A New Technique for the Extraction of Arbuscular Mycorrhizae Fungal Spores from Rhizosphere
Abstract
1. Introduction
2. Materials and Methods
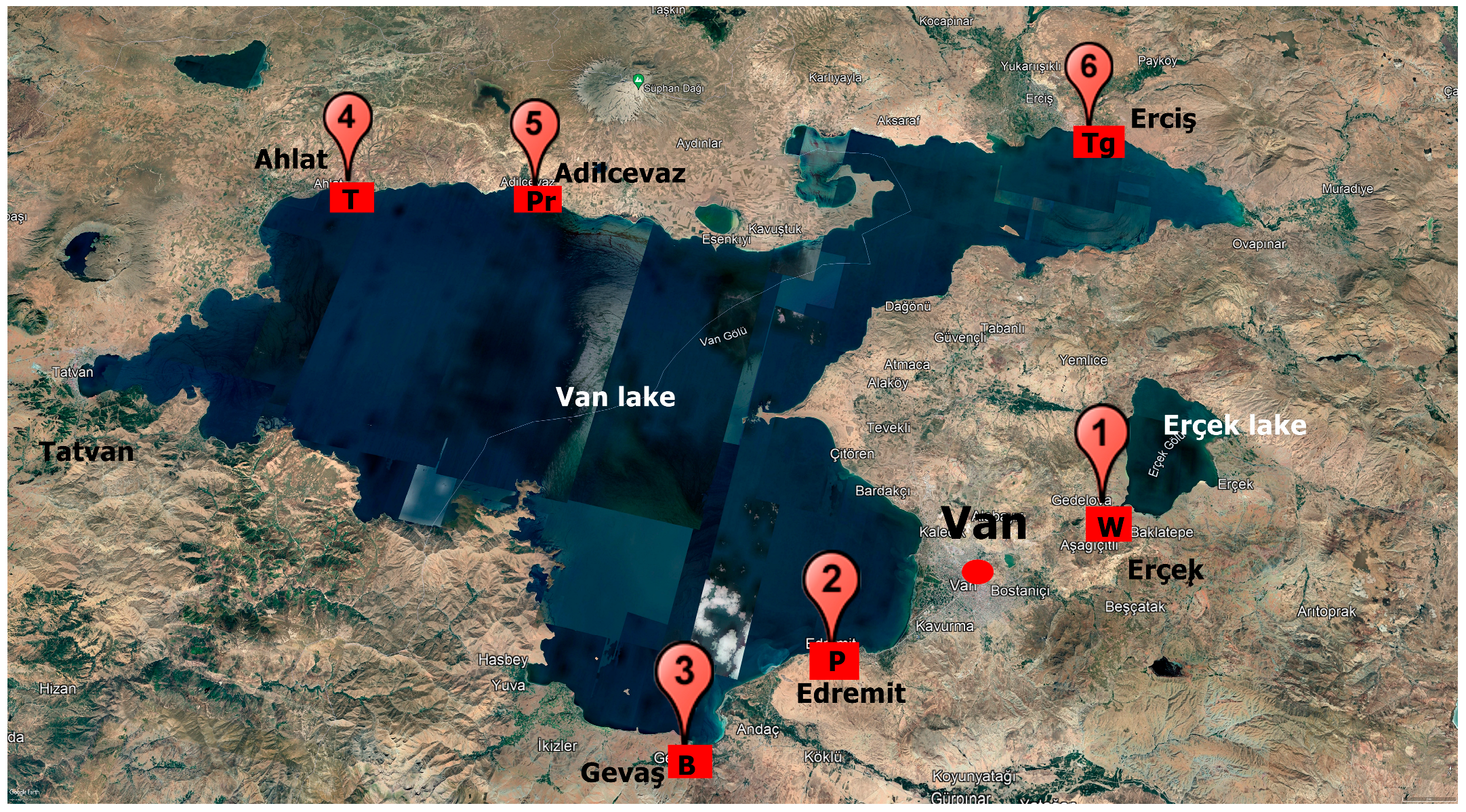
2.1. Site Description
2.2. Rhizosphere Sampling
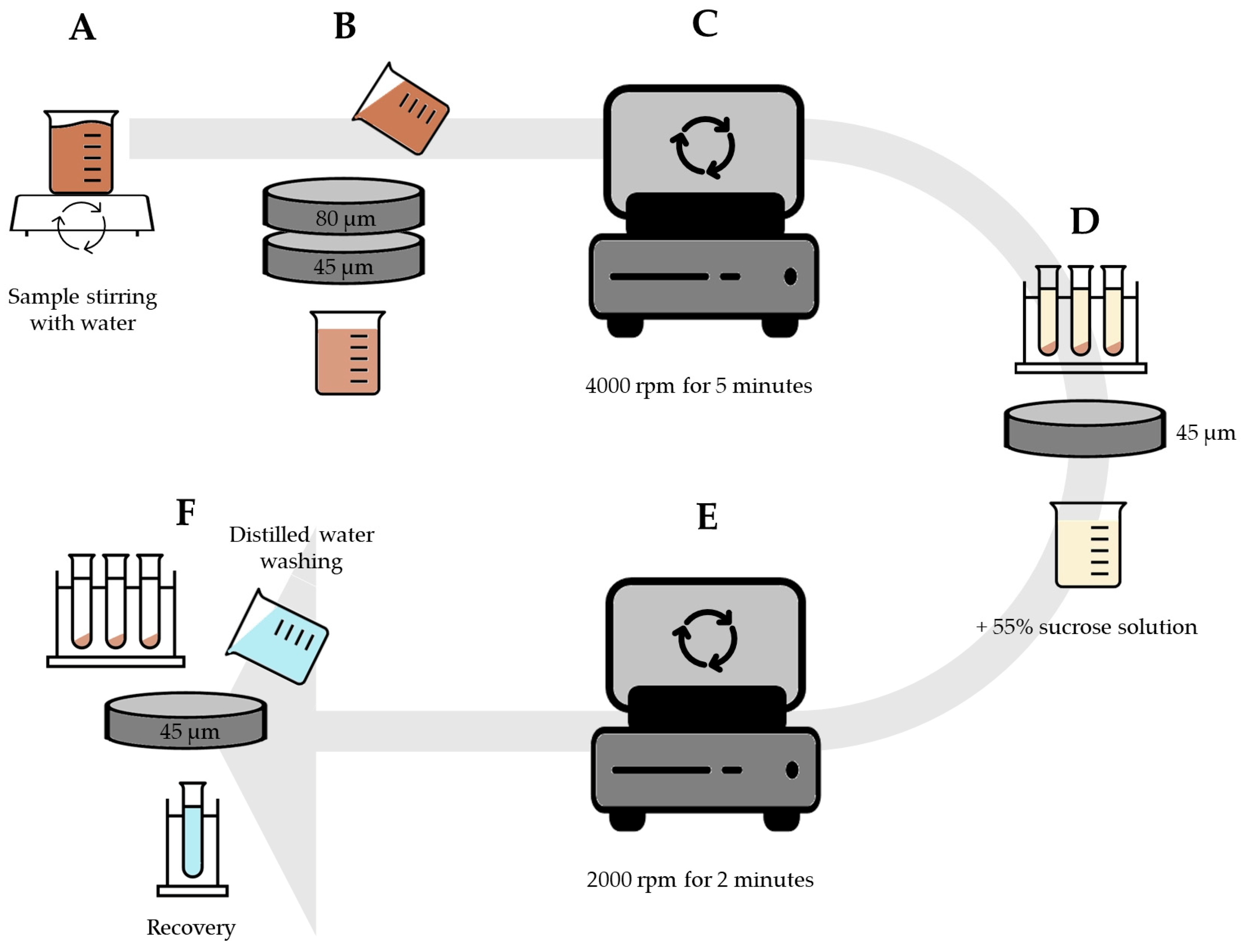
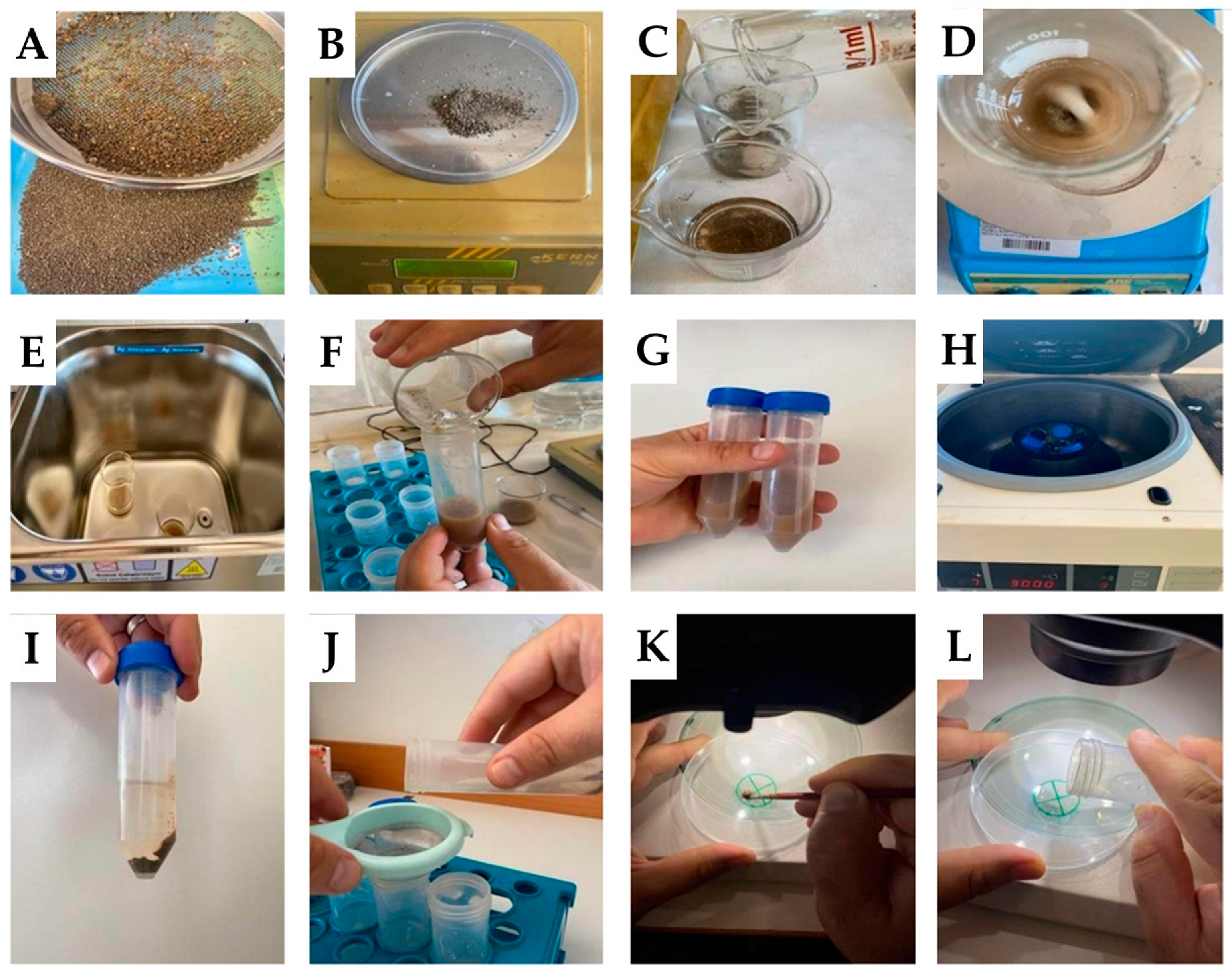
2.3. Wet-Sieving Technique (WST)
2.4. Ultrasound Wet-Sieving Technique (UWST)
2.5. Ultrasound Centrifuge Technique (UCT)
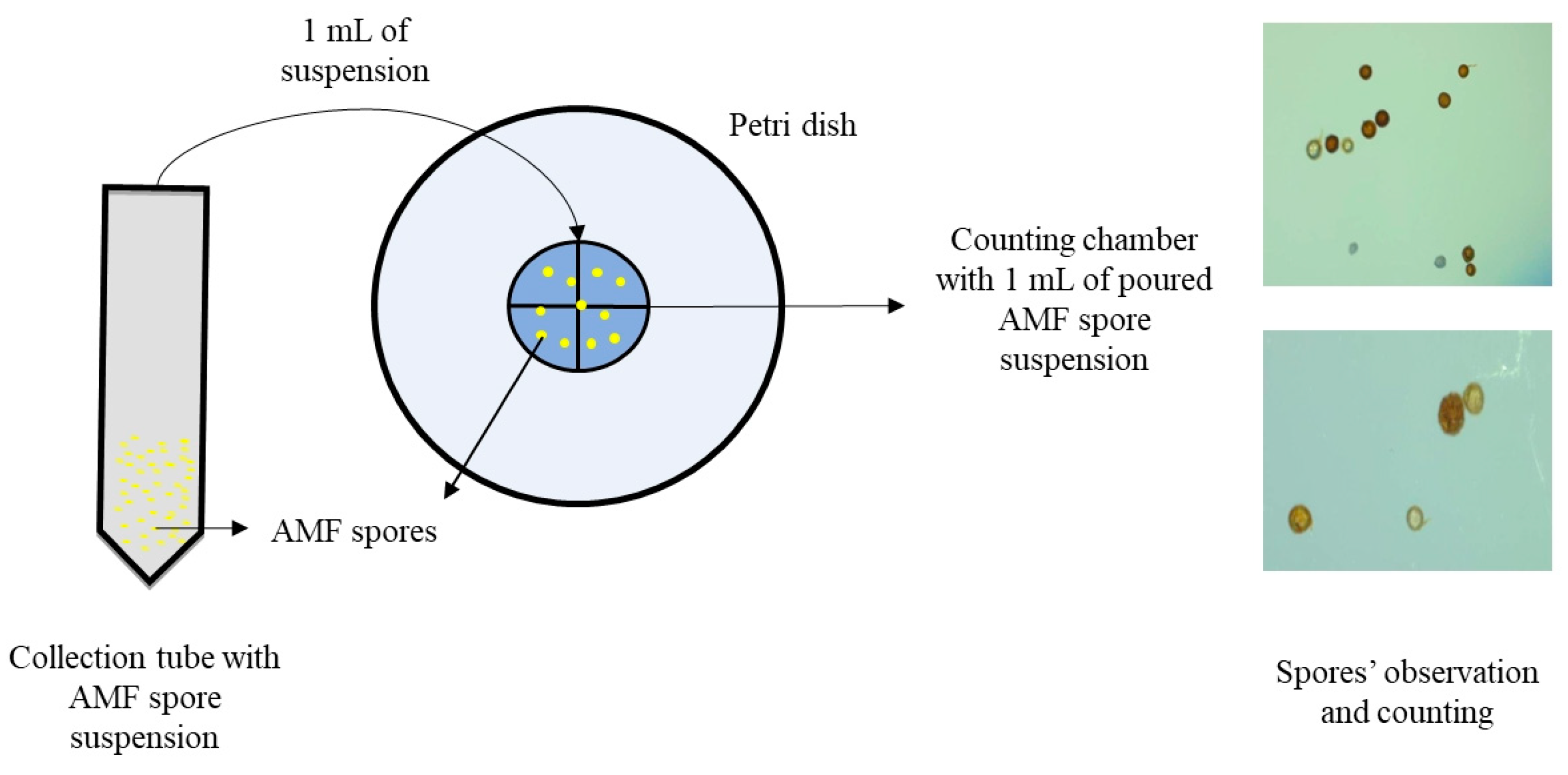
2.6. AMF Spore Enumeration
- TSN: Total AMF spore numbers in 1 g of rhizosphere
- SN: AMF spore numbers in 1 mL of spore suspension
- W: Amount of water used (mL)
- S: Amount of soil used (g)
2.7. Statistical Analysis
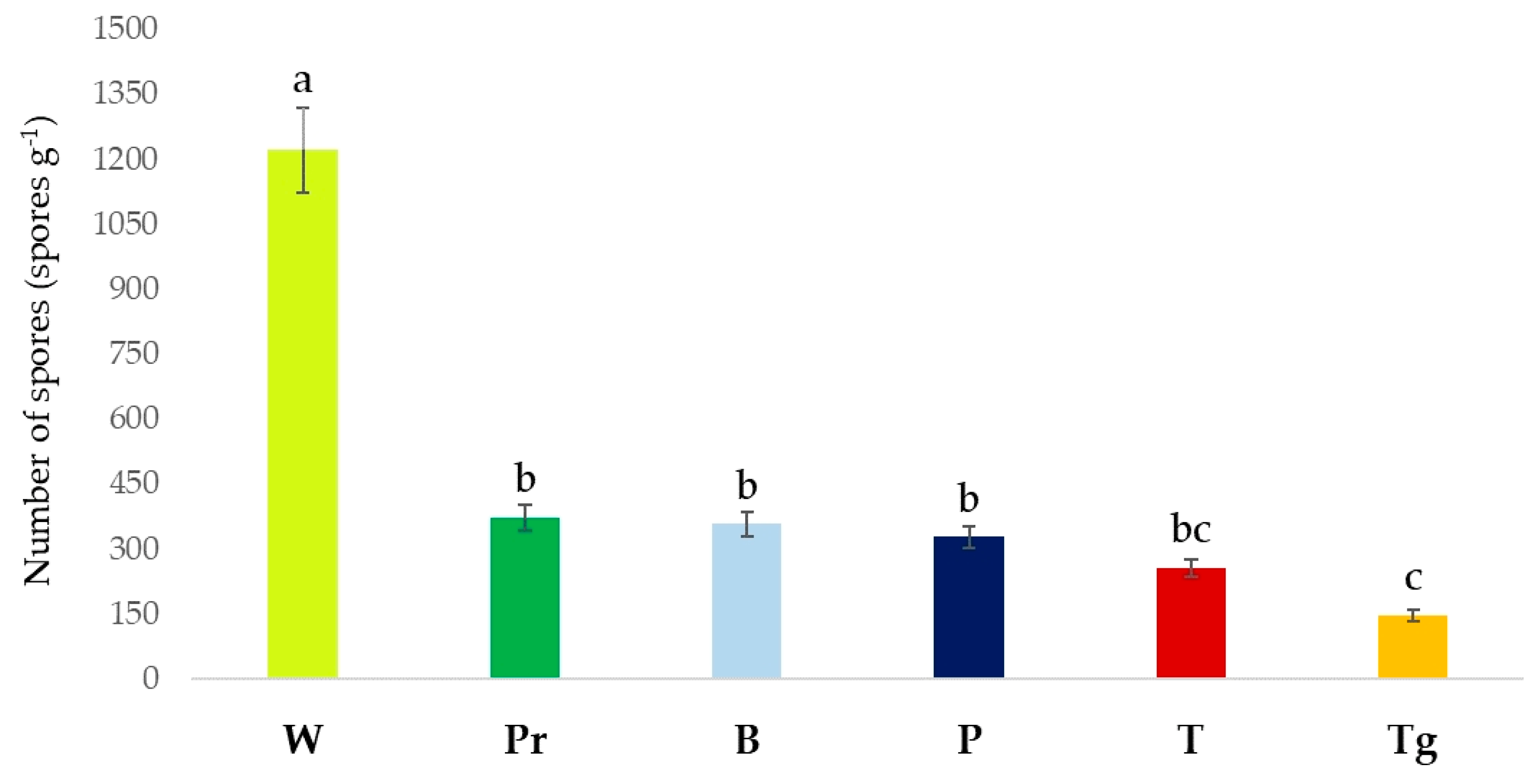
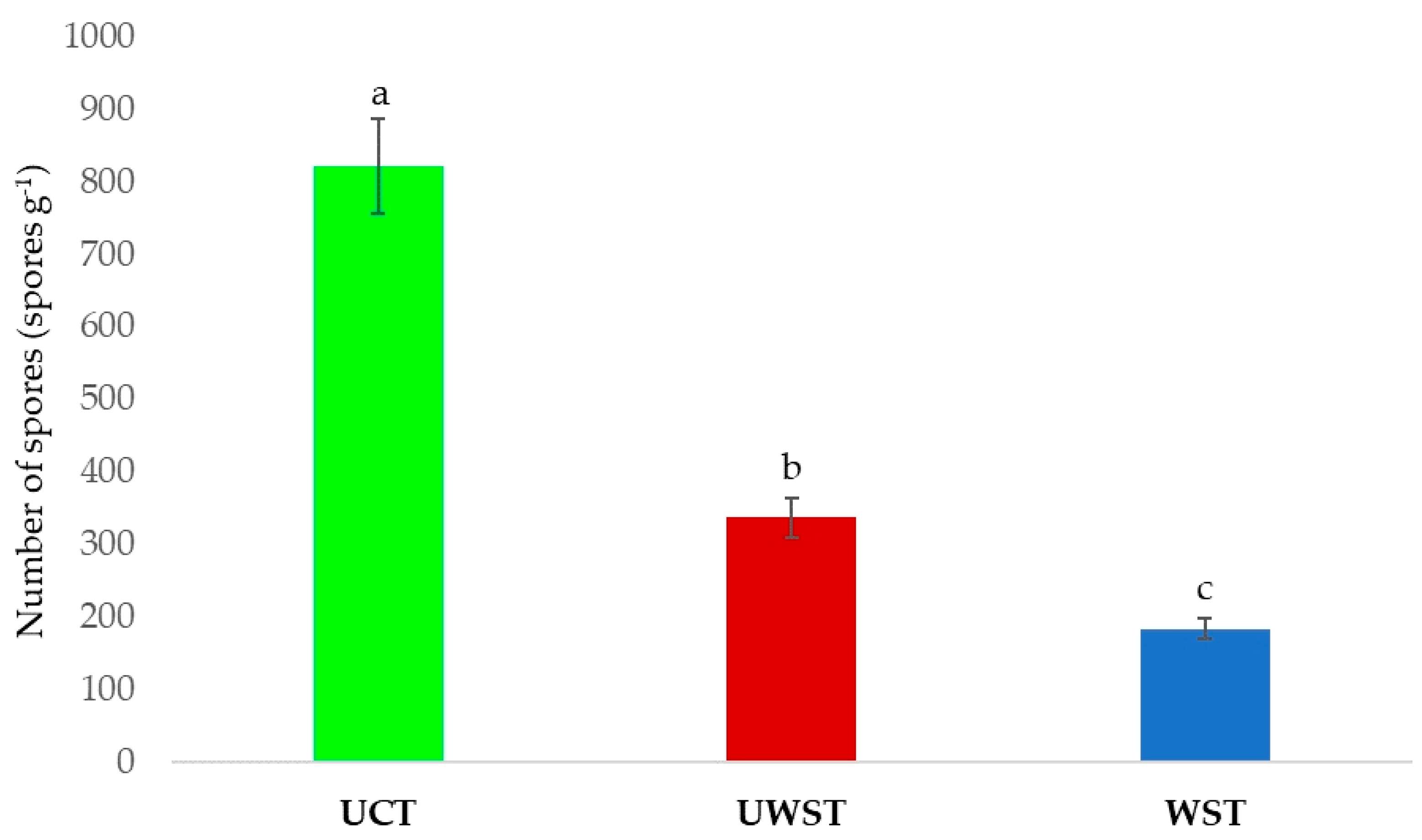
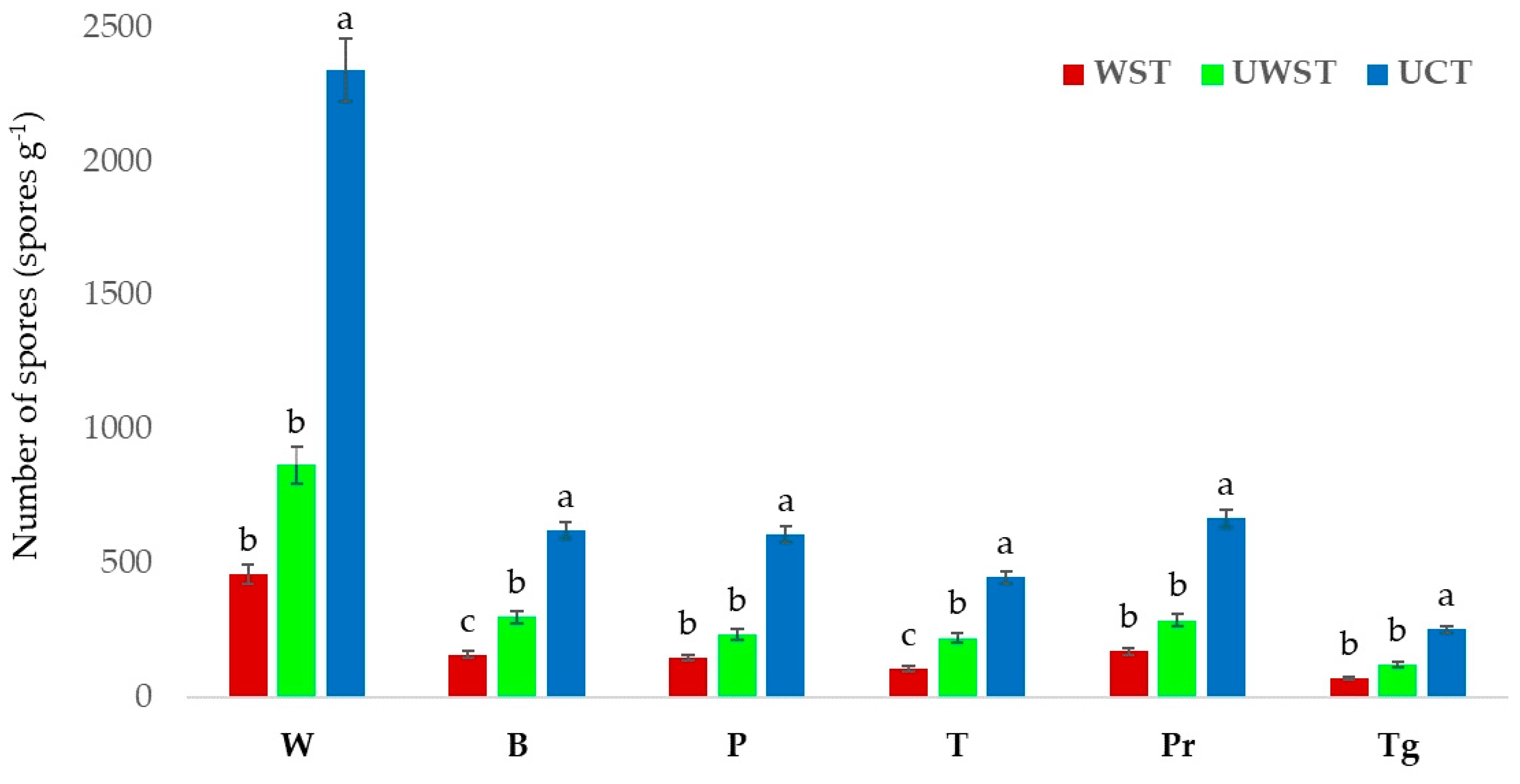
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Q.-S.; Silva, F.S.B.; Hijri, M.; Kapoor, R. Editorial: Arbuscular Mycorrhiza-Mediated Augmentation of Plant Secondary Metabolite Production. Front. Plant Sci. 2023, 14, 1150900. [Google Scholar] [CrossRef] [PubMed]
- Boya, S.; Puttaswamy, P.; Mahadevappa, N.; Sharma, B.; Othumbamkat, R. Enumerating Indigenous Arbuscular Mycorrhizal Fungi (AMF) Associated with Three Permanent Preservation Plots of Tropical Forests in Bangalore, Karnataka, India. Bacteria 2023, 2, 70–80. [Google Scholar] [CrossRef]
- Wilkes, T.I. Arbuscular Mycorrhizal Fungi in Agriculture. Encyclopedia 2021, 1, 1132–1154. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Shachar-Hill, Y.; Pfeffer, P.E.; Douds, D.; Osman, S.F.; Doner, L.W.; Ratcliffe, R.G. Partitioning of Intermediary Carbon Metabolism in Vesicular-Arbuscular Mycorrhizal Leek. Plant Physiol. 1995, 108, 7–15. [Google Scholar] [CrossRef]
- Mitra, D.; Djebaili, R.; Pellegrini, M.; Mahakur, B.; Sarker, A.; Chaudhary, P.; Khoshru, B.; Del Gallo, M.; Kitouni, M.; Barik, D.P.; et al. Arbuscular Mycorrhizal Symbiosis: Plant Growth Improvement and Induction of Resistance under Stressful Conditions. J. Plant Nutr. 2021, 44, 1993–2028. [Google Scholar] [CrossRef]
- Mitra, D.; Saritha, B.; Janeeshma, E.; Gusain, P.; Khoshru, B.; Abo Nouh, F.A.; Rani, A.; Olatunbosun, A.N.; Ruparelia, J.; Rabari, A.; et al. Arbuscular Mycorrhizal Fungal Association Boosted the Arsenic Resistance in Crops with Special Responsiveness to Rice Plant. Environ. Exp. Bot. 2022, 193, 104681. [Google Scholar] [CrossRef]
- Sui, X.-L.; Li, A.-R.; Chen, Y.; Guan, K.-Y.; Zhuo, L.; Liu, Y.-Y. Arbuscular Mycorrhizal Fungi: Potential Biocontrol Agents against the Damaging Root Hemiparasite Pedicularis Kansuensis? Mycorrhiza 2014, 24, 187–195. [Google Scholar] [CrossRef]
- Boyno, G.; Demir, S.; Danesh, Y.R. Effects of Some Biological Agents on the Growth and Biochemical Parameters of Tomato Plants Infected with Alternaria Solani (Ellis & Martin) Sorauer. Eur. J. Plant Pathol. 2022, 162, 19–29. [Google Scholar] [CrossRef]
- Ortiz, N.; Armada, E.; Duque, E.; Roldán, A.; Azcón, R. Contribution of Arbuscular Mycorrhizal Fungi and/or Bacteria to Enhancing Plant Drought Tolerance under Natural Soil Conditions: Effectiveness of Autochthonous or Allochthonous Strains. J. Plant Physiol. 2015, 174, 87–96. [Google Scholar] [CrossRef]
- Singh, P.; Chauhan, P.K.; Upadhyay, S.K.; Singh, R.K.; Dwivedi, P.; Wang, J.; Jain, D.; Jiang, M. Mechanistic Insights and Potential Use of Siderophores Producing Microbes in Rhizosphere for Mitigation of Stress in Plants Grown in Degraded Land. Front. Microbiol. 2022, 13, 898979. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Tullio, M.; Rivera, C.M.; Rea, E. Alleviation of Salt Stress by Arbuscular Mycorrhizal in Zucchini Plants Grown at Low and High Phosphorus Concentration. Biol. Fertil. Soils 2008, 44, 501–509. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and Sustainable Agriculture: Exploring Arbuscular Mycorrhizal Fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881. [Google Scholar] [CrossRef]
- Boyno, G.; Demir, S. Plant-Mycorrhiza Communication and Mycorrhizae in Inter-Plant Communication. Symbiosis 2022, 86, 155–168. [Google Scholar] [CrossRef]
- Lennon, J.T.; Jones, S.E. Microbial Seed Banks: The Ecological and Evolutionary Implications of Dormancy. Nat. Rev. Microbiol. 2011, 9, 119–130. [Google Scholar] [CrossRef]
- Silva-Flores, P.; Bueno, C.G.; Neira, J.; Palfner, G. Factors Affecting Arbuscular Mycorrhizal Fungi Spore Density in the Chilean Mediterranean-Type Ecosystem. J. Soil Sci. Plant Nutr. 2019, 19, 42–50. [Google Scholar] [CrossRef]
- Sanders, I.R.; Alt, M.; Groppe, K.; Boller, T.; Wiemken, A. Identification of Ribosomal DNA Polymorphisms among and within Spores of the Glomales: Application to Studies on the Genetic Diversity of Arbuscular Mycorrhizal Fungal Communities. New Phytol. 1995, 130, 419–427. [Google Scholar] [CrossRef]
- Sasvári, Z.; Magurno, F.; Galanics, D.; Hang, T.T.N.; Ha, T.T.H.; Luyen, N.D.; Huong, L.M.; Posta, K. Isolation and Identification of Arbuscular Mycorrhizal Fungi from Agricultural Fields of Vietnam. Am. J. Plant Sci. 2012, 03, 1796–1801. [Google Scholar] [CrossRef]
- Varga, S.; Finozzi, C.; Vestberg, M.; Kytöviita, M.-M. Arctic Arbuscular Mycorrhizal Spore Community and Viability after Storage in Cold Conditions. Mycorrhiza 2015, 25, 335–343. [Google Scholar] [CrossRef]
- Hanlon, M.T.; Coenen, C. Genetic Evidence for Auxin Involvement in Arbuscular Mycorrhiza Initiation. New Phytol. 2011, 189, 701–709. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.S.A.; Aqil, F.; Singh, M. Microbial Applications in Agriculture and the Environment: A Broad Perspective. In Microbes and Microbial Technology; Springer: New York, NY, USA, 2011; pp. 1–27. [Google Scholar]
- Gerdemann, J.W.; Nicolson, T.H. Spores of Mycorrhizal Endogone Species Extracted from Soil by Wet Sieving and Decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Jenkins, W.R.B. A Rapid Centrifugal-Flotation Technique for Separating Nematodes from Soil. Plant Dis. Report. 1964, 48, 692. [Google Scholar]
- Walker, C.; Mize, C.W.; McNabb, H.S., Jr. Populations of Endogonaceous Fungi at Two Locations in Central Iowa. Can. J. Bot. 1982, 60, 2518–2529. [Google Scholar] [CrossRef]
- Vilariño, A.; Arines, J. An Instrumental Modification of Gerdemann and Nicolson’s Method for Extracting VAM Fungal Spores from Soil Samples. Plant Soil 1990, 121, 211–215. [Google Scholar] [CrossRef]
- An, Z.-Q.; Hendrix, J.W.; Hershman, D.E.; Henson, G.T. Evaluation of the “Most Probable Number” (MPN) and Wet-Sieving Methods for Determining Soil-Borne Populations of Endogonaceous Mycorrhizal Fungi. Mycologia 1990, 82, 576–581. [Google Scholar] [CrossRef]
- Pacioni, G. Wet-Sieving and Decanting Techniques for the Extraction of Spores of Vesicular-Arbuscular Fungi. Tech. Mycorrhizal Res. Methods Microbiol. 1994, 24, 317–322. [Google Scholar]
- Shamini, S.; Amutha, K. Techniques for Extraction of Arbuscular Mycorrhizal Fungi Spores. Int. J. Front. Sci. Technol. 2014, 2, 1–6. [Google Scholar]
- Srisom, K.; Tittabutr, P.; Teaumroong, N.; Lapwong, Y.; Phatthanakun, R.; Sirivisoot, S.; Kuntanawat, P. New Method for Arbuscular Mycorrhizal Fungus Spore Separation Using a Microfluidic Device Based on Manual Temporary Flow Diversion. Mycorrhiza 2020, 30, 789–796. [Google Scholar] [CrossRef]
- Walker, C. Arbuscular Mycorrhiza in the Living Collections at the Royal Botanic Garden Edinburgh. Int. J. Bot. Gard. Hortic. 2013, 11, 143–157. [Google Scholar] [CrossRef]
- Gallego-Juárez, J.A.; Graff, K.F.; Lucas, M. Power Ultrasonics: Applications of High-Intensity Ultrasound; Woodhead Publishing: Sawston, UK, 2023; ISBN 0323851444. [Google Scholar]
- Satomura, T. Extraction of Arbuscular Mycorrhizal Fungus Spores from Clayey Soil. Tropics 2007, 16, 337–341. [Google Scholar] [CrossRef][Green Version]
- Stamou, G.P.; Konstadinou, S.; Monokrousos, N.; Mastrogianni, A.; Orfanoudakis, M.; Hassiotis, C.; Menkissoglu-Spiroudi, U.; Vokou, D.; Papatheodorou, E.M. The Effects of Arbuscular Mycorrhizal Fungi and Essential Oil on Soil Microbial Community and N-Related Enzymes during the Fungal Early Colonization Phase. AIMS Microbiol. 2017, 3, 938–959. [Google Scholar] [CrossRef] [PubMed]
- Ellouze, W.; Hamel, C.; Cruz, A.F.; Ishii, T.; Gan, Y.; Bouzid, S.; St-Arnaud, M. Phytochemicals and Spore Germination: At the Root of AMF Host Preference? Appl. Soil Ecol. 2012, 60, 98–104. [Google Scholar] [CrossRef]
- Moukarzel, R.; Ridgway, H.J.; Guerin-Laguette, A.; Jones, E.E. Grapevine Rootstocks Drive the Community Structure of Arbuscular Mycorrhizal Fungi in New Zealand Vineyards. J. Appl. Microbiol. 2021, 131, 2941–2956. [Google Scholar] [CrossRef]
- Iannucci, A.; Canfora, L.; Nigro, F.; De Vita, P.; Beleggia, R. Relationships between Root Morphology, Root Exudate Compounds and Rhizosphere Microbial Community in Durum Wheat. Appl. Soil Ecol. 2021, 158, 103781. [Google Scholar] [CrossRef]
- Mukhongo, R.W.; Ebanyat, P.; Masso, C.; Tumuhairwe, J.B. Composition and Spore Abundance of Arbuscular Mycorrhizal Fungi in Sweet Potato Producing Areas in Uganda. Front. Soil Sci. 2023, 3, 1152524. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, X.; Guo, R.; Guo, J. Response of AM Fungi Spore Population to Elevated Temperature and Nitrogen Addition and Their Influence on the Plant Community Composition and Productivity. Sci. Rep. 2016, 6, 24749. [Google Scholar] [CrossRef]
- Karmakar, A.; Mandal, P.; Adhikary, R.; Mandal, V. Assessment of Rhizospheric Arbuscular Mycorrhizae Spores in Relation to Soil Characters in the Rice Fields of Malda District, India. Russ. Agric. Sci. 2020, 46, 48–55. [Google Scholar] [CrossRef]
- Susila, E.; Chan, S.R.O.S.; Achmad, B.S.; Maulina, F. Exploration and Morphology Identification of Spores Asbuscular Mycorrhizal Fungi from Horticultural Plantation. J. Appl. Agric. Sci. Technol. 2022, 6, 20–30. [Google Scholar] [CrossRef]
- Azcón-Aguilar, C.; Palenzuela, J.; Roldán, A.; Bautista, S.; Vallejo, R.; Barea, J.M. Analysis of the Mycorrhizal Potential in the Rhizosphere of Representative Plant Species from Desertification-Threatened Mediterranean Shrublands. Appl. Soil Ecol. 2003, 22, 29–37. [Google Scholar] [CrossRef]
- Diop, I.; Ndoye, F.; Diédhiou, A.G.; Krasova-Wade, T.; Dorego, F.; Noba, K.; Ambrosi, J.P.; Kane, A. Diversity and Spore Density of Arbuscular Mycorrhizal Fungi in the Rhizosphere of Cowpea (Vigna unguiculata [L.] Walp.) Cultivated in Different Soils in Senegal. J. Anim. Plant Sci. 2021, 48, 8552–8565. [Google Scholar]
- Morton, J.B.; Benny, G.L. Revised Classification of Arbuscular Mycorrhizal Fungi (Zygomycetes): A New Order, Glomales, Two New Suborders, Glomineae and Gigasporineae, and Two New Families, Acaulosporaceae and Gigasporaceae, with an Emendation of Glomaceae. Mycotaxon 1990, 37, 471–491. [Google Scholar]

| Techniques | Magnetic Stirrer | 45 and 80 µm Sieves * | Ultrasound (Total) | Centrifuge (Total) | Filtration Through Filter Papers * | 45 µm Sieve * | Total Time |
|---|---|---|---|---|---|---|---|
| WST | 1 min | ~3 min | - | 7 min | - | ~2 min | 13 min |
| UWST | 1 min | ~3 min | 1.5 min | 7 min | ~3 min | - | 15.5 min |
| UCT | 5 min | - | 0.5 min | 3 min | - | - | 8.5 min |
| Degrees of Freedom | Mean Square | |
|---|---|---|
| A | 5 | 1,356,710.163 ** |
| B | 2 | 1,998,088.963 ** |
| A × B | 10 | 322,316.430 ** |
| Error | 36 | |
| Total | 53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyno, G.; Demir, S.; Rezaee Danesh, Y.; Durak, E.D.; Çevik, R.; Farda, B.; Djebaili, R.; Pellegrini, M. A New Technique for the Extraction of Arbuscular Mycorrhizae Fungal Spores from Rhizosphere. J. Fungi 2023, 9, 845. https://doi.org/10.3390/jof9080845
Boyno G, Demir S, Rezaee Danesh Y, Durak ED, Çevik R, Farda B, Djebaili R, Pellegrini M. A New Technique for the Extraction of Arbuscular Mycorrhizae Fungal Spores from Rhizosphere. Journal of Fungi. 2023; 9(8):845. https://doi.org/10.3390/jof9080845
Chicago/Turabian StyleBoyno, Gökhan, Semra Demir, Younes Rezaee Danesh, Emre Demirer Durak, Rojbin Çevik, Beatrice Farda, Rihab Djebaili, and Marika Pellegrini. 2023. "A New Technique for the Extraction of Arbuscular Mycorrhizae Fungal Spores from Rhizosphere" Journal of Fungi 9, no. 8: 845. https://doi.org/10.3390/jof9080845
APA StyleBoyno, G., Demir, S., Rezaee Danesh, Y., Durak, E. D., Çevik, R., Farda, B., Djebaili, R., & Pellegrini, M. (2023). A New Technique for the Extraction of Arbuscular Mycorrhizae Fungal Spores from Rhizosphere. Journal of Fungi, 9(8), 845. https://doi.org/10.3390/jof9080845











