Iron Starvation Induces Ferricrocin Production and the Reductive Iron Acquisition System in the Chromoblastomycosis Agent Cladophialophora carrionii
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Growth Conditions
2.2. In Silico Analysis of Siderophore and RIA-Related Sequences
2.3. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.4. Overlay-Chrome Azurol S (O-CAS) Assay
2.5. Ferric Perchlorate Assay
2.6. Siderophore Identification
3. Results
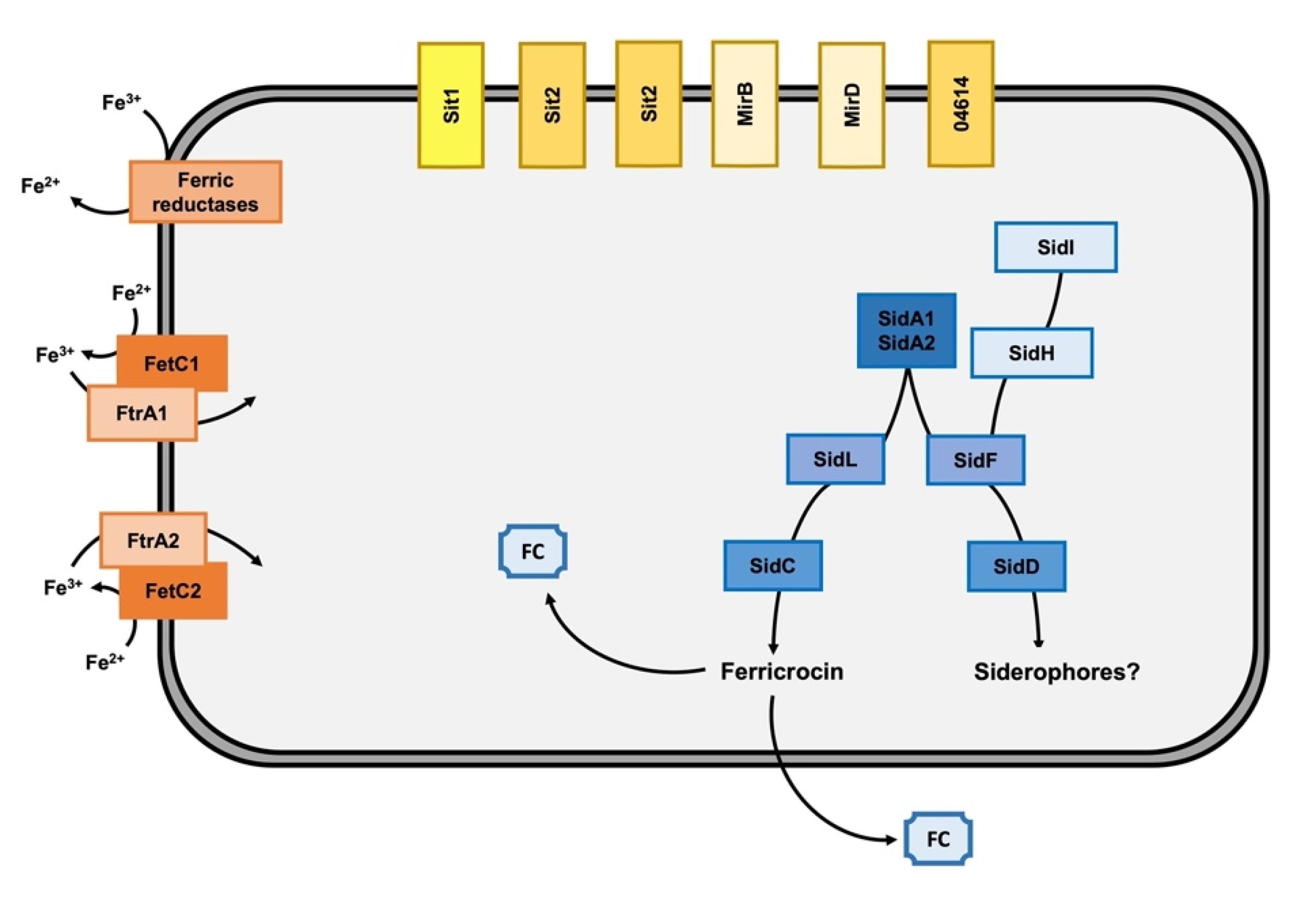
3.1. Genes for High-Affinity Iron Acquisition Are Conserved in C. carrionii
3.2. Iron Availability Regulates the Expression of Iron Acquisition Genes
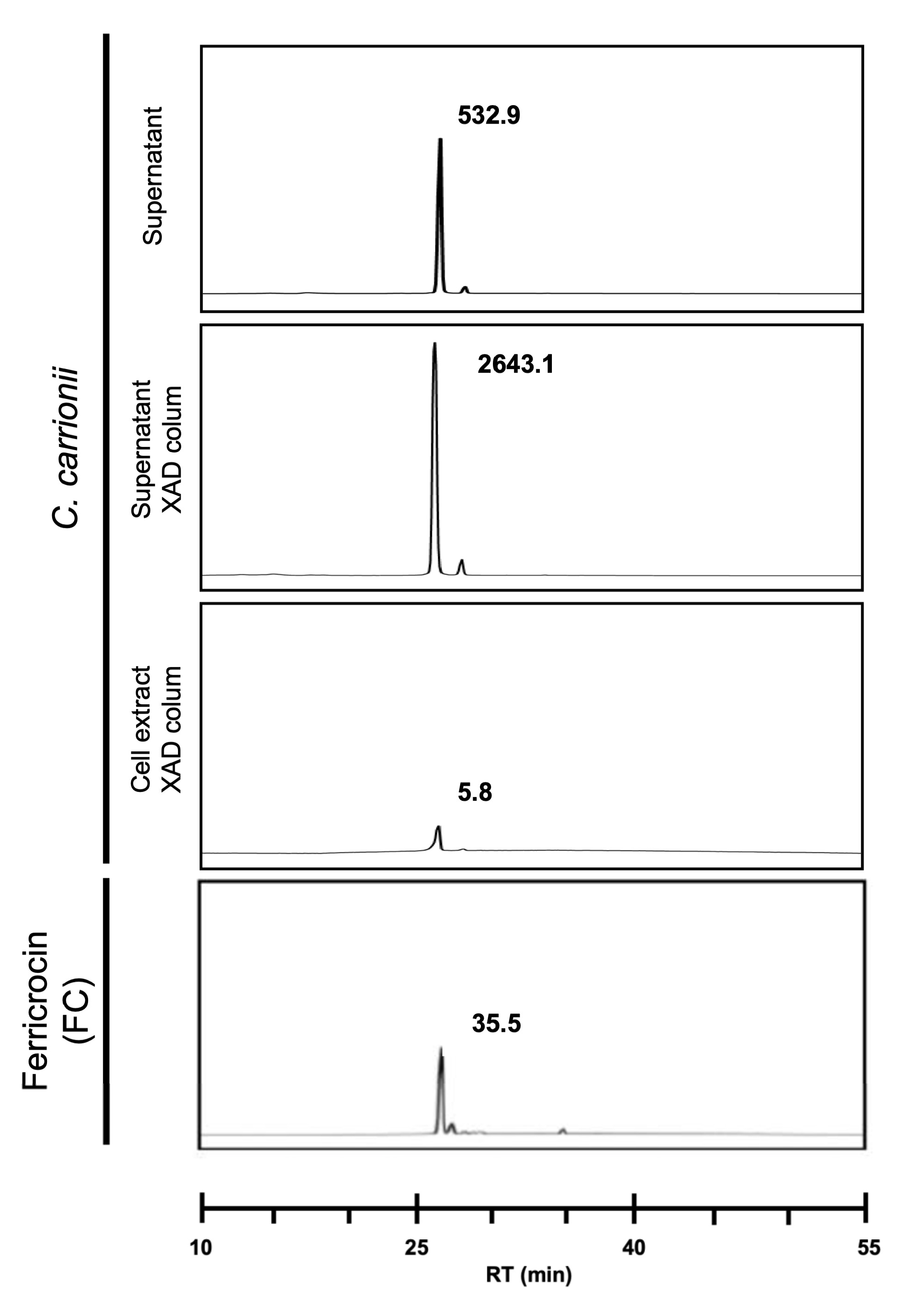
3.3. C. carrionii Produces and Secretes Hydroxamate Siderophores under Low-Iron Conditions
3.4. Ferricrocin Is Produced by C. carrionii as an Intra- and Extracellular Siderophore
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cairo, G.; Bernuzzi, F.; Recalcati, S. A precious metal: Iron, an essential nutrient for all cells. Genes Nutr. 2006, 1, 25–39. [Google Scholar] [CrossRef]
- Ilbert, M.; Bonnefoy, V. Insight into the evolution of the iron oxidation pathways. Biochim. Biophys. Acta 2013, 1827, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Kosman, D.J. Molecular mechanisms of iron uptake in fungi. Mol. Microbiol. 2003, 47, 1185–1197. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Appelberg, R. Macrophage nutriprive antimicrobial mechanisms. J. Leukoc. Biol. 2006, 79, 1117–1128. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Cassat, J.E.; Skaar, E.P. Iron in infection and immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef]
- Neilands, J.B. Siderophores. Arch. Biochem. Biophys. 1993, 302, 1–3. [Google Scholar] [CrossRef]
- Haas, H.; Eisendle, M.; Turgeon, B.G. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 2008, 46, 149–187. [Google Scholar] [CrossRef] [PubMed]
- Schrettl, M.; Bignell, E.; Kragl, C.; Sabiha, Y.; Loss, O.; Eisendle, M.; Wallner, A.; Arst, H.N., Jr.; Haynes, K.; Haas, H. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 2007, 3, 1195–1207. [Google Scholar] [CrossRef]
- Yasmin, S.; Alcazar-Fuoli, L.; Grundlinger, M.; Puempel, T.; Cairns, T.; Blatzer, M.; Lopez, J.F.; Grimalt, J.O.; Bignell, E.; Haas, H. Mevalonate governs interdependency of ergosterol and siderophore biosyntheses in the fungal pathogen Aspergillus fumigatus. Proc. Natl. Acad. Sci. USA 2012, 109, E497–E504. [Google Scholar] [CrossRef] [PubMed]
- Pao, S.S.; Paulsen, I.T.; Saier, M.H., Jr. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 1998, 62, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.C.; Protchenko, O. Response to iron deprivation in Saccharomyces cerevisiae. Eukaryot. Cell 2008, 7, 20–27. [Google Scholar] [CrossRef]
- Schrettl, M.; Bignell, E.; Kragl, C.; Joechl, C.; Rogers, T.; Arst, H.N., Jr.; Haynes, K.; Haas, H. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J. Exp. Med. 2004, 200, 1213–1219. [Google Scholar] [CrossRef]
- Ramanan, N.; Wang, Y. A high-affinity iron permease essential for Candida albicans virulence. Science 2000, 288, 1062–1064. [Google Scholar] [CrossRef]
- Eck, R.; Hundt, S.; Hartl, A.; Roemer, E.; Kunkel, W. A multicopper oxidase gene from Candida albicans: Cloning, characterization and disruption. Microbiology 1999, 145 Pt 9, 2415–2422. [Google Scholar] [CrossRef]
- Jung, W.H.; Sham, A.; Lian, T.; Singh, A.; Kosman, D.J.; Kronstad, J.W. Iron source preference and regulation of iron uptake in Cryptococcus neoformans. PLoS Pathog. 2008, 4, e45. [Google Scholar] [CrossRef]
- Jung, W.H.; Hu, G.; Kuo, W.; Kronstad, J.W. Role of ferroxidases in iron uptake and virulence of Cryptococcus neoformans. Eukaryot. Cell 2009, 8, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, H.; Lessing, F.; Winterberg, B.; Schirawski, J.; Kamper, J.; Muller, P.; Kahmann, R. A ferroxidation/permeation iron uptake system is required for virulence in Ustilago maydis. Plant Cell 2006, 18, 3332–3345. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Kim, J.H.; Cho, J.H.; Chang, H.I.; Kim, S.W.; Paik, H.D.; Kang, C.W.; Kim, T.H.; Sung, H.C.; Yun, C.W. Physical and functional interaction of FgFtr1-FgFet1 and FgFtr2-FgFet2 is required for iron uptake in Fusarium graminearum. Biochem. J. 2007, 408, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, G.; Jiang, H.; Chi, Z.; Chi, Z. An insight into the iron acquisition and homeostasis in Aureobasidium melanogenum HN6.2 strain through genome mining and transcriptome analysis. Funct. Integr. Genomics 2019, 19, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Gostincar, C.; Ohm, R.A.; Kogej, T.; Sonjak, S.; Turk, M.; Zajc, J.; Zalar, P.; Grube, M.; Sun, H.; Han, J.; et al. Genome sequencing of four Aureobasidium pullulans varieties: Biotechnological potential, stress tolerance, and description of new species. BMC Genomics 2014, 15, 549. [Google Scholar] [CrossRef] [PubMed]
- Holinsworth, B.; Martin, J.D. Siderophore production by marine-derived fungi. Biometals 2009, 22, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chi, Z.; Liu, G.; Buzdar, M.A.; Chi, Z.; Gu, Q. Chemical and biological characterization of siderophore produced by the marine-derived Aureobasidium pullulans HN6.2 and its antibacterial activity. Biometals 2009, 22, 965–972. [Google Scholar] [CrossRef]
- Wang, W.L.; Chi, Z.M.; Chi, Z.; Li, J.; Wang, X.H. Siderophore production by the marine-derived Aureobasidium pullulans and its antimicrobial activity. Bioresour. Technol. 2009, 100, 2639–2641. [Google Scholar] [CrossRef]
- Ameen, M. Chromoblastomycosis: Clinical presentation and management. Clin. Exp. Dermatol. 2009, 34, 849–854. [Google Scholar] [CrossRef]
- WHO. World Health Organization: Neglected Tropical Diseases; 2017. Available online: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_1 (accessed on 10 March 2023).
