Electrostatic Atomized Water Particles Induce Disease Resistance in Muskmelon (Cucumis melo L.) against Postharvest Fruit Rot Caused by Fusarium incarnatum
Abstract
1. Introduction
2. Materials and Methods
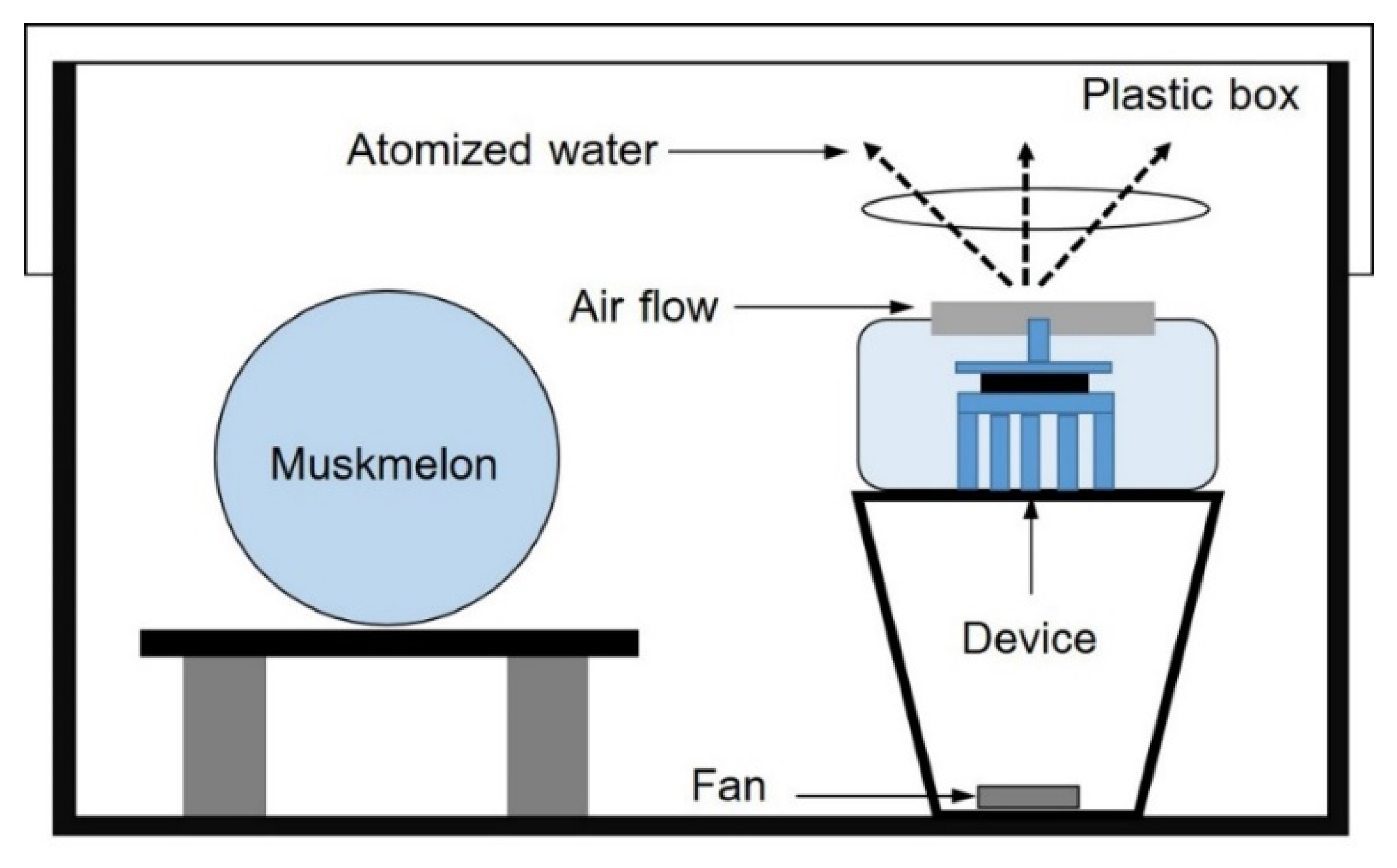
2.1. Plant Material and Pretreatment of Muskmelon with EAWPs
2.2. Pathogen Inoculation and Disease Assessment
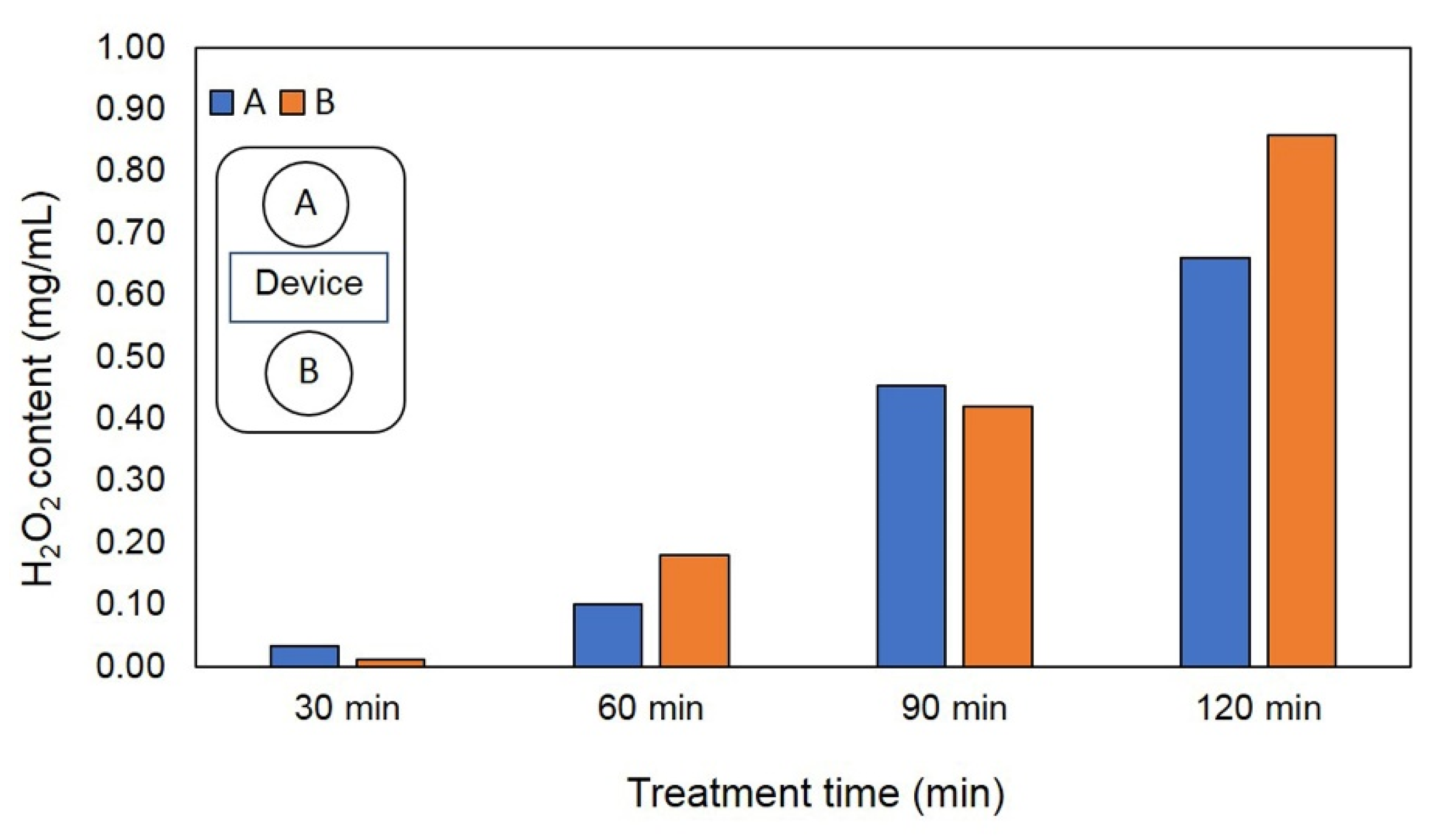
2.3. Formation of H2O2 from EAWPs
2.4. qRT-PCR of PR Protein Genes
2.5. Enzyme Assay
2.6. Scanning Electron Microscopy Observation
2.7. Postharvest Quality Measurement
2.8. Statistical Analysis
3. Results
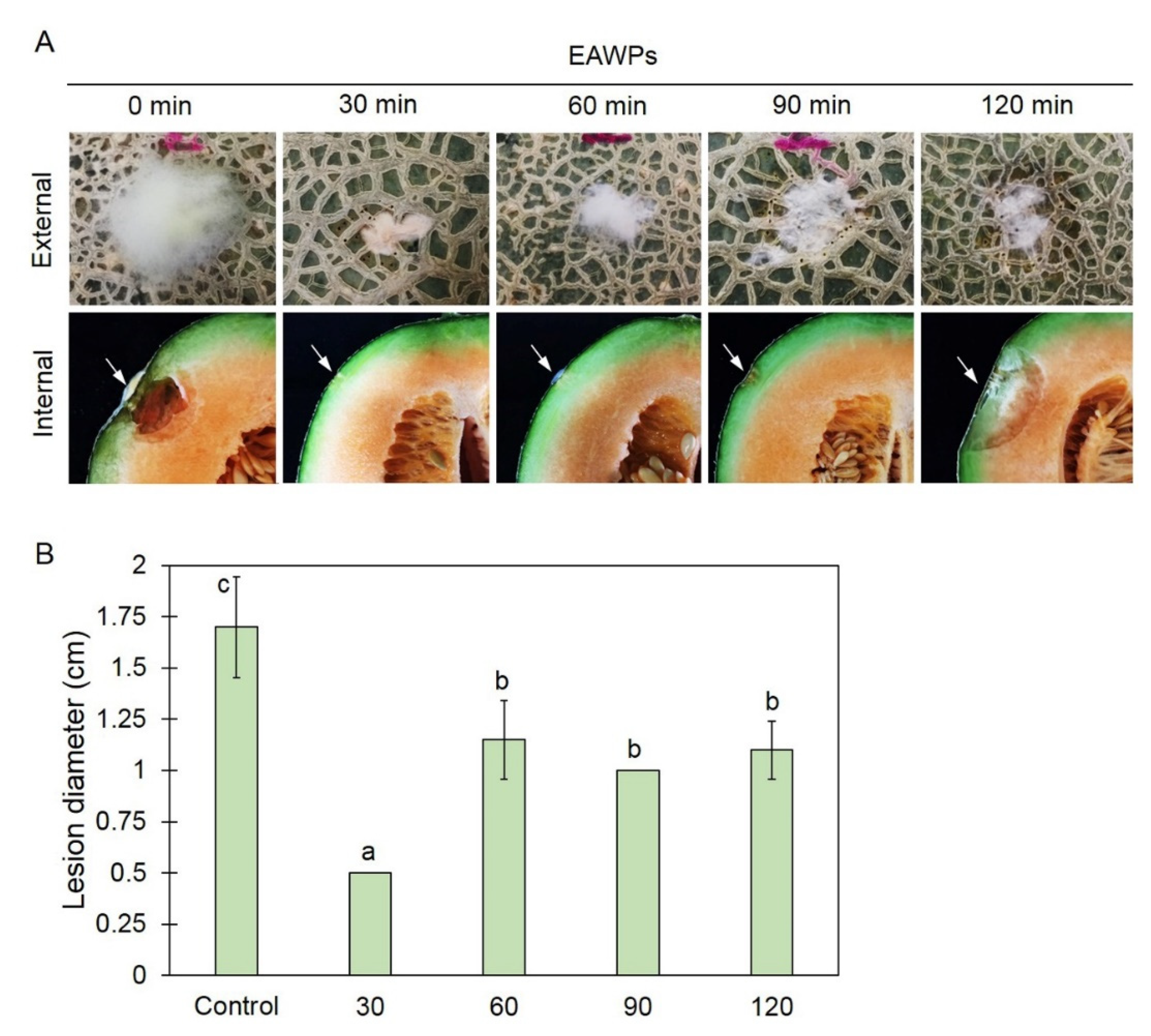
3.1. Application of EAWPs Limits Disease Progression
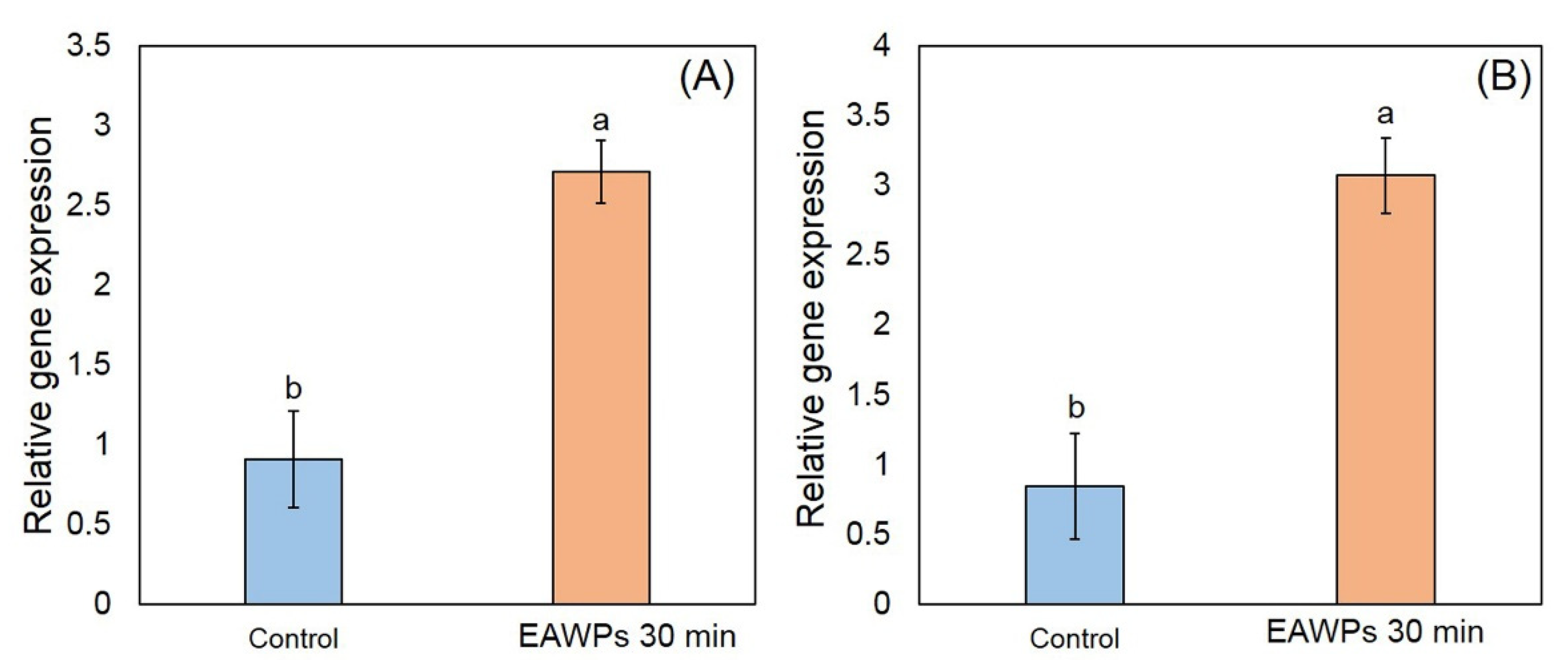
3.2. Overexpression of PR Protein Genes by EAWPs in Muskmelon
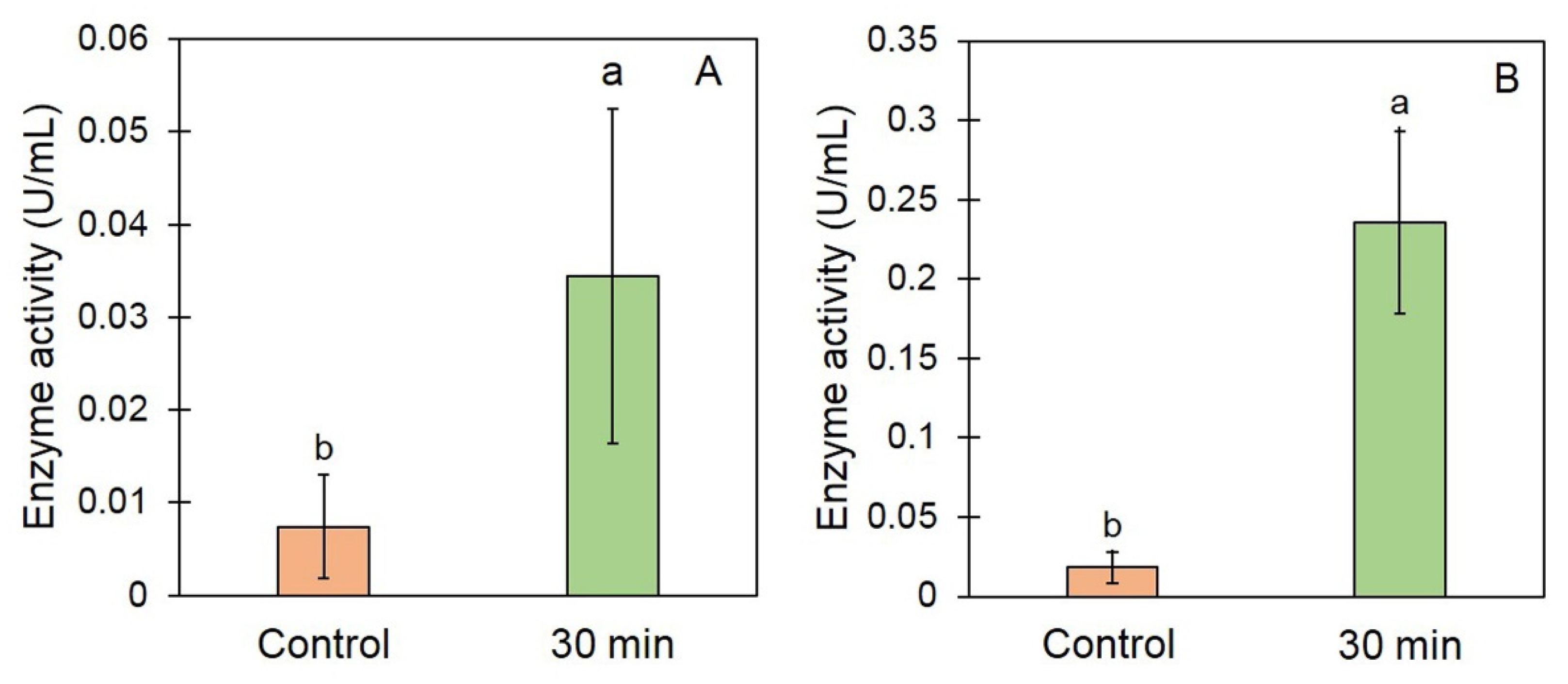
3.3. Effect of EAWPs on Defense-Related Enzymes and CWDEs’ Activity in Muskmelon
3.4. Effect of EAWP-Treated Muskmelon Crude Enzymes on Morphological Changes in Fusarium incarnatum
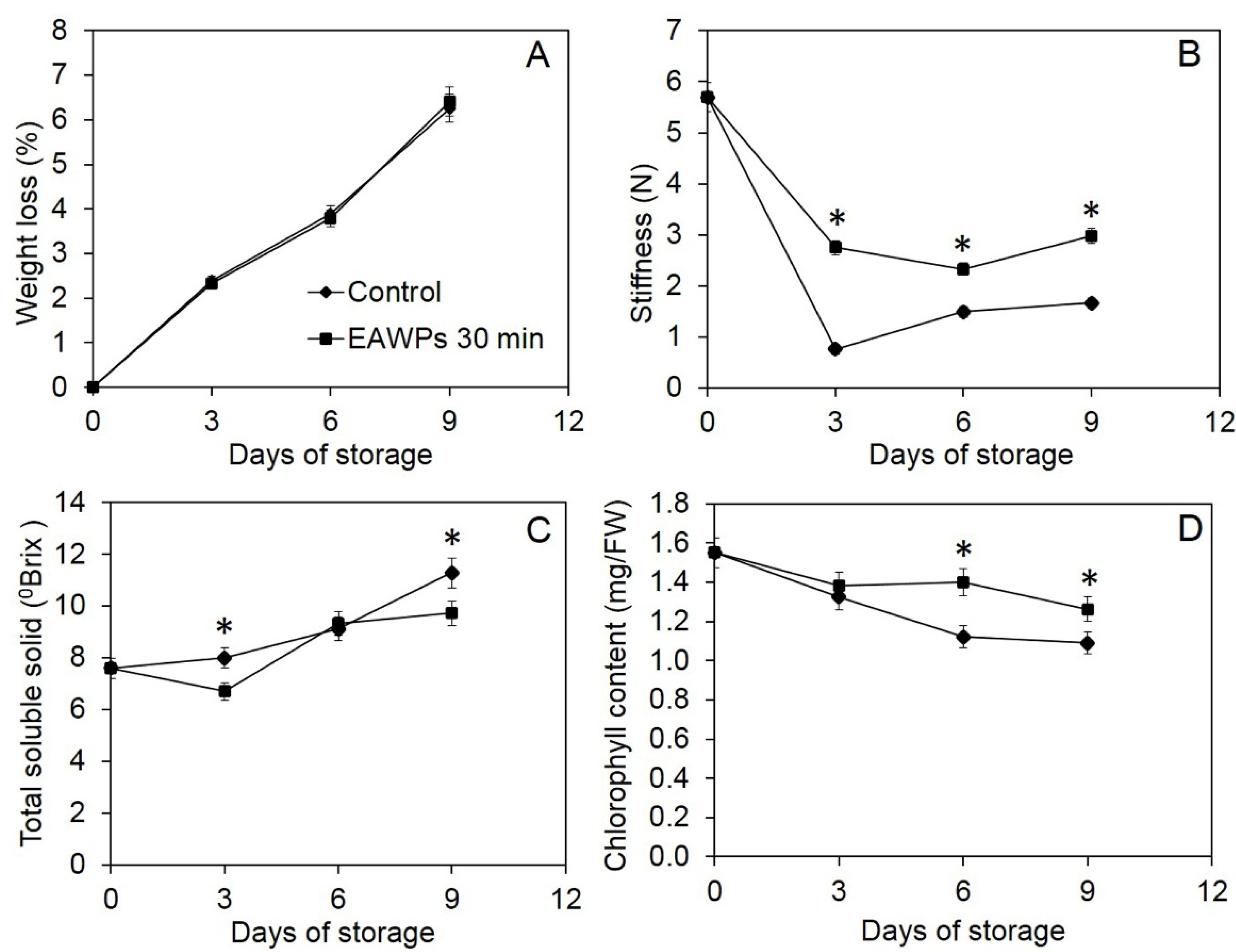
3.5. Effect of EAWPs on Postharvest Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lester, G.E.; Hodges, D.M. Antioxidants associated with fruit senescence and human health: Novel orange fleshed non-netted honey dew melon genotype comparisons following different seasonal productions and cold storage durations. Postharvest Biol. Technol. 2008, 48, 347–354. [Google Scholar] [CrossRef]
- Pornsuriya, C.; Chitphithak, I. Blue mold caused by Penicillium oxalicum on muskmelon (Cucumis melo) in Thailand. Australas. Plant Dis. Notes 2018, 13, 46. [Google Scholar] [CrossRef]
