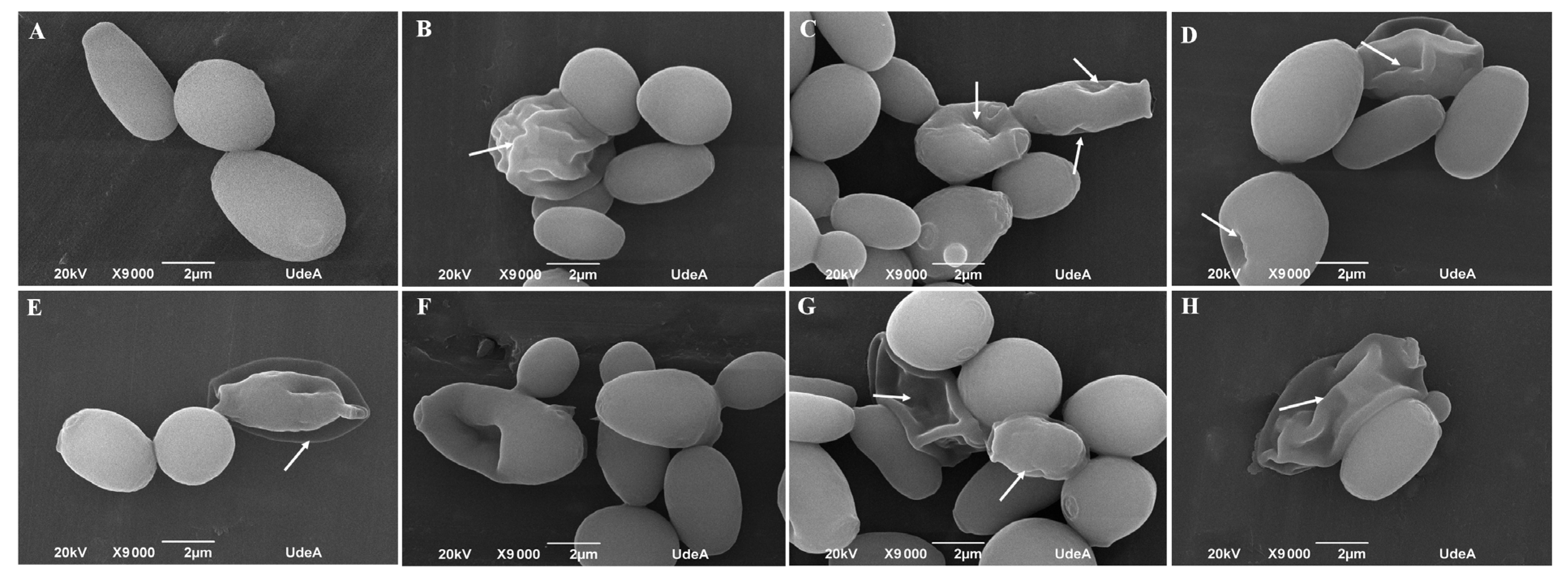
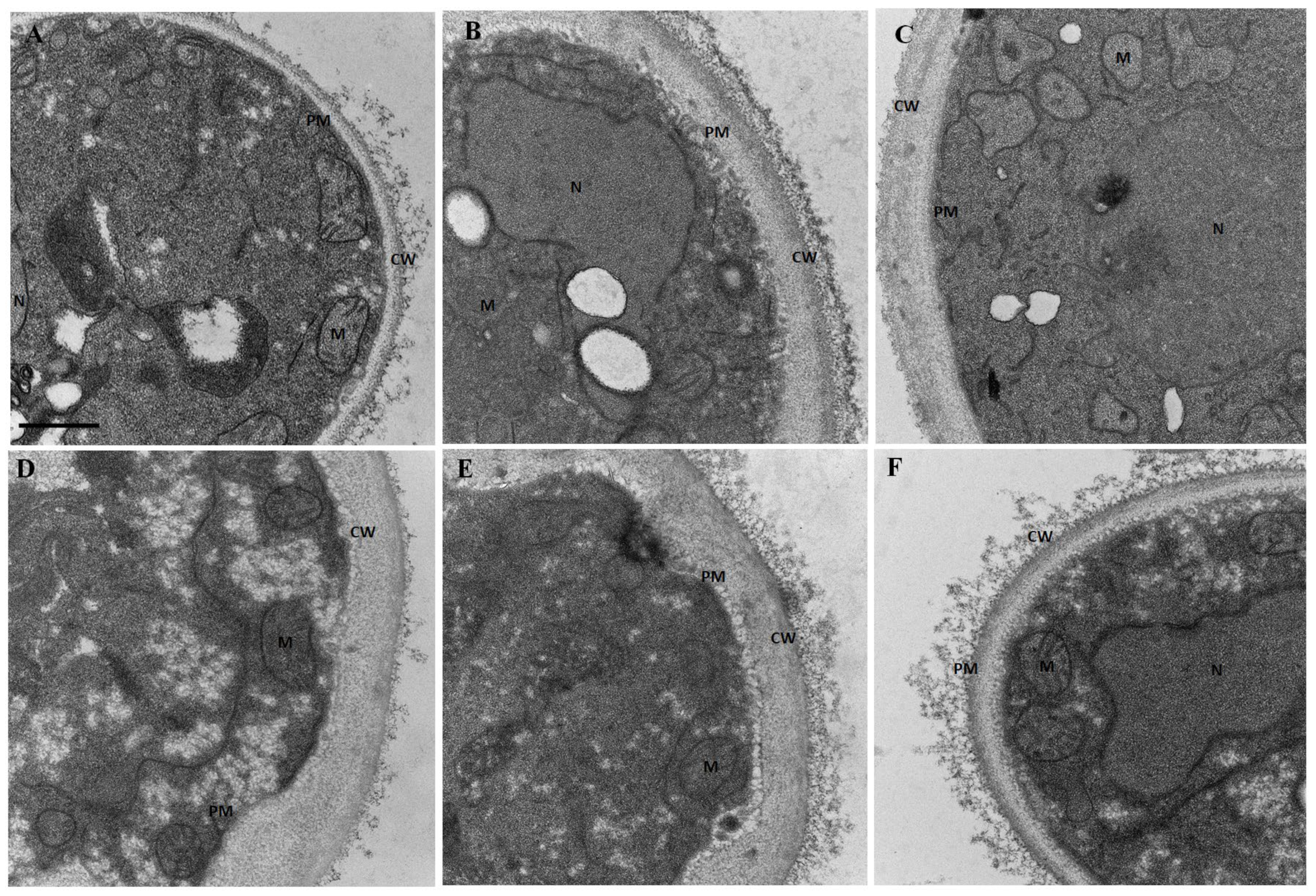
2.9. Morphology Assessment by Scanning Electron Microscopy
2.10. Ultrastructure Assessment by Transmission Electron Microscopy
3. Results
3.1. Lippia Origanoides EO or Thymol in Combination with FLC Reduce Mitochondrial Membrane Potential (∆ψm) but Do Not Affect the Plasmatic Membrane
3.2. Thymol Used Alone and in Combination with FLC Increased Mitochondrial Superoxide Production
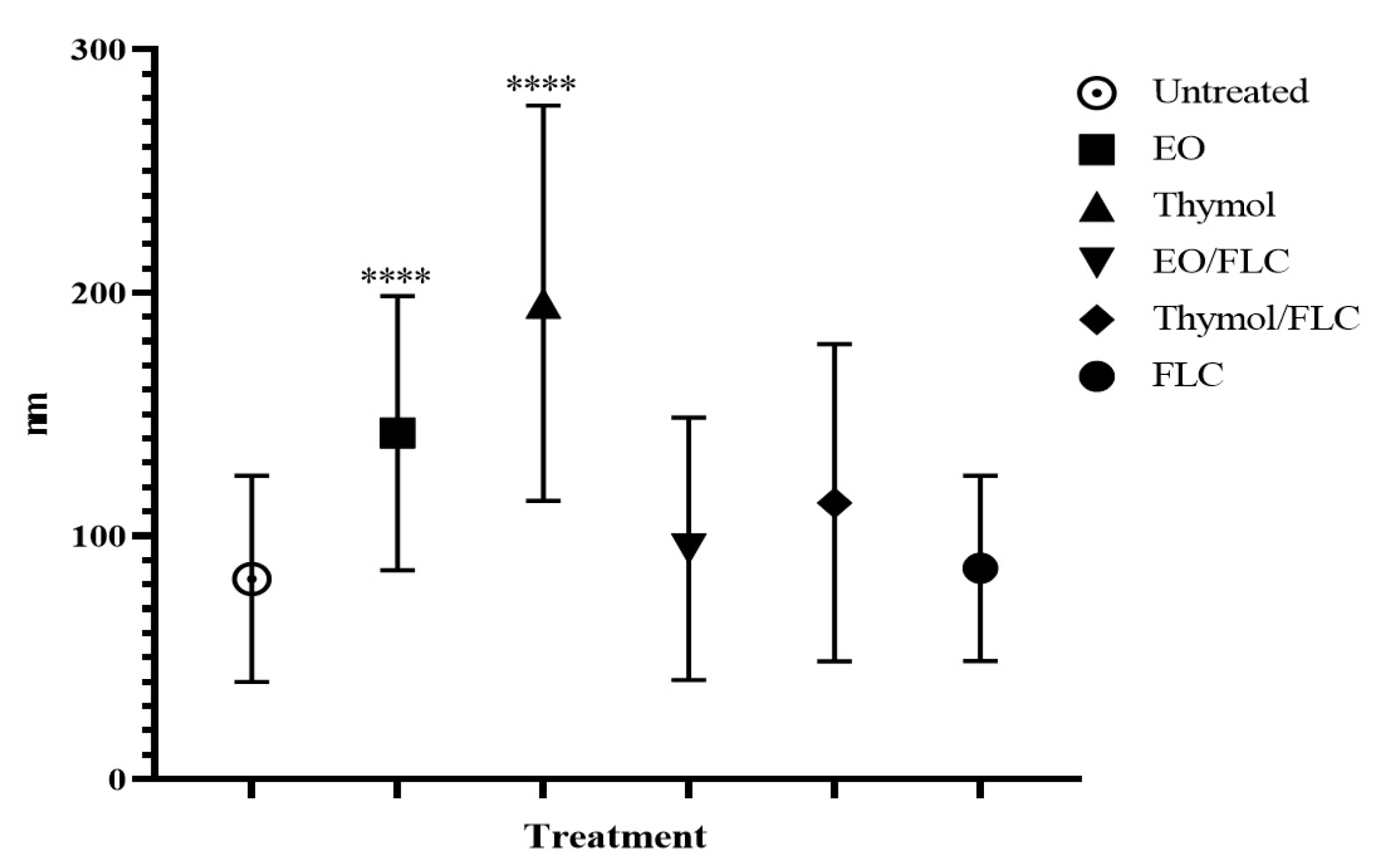
3.3. Lippia Origanoides EO or Thymol in Combination with FLC Causes Nuclear Alterations
3.4. Lippia origanoides EO or Thymol in Combination with FLC Causes Alterations in the Cell Cycle
3.5. Lippia origanoides EO or Thymol in Combination with FLC Cause Both Superficial and Ultrastructural Alterations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arendrup, M.C. Candida and candidaemia. Susceptibility and epidemiology. Dan. Med. J. 2013, 60, B4698. [Google Scholar]
- Riera, F.O.; Caeiro, J.P.; Angiolini, S.C.; Vigezzi, C.; Rodriguez, E.; Icely, P.A.; Sotomayor, C.E. Invasive Candidiasis: Update and current challenges in the management of this mycosis in South America. Antibiotics 2022, 11, 877. [Google Scholar] [CrossRef]