- Queiroz-Telles, F.; de Hoog, S.; Santos, D.W.; Salgado, C.G.; Vicente, V.A.; Bonifaz, A.; Roilides, E.; Xi, L.; Azevedo, C.M.; da Silva, M.B.; et al. Chromoblastomycosis. Clin. Microbiol. Rev. 2017, 30, 233–276. [Google Scholar] [CrossRef]
- Queiroz-Telles, F. Chromoblastomycosis: A Neglected Tropical Disease. Rev. Inst. Med. Trop. Sao Paulo 2015, 57 (Suppl. S19), 46–50. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Netea, M.G.; Mouton, J.W.; Melchers, W.J.; Verweij, P.E.; de Hoog, G.S. Black yeasts and their filamentous relatives: Principles of pathogenesis and host defense. Clin. Microbiol. Rev. 2014, 27, 527–542. [Google Scholar] [CrossRef]
- Badali, H.; Gueidan, C.; Najafzadeh, M.J.; Bonifaz, A.; van den Ende, A.H.; de Hoog, G.S. Biodiversity of the genus Cladophialophora. Stud. Mycol. 2008, 61, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, D.; Corbellini, V.A.; Mendes, S.D.C.; Fernandes, E.K.; Lazzarotto, L.; Ribeiro, A.C.; Zanette, R.A.; Scroferneker, M.L. Melanin: Quantification and protection against oxidative stress in chromoblastomycosis agents. Med. Mycol. 2019, 57, 260–263. [Google Scholar] [CrossRef]
- De Hoog, G. Evolution of black yeasts: Possible adaptation to the human host. Antonie Van Leeuwenhoek 1993, 63, 105–109. [Google Scholar] [CrossRef]
- Florencio, C.S.; Brandao, F.A.S.; Teixeira, M.M.; Bocca, A.L.; Felipe, M.S.S.; Vicente, V.A.; Fernandes, L. Genetic manipulation of Fonsecaea pedrosoi using particles bombardment and Agrobacterium mediated transformation. Microbiol. Res. 2018, 207, 269–279. [Google Scholar] [CrossRef]
- Restrepo, A.; Jimenez, B.E. Growth of Paracoccidioides brasiliensis yeast phase in a chemically defined culture medium. J. Clin. Microbiol. 1980, 12, 279–281. [Google Scholar] [CrossRef]
- Omasits, U.; Ahrens, C.H.; Muller, S.; Wollscheid, B. Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 2014, 30, 884–886. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Bookout, A.L.; Cummins, C.L.; Mangelsdorf, D.J.; Pesola, J.M.; Kramer, M.F. High-throughput real-time quantitative reverse transcription PCR. Curr. Protoc. Mol. Biol. 2006, 15, 15–18. [Google Scholar] [CrossRef]
- Perez-Miranda, S.; Cabirol, N.; George-Tellez, R.; Zamudio-Rivera, L.S.; Fernandez, F.J. O-CAS, a fast and universal method for siderophore detection. J. Microbiol. Methods 2007, 70, 127–131. [Google Scholar] [CrossRef]
- Cox, C.D. Deferration of laboratory media and assays for ferric and ferrous ions. Methods Enzymol. 1994, 235, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Atkin, C.L.; Neilands, J.B.; Phaff, H.J. Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. J. Bacteriol. 1970, 103, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Silva-Bailão, M.G.; Bailão, E.F.; Lechner, B.E.; Gauthier, G.M.; Lindner, H.; Bailão, A.M.; Haas, H.; Soares, C.M.A. Hydroxamate production as a high affinity iron acquisition mechanism in Paracoccidioides spp. PLoS ONE 2014, 9, e105805. [Google Scholar] [CrossRef]
- Aguiar, M.; Orasch, T.; Shadkchan, Y.; Caballero, P.; Pfister, J.; Sastre-Velasquