Abstract
Cryptococcus neoformans is an invasive fungus that causes both acute and chronic infections, especially in immunocompromised patients. Owing to the increase in the prevalence of drug-resistant pathogenic fungi and the limitations of current treatment strategies, drug repositioning has become a feasible strategy to accelerate the development of new drugs. In this study, the minimum inhibitory concentration of vitamin D3 (VD3) against C. neoformans was found to be 0.4 mg/mL by broth microdilution assay. The antifungal activities of VD3 were further verified by solid dilution assays and “time-kill” curves. The results showed that VD3 reduced fungal cell adhesion and hydrophobicity and inhibited biofilm formation at various developmental stages, as confirmed by crystal violet staining and the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide assay. Fluorescence staining of cellular components and a stress susceptibility assay indicated that VD3 compromised cell integrity. Reverse transcription quantitative PCR demonstrated that VD3 treatment upregulated the expression of fungal genes related to cell wall synthesis (i.e., CDA3, CHS3, FKS1, and AGS1). Moreover, VD3 enhanced cell membrane permeability and caused the accumulation of intracellular reactive oxygen species. Finally, VD3 significantly reduced the tissue fungal burden and prolonged the survival of Galleria mellonella larvae infected with C. neoformans. These results showed that VD3 could exert significant antifungal activities both in vitro and in vivo, demonstrating its potential application in the treatment of cryptococcal infections.
1. Introduction
In addition to the serious threats posed by various viral outbreaks and drug-resistant bacterial infections, fungal infections have also received widespread attention in recent years. Fungal diseases affect more than 1 billion people worldwide annually, resulting in over 1.5 million deaths [1]. Cryptococcus neoformans is a particularly harmful species of invasive fungi, the latest report estimated that there were 152,000 cases (111,000–185,000) of cryptococcal meningitis (CM), resulting in 112,000 cryptococcal-related deaths (79,000–134,000) globally each year [2]. It is a ubiquitous opportunistic fungal pathogen, and its infection results from the inhalation of airborne spores. While C. neoformans is rapidly cleared by the immune system in healthy individuals, it can cause lung infections in immunocompromised patients, organ transplant recipients, cancer patients receiving chemotherapy, and individuals with advanced human immunodeficiency virus (HIV) infections. In more serious cases, C. neoformans can spread to the brain, causing CM [3,4]. HIV-associated CM is responsible for approximately 70% of mortality in developing African countries [5] and 150,000–200,000 deaths worldwide each year [6].
Clinically, the standard treatment regimen for C. neoformans infection consists of a short course of amphotericin B combined with flucytosine, followed by fluconazole (FCZ) consolidation monotherapy [7]. However, up to 60% of patients experience relapse after the standard treatment regimen, and the clinical resistance to FCZ continues to increase [8]. A previous study reported that up to 25% of colonies derived from clinical isolates were resistant to FCZ, and 11 of 13 patients exhibited heteroresistance within 7 days of FCZ monotherapy [9]. Amphotericin B resistance in C. neoformans is rare; however, intravenous administration and the high host toxicity limit its use [10]. The limited availability of antifungal agents and the emergence of antifungal resistance highlight the urgent need for new strategies against aggressive fungal infections. Repositioning and re-development of existing drugs, rather than the time-consuming and expensive process of developing new drugs, can offer a quick alternative strategy to address this problem [11,12].
Vitamin D3 (VD3) regulates calcium and phosphorus homeostasis in bone metabolism and has been suggested to exhibit some antiviral activities. For example, VD3 has been reported to attenuate rotavirus infection by regulating autophagy maturation and porcine antimicrobial peptide gene expression [13]. Moreover, VD3, which is highly fat-soluble, has been noted to affect the cell membrane integrity of Candida albicans, thereby presenting antifungal activities [14]. A previous study by our group confirmed that VD3 exhibits significant anti-C. albicans activity both in vivo and in vitro [15]. The present study is the first to investigate the antifungal effect and mode of action of VD3 against C. neoformans. The results obtained confirmed that the antifungal effect of VD3 against C. neoformans coincides with reduced biofilm formation, compromised cell wall integrity, and increased generation of reactive oxygen species (ROS). Thus, VD3 could be a potential antifungal drug for the prevention and treatment of C. neoformans infections.
2. Materials and Methods
2.1. Chemicals, Reagents, and Culture Conditions
VD3 (67-97-0, Macklin, Shanghai, China) and 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) (111072-31-2, Macklin, Shanghai, China) were used in this study. Roswell Park Memorial Institute (RPMI) 1640 medium was obtained from HyClone Laboratories, Inc. (South Logan, UT, USA). Calcium fluoride white (CFW), sodium dodecyl sulfate (SDS), and Congo red were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). Before each experiment, a stock solution of VD3 dissolved in dimethyl sulfoxide (DMSO) at 50 mg/mL was prepared and diluted to the target concentration with RPMI 1640 medium or YPD medium (1% yeast extract, 2% peptone, and 2% dextrose) containing 0.05% Tween 80. C. neoformans strain H99 was stored in 30% glycerol at −80 °C and cultured overnight in YPD medium at 37 °C and 200 rpm prior to use.
2.2. Broth Microdilution Assay
The minimum inhibitory concentration (MIC) of VD3 against C. neoformans was determined using the Clinical and Laboratory Standards Institute Standard M27-A3 Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts [16] with minor modifications. Activated C. neoformans cells were resuspended in RPMI 1640 medium to a final concentration of 5 × 103 cells/mL. Various concentrations of VD3 (0.0, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, and 0.8 mg/mL) were prepared as described in our previous report [15]. A positive drug-free control and a negative yeast-free control were established. The test strains and controls were incubated at 37 °C for 72 h. The optical density at 600 nm (OD600) was measured using a Varioskan™ LUX multimode microplate reader (Thermo Fisher Scientific, Waltham, MA, USA) to determine the minimum concentration needed to inhibit the growth of 90% (MIC90) of isolates.
2.3. Spot Dilution and Stress Susceptibility Assay
A spot dilution assay was performed to determine the antifungal effect of VD3 as described previously [17,18]. In brief, 3-microliter aliquots of C. neoformans strain H99 at various concentrations (102, 103, and 104 cells/mL) were spotted onto YPD plates containing different concentrations of VD3 (0.1, 0.2, 0.3, and 0.4 mg/mL) and incubated at 37 °C for 72 h. In the control group, an equal volume of DMSO was added. After incubation, the plates were photographed using a digital camera (Canon Inc., Tokyo, Japan).
For the stress susceptibility assay, an overnight culture of C. neoformans strain H99 was washed and diluted to 1 × 107 cells/mL in phosphate-buffered saline (PBS). The cell wall stress susceptibility was tested using 100 μg/mL CFW, 0.01% SDS, and 0.2% Congo red, as described previously [19] with slight modifications. Sensitivity to oxidative stress was evaluated with 1 mM H2O2 (Sigma-Aldrich Corporation). In brief, the cell suspensions were serially diluted in YPD medium containing different concentrations of VD3 (0, 0.4, and 0.8 mg/mL) with or without the stressors, incubated at 37 °C for 3 days, and photographed.