- Harada, K.; Maeda, T. Case of onychomycosis due to Candida tropicalis successfully treated using fosravuconazole. J. Dermatol. 2020, 47, e348–e349. [Google Scholar] [CrossRef]
- Maikan, H.K.; Jabbar, S.; Al-Haishawi, H. Isolation and identification of Candida tropicalis as a cause of cutaneous candidiasis in Kalar District, Iraq. Arch. Razi Inst. 2022, 77, 1377–1382. [Google Scholar] [CrossRef]
- Barantsevich, N.; Barantsevich, E. Diagnosis and treatment of invasive candidiasis. Antibiotics 2022, 11, 718. [Google Scholar] [CrossRef]
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and invasive candidiasis. Infect. Dis. Clin. N. Am. 2021, 35, 389–413. [Google Scholar] [CrossRef]
- Fisher, M.C.; Denning, D.W. The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023, 21, 211–212. [Google Scholar] [CrossRef]
- Spruijtenburg, B.; Baqueiro, C.C.S.Z.; Colombo, A.L.; Meijer, E.F.J.; de Almeida, J.N.J.; Berrio, I.; Fernández, N.B.; Chaves, G.M.; Meis, J.F.; de Groot, T. Short Tandem Repeat Genotyping and Antifungal Susceptibility Testing of Latin American Candida tropicalis Isolates. J. Fungi 2023, 9, 207. [Google Scholar] [CrossRef]