- Nuangmek, W.; Aiduang, W.; Suwannarach, N.; Kumla, J.; Kiatsiriroat, T.; Lumyong, S. First report of fruit rot on cantaloupe caused by Fusarium equiseti in Thailand. J. Gen. Plant Pathol. 2019, 85, 295–300. [Google Scholar] [CrossRef]
- Ruangwong, O.-U.; Wonglom, P.; Phoka, N.; Suwannarach, N.; Lumyong, S.; Ito, S.-I.; Sunpapao, A. Biological control activity of Trichoderma asperelloides PSU-P1 against gummy stem blight in muskmelon (Cucumis melo). Physiol. Mol. Plant Pathol. 2021, 115, 101663. [Google Scholar] [CrossRef]
- Wonglom, P.; Sunpapao, A. Fusarium incarnatum is associated with postharvest fruit rot of muskmelon (Cucumis melo). J. Phytopath. 2020, 168, 204–210. [Google Scholar] [CrossRef]
- Perincherry, L.; Urbaniak, M.; Pawłowicz, I.; Kotowska, K.; Waśkiewicz, A.; Stępień, Ł. Dynamics of Fusarium mycotoxins and lytic enzymes during pea plants’ infection. Int. J. Mol. Sci. 2021, 22, 9888. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signaling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef]
- Ryals, J.A.; Neuenschwander, U.H.; Willits, M.G.; Molina, A.; Steiner, H.Y.; Hunt, M.D. Systemic acquired resistance. Plant Cells 1996, 8, 1809–1819. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Bienert, G.P.; Møller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Van Breusegem, F.; Bailey-Serres, J.; Mittler, R. Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiol. 2008, 147, 978–984. [Google Scholar] [CrossRef]
- Kuźniak, E.; Urbanek, H. The involvement of hydrogen peroxide in plant responses to stresses. Acta Physiol. Plant 2000, 22, 195–203. [Google Scholar] [CrossRef]
- Mori, Y.; Fukumoto, Y. Production of silica particles by electrostatic atomization. Kona 2002, 20, 238–245. [Google Scholar] [CrossRef]
- Shimokage, T.; Saimoto, M.; Okumoto, S.; Miyata, T.; Yamauchi, T. Method of analyzing components in fine water droplets generated by electrostatic atomization. Matsushita Electr. Works Tech. Rep. 2005, 53, 11–16. [Google Scholar]
- Yamauchi, T.; Suda, H.; Matsui, Y. Development of home appliances using electrostatic atomization. J. Aerosol Res. 2007, 22, 5–10. [Google Scholar]
- Yamauchi, T.; Takamura, K.; Shigyo, M.; Migita, C.T.; Masuda, Y.; Maekawa, T. Control of degreening in postharvest green sour citrus fruit by electrostatic atomized water particles. Food Chem. 2014, 156, 160–164. [Google Scholar] [CrossRef]
- Salaemae, N.; Pongprasert, N.; Boonyaritthongchai, P.; Wongs-Aree, C.; Kaewsuksaeng, S.; Shigyo, M.; Yamauchi, N.; Srilaong, V. Electrostatic atomized water particles delay postharvest senescence of ‘Namwa’ banana (Musa × paradisiana). Fruits 2021, 76, 72–79. [Google Scholar] [CrossRef]
- Nomura, M.; Aiamla-Or, S.; Tanaka, S.; Shigyo, M.; Masuda, Y.; Yamauchi, N. Effect of reactive oxygen species on quality maintenance of broccoli florets with electrostatic atomized water particle treatment. Food Chem. 2017, 237, 749–755. [Google Scholar] [CrossRef]
- Lwin, W.W.; Srilaong, V.; Boonyaritthongchai, P.; Wongs-Aree, C.; Pongprasert, N. Electrostatic atomised water particles reduces postharvest lignification and maintain asparagus quality. Scientia Hort. 2020, 271, 109487. [Google Scholar] [CrossRef]
- Imada, K.; Tanaka, S.; Masuda, Y.; Maekawa, T.; Ito, S.I. Induction of disease resistance against Botrytis cinerea in tomato (Solanum lycopersicum L.) by using electrostatic atomized water particles. Physiol. Mol. Plant Pathol. 2015, 89, 1–7. [Google Scholar] [CrossRef]
- Kachroo, A.; He, Z.; Patkar, R.; Zhu, Q.; Zhong, J.; Li, D.; Ronald, P.; Lamb, C.; Chattoo, B.B. Induction of H2O2in transgenic rice leads to cell death and enhanced resistance to both bacterial and fungal pathogens. Transgenic Res. 2003, 12, 577–586. [Google Scholar] [CrossRef]
- Pornsuriya, C.; Ito, S.; Sunpapao, A. First report of leaf spot on lettuce caused by Curvularia aeria. J. Gen. Plant Pathol. 2018, 84, 296–299. [Google Scholar] [CrossRef]
- Sagisaka, S. The occurrence of peroxide in a perennial plant, Populus gelrica. Plant Physiol. 1976, 57, 308–309. [Google Scholar] [CrossRef]
- Phoka, N.; Suwannarach, N.; Lumyong, S.; Ito, S.-I.; Matsui, K.; Arikit, S.; Sunpapao, A. Role of volatiles from the endophytic fungus Trichoderma asperelloides PSU-P1 in biocontrol potential and in promoting the plant growth of Arabidopsis thaliana. J. Fungi 2020, 6, 341. [Google Scholar] [CrossRef]
- Dumhai, R.; Wanchana, S.; Saensuk, C.; Choowongkomon, K.; Mahatheeranont, S.; Kraithong, T.; Toojinda, T.; Vanavichit, A.; Arikit, S. Discovery of a novel CnAMADH2 allele associated with higher levels of 2-acetyl-1-pyroline (2AP) in yellow dwarf coconut (Cocos nucifera L.). Sci. Hort. 2019, 243, 490–497. [Google Scholar] [CrossRef]