2.4. Time-Kill Assay
The time-kill assay was performed as described previously [15]. An overnight culture of C. neoformans strain H99 was washed, diluted to 5 × 105 cells/mL in YPD medium containing 0.4 mg/mL VD3 or DMSO, and incubated at 37 °C and 200 rpm. A portion of the cell suspension was collected at 2, 4, 8, 12, 16, and 24 h, respectively, diluted with PBS, and spread onto YPD plates. After incubation of the plates at 37 °C for 72 h, the number of colony-forming units (CFUs) was quantified. The experiment was repeated three times on separate days, and the data were averaged for analysis.
2.5. Biofilm Inhibition Assay
The effects of VD3 on biofilm formation by C. neoformans strain H99 were assessed by crystal violet (CV) staining and XTT assay [20,21]. In brief, the C. neoformans cells were washed and diluted to 106 cells/mL in RPMI 1640 medium and incubated in 96-well plates at 37 °C for 90 min (initial phase), 12 h (developmental phase), and 48 h (maturation phase). Furthermore, the medium and non-adherent cells were discarded. Subsequently, RPMI 1640 medium containing 0.4 and 0.8 mg/mL VD3 or DMSO was added to the wells and incubated at 37 °C for 6 h. After incubation, the medium was discarded, the cells were rinsed with PBS, and fresh RPMI 1640 medium (200 μL) was added to each well and incubated for 48 h. Moreover, each well was washed twice with PBS, and the biofilm mass and metabolic activity of the cells were measured at OD595 and OD490, respectively. The biofilm activity was calculated as (OD3 − OB)/(OC − OB) × 100%, where OD3, OC, and OB are the OD values of VD3, DMSO, and blank groups, respectively.
The three-dimensional structure of the biofilm produced by C. neoformans after VD3 treatment was visualized using a confocal laser scanning microscope (TCS SP8; Leica Microsystems GmbH, Wetzlar, Germany) [21]. In brief, the yeast cells were washed and diluted to 106 cells/mL in RPMI 1640 medium containing 0.4 mg/mL VD3 or DMSO in a 6-well plate and incubated at 37 °C for 48 h. After incubation, the supernatant was discarded, and the cells were rinsed with PBS, stained with CFW at 37 °C for 10 min, and imaged.
2.6. Adhesion Assay and Cell Surface Hydrophobicity Analysis
To explore the effect of VD3 on the adhesion activity of C. neoformans, an adhesion assay was performed as previously described [22], with slight modifications. In brief, the yeast cells were diluted to 106 cells/mL in RPMI 1640 medium containing 0.4 and 0.8 mg/mL VD3 or DMSO in a 96-well plate and incubated at 37 °C for 4 h. Subsequently, the medium along with non-adherent cells were discarded, the wells were rinsed thrice with PBS, and 200 μL of fresh RPMI 1640 medium were added to each well and incubated for 48 h. Finally, each well was washed twice with PBS. Early adhesion activity was confirmed by CV staining and the XTT assay, similarly. The adhesion activity was calculated as (OD3 − OB)/(OC − OB) × 100%, where OD3, OC, and OB are the OD values of VD3, DMSO, and blank groups, respectively.
Cell surface hydrophobicity (CSH) analysis was performed as previously described [23] with some modifications. In brief, the yeast cells were washed and diluted to 106 cells/mL in YPD medium containing 0.4 and 0.8 mg/mL VD3 or DMSO in a 96-well plate and incubated for 6 h at 37 °C and 200 rpm. Furthermore, the cells were collected, resuspended in 2.45 mL of PBS, and the absorbance of 200 μL of the cell suspension was determined at OD600 (D0). Subsequently, 3 mL of chloroform were added to the cell suspension and mixed for 3 min, and the mixture was allowed to stand for 30 min. After that, the absorbance of 200 μL of the supernatant was measured at OD600 (D1). The CSH was calculated as (D0 − D1)/D0.
2.7. Cell Wall Staining, Microscopy, and Quantification of Cellular Components
The C. neoformans cells treated without or with 0.4 and 0.8 mg/mL VD3 were incubated at 37 °C and 200 rpm for 6 h. Furthermore, the cells were washed with PBS, resuspended to a concentration of 5 × 107 cells/mL, fixed with 4% paraformaldehyde at room temperature for 15 min, washed again with PBS, and stained as described previously [24]. The chitooligomers of the cell wall were stained with 100 µg/mL fluorescein isothiocyanate-labeled wheat germ agglutinin (Sigma-Aldrich Corporation, Bengaluru, India) at 37 °C for 35 min in the dark. Chitin was stained by incubating the cells with 5 μg/mL CFW at 37 °C for 10 min in the dark, and chitosan was stained by incubating the cells with 300 μg/mL Eosin Y (Shanghai Macklin Biochemical Co., Ltd., Shanghai, China) at 37 °C for 10 min. After staining, the cells were washed twice with PBS and observed at 40× under a fluorescence microscope (BX63; Olympus Corporation, Tokyo, Japan).
Staining of β-1,3-glucan was performed as described previously [25]. In brief, The C. neoformans cells treated without or with 0.4 and 0.8 mg/mL VD3 were incubated at 37 °C and 200 rpm for 6 h. Furthermore, the cells were washed with PBS, resuspended to a concentration of 5 × 107 cells/mL, and then the OD600 was measured. To determine the total glucan content, cells were stained with 0.1% aniline blue (Wako Pure Chemical Industries, Ltd., Osaka, Japan) at 80 °C for 15 min in the dark. Subsequently, fluorescence was measured at excitation and emission wavelengths of 400 and 460 nm, respectively, using a plate reader. Furthermore, the yeast cells were stained with 5 μg/mL CFW as described previously [26], and the total chitin content was quantified by measuring fluorescence at excitation and emission wavelengths of 365 and 435 nm, respectively, using a plate reader. The change in fluorescence (∆F) was calculated as [F(test) − F(blank)], where F(test) is the fluorescence of the test sample and F(blank) is the fluorescence of the test group without dye solution.
2.8. Fungal Membrane Integrity and Intracellular Content of Reactive Oxygen Species (ROS)
The membrane integrity of C. neoformans cells was assessed by staining the cells with propidium iodide (PI) (Solarbio Science and Technology Co., Ltd., Beijing, China) as described previously [22]. The C. neoformans cells treated without or with 0.4 and 0.8 mg/mL VD3 were incubated at 37 °C and 200 rpm for 6 h. Furthermore, the cells were washed with PBS, resuspended to a concentration of 5 × 107 cells/mL, and then the OD600 was measured. Cells were stained with 5 μM PI for 10 min at 37 °C in the dark. Following that, the cells were washed with PBS, and the fluorescence was measured at excitation and emission wavelengths of 535 and 617 nm, respectively, using a plate reader, and the cells were observed at 40× under a fluorescence microscope.