- Cortés, J.A.; Ruiz, J.F.; Melgarejo-Moreno, L.N.; Lemos, E.V. Candidemia in Colombia. Biomedica 2020, 40, 195–207. [Google Scholar] [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Forastiero, A.; Mesa-Arango, A.C.; Alastruey-Izquierdo, A.; Alcazar-Fuoli, L.; Bernal-Martinez, L.; Pelaez, T.; Lopez, J.F.; Grimalt, J.O.; Gomez-Lopez, A.; Cuesta, I.; et al. Candida tropicalis antifungal cross-resistance is related to different azole target (Erg11p) modifications. Antimicrob. Agents Chemother. 2013, 57, 4769–4781. [Google Scholar] [CrossRef]
- de Oliveira, J.S.; Pereira, V.S.; Castelo-Branco, D.S.C.M.; Cordeiro, R.A.; Sidrim, J.J.C.; Brilhante, R.S.N.; Rocha, M.F.G. The yeast, the antifungal, and the wardrobe: A journey into antifungal resistance mechanisms of Candida tropicalis. Can. J. Microbiol. 2020, 66, 377–388. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Watanabe, A.; Kamei, K. Azole and echinocandin resistance mechanisms and genotyping of Candida tropicalis in Japan: Cross-boundary dissemination and animal-human transmission of C. tropicalis infection. Clin. Microbiol. Infect. 2022, 28, 302.e5–302.e8. [Google Scholar] [CrossRef]
- Szymański, M.; Chmielewska, S.; Czyżewska, U.; Malinowska, M.; Tylicki, A. Echinocandins—Structure, mechanism of action and use in antifungal therapy. J. Enzym. Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef]
- Lo Re, V., 3rd; Carbonari, D.M.; Lewis, J.D.; Forde, K.A.; Goldberg, D.S.; Reddy, K.R.; Haynes, K.; Roy, J.A.; Sha, D.; Marks, A.R.; et al. Oral azole antifungal medications and risk of acute liver injury, overall and by chronic liver disease status. Am. J. Med. 2016, 129, 283–291.e5. [Google Scholar] [CrossRef] [PubMed]
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-derived products as antibacterial and antifungal agents in human health care. Curr. Med. Chem. 2019, 26, 5501–5541. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Mesa Vanegas, A.M. Una visión histórica en el desarrollo de fármacos a partir de productos naturales. Rev. Mex. Ciencias Farm. 2017, 48, 16–27. Available online: https://www.redalyc.org/articulo.oa?id=57956616003 (accessed on 20 May 2023).
