Cyanobacterial Variability in Lichen Cephalodia
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; Cabi: Wallingford, UK, 2008; p. 640. [Google Scholar]
- Ahmadjian, V. Lichens are more important than you think. BioScience 1995, 45, 123–124. [Google Scholar] [CrossRef]
- Kershaw, K.A. Physiological Ecology of Lichens; Cambridge University Press: Cambridge, UK, 1985; p. 293. [Google Scholar]
- Rai, A.N. Nitrogen metabolism. In CRC Handbook of Lichenology, 1st ed.; Galun, M., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1998; Volume 1, pp. 201–237. [Google Scholar]
- Stanton, D.E.; Ormond, A.; Koch, N.M.; Colesie, C. Lichen ecophysiology in a changing climate. Am. J. Bot. 2023, 110, e16131. [Google Scholar] [CrossRef] [PubMed]
- Peksa, O.; Škaloud, P. Do photobionts influence the ecology of lichens? A case study of environmental preferences in symbiotic green alga Asterochloris (Trebouxiophyceae). Mol. Ecol. 2011, 20, 3936–3948. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mendoza, F.; Domaschke, S.; García, M.A.; Jordan, P.; Martín, M.P.; Printzen, C. Population structure of mycobionts and photobionts of the widespread lichen Cetraria aculeata. Mol. Ecol. 2011, 20, 1208–1232. [Google Scholar] [CrossRef] [PubMed]
- Rolshausen, G.; Grande, F.D.; Sadowska-Deś, A.D.; Otte, J.; Schmitt, I. Quantifying the climatic niche of symbiont partners in a lichen symbiosis indicates mutualist mediated niche expansions. Ecography 2018, 41, 1380–1392. [Google Scholar] [CrossRef]
- Rolshausen, G.; Hallman, U.; Grande, F.D.; Otte, J.; Knudsen, K.; Schmitt, I. Expanding the mutualistic niche: Parallel symbiont turnover along climatic gradients. Proc. R. Soc. B 2020, 287, 20192311. [Google Scholar] [CrossRef]
- Muggia, L.; Pérez-Ortega, S.; Kopun, T.; Zellnig, G.; Grube, M. Photobiont selectivity leads to ecological tolerance and evolutionary divergence in a polymorphic complex of lichenized fungi. Ann. Bot. 2014, 114, 463–475. [Google Scholar] [CrossRef]