The intracellular ROS content was evaluated by staining the yeast cells with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) (Sigma-Aldrich Corporation) as described previously [27]. In brief, the cells treated without or with 0.4 and 0.8 mg/mL VD3 were incubated at 37 °C and 200 rpm for 6 h. Furthermore, the cells were washed with PBS, resuspended to a concentration of 5 × 107 cells/mL, and then the OD600 was measured. Cells were stained with 10 μM DCFH-DA for 30 min at 37 °C in the dark. The fluorescence was measured at excitation and emission wavelengths of 485 and 530 nm, respectively, using a plate reader, and the cells were observed at 40× under a fluorescence microscope.
2.9. RNA Isolation and Reverse Transcription Quantitative PCR
Overnight cultures of C. neoformans (106 cells/mL) were treated with or without 0.4 mg/mL VD3 at 37 °C and 200 rpm for 6 h. Furthermore, the cells were collected by centrifugation at 4400 rpm at 4 °C for 3 min. The total RNA was isolated using yeast processing reagent (TaKaRa, Dalian, China), reverse-transcribed into cDNA using PrimeScript™ RT Reagent Kit with gDNA Eraser (Takara Bio, Inc., Shiga, Japan), and amplified by reverse transcription quantitative PCR (RT-qPCR) with TB Green Premix Ex Taq™ Ⅱ Master Mix (Takara Bio, Inc., Dalian, China). The primers used in this study are listed in Supplemental Table S1. The relative expression levels of genes were calculated using 2−ΔΔCT method against glycerol-3-phosphate dehydrogenase 1 (GPD1) as the housekeeping gene [24,28].
2.10. Antifungal Efficacy of VD3 In Vivo
The larvae of the honeycomb moth (Galleria mellonella) were used to construct a fungal infection model for survival analysis and the determination of fungal burden [29]. Prior to the experiment, the G. mellonella larvae (average body weight, 300 mg) were kept in the dark at 37 °C, and C. neoformans strain H99 cells were washed, resuspended in normal saline, and diluted to 2 × 107 cells/mL. Moreover, the larvae were infected with 10 μL of C. neoformans cells or normal saline (Control group) using a Hamilton syringe. After infection for 2 h, the larvae were injected with 10 μL of the drug or normal saline containing DMSO (Cn group). The experimental groups were treated with 0.5, 1, 5, 10, and 20 mg/kg VD3, respectively. The positive control group was treated with 10 mg/kg of FCZ. To quantify the fungal burden, five larvae per group were homogenized after treatment for 24 h, serially diluted 10 folds, inoculated onto YPD plates, and incubated for 72 h at 37 °C. Subsequently, the fungal CFUs were quantified. For survival analysis, 20 larvae per group were monitored daily for 5 days at 37 °C in the dark. A one-way analysis of variance (ANOVA) was used to assess the differences in fungal burden among the groups. Survival curves were analyzed by the Kaplan-Meier method (log-rank test) using GraphPad Prism 9.0 software (GraphPad Software, Inc., San Diego, CA, USA).
2.11. Statistical Analysis
The experiments were repeated three times. All statistical analyses were performed using GraphPad Prism 9.0 software. As the data were normally distributed, the differences between the groups were compared with a t-test, log-rank test, or one-way ANOVA. A probability (p) value < 0.05 was considered statistically significant.
3. Results
3.1. Antifungal Effects of VD3 In Vitro
A previous study by our group confirmed that VD3 had significant antifungal activities against C. albicans both in vitro and in vivo [15]. In the present study, the broth microdilution method confirmed that the MIC90 of VD3 was 0.4 mg/mL against C. neoformans (Figure 1A). The results of the spot dilution assay showed that the growth of C. neoformans was inhibited by VD3 on agar medium (Figure 1B). The “time-kill” curve revealed that VD3 hindered the growth of C. neoformans in the lag, logarithmic, and stationary phases (Figure 1C). These results indicated that VD3 exerted significant antifungal activity against C. neoformans in vitro.
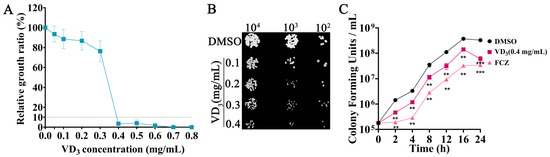
Figure 1.
Growth inhibition of C. neoformans by VD3 in vitro. (A) Growth inhibition of C. neoformans by VD3 is evaluated by the broth microdilution method. (B) Growth of C. neoformans on solid YPD plates containing different concentrations of VD3. (C) Time-kill curves of C. neoformans (initial inoculum concentration of 105 CFU/mL) treated with 0.4 mg/mL VD3. VD3, Vitamin D3; FCZ, fluconazole. Data were analyzed by one-way ANOVA (**, p < 0.01; ***, p < 0.001).
3.2. VD3-Inhibited Biofilm Formation by C. neoformans
A previous investigation found that C. neoformans biofilms are resistant to antimicrobial agents and host defense mechanisms, causing significant morbidity and mortality [30]. Therefore, the effects of VD3 against biofilm formation by C. neoformans were evaluated in the present study. The results showed that VD3 significantly inhibited biofilm formation at all fungal growth phases and destroyed mature biofilm (Figure 2A,B). Furthermore, VD3 significantly hindered the early adhesion activity of C. neoformans (Figure 2C,D). Subsequently, the effects of VD3 on biofilm structure were assessed using a confocal laser scanning microscope. When compared with the DMSO group, the total amount of C. neoformans biofilm was significantly reduced in the VD3 groups, and damage to the biofilm was indicated by decreased density and scattered distribution (Figure 2E). Moreover, the CSH analysis results showed that VD3 reduced the CSH of C. neoformans (Figure 2F).
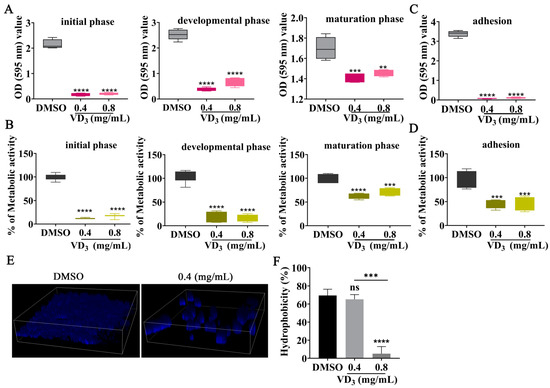
Figure 2.