- Zida, A.; Bamba, S.; Yacouba, A.; Ouedraogo-Traore, R.; Guiguemdé, R.T. Anti-Candida albicans natural products, sources of new antifungal drugs: A review. J. Mycol. Med. 2017, 27, 1–19. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Rolta, R.; Dev, K.; Sourirajan, A. Synergistic potential of essential oils with antibiotics to combat fungal pathogens: Present status and future perspectives. Phytother. Res. 2021, 35, 6089–6100. [Google Scholar] [CrossRef] [PubMed]
- Donadu, M.G.; Peralta-Ruiz, Y.; Usai, D.; Maggio, F.; Molina-Hernandez, J.B.; Rizzo, D.; Bussu, F.; Rubino Zanetti, S.; Paparella, A. Colombian essential oil of Ruta graveolens against nosocomial antifungal resistant Candida strains. J. Fungi 2021, 7, 383. [Google Scholar] [CrossRef]
- Jafri, H.; Ahmad, I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef] [PubMed]
- Samber, N.; Khan, A.; Varma, A.; Manzoor, N. Synergistic anti-candidal activity and mode of action of Mentha piperita essential oil and its major components. Pharm. Biol. 2015, 53, 1496–1504. [Google Scholar] [CrossRef]
- Spengler, G.; Gajdács, M.; Donadu, M.G.; Usai, M.; Marchetti, M.; Ferrari, M.; Mazzarello, V.; Zanetti, S.; Nagy, F.; Kovács, R. Evaluation of the antimicrobial and antivirulent potential of essential oils isolated from Juniperus oxycedrus L. ssp. macrocarpa aerial parts. Microorganisms 2022, 10, 758. [Google Scholar] [CrossRef]
- Tran, H.N.H.; Graham, L.; Adukwu, E.C. In vitro antifungal activity of Cinnamomum zeylanicum bark and leaf essential oils against Candida albicans and Candida auris. Appl. Microbiol. Biotechnol. 2020, 104, 8911–8924. [Google Scholar] [CrossRef]
- Borotová, P.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Tvrdá, E.; Kačániová, M. Chemical and biological characterization of Melaleuca alternifolia essential oil. Plants 2022, 11, 558. [Google Scholar] [CrossRef]
- Niu, C.; Wang, C.; Yang, Y.; Chen, R.; Zhang, J.; Chen, H.; Zhuge, Y.; Li, J.; Cheng, J. Carvacrol induces Candida albicans apoptosis associated with Ca2+/calcineurin pathway. Front. Cell. Infect. Microbiol. 2020, 10, 192. [Google Scholar] [CrossRef]
- Konuk, H.B.; Ergüden, B. Phenolic -OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity. Folia Microbiol. 2020, 65, 775–783. [Google Scholar] [CrossRef]
- Contreras Martínez, O.I.; Angulo Ortíz, A.; Santafé Patiño, G. Mechanism of antifungal action of monoterpene isoespintanol against Clinical isolates of Candida tropicalis. Molecules 2022, 27, 5808. [Google Scholar] [CrossRef]
- Bernal, R.; Gradstein, S.R.; Celis, M. (Eds.) Catálogo de Plantas y Líquenes de Colombia, Bogotá. 2020. Available online: https://ipt.biodiversidad.co/sib/resource?r=catalogo_plantas_liquenes (accessed on 22 May 2022). [CrossRef]
- Sistema de Información Sobre Biodiversidad de Colombia—Biodiversidad de Colombia en Cifras. 2022. Available online: https://biodiversidad.co/post/2022/biodiversidad-colombia-cifras-2022/ (accessed on 22 May 2022).
- Meneses, R.; Ocazionez, R.E.; Martínez, J.R.; Stashenko, E.E. Inhibitory effect of essential oils obtained from plants grown in Colombia on yellow fever virus replication in vitro. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 8. [Google Scholar] [CrossRef]
- Espinel-Mesa, D.X.; González Rugeles, C.I.; Mantilla Hernández, J.C.; Stashenko, E.E.; Villegas-Lanau, C.A.; Quimbaya Ramirez, J.J.; García Sánchez, L.T. Immunomodulation and antioxidant activities as possible trypanocidal and cardioprotective mechanisms of major terpenes from Lippia alba essential oils in an experimental model of chronic Chagas disease. Antioxidants 2021, 10, 1851. [Google Scholar] [CrossRef] [PubMed]
- Raman, V.; Fuentes Lorenzo, J.L.; Stashenko, E.E.; Levy, M.; Levy, M.M.; Camarillo, I.G. Lippia origanoides extract induces cell cycle arrest and apoptosis and suppresses NF-κB signaling in triple-negative breast cancer cells. Int. J. Oncol. 2017, 51, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.E.; Slowing, K.; Carretero, E.; Sánchez Mata, D.; Villar, A. Lippia: Traditional uses, chemistry and pharmacology: A review. J. Ethnopharmacol. 2001, 76, 201–214. [Google Scholar] [CrossRef]
- Pérez Zamora, C.M.; Torres, C.A.; Nuñez, M.B. Antimicrobial activity and chemical composition of essential oils from Verbenaceae species growing in South America. Molecules 2018, 23, 544. [Google Scholar] [CrossRef]
- Sarrazin, S.L.F.; da Silva, L.A.; de Assunção, A.P.F.; Oliveira, R.B.; Calao, V.Y.P.; da Silva, R.; Stashenko, E.E.; Maia, J.G.S.; Mourão, R.H.V. Antimicrobial and seasonal evaluation of the carvacrol-chemotype oil from Lippia origanoides Kunth. Molecules 2015, 20, 1860–1871. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Zapata, C.; Loaiza-Oliva, M.; Martínez-Pabón, M.C.; Stashenko, E.E.; Mesa-Arango, A.C. In Vitro activity of essential oils distilled from Colombian plants against Candida auris and other Candida Species with different antifungal susceptibility profiles. Molecules 2022, 27, 6837. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Lewis, R.E. Toward more effective antifungal therapy: The prospects of combination therapy. Br. J. Haematol. 2004, 126, 165–175. [Google Scholar] [CrossRef]
- Spitzer, M.; Robbins, N.; Wright, G.D. Combinatorial strategies for combating invasive fungal infections. Virulence 2017, 8, 169–185. [Google Scholar] [CrossRef]
- Lu, M.; Li, T.; Wan, J.; Li, X.; Yuan, L.; Sun, S. Antifungal effects of phytocompounds on Candida species alone and in combination with fluconazole. Int. J. Antimicrob. Agents 2017, 49, 125–136. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Manzoor, N. Reversal of efflux mediated antifungal resistance underlies synergistic activity of two monoterpenes with fluconazole. Eur. J. Pharm. Sci. 2013, 48, 80–86. [Google Scholar] [CrossRef]
- Liu, S.; Hou, Y.; Chen, X.; Gao, Y.; Li, H.; Sun, S. Combination of fluconazole with non-antifungal agents: A promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery. Int. J. Antimicrob. Agents 2014, 43, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, H.; Jiang, D.; Yang, Y.; Tang, W.; Xu, K. The antifungal activity of essential oil from Melaleuca leucadendra (L.) L. grown in China and its synergistic effects with conventional antibiotics against Candida. Nat. Prod. Res. 2019, 33, 2545–2548. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Reference Method for Broth of Yeasts; Dilution Antifungal Susceptibility Testing Approved Standard, 4th ed.; CLSI Document M27; CLSI: Wayne, PA, USA, 2017; Volume 37. [Google Scholar]
- Fivelman, Q.L.; Adagu, I.S.; Warhurst, D.C. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 2004, 48, 4097–4102. [Google Scholar] [CrossRef]
- Loewe, S.; Muischnek, H. Über Kombinationswirkungen. Arch. Exp. Pathol. Pharmakol. 1926, 114, 313–326. [Google Scholar] [CrossRef]
- Bidaud, A.-L.; Schwarz, P.; Herbreteau, G.; Dannaoui, E. Techniques for the assessment of in vitro and in vivo antifungal combinations. J. Fungi 2021, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Y.; Pei, L.; Liu, Q.; Chen, S.; Dou, H.; Shu, G.; Yuan, Z.X.; Lin, J.; Peng, G. Isobologram analysis: A comprehensive review of methodology and current research. Front. Pharmacol. 2019, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bio-Protocol 2019, 9, e3128. [Google Scholar] [CrossRef]
- Shaban, S.; Patel, M.; Ahmad, A. Improved efficacy of antifungal drugs in combination with monoterpene phenols against Candida auris. Sci Rep. 2020, 10, 1162. [Google Scholar] [CrossRef] [PubMed]
- Loaiza Oliva, M.; Morales Uchima, S.M.; Puerta Suárez, J.; Mesa Arango, A.C.; Martínez Pabón, M.C. Lippia origanoides derivatives in vitro evaluation on polymicrobial biofilms: Streptococcus mutans, Lactobacillus rhamnosus and Candida albicans. Arch. Oral Biol. 2023, 148, 105656. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Rueda, C.; Román, E.; Quintin, J.; Terrón, M.C.; Luque, D.; Netea, M.G.; Pla, J.; Zaragoza, O. Cell wall changes in amphotericin B-resistant strains from Candida tropicalis and relationship with the immune responses elicited by the host. Antimicrob. Agents Chemother. 2016, 60, 2326–2335. [Google Scholar] [CrossRef] [PubMed]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.G.; Nakamura, C.V.; Filho, B.P.D. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef]
- Sokolov, S.S.; Popova, M.M.; Pohl, P.; Horner, A.; Akimov, S.A.; Kireeva, N.; Knorre, D.A.; Batishchev, O.V.; Severin, F.F. Structural role of plasma membrane sterols in osmotic stress tolerance of yeast Saccharomyces cerevisiae. Membranes 2022, 12, 1278. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, J.; Yu, L.; Wang, C.; Yang, Y.; Rong, X.; Xu, K.; Chu, M. Antifungal Activity of Coumarin Against Candida albicans Is Related to Apoptosis. Front. Cell. Infect. Microbiol. 2018, 8, 445. [Google Scholar] [CrossRef]
- Thakre, A.; Zore, G.; Kodgire, S.; Kazi, R.; Mulange, S.; Patil, R.; Shelar, A.; Santhakumari, B.; Kulkarni, M.; Kharat, K. Limonene inhibits Candida albicans growth by inducing apoptosis. Med. Mycol. 2018, 56, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Shekhova, E.; Kniemeyer, O.; Brakhage, A.A. Induction of mitochondrial reactive oxygen species production by itraconazole, terbinafine, and amphotericin B as a mode of action against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2017, 61, e00978-17. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Román, E.; Sánchez-Fresneda, R.; Casas, C.; Herrero, E.; Argüelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The production of reactive oxygen species is a universal action mechanism of amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef]
- Sangalli-Leite, F.; Scorzoni, L.; Mesa-Arango, A.C.; Casas, C.; Herrero, E.; Gianinni, M.J.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M.; Zaragoza, O. Amphotericin B mediates killing in Cryptococcus neoformans through the induction of a strong oxidative burst. Microbes Infect. 2011, 13, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Calderone, R. Exploiting mitochondria as targets for the development of new antifungals. Virulence 2017, 8, 159–168. [Google Scholar] [CrossRef]
- Thomson, G.J.; Hernon, C.; Austriaco, N.; Shapiro, R.S.; Belenky, P.; Bennett, R.J. Metabolism-induced oxidative stress and DNA damage selectively trigger genome instability in polyploid fungal cells. EMBO J. 2019, 38, 1–17. [Google Scholar] [CrossRef]
- Andalis, A.A.; Storchova, Z.; Styles, C.; Galitski, T.; Pellman, D.; Fink, G.R. Defects arising from whole-genome duplications in Saccharomyces cerevisiae. Genetics 2004, 167, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Mayer, V.W.; Aguilera, A. High levels of chromosome instability in polyploids of Saccharomyces cerevisiae. Mutat. Res. 1990, 231, 177–186. [Google Scholar] [CrossRef] [PubMed]


| Plant Species and Chemotype | Collection Site | Voucher Number | Principal Compounds (Relative Amount, %) |
|---|---|---|---|
| Lippia origanoides (Thymol + p-cymene) chemotype | Bucaramanga—Santander, Colombia | UIS * herbarium 22039 | Thymol (49.4), p-cymene (19.1), γ-terpinene (9.2), β-myrcene (5.2), α-terpinene (2.9), carvacrol (2.7), thymyl methyl ether (1.8), trans-β-caryophyllene (1.6), cis-β-ocimene (1.2), and limonene (0.9). |
| Combination | FLC MIC (X) | Thymol or EO MIC (X) |
|---|---|---|
| 1 | 0 | 8 |
| 2 | 0.5 | 4 |
| 3 | 1 | 2 |
| 4 | 2 | 1 |
| 5 | 4 | 0.5 |
| 6 | 8 | 0 |
| Strain | Compound/ Combination | GM-MIC (µg/mL) | FICI | Interaction Type | No. Times ↓ MIC | ||
|---|---|---|---|---|---|---|---|
| FLC | EO | Thymol | |||||
| C. tropicalisATCC 200956 * | FLC | >64 | - | - | - | - | - |
| EO | 128 | - | - | - | - | - | |
| Thymol | 128 | - | - | - | - | - | |
| EO/FLC | 32/2 | 0.28 | Synergistic | 32 | 4 | - | |
| Thymol/FLC | 32/2 | 0.28 | Synergistic | 32 | - | 4 | |
| C. tropicalisATCC 750 ** | FLC | 2 | - | - | - | - | - |
| EO | 512 | - | - | - | - | - | |
| Thymol | 256 | - | - | - | - | - | |
| EO/FLC | 181/0.25 | 0.53 | Additive | 8.0 | 2.8 | - | |
| Thymol/FLC | 42/0.60 | 0.47 | Synergistic | 3.3 | - | 6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapata-Zapata, C.; Rojas-López, M.; García, L.T.; Quintero, W.; Terrón, M.C.; Luque, D.; Mesa-Arango, A.C. Lippia origanoides Essential Oil or Thymol in Combination with Fluconazole Produces Damage to Cells and Reverses the Azole-Resistant Phenotype of a Candida tropicalis Strain. J. Fungi 2023, 9, 888. https://doi.org/10.3390/jof9090888
Zapata-Zapata C, Rojas-López M, García LT, Quintero W, Terrón MC, Luque D, Mesa-Arango AC. Lippia origanoides Essential Oil or Thymol in Combination with Fluconazole Produces Damage to Cells and Reverses the Azole-Resistant Phenotype of a Candida tropicalis Strain. Journal of Fungi. 2023; 9(9):888. https://doi.org/10.3390/jof9090888
Chicago/Turabian StyleZapata-Zapata, Carolina, Mauricio Rojas-López, Liliana T. García, Wendy Quintero, María C. Terrón, Daniel Luque, and Ana C. Mesa-Arango. 2023. "Lippia origanoides Essential Oil or Thymol in Combination with Fluconazole Produces Damage to Cells and Reverses the Azole-Resistant Phenotype of a Candida tropicalis Strain" Journal of Fungi 9, no. 9: 888. https://doi.org/10.3390/jof9090888
APA StyleZapata-Zapata, C., Rojas-López, M., García, L. T., Quintero, W., Terrón, M. C., Luque, D., & Mesa-Arango, A. C. (2023). Lippia origanoides Essential Oil or Thymol in Combination with Fluconazole Produces Damage to Cells and Reverses the Azole-Resistant Phenotype of a Candida tropicalis Strain. Journal of Fungi, 9(9), 888. https://doi.org/10.3390/jof9090888