- Wonglom, P.; Ito, S.; Sunpapao, A. Volatile organic compounds emitted from endophytic fungus Trichoderma asperellum T1 mediate antifungal activity, defense response and plant growth promoting ability in lettuce (Lactuca sativa L.). Fungal Ecol. 2020, 43, 100867. [Google Scholar] [CrossRef]
- Thaochan, N.; Pornsuriya, C.; Chairin, T.; Sunpapao, A. Roles of systemic fungicide in antifungal activity and induced defense responses in rubber tree (Hevea brasiliensis) against leaf fall disease caused by Neopestalotiopsis cubana. Physiol. Mol. Plant Pathol. 2020, 111, 101511. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Annl. Biochem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Wonglom, P.; Daengsuwan, W.; Ito, S.; Sunpapao, A. Biological control of Sclerotium fruit rot of snake fruit and stem rot of lettuce by Trichoderma sp. T76-12/2 and the mechanisms involved. Physiol. Mol. Plant Pathol. 2019, 107, 1–7. [Google Scholar] [CrossRef]
- Kaewsuksaeng, S.; Tatmala, N.; Shigyo, M.; Tanaka, S.; Yamauchi, N. Application of electrostatic atomized water particle suppress calyx discoloration in relation to postharvest quality of mangosteen (Garcinia mangostana L.). Sci. Hort. 2019, 250, 380–387. [Google Scholar] [CrossRef]
- Sunpapao, A.; Suwannarach, N.; Kumla, J.; Dumhai, R.; Riangwong, K.; Sanguansub, S.; Wanchana, S.; Arikit, S. Morphological and molecular identification of plant pathogenic fungi associated with dirty panicle disease in coconuts (Cocos nucifera) in Thailand. J. Fungi 2022, 8, 335. [Google Scholar] [CrossRef]
- Yan, K.; Han, G.; Ren, C.; Zhao, S.; Wu, X.; Bian, T. Fusarium solani infection depressed photosystem performance by inducing foliage wilting in apple seedlings. Front. Plant Sci. 2018, 9, 479. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Van De Poel, B.; Höfte, M.; Ende, W.V.D. Sweet immunity: Inulin boosts resistance of lettuce (Lactuca sativa) against grey mold (Botrytis cinerea) in an ethylene-dependent manner. Int. J. Mol. Sci. 2019, 20, 1052. [Google Scholar] [CrossRef]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.-Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef]
- Antunes, F.; Brito, P.M. Quantitative biology of hydrogen peroxide signaling. Redox Biol. 2017, 13, 1–7. [Google Scholar] [CrossRef]
- Vandenbroucke, K.; Robbens, S.; Vandepoele, K.; Inzé, D.; Van de Peer, Y.; Van Breusegem, F. Hydrogen peroxide-induced gene expression across kingdoms: A comparative analysis. Mol. Biol. Evol. 2008, 25, 507–516. [Google Scholar] [CrossRef]
- Niki, T.; Mitsuhara, I.; Seo, S.; Ohtsubo, N.; Ohashi, Y. Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tobacco laves. Plant Cell Physiol. 1998, 39, 500–507. [Google Scholar] [CrossRef]
- Jaakola, L.; Koskimäki, J.J.; Riihinen, K.R.; Tolvanen, A.; Hohtola, A. Effect of wounding on chalcone synthase and pathogenesis related PR-10 gene expression and content of phenolic compounds in bilberry leaves. Biol. Plant. 2008, 52, 391–395. [Google Scholar] [CrossRef]
- Lin, W.; Hu, X.; Zhang, W.; Rogers, W.J.; Cai, W. Hydrogen peroxide mediates defence responses induced by chitosans of different molecular weights in rice. J. Plant Physiol. 2005, 162, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Ravi, I.; Sharma, V. Induction of β-1,3- glucanase and chitinase activity in the defense response of Eruca sativa plants against the fungal pathogen Alternaria brassicicola. J. Plant Interact. 2013, 8, 155–161. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Y.; Liu, Q.; Deng, S.; Jin, X.; Yin, Y.; Guo, J.; Li, N.; Liu, Y.; Han, S.; et al. A Na2CO3-responsive chitinase gene from Leymus chinensis improve pathogen resistance and saline-alkali stress tolerance in transgenic tobacco and maize. Front. Plant Sci. 2020, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Baiyee, B.; Ito, S.; Sunapapo, A. Trichoderma asperellum T1 mediated antifungal activity and induced defense response against leaf spot fungi in lettuce (Lactuca sativa L.). Physiol. Mol. Plant Pathol. 2019, 106, 96–101. [Google Scholar] [CrossRef]
- Intana, W.; Wonglom, P.; Suwannarach, N.; Sunpapao, A. Trichoderma asperelloides PSU-P1 induced expression of pathogenesis-related protein genes against gummy stem blight of muskmelon (Cucumis melo) in field evaluation. J. Fungi 2022, 8, 156. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, H.; Zhang, H.; Zhang, X.; Apaliya, M.T.; Zheng, X.; Mahunu, G.K. Effect of Yarrowia lipolytica on postharvest decay of grapes caused by Talaromyces regulosus and the protein expression profile of the T. regulosus. Postharvest Biol. Technol. 2017, 126, 15–22. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Sun, Y.; Zhao, L.; Zheng, X.; Yang, Q.; Zhang, X. Investigating proteome and transcriptome defense response of apples induced by Yarrowia lipolytica. Mol. Plant Microbe. Interact. 2017, 30, 301–311. [Google Scholar] [CrossRef]

| Gene | Accession No. | Primer | Sequence (5′→3′) | Product Size (bp) |
|---|---|---|---|---|
| CmCHI 1 | AF241538 | Chi-F | AGGATCCACAATGTCCAAGC | 160 |
| Chi-R | CCGGTGGTTTGATGAGAAGT | |||
| CmGLU | AF459794 | Glu-F | AGAATGGTGGAGGATCGTTG | 182 |
| Glu-R | GTCAGACATGGCGAACACAT | |||
| CmACT | AB033599 | ACT-F | TGTTGGTCGACCTCGTCATA | 234 |
| ACT-R | GGGTTGAGTGGTGCTTCAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewsuksaeng, S.; Wonglom, P.; Sunpapao, A. Electrostatic Atomized Water Particles Induce Disease Resistance in Muskmelon (Cucumis melo L.) against Postharvest Fruit Rot Caused by Fusarium incarnatum. J. Fungi 2023, 9, 745. https://doi.org/10.3390/jof9070745
Kaewsuksaeng S, Wonglom P, Sunpapao A. Electrostatic Atomized Water Particles Induce Disease Resistance in Muskmelon (Cucumis melo L.) against Postharvest Fruit Rot Caused by Fusarium incarnatum. Journal of Fungi. 2023; 9(7):745. https://doi.org/10.3390/jof9070745
Chicago/Turabian StyleKaewsuksaeng, Samak, Prisana Wonglom, and Anurag Sunpapao. 2023. "Electrostatic Atomized Water Particles Induce Disease Resistance in Muskmelon (Cucumis melo L.) against Postharvest Fruit Rot Caused by Fusarium incarnatum" Journal of Fungi 9, no. 7: 745. https://doi.org/10.3390/jof9070745
APA StyleKaewsuksaeng, S., Wonglom, P., & Sunpapao, A. (2023). Electrostatic Atomized Water Particles Induce Disease Resistance in Muskmelon (Cucumis melo L.) against Postharvest Fruit Rot Caused by Fusarium incarnatum. Journal of Fungi, 9(7), 745. https://doi.org/10.3390/jof9070745