Inhibitory effects of VD3 against C. neoformans biofilm formation. (A) Biomass and (B) metabolic activity of C. neoformans biofilm at the initial phase (90 min), developmental phase (12 h), and maturation phase (48 h) as determined by CV staining and XTT assay. Adhesion (4 h) activity of C. neoformans was evaluated by (C) CV staining and (D) XTT assay. (E) CFW staining of C. neoformans cells and the three-dimensional structure of the biofilm. (F) Effects of VD3 on CSH. Data were analyzed by one-way ANOVA (ns, p > 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001).
3.3. VD3 Impact on Cell Wall Integrity of C. neoformans
The cell wall of Cryptococcus is a double-layer structure surrounding the plasma membrane, which is an optimal target for antifungal drugs [31]. Therefore, we are investigating whether VD3 impacts cell wall composition and cell wall integrity. When compared with the DMSO group, both CFW fluorescence (Figure 3A) and quantitative results (Figure 3B) demonstrated a decrease in the total chitin content, and chitooligomers staining (by WGA, green fluorescence) was enhanced in the C. neoformans cell wall after VD3 treatment (Figure 3A), along with localized changes from the cell tip and buds to the entire cell wall (red arrows). In addition, as shown in Figure 3C, VD3 groups showed increased staining intensity of chitosan (by Eosin Y, green fluorescence). Meanwhile, β-1,3-glucan levels increased after VD3 treatment (Figure 3D). RT-qPCR analysis revealed that the expression levels of CHS3, CHS4, and CHS5 (encoding chitin synthase, the key enzyme involved in chitin synthesis), along with CDA3 and CDA4 (encoding chitin deacetylase, the key enzyme involved in the synthesis of chitosan from chitin) were significantly upregulated after VD3 treatment (Figure 3E); however, there were no significant differences in the expression of CHI22 (associated with chitooligomer synthesis).
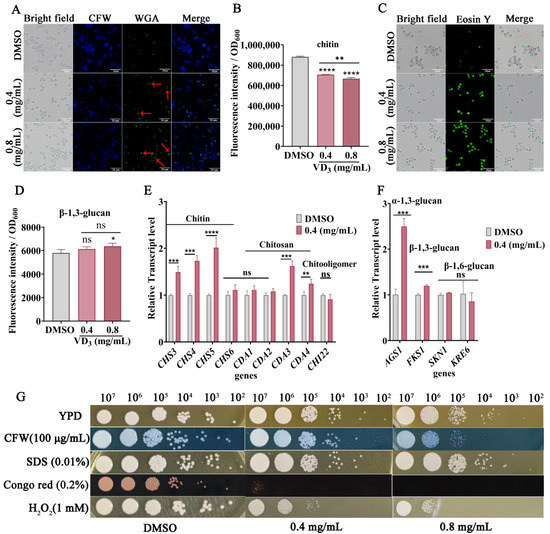
Figure 3.
Effects of VD3 on cell wall composition and structure of C. neoformans. The yeast cells were cultured in YPD medium containing 0.4 and 0.8 mg/mL VD3 or DMSO for 6 h. (A) Total chitin was stained with CFW, and exposed chitooligomers were stained with fluorescein isothiocyanate-labeled wheat germ agglutinin. Cryptococcus cells with altered localization of exposed chitooligomers were indicated by red arrows. (B) Total chitin levels of cells stained with CFW were determined using a microplate reader. (C) Cell wall chitosan was labeled with Eosin Y and observed under a fluorescence microscope at 40×. Bar, 20 μm. (D) The fluorescence intensity of aniline blue was determined to evaluate β-1,3-glucan levels. (E) Transcription of genes related to chitin and chitosan syntheses. (F) Quantification of glucan biosynthesis by RT-qPCR analysis. The relative expression levels of the genes were calculated using 2−ΔΔCT method against GPD1 as the housekeeping gene. (G) Ten-fold serial dilutions (107, 106, 105, 104, 103, and 102) of C. neoformans cells were spotted onto YPD medium containing different concentrations of VD3 (0, 0.4, and 0.8 mg/mL) with or without CFW (100 μg/mL), SDS (0.01%), Congo red (0.2%), and H2O2 (1 mM). The plates were incubated at 37 °C for 72 h. Data were analyzed by one-way ANOVA or t-test (ns, p > 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001).
The RT-qPCR results showed that the expression levels of FKS1 (encoding β-1,3-glucan synthase) and AGS1 (encoding α-1,3-glucan synthase) were significantly upregulated after VD3 treatment, whereas no significant changes were noted in the expression levels of SKN1 and KRE6 (involved in the synthesis of β-1,6-glucan) (Figure 3F). Furthermore, VD3 treatment altered cell wall structure as detected by staining with CFW, Eosin Y, and WGA, and this coincided with increased susceptibility to cell wall perturbing agents, particularly CFW and Congo red, although there was no increased sensitivity to SDS in combination with VD3 (Figure 3G). In short, these results demonstrated that VD3 compromised cell wall integrity.
3.4. VD3-Altered Cell Membrane Permeability of C. neoformans
To further explore the structural damage caused by VD3, C. neoformans cell membrane permeability was evaluated by PI staining. In principle, PI can permeate damaged fungal cell membranes, bind to nucleic acids, and emit red fluorescence [28]. As shown in Figure 4A,B, the untreated fungal cells did not emit red fluorescence, whereas the VD3-treated fungal cells emitted strong red fluorescence, with the fluorescence intensity increasing. These results suggested that VD3 compromised fungal cell membrane integrity.
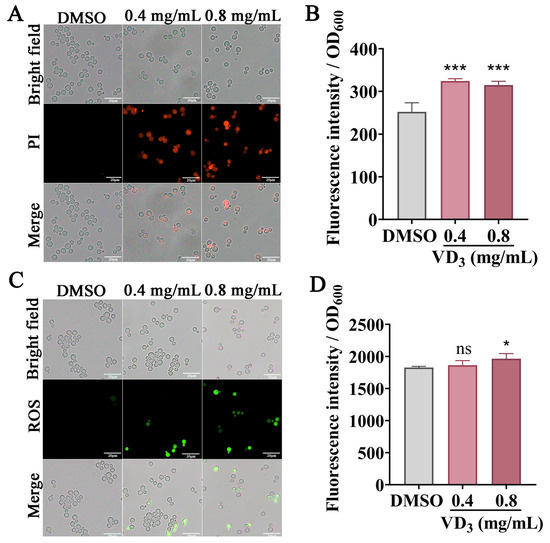
Figure 4.
Effects of VD3 on C. neoformans cell membrane permeability and ROS accumulation. C. neoformans cells were treated with or without VD3 at 37 °C for 6 h. PI combined with nucleic acids emitted red fluorescence. (A) Images captured using a fluorescence microscope. (B) Fluorescence was measured using a microplate reader. (C,D) Green fluorescence of DCFH-DA indicates ROS accumulation. Bar, 20 μm. Data were analyzed by one-way ANOVA (ns, p > 0.05; *, p < 0.05; ***, p < 0.001).
3.5. VD3-Induced Intracellular ROS Accumulation in C. neoformans
A DCFH-DA probe was used to measure the ROS levels in C. neoformans cells. As shown in Figure 4C, when compared with the DMSO group, the green fluorescence intensity increased after VD3 treatment, especially after 0.8 mg/mL VD3 treatment (Figure 4D). Furthermore, hydrogen peroxide can induce cellular oxidative damage; we tested the cellular sensitivity to oxidative stress after VD3 treatment. As shown in Figure 3G, VD3 significantly restricted cell growth on the agar medium after adding 1 mM H2O2. These results suggested that VD3 induced the accumulation of intracellular ROS and caused oxidation and an antioxidant imbalance in C. neoformans cells.
3.6. Antifungal Activity of VD3 In Vivo
The G. mellonella larval infection model is commonly used to study the pathogenesis of C. neoformans in vivo [32]. To evaluate the in vivo antifungal effects of VD3, the burden of C. neoformans in tissues and the survival of infected G. mellonella larvae were analyzed. When compared with the C. neoformans-infected group, the fungal burden of all VD3-treated groups was significantly reduced (Figure 5A). Survival analysis showed that the mortality rate of G. mellonella larvae infected with C. neoformans and without VD3 treatment was 75% on day 1, which increased to 100% within 5 days. However, on the fifth day of infection, the survival rates for 0.5, 1, 5, 10, and 20 mg/kg VD3 groups and FCZ groups were 30%, 15%, 20%, 20%, 40%, and 30%, respectively. Treatments with 0.5 or 20 mg/kg VD3 or 10 mg/kg FCZ significantly prolonged the survival of the larvae (Figure 5B). These results demonstrated that VD3 exhibited significant antifungal effects in vivo.
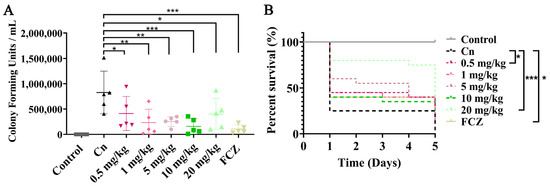
Figure 5.
Antifungal effects of VD3 in vivo. The G. mellonella larvae were infected with 2 × 105 C. neoformans cells and treated 2 h later. Control group, larvae injected with normal saline and treated with normal saline containing DMSO; Cn group, larvae injected with C. neoformans suspended in normal saline and treated with normal saline containing DMSO; VD3 groups, larvae injected with C. neoformans and treated with various concentrations of VD3 (0.5, 1, 5, 10, and 20 mg/kg, respectively); FCZ group, larvae injected with C. neoformans and treated with 10 mg/kg FCZ. The larvae were incubated in the dark at 37 °C. (A) The fungal burden in the tissues of G. mellonella larvae (5 larvae per group) was investigated after 24 h of treatment. (B) Larval survival curves (20 larvae per group) after 5 days of treatment. Data on fungal burden were analyzed by one-way ANOVA, and the survival curves were examined using the Kaplan-Meier method (log-rank test) (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
4. Discussion
The significant increase in C. neoformans-related infections and deaths suggests that the development of novel antifungal drugs has not kept pace with the increase in drug resistance among fungi [33]. Consequently, drug repurposing has become a novel research direction [34]. The present study is the first to investigate the antifungal effects and mechanism of VD3 against C. neoformans infection. The results demonstrated that VD3 exhibited significant anti-biofilm activities, compromised the cell wall, enhanced cell membrane permeability, and induced intracellular ROS accumulation in C. neoformans. A simplified illustration model with the phenotypic characteristics observed in this study by VD3 in C. neoformans is summarized in Figure 6.
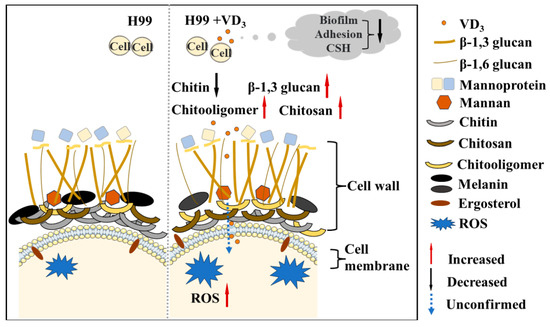
Figure 6.
Model: Effects of VD3 on C. neoformans biofilm, cell integrity, and ROS accumulation. VD3 inhibited biofilm formation, hindered early adhesion activity, and reduced the CSH of C. neoformans. Furthermore, VD3 enhanced cell membrane permeability, impacted cell wall integrity, and caused the accumulation of intracellular ROS. We observed a decrease in cell wall chitin (black solid arrow) and an increase in β-1,3 glucan, chitooligomers, and chitosan staining intensity by VD3 (red solid arrow); however, whether VD3 can destroy the cell surface and play an antifungal role in the cell remains to be confirmed (blue dotted arrow). CSH, cell surface hydrophobicity; ROS, reactive oxygen species.
In this study, VD3 increased intracellular ROS levels and significantly increased cell H2O2 sensibility (Figure 3G). Optimal levels of intracellular ROS ensure appropriate physiological and biochemical activities in the cells, and intracellular ROS signal transduction might possibly be related to the biosynthesis of enzymes involved in maintaining the integrity of the cell wall and cell membrane [35]. However, excessive ROS (i.e., H2O2, oxides, and hydroxide) accumulation can be toxic to the cell membrane, DNA, and other structures, resulting in abnormal energy metabolism and apoptosis and making cells more susceptible to the environment [36]. The result was consistent with the universal action mechanism of amphotericin B against C. neoformans [37]. SDS can disrupt cell membranes and activate cell wall integrity signaling [38]. Interestingly, although there was no effect of VD3 on cell sensitivity under 0.01% SDS, we observed that VD3 made cells more resistant to high concentrations of SDS on agar medium (Figure S1). This may be caused by VD3 interfering with SDS. Further investigations are needed to explore this finding.
During the infection process, invasive pathogenic fungi often produce biofilms to facilitate the production of multiple aggregates of surface-attached cells that promote survival in harsh environments and increase resistance to external stressors [39]. Clinically, C. neoformans has also been reported to form biofilms on medical devices [40] and exhibit increased virulence in vivo [41], and its biofilm formation ability is closely related to chronic infection and pathogenesis [42]. Moreover, biofilm formation has been linked to increased fungal resistance to host immunity and antifungal therapies [43]. The formation of biofilm by C. neoformans is a complex biological process with coordinated stages, including surface adhesion, microcolony formation, exopolymeric matrix production, and maturation phases [30]. In the present study, VD3 exhibited significant antibiofilm activity in C. neoformans (Figure 2), similar to that reported in C. albicans [15]. In addition, comparable antibiofilm activities have also been exhibited by amphotericin B in fungi [44]. However, the effective concentrations of amphotericin B are considerably toxic, and biofilm formation can significantly reduce the efficacy of antifungal drugs against cryptococcal infections [45]. To resolve these issues, for the treatment of chronic cryptococcal infections, new drugs are often explored in combination with conventional clinical agents to increase their drug susceptibility and reduce their toxicity [46].
The fungal cell wall is a dynamic structure and is considered an ideal target for antifungal drugs [47]. The C. neoformans cell wall primarily consists of glucose polymers composed of acetylglucosamine, chitosan, and α-/β-glucans, in addition to mannoproteins [48]. Chitosan is the deacetylation product of chitin, synthesized by enzymes encoded by CDA, while chitooligomers are the products of chitin consumption by CHI22-encoded endochitinase [25]. No significant changes in the CHI22 transcription levels were noted, whereas an increase in exposed chitooligomers was observed by fluorescent staining (Figure 3). VD3 may increase exposure to chitooligomers by altering the cell wall composition and structure. Abnormally increased exposure to chitooligomers can lead to harmful immune responses [49]. Furthermore, in the present study, upregulation of CDA3 and CDA4 involved in the chitosan synthesis pathway led to increased chitosan production, whereas upregulation of CHS3, CHS4, and CHS5 involved in the chitin synthesis pathway did not result in increased chitin levels, which might be attributed to insufficient synthesis and excessive consumption of chitin for a long period of time. This imbalance in the regulation of chitin synthesis/degradation can affect cell replication [50]. It must be noted that chitin, glucan, and chitosan are the main antigens on the cell surface. Moreover, fungal pathogens evade host immune recognition by masking β-1,3-glucan on the cell wall surface [51,52]. Thus, further investigations are needed to confirm whether these changes in the fungal cell wall caused by VD3 can affect host-pathogen interactions [49]. Moreover, as C. neoformans is naturally resistant to echinocandin, which acts on β-1,3-glucan of the cell wall, it is necessary to determine whether the changes in the β-1,3-glucan content of C. neoformans after VD3 treatment affect the resistance of the fungal cells to echinocandin.
Fungal CSH is an important cellular biophysical parameter that affects cell-cell and cell-surface interactions [53]. High fungal CSH may promote virulence through more complex mechanisms [54]. However, the mechanism of CSH and its direct correlation with the virulence of C. neoformans have not been established. In the present study, VD3 reduced the CSH of C. neoformans, similar to the effect of VD3 on C. albicans [15]. Melanin is an important component associated with C. neoformans virulence [55], and VD3 treatment partially decreased melanin production by C. neoformans on melanin-induced medium when compared with the DMSO group (Figure S2A). In addition, the expression of LAC2, which is related to melanin biosynthesis, was also significantly downregulated after VD3 treatment (Figure S2B). These results confirmed that VD3 partially reduced the virulence of C. neoformans through a complex mechanism.
In addition, according to human high VD3 supplementation regimens (4000–8000 IU/day) [56], taking large amounts over a long period poses a risk of poisoning. Therefore, in the case of effective therapeutics, reducing the dosage and the course of treatment are recommended. C. neoformans was significantly inhibited by 0.4 mg/mL VD3 in vitro, and in cytotoxicity tests with VD3, our investigations confirmed that 0.1–0.6 mg/mL VD3 was nontoxic to HepG2 cells in vitro (unpublished data). Moreover, the liver injury was reduced after treatment with 0.6 mg/kg of VD3 in mice with a fungal infection [15]. In this study, after treatment of infected G. mellonella larvae with 0.5 and 20 mg/kg of VD3 for 24 h, the fungal burden was significantly reduced, and the survival of the larvae was significantly prolonged. Further investigations are needed to confirm the antifungal activity and toxicity of 0.5–20 mg/kg VD3 in mice. It is presumed that VD3 might be rapidly metabolized in vivo into its active form, calcitriol (1,25-dihydroxycholecalciferol), which could induce antifungal effects through regulation of the host immune response [57,58]. In addition, remodeling of the fungal cell wall composition and structure caused by VD3 might also induce a host immune response. Therefore, further research is needed to confirm these assumptions and understand the mechanism of the antifungal activities of VD3.
5. Conclusions
To the best of our knowledge, this study is the first to investigate the antifungal activities of VD3 against C. neoformans both in vitro and in vivo. The results confirmed that the antifungal effect of VD3 against C. neoformans coincided with reduced biofilm formation, compromised cell wall integrity, increased generation of ROS, and offered new insights into the role of VD3 in cell wall remodeling and induction of host immune response. Nonetheless, further in vivo investigations of VD3 for the treatment of cryptococcal infections are needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof9070772/s1. Table S1. Nucleotide sequences of primers used for RT-qPCR analysis. Figure S1. VD3 reduced the stress of SDS with high concentrations. Figure S2. Effects of VD3 on melanin formation.
Author Contributions
J.H. and Z.S. performed the experiments, conceptualized, wrote, and edited the manuscript; J.L., A.G. and W.X. revised the manuscript. J.Z. and C.X. proofread it. J.Z. revised the manuscript and was responsible for the final version of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported financially by the Sichuan Science and Technology Program (2023NSFSC0529, 2023NSFSC1698, 2022NSFSC1539, and 2022YFS0629), the Technology Strategic Cooperation Project of Luzhou Municipal People’s Government at the Southwest Medical University (2018LZNYD-ZK26), and the Foundation of Southwest Medical University (2022QN042, 2022QN085, 2022QN102, and 2022QN118).
Institutional Review Board Statement
The animal study protocol was approved by the Southwest Medical University Institutional Animal Care and Use Committee (protocol 20220817-016 approval on 17 August 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in the article and the Supplementary Materials, those are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Botts, M.R.; Hull, C.M. Dueling in the lung: How Cryptococcus spores race the host for survival. Curr. Opin. Microbiol. 2010, 13, 437–442. [Google Scholar] [CrossRef]
- Walsh, N.M.; Botts, M.R.; McDermott, A.J.; Ortiz, S.C.; Wüthrich, M.; Klein, B.; Hull, C.M. Infectious particle identity determines dissemination and disease outcome for the inhaled human fungal pathogen Cryptococcus. PLoS Pathog. 2019, 15, e1007777. [Google Scholar] [CrossRef] [PubMed]
- Loyse, A.; Burry, J.; Cohn, J.; Ford, N.; Chiller, T.; Ribeiro, I.; Koulla-Shiro, S.; Mghamba, J.; Ramadhani, A.; Nyirenda, R.; et al. Leave no one behind: Response to new evidence and guidelines for the management of cryptococcal meningitis in low-income and middle-income countries. Lancet Infect. Dis. 2019, 19, e143–e147. [Google Scholar] [CrossRef]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 17, e334–e343. [Google Scholar] [CrossRef]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef]
- Mpoza, E.; Rhein, J.; Abassi, M. Emerging fluconazole resistance: Implications for the management of cryptococcal meningitis. Med. Mycol. Case Rep. 2017, 19, 30–32. [Google Scholar] [CrossRef]
- Stone, N.R.; Rhodes, J.; Fisher, M.C.; Mfinanga, S.; Kivuyo, S.; Rugemalila, J.; Segal, E.S.; Needleman, L.; Molloy, S.F.; Kwon-Chung, J.; et al. Dynamic ploidy changes drive fluconazole resistance in human cryptococcal meningitis. J. Clin. Investig. 2019, 129, 999–1014. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes-Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef]
- Avram, S.; Bologa, C.G.; Holmes, J.; Bocci, G.; Wilson, T.B.; Nguyen, D.T.; Curpan, R.; Halip, L.; Bora, A.; Yang, J.J.; et al. DrugCentral 2021 supports drug discovery and repositioning. Nucleic Acids Res. 2021, 49, D1160–D1169. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, F.; Zeng, M.; Mao, Y.; Song, Z. Drug repurposing strategies in the development of potential antifungal agents. Appl. Microbiol. Biotechnol. 2021, 105, 5259–5279. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Liang, X.; Chen, D.; Mao, X.; Yu, J.; Zheng, P.; He, J.; Huang, Z.; Yu, B. Vitamin D3 supplementation alleviates rotavirus infection in pigs and IPEC-J2 cells via regulating the autophagy signaling pathway. J. Steroid Biochem. Mol. Biol. 2016, 163, 157–163. [Google Scholar] [CrossRef]
- Bouzid, D.; Merzouki, S.; Bachiri, M.; Ailane, S.E.; Zerroug, M.M. Vitamin D3 a new drug against Candida albicans. J. Mycol. Med. 2017, 27, 79–82. [Google Scholar] [CrossRef]
- Lei, J.; Xiao, W.; Zhang, J.; Liu, F.; Xin, C.; Zhou, B.; Chen, W.; Song, Z. Antifungal activity of vitamin D3 against Candida albicans in vitro and in vivo. Microbiol. Res. 2022, 265, 127200. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.; Alexander, B.D.; Andes, D.; Arthington-Skaggs, B.; Brown, S.D.; Chaturvedi, V.; Ghannoum, M.A.; Espinel-Ingroff, A.; Knapp, C.C.; Ostrosky-Zeichner, L.; et al. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast, 3rd ed.; Approved Standard M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Mamoon, K.; Thammasit, P.; Iadnut, A.; Kitidee, K.; Anukool, U.; Tragoolpua, Y.; Tragoolpua, K. Unveiling the Properties of Thai Stingless Bee Propolis via Diminishing Cell Wall-Associated Cryptococcal Melanin and Enhancing the Fungicidal Activity of Macrophages. Antibiotics 2020, 9, 420. [Google Scholar] [CrossRef]
- Feretzaki, M.; Hardison, S.E.; Wormley, F.L., Jr.; Heitman, J. Cryptococcus neoformans hyperfilamentous strain is hypervirulent in a murine model of cryptococcal meningoencephalitis. PLoS ONE 2014, 9, e104432. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Walsh, B.; Ragsdale, A.; Lam, W.; Upadhya, R.; Xu, E.; Lodge, J.K.; Donlin, M.J. Membrane Integrity Contributes to Resistance of Cryptococcus neoformans to the Cell Wall Inhibitor Caspofungin. mSphere 2022, 7, e0013422. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Yang, Y.; Zhang, C.; Chen, H.-Y.; Chen, F.; Wang, K.-J. A Novel Antimicrobial Peptide Sparamosin26-54 From the Mud Crab Scylla paramamosain Showing Potent Antifungal Activity Against Cryptococcus neoformans. Front. Microbiol. 2021, 12, 746006. [Google Scholar] [CrossRef]
- Xin, C.; Wang, F.; Zhang, J.; Zhou, Q.; Liu, F.; Zhao, C.; Song, Z. Secretions from Serratia marcescens inhibit the growth and biofilm formation of Candida spp. and Cryptococcus neoformans. J. Microbiol. 2023, 61, 221–232. [Google Scholar] [CrossRef]
- Qian, W.; Wang, W.; Zhang, J.; Fu, Y.; Liu, Q.; Li, X.; Wang, T.; Zhang, Q. Exploitation of the antifungal and antibiofilm activities of plumbagin against Cryptococcus neoformans. Biofouling 2022, 38, 558–574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, J.; Sun, J.; Zhu, X.; Zhou, L.; Lu, Z.; Lu, Y. C16-Fengycin A affect the growth of Candida albicans by destroying its cell wall and accumulating reactive oxygen species. Appl. Microbiol. Biotechnol. 2019, 103, 8963–8975. [Google Scholar] [CrossRef] [PubMed]
- Kalem, M.C.; Subbiah, H.; Leipheimer, J.; Glazier, V.E.; Panepinto, J.C. Puf4 Mediates post-transcriptional regulation of cell wall biosynthesis and caspofungin resistance in Cryptococcus neoformans. mBio 2021, 12, e03225-20. [Google Scholar] [CrossRef]
- Silva, V.K.A.; Bhattacharya, S.; Oliveira, N.K.; Savitt, A.G.; Zamith-Miranda, D.; Nosanchuk, J.D.; Fries, B.C. Replicative aging remodels the cell wall and is associated with increased intracellular trafficking in human pathogenic yeasts. mBio 2021, 13, e0019022. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, H.C.; Castelli, R.F.; Reis, F.C.G.; Samby, K.; Nosanchuk, J.D.; Alves, L.R.; Rodrigues, M.L. Screening of the Pandemic Response Box Reveals an Association between Antifungal Effects of MMV1593537 and the Cell Wall of Cryptococcus neoformans, Cryptococcus deuterogattii, and Candida auris. Microbiol. Spectr. 2022, 10, e0060122. [Google Scholar] [CrossRef]
- Sá, N.P.d.; Lima, C.M.d.; Lino, C.I.; Barbeira, P.J.S.; Baltazar, L.d.M.; Santos, D.A.; Oliveira, R.B.d.; Mylonakis, E.; Fuchs, B.B.; Johann, S. Heterocycle Thiazole Compounds Exhibit Antifungal Activity through Inc rease in the Production of Reactive Oxygen Species in the Cryptococcus neoformans-Cryptococcus gattii Species Complex. Antimicrob. Agents Chemother. 2017, 61, e02700-16. [Google Scholar] [CrossRef]
- Lei, J.; Huang, J.; Xin, C.; Liu, F.; Zhang, J.; Xie, Y.; Mao, Y.; Chen, W.; Song, Z. Riboflavin targets the cellular metabolic and ribosomal pathways of Candida albicans in vitro and exhibits efficacy against oropharyngeal candidiasis. Microbiol. Spectr. 2023, 11, e0380122. [Google Scholar] [CrossRef]
- Li, L.; Wu, H.; Zhu, S.; Ji, Z.; Chi, X.; Xie, F.; Hao, Y.; Lu, H.; Yang, F.; Yan, L.; et al. Discovery of Novel 7-Hydroxy-5-oxo-4,5-dihydrothieno [3,2-b]pyridine-6-carboxamide Derivatives with Potent and Selective Antifungal Activity against Cryptococcus Species. J. Med. Chem. 2022, 65, 11257–11269. [Google Scholar] [CrossRef]
- Martinez, L.R.; Casadevall, A. Biofilm Formation by Cryptococcus neoformans. Microbiol. Spectr. 2015, 3, 135–147. [Google Scholar] [CrossRef]
- Upadhya, R.; Baker, L.G.; Lam, W.C.; Specht, C.A.; Donlin, M.J.; Lodge, J.K. Cryptococcus neoformans Cda1 and Its Chitin Deacetylase Activity Are Required for Fungal Pathogenesis. mBio 2018, 9, e02087-18. [Google Scholar] [CrossRef]
- Mylonakis, E.; Moreno, R.; El Khoury, J.B.; Idnurm, A.; Heitman, J.; Calderwood, S.B.; Ausubel, F.M.; Diener, A. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immun. 2005, 73, 3842–3850. [Google Scholar] [CrossRef]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.G.; Tan, S.-X.; Dawes, I.W. Reactive oxygen species and yeast apoptosis. Biochim. Biophys. Acta 2008, 1783, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Román, E.; Sánchez-Fresneda, R.; Casas, C.; Herrero, E.; Argüelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The Production of Reactive Oxygen Species Is a Universal Action Mechanism of Amphotericin B against Pathogenic Yeasts and Contributes to the Fungicidal Effect of This Drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, L.; Ikui, A.E. Tryptophan confers resistance to SDS-associated cell membrane stress in Saccharomyces cerevisiae. PLoS ONE 2019, 14, e0199484. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Casadevall, A. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl. Environ. Microbiol. 2007, 73, 4592–4601. [Google Scholar] [CrossRef]
- Martinez, L.R.; Casadevall, A. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect. Immun. 2005, 73, 6350–6362. [Google Scholar] [CrossRef] [PubMed]
- Benaducci, T.; Sardi Jde, C.; Lourencetti, N.M.; Scorzoni, L.; Gullo, F.P.; Rossi, S.A.; Derissi, J.B.; de Azevedo Prata, M.C.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J. Virulence of Cryptococcus sp. Biofilms In Vitro and In Vivo using Galleria mellonella as an Alternative Model. Front. Microbiol. 2016, 7, 290. [Google Scholar] [CrossRef]
- Aslanyan, L.; Sanchez, D.A.; Valdebenito, S.; Eugenin, E.A.; Ramos, R.L.; Martinez, L.R. The Crucial Role of Biofilms in Cryptococcus neoformans Survival within Macrophages and Colonization of the Central Nervous System. J. Fungi 2017, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Pierce, C.; Witt, C.; Wormley, F.L., Jr. Biofilm formation by Cryptococcus neoformans under distinct environmental conditions. Mycopathologia 2009, 167, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Casadevall, A. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 2006, 50, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Delattin, N.; Cammue, B.P.; Thevissen, K. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med. Chem. 2014, 6, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.S.N.; Silva, J.A.T.; Araújo, G.D.S.; Pereira, V.S.; Gotay, W.J.P.; Oliveira, J.S.; Guedes, G.M.M.; Pereira-Neto, W.A.; Castelo-Branco, D.; Cordeiro, R.A.; et al. Darunavir inhibits Cryptococcus neoformans/Cryptococcus gattii species complex growth and increases the susceptibility of biofilms to antifungal drugs. J. Med. Microbiol. 2020, 69, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Ibe, C.; Munro, C.A. Fungal cell wall: An underexploited target for antifungal therapies. PLoS Pathog. 2021, 17, e1009470. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, 267–292. [Google Scholar] [CrossRef]
- Ost, K.S.; Esher, S.K.; Leopold Wager, C.M.; Walker, L.; Wagener, J.; Munro, C.; Wormley, F.L., Jr.; Alspaugh, J.A. Rim Pathway-Mediated Alterations in the Fungal Cell Wall Influence Immune Recognition and Inflammation. mBio 2017, 8, e02290-16. [Google Scholar] [CrossRef]
- Latgé, J.-P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007, 66, 279–290. [Google Scholar] [CrossRef]
- Gantner, B.N.; Simmons, R.M.; Underhill, D.M. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005, 24, 1277–1286. [Google Scholar] [CrossRef]
- Lim, J.; Coates, C.J.; Seoane, P.I.; Garelnabi, M.; Taylor-Smith, L.M.; Monteith, P.; Macleod, C.L.; Escaron, C.J.; Brown, G.D.; Hall, R.A.; et al. Characterizing the Mechanisms of Nonopsonic Uptake of Cryptococci by Macrophages. J. Immunol. 2018, 200, 3539–3546. [Google Scholar] [CrossRef] [PubMed]
- Vij, R.; Danchik, C.; Crawford, C.; Dragotakes, Q.; Casadevall, A. Variation in Cell Surface Hydrophobicity among Cryptococcus neoformans Strains Influences Interactions with Amoebas. mSphere 2020, 5, e00310-20. [Google Scholar] [CrossRef] [PubMed]
- Danchik, C.; Casadevall, A. Role of Cell Surface Hydrophobicity in the Pathogenesis of Medically-Significant Fungi. Front. Cell Infect. Microbiol. 2021, 10, 594973. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, N.; Tu, J.; Ji, C.; Han, G.; Sheng, C. Discovery of Simplified Sampangine Derivatives with Potent Antifungal Activities against Cryptococcal Meningitis. ACS Infect. Dis. 2019, 5, 1376–1384. [Google Scholar] [CrossRef]
- Goto, R.L.; Tablas, M.B.; Prata, G.B.; Espírito Santo, S.G.; Fernandes, A.A.H.; Cogliati, B.; Barbisan, L.F. Vitamin D3 supplementation alleviates chemically-induced cirrhosis-associated hepatocarcinogenesis. J. Steroid Biochem. Mol. Biol. 2022, 215, 106022. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Mulligan, J.K.; Pasquini, W.N.; Carroll, W.W.; Williamson, T.; Reaves, N.; Patel, K.J.; Mappus, E.; Schlosser, R.J.; Atkinson, C. Dietary vitamin D3 deficiency exacerbates sinonasal inflammation and alters local 25(OH)D3 metabolism. PLoS ONE 2017, 12, e0186374. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).