- Leavitt, S.D.; Kraichak, E.; Nelsen, M.P.; Altermann, S.; Divakar, P.K.; Alors, D.; Esslinger, T.L.; Crespo, A.; Lumbsch, T. Fungal specificity and selectivity for algae play a major role in determining lichen partnerships across diverse ecogeographic regions in the lichen-forming family Parmeliaceae (Ascomycota). Mol. Ecol. 2015, 24, 3779–3797. [Google Scholar] [CrossRef]
- Magain, N.; Miadlikowska, J.; Goffinet, B.; Sérusiaux, E.; Lutzoni, F. Macroevolution of specificity in cyanolichens of the genus Peltigera Section Polydactylon (Lecanoromycetes, Ascomycota). Syst. Biol. 2017, 66, 74–99. [Google Scholar]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 6, 9. [Google Scholar] [CrossRef][Green Version]
- Sahli, H.F.; Conner, J.K. Characterizing ecological generalization in plant-pollination systems. Oecologia 2006, 148, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Arribas, C.; Prieto, M.; Aragón, G.; López-Angulo, J.; Escudero, A.; Martínez, I. Specialization: A new multidimensional and integrative perspective. Ecology 2023, 13, e10296. [Google Scholar]
- Fox, L.R.; Morrow, P.A. Specialization: Species property or local phenomenon? Science 1981, 211, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Fedrowitz, K.; Kaasalainen, U.; Rikkinen, J. Geographic mosaic of symbiont selectivity in a genus of epiphytic cyanolichens. Ecol. Evol. 2012, 2, 2291–2303. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Magain, N.; Miadlikowska, J.; Coyle, J.R.; Truong, C.; Lutzoni, F. Bioclimatic factors at an intrabiome scale are more limiting than cyanobiont availability for the lichen-forming genus Peltigera. Am. J. Bot. 2018, 105, 1198–1211. [Google Scholar] [CrossRef]
- Herrera, P.; Suárez, J.P.; Sánchez-Rodríguez, A.; Molina, M.C.; Prieto, M.; Méndez, M. Many broadly-shared mycobionts characterize mycorrhizal interactions of two coexisting epiphytic orchids in a high elevation tropical forest. Fungal Ecol. 2019, 39, 26–36. [Google Scholar] [CrossRef]
- Rodríguez-Arribas, C.; Martínez, I.; Aragón, G.; Zamorano-Elgueta, C.; Cavieres, L.; Prieto, M. Specialization patterns in symbiotic associations: A community perspective over spatial scales. Ecol. Evol. 2023, 13, e10296. [Google Scholar] [CrossRef]
- Paulsrud, P.; Rikkinen, J.; Lindblad, P. Cyanobiont specificity in some Nostoc-containing lichens and in a Peltigera aphthosa photosymbiodeme. New Phytol. 1998, 139, 517–524. [Google Scholar] [CrossRef]
- Piercey-Normore, M.D.; DePriest, P.T. Algal switching among lichen symbioses. Am. J. Bot. 2001, 88, 1490–1498. [Google Scholar] [CrossRef]
- Myllys, L.; Stenroos, S.; Thell, A.; Kuusinen, M. High cyanobiont selectivity of epiphytic lichens in old growth boreal forest of Finland. New Phytol. 2007, 173, 621–629. [Google Scholar] [CrossRef]
- O’Brien, H.E.; Miadlikowska, J.; Lutzoni, F. Assessing population structure and host specialization in lichenized cyanobacteria. New Phytol. 2013, 198, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, N.; Lumbsch, T.; Green, T.G.A.; Turk, R.; Pintado, A.; Sancho, L.; Schroeter, B. Lichen fungi have low cyanobiont selectivity in maritime Antarctica. New Phytol. 2003, 160, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Muggia, L.; Vancurova, L.; Škaloud, P.; Peksa, O.; Wedin, M.; Grube, M. The symbiotic playground of lichen thalli—A highly flexible photobiont association in rock-inhabiting lichens. FEMS Microbiol. Ecol. 2013, 85, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Deś, A.D.; Dal Grande, F.; Lumbsch, H.T.; Beck, A.; Otte, J.; Hur, J.S.; Kim, J.A.; Schmitt, I. Integrating coalescent and phylogenetic approaches to delimit species in the lichen photobiont Trebouxia. Mol. Phylogenet. Evol. 2014, 76, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Paulsrud, P.; Rikkinen, J.; Lindblad, P. Field investigations on cyanobacterial specificity in Peltigera aphthosa. New Phytol. 2001, 152, 117–123. [Google Scholar] [CrossRef]
- Pardo De la Hoz, C.J.; Magain, N.; Lutzoni, F.; Goward, T.; Restrepo, S.; Miadlikowska, J. Contrasting symbiotic patterns in two closely related lineages of trimembered lichens of the genus Peltigera. Front. Microbiol. 2019, 9, 2770. [Google Scholar] [CrossRef]
- Elvebakk, A.; Papaefthimiou, D.; Robertsen, E.; Liaimer, A. Phylogenetic patterns among Nostoc cyanobionts within bi- and tripartite lichens of the genus Pannaria. J. Phycol. 2008, 44, 1049–1059. [Google Scholar] [CrossRef]
- Paulsrud, P.; Rikkinen, J.; Lindblad, P. Spatial patterns of photobiont diversity in some Nostoc-containing lichens. New Phytol. 2000, 146, 291–299. [Google Scholar] [CrossRef]
- Bačkor, M.; Peksa, O.; Škaloud, P.; Bačkorová, M. Photobiont diversity in lichens from metal-rich substrata based on ITS rDNA sequences. Ecotoxicol. Environ. Saf. 2010, 73, 603–612. [Google Scholar] [CrossRef]
- Moya, P.; Molins, A.; Martinez-Alberola, F.; Muggia, L.; Barreno, E. Unexpected associated microalgal diversity in the lichen Ramalina farinacea is uncovered by pyrosequencing analyses. PLoS ONE 2017, 12, 1–21. [Google Scholar] [CrossRef]
- Vančurová, L.; Muggia, L.; Peksa, O.; Řídká, T.; Škaloud, P. The complexity of symbiotic interactions influences the ecological amplitude of the host: A case study in Stereocaulon (lichenized Ascomycota). Mol. Ecol. 2018, 27, 3016–3033. [Google Scholar] [CrossRef] [PubMed]
- Onuț-Brännström, I.; Benjamin, M.; Scofield, D.G.; Heiðmarsson, S.; Andersson, M.G.I.; Lindström, E.S.; Johannesson, H. Sharing of photobionts in sympatric populations of Thamnolia and Cetraria lichens: Evidence from high-throughput sequencing. Sci. Rep. 2018, 8, 4406. [Google Scholar] [CrossRef] [PubMed]
- Peksa, O.; Gebouská, T.; Škvorová, Z.; Vančurová, L.; Škaloud, P. The guilds in green algal lichens-an insight into the life of terrestrial symbiotic communities. FEMS Microbiol. Ecol. 2022, 98, fiac008. [Google Scholar] [CrossRef] [PubMed]
- Rikkinen, J.; Oksanen, I.; Lohtander, K. Lichen guilds share related cyanobacterial symbionts. Science 2002, 297, 357. [Google Scholar] [CrossRef]
- Rikkinen, J. Ecological and evolutionary role of photobiont-mediated guilds in lichens. Symbiosis 2003, 34, 99–110. [Google Scholar]
- Yahr, R.; Vilgalys, R.; DePriest, P.T. Geographic variation in algal partners of Cladonia subtenuis (Cladoniaceae) highlights the dynamic nature of a lichen symbiosis. New Phytol. 2006, 171, 847–860. [Google Scholar] [CrossRef]
- Otálora, M.A.G.; Martínez, I.; O’Brien, H.; Molina, M.C.; Aragón, G.; Lutzoni, F. Multiple origins of high reciprocal specificity at an intercontinental spatial scale among gelatinous lichens (Collemataceae, Lecanoromycetes). Mol. Phylogenet. Evol. 2010, 56, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Dal Grande, F.; Divakar, P.K.; Otte, J.; Crespo, A.; Schmitt, I. Fungal–algal association patterns in lichen symbiosis linked to macroclimate. New Phytol. 2017, 214, 317–329. [Google Scholar] [CrossRef]
- Mark, K.; Laanisto, L.; Bueno, C.G.; Niinemets, Ü.; Keller, C.; Scheidegger, C. Contrasting co-occurrence patterns of photobiont and cystobasidiomycete yeast associated with common epiphytic lichen species. New Phytol. 2020, 227, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Rudi, K.; Skulberg, O.M.; Jakobsen, K.S. Evolution of cyanobacteria by exchange of genetic material among phyletically related strains. J. Bacteriol. 1998, 180, 3453–3461. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; Snell, Q.; Walker, P.; Posada, D.; Crandall, K. TCS: Estimating gene genealogies. In Proceedings of the Parallel and Distributed Processing Symposium, International Proceedings, Ft. Lauderdale, FL, USA, 15–19 April 2002; Volume 2, p. 184. [Google Scholar]
- Piercey-Normore, M.D.; Coxson, D.; Goward, T.; Goffinet, B. Phylogenetic position of a Pacific North American endemic cyanolichen, Nephroma occultum (Ascomycota, Peltigerales). Lichenologist 2006, 38, 441–456. [Google Scholar] [CrossRef]
- del Campo, E.M.; Gimeno, J.; de Nova, J.P.G.; Casano, L.M.; Gasulla, F.; García-Breijo, F.; Reig, F.J.; Barreno, E. South European populations of Ramalina farinacea (L.) Ach. Share different Trebouxia algae. Bibl. Lichenol. 2010, 105, 247–256. [Google Scholar]
- Blázquez, M.; Hernández-Moreno, L.S.; Gasulla, F.; Pérez-Vargas, I.; Pérez-Ortega, S. The role of photobionts as drivers of diversification in an island radiation of lichen-forming Fungi. Front. Microbiol. 2022, 12, 784182. [Google Scholar] [CrossRef] [PubMed]
- Jordan, W.P.; Rickson, F.R. Cyanophyte cephalodia in the lichen genus Nephroma. Am. J. Bot. 1971, 58, 562–568. [Google Scholar] [CrossRef]
- Tschermak-Woess, E. The algal partner. In CRC Handbook of Lichenology, 1st ed.; Galun, M., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1998; Volume 1, pp. 39–92. [Google Scholar]
- Casano, L.M.; del Campo, E.M.; García-Breijo, F.J.; Reig-Armiñana, J.; Gasulla, F.; Del Hoyo, A.; Guéra, A.; Barreno, E. Two Trebouxia algae with different physiological performances are ever-present in lichen thalli of Ramalina farinacea. Coexistence versus competition? Environ. Microbiol. 2011, 13, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Lehr, H.; Galun, M.; Ott, S.; Jahns, H.M.; Fleminger, G. Cephalodia of the lichen Peltigera aphthosa (L.) Willd. Specific recognition of the compatible photobiont. Symbiosis 2000, 29, 357–365. [Google Scholar]
- Rikkinen, J. Molecular studies on cyanobacterial diversity in lichen symbioses. MycoKeys 2013, 6, 3–32. [Google Scholar] [CrossRef]
- O’Brien, H.E.; Miadlikowska, J.; Lutzoni, F. Assessing host specialization in symbiotic cyanobacteria associated with four closely related species of the lichen fungus Peltigera. Eur. J. Phycol. 2005, 40, 363–378. [Google Scholar] [CrossRef]
- Cardós, J.L.H.; Prieto, M.; Jylhä, M.; Aragón, G.; Molina, M.C.; Martínez, I.; Rikkinen, J. A case study on the re-establishment of the cyanolichen symbiosis: Where do the compatible photobionts come from? An. Bot. 2019, 124, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.N.; Soderback, E.; Bergman, B. Cyanobacterium–plant symbioses. New Phytol. 2000, 147, 449–481. [Google Scholar] [CrossRef] [PubMed]
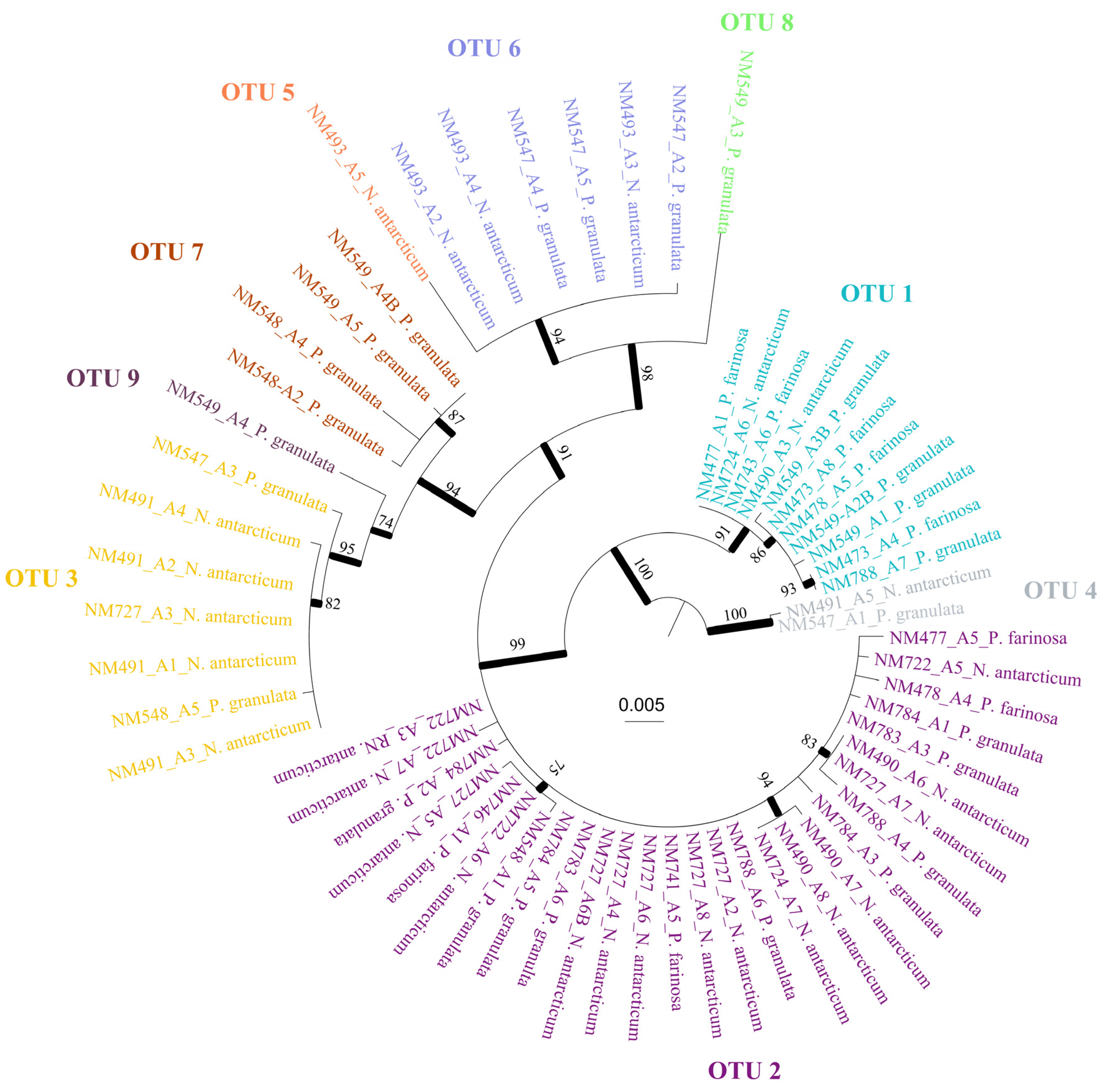
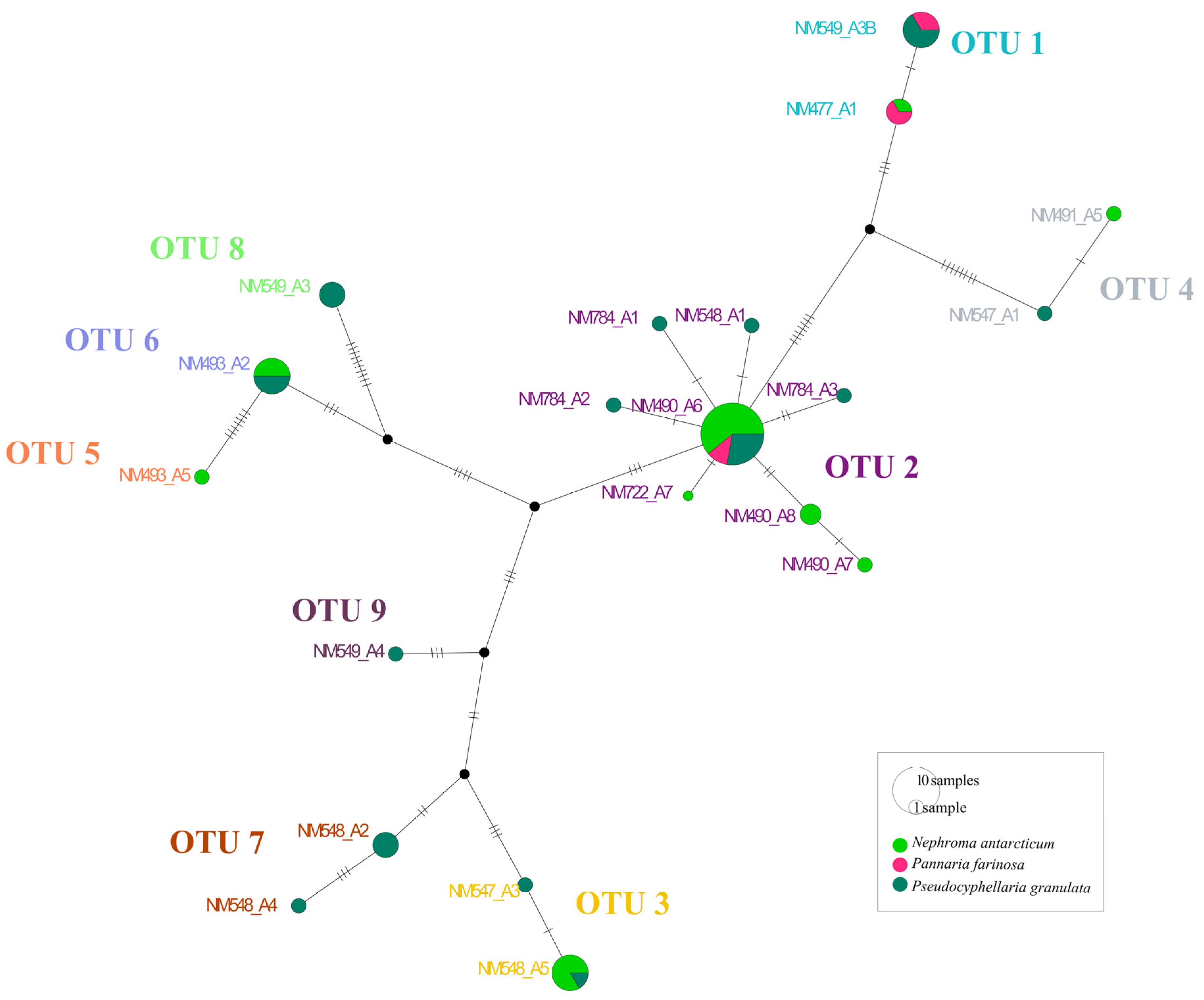
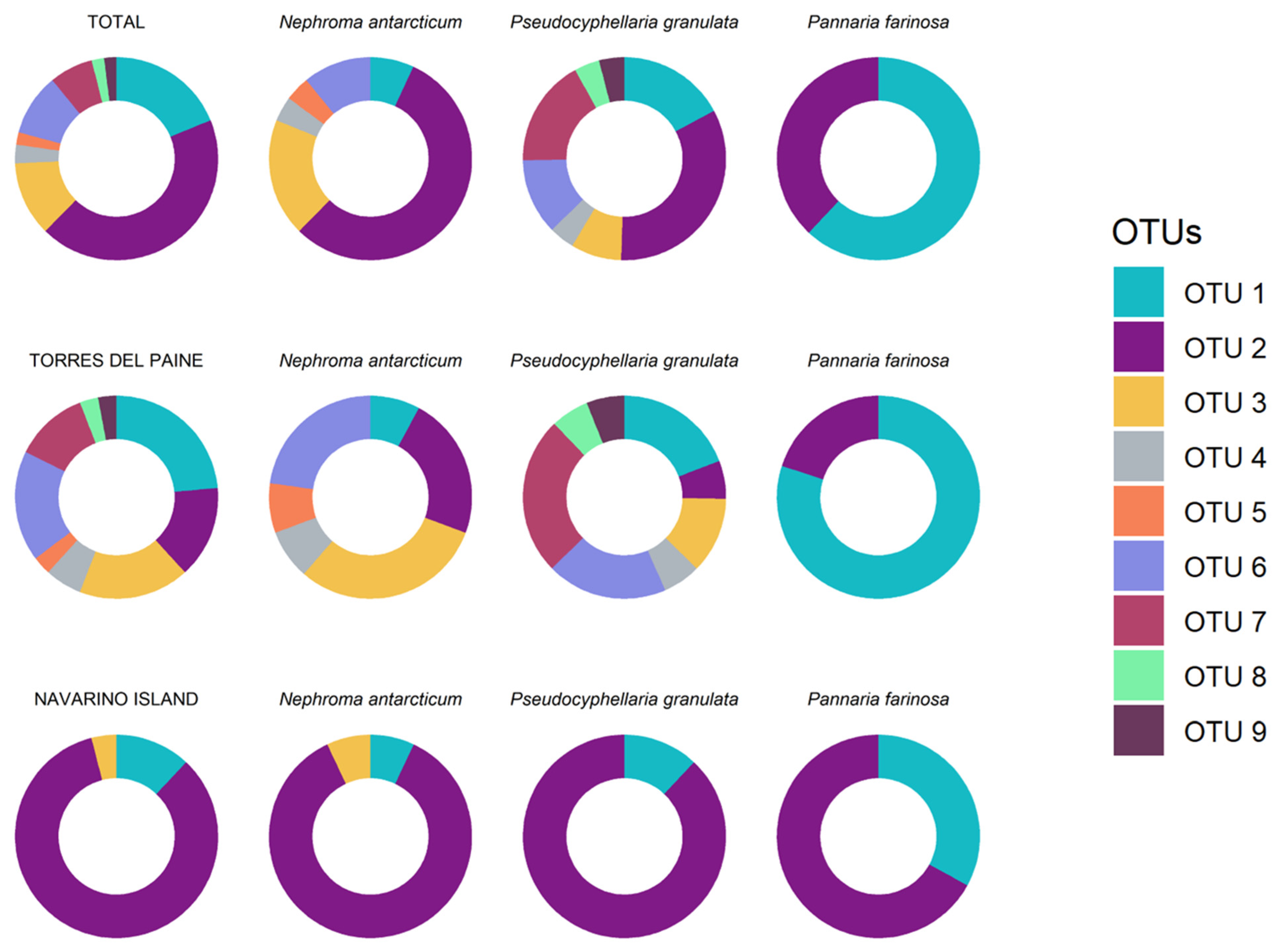
| Species | Thallus | Cephalodia | OTU ASAP | Forest | Voucher | GenBank |
|---|---|---|---|---|---|---|
| Nephroma antarcticum | NM490 | A3 | 1 | TP | ARAN20283 | OR344442 |
| Nephroma antarcticum | NM490 | A6 | 2 | TP | OR344444 | |
| Nephroma antarcticum | NM490 | A7 | 2 | TP | OR344445 | |
| Nephroma antarcticum | NM490 | A8 | 2 | TP | OR344446 | |
| Nephroma antarcticum | NM491 | A3 | 3 | TP | ARAN20284 | OR344447 |
| Nephroma antarcticum | NM491 | A1 | 3 | TP | OR344449 | |
| Nephroma antarcticum | NM491 | A2 | 3 | TP | OR344450 | |
| Nephroma antarcticum | NM491 | A4 | 3 | TP | OR344451 | |
| Nephroma antarcticum | NM491 | A5 | 4 | TP | OR344448 | |
| Nephroma antarcticum | NM493 | A5 | 5 | TP | ARAN20285 | OR344452 |
| Nephroma antarcticum | NM493 | A2 | 6 | TP | OR344454 | |
| Nephroma antarcticum | NM493 | A3 | 6 | TP | OR344455 | |
| Nephroma antarcticum | NM493 | A4 | 6 | TP | OR344456 | |
| Nephroma antarcticum | NM722 | A3 | 2 | IN | ARAN20292 | OR344474 |
| Nephroma antarcticum | NM722 | A6 | 2 | IN | OR344476 | |
| Nephroma antarcticum | NM722 | A5 | 2 | IN | OR344475 | |
| Nephroma antarcticum | NM722 | A7 | 2 | IN | OR344477 | |
| Nephroma antarcticum | NM724 | A6 | 1 | IN | ARAN20293 | OR344478 |
| Nephroma antarcticum | NM724 | A7 | 2 | IN | OR344479 | |
| Nephroma antarcticum | NM727 | A3 | 3 | IN | ARAN20294 | OR344481 |
| Nephroma antarcticum | NM727 | A2 | 2 | IN | OR344480 | |
| Nephroma antarcticum | NM727 | A4 | 2 | IN | OR344482 | |
| Nephroma antarcticum | NM727 | A6 | 2 | IN | OR344484 | |
| Nephroma antarcticum | NM727 | A8 | 2 | IN | OR344486 | |
| Nephroma antarcticum | NM727 | A5 | 2 | IN | OR344483 | |
| Nephroma antarcticum | NM727 | A7 | 2 | IN | OR344485 | |
| Pannaria farinosa | NM473 | A4 | 1 | TP | OR344431 | |
| Pannaria farinosa | NM473 | A8 | 1 | TP | ARAN20286 | OR344432 |
| Pannaria farinosa | NM477 | A1 | 1 | TP | ARAN20287 | OR344433 |
| Pannaria farinosa | NM477 | A2 | 2 | TP | OR344434 | |
| Pannaria farinosa | NM478 | A5 | 1 | TP | ARAN20288 | OR344441 |
| Pannaria farinosa | NM741 | A5 | 2 | IN | ARAN20295 | OR344487 |
| Pannaria farinosa | NM743 | A6 | 1 | IN | ARAN20296 | OR344488 |
| Pannaria farinosa | NM746 | A1 | 2 | IN | ARAN20297 | OR344489 |
| Pseudocyphellaria granulata | NM547 | A4 | 6 | TP | ARAN20289 | OR344460 |
| Pseudocyphellaria granulata | NM547 | A5 | 6 | TP | OR344461 | |
| Pseudocyphellaria granulata | NM547 | A2 | 6 | TP | OR344458 | |
| Pseudocyphellaria granulata | NM547 | A3 | 3 | TP | OR344459 | |
| Pseudocyphellaria granulata | NM547 | A1 | 4 | TP | OR344457 | |
| Pseudocyphellaria granulata | NM548 | A5 | 3 | TP | ARAN20290 | OR344465 |
| Pseudocyphellaria granulata | NM548 | A2 | 7 | TP | OR344462 | |
| Pseudocyphellaria granulata | NM548 | A4 | 7 | TP | OR344463 | |
| Pseudocyphellaria granulata | NM548 | A1 | 2 | TP | OR344464 | |
| Pseudocyphellaria granulata | NM549 | A4B | 7 | TP | ARAN20291 | OR344469 |
| Pseudocyphellaria granulata | NM549 | A5 | 7 | TP | OR344470 | |
| Pseudocyphellaria granulata | NM549 | A3 | 8 | TP | OR344471 | |
| Pseudocyphellaria granulata | NM549 | A4 | 9 | TP | OR344472 | |
| Pseudocyphellaria granulata | NM549 | A2B | 1 | TP | OR344467 | |
| Pseudocyphellaria granulata | NM549 | A3B | 1 | TP | OR344468 | |
| Pseudocyphellaria granulata | NM549 | A1 | 1 | TP | OR344466 | |
| Pseudocyphellaria granulata | NM783 | A3 | 2 | IN | ARAN20298 | OR344491 |
| Pseudocyphellaria granulata | NM783 | A6 | 2 | IN | OR344492 | |
| Pseudocyphellaria granulata | NM784 | A5 | 2 | IN | ARAN20299 | OR344496 |
| Pseudocyphellaria granulata | NM784 | A2 | 2 | IN | OR344494 | |
| Pseudocyphellaria granulata | NM784 | A1 | 2 | IN | OR344493 | |
| Pseudocyphellaria granulata | NM784 | A3 | 2 | IN | OR344495 | |
| Pseudocyphellaria granulata | NM788 | A7 | 1 | IN | ARAN20300 | OR344500 |
| Pseudocyphellaria granulata | NM788 | A6 | 2 | IN | OR344499 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto, M.; Montané, N.; Aragón, G.; Martínez, I.; Rodríguez-Arribas, C. Cyanobacterial Variability in Lichen Cephalodia. J. Fungi 2023, 9, 826. https://doi.org/10.3390/jof9080826
Prieto M, Montané N, Aragón G, Martínez I, Rodríguez-Arribas C. Cyanobacterial Variability in Lichen Cephalodia. Journal of Fungi. 2023; 9(8):826. https://doi.org/10.3390/jof9080826
Chicago/Turabian StylePrieto, Maria, Natalia Montané, Gregorio Aragón, Isabel Martínez, and Clara Rodríguez-Arribas. 2023. "Cyanobacterial Variability in Lichen Cephalodia" Journal of Fungi 9, no. 8: 826. https://doi.org/10.3390/jof9080826
APA StylePrieto, M., Montané, N., Aragón, G., Martínez, I., & Rodríguez-Arribas, C. (2023). Cyanobacterial Variability in Lichen Cephalodia. Journal of Fungi, 9(8), 826. https://doi.org/10.3390/jof9080826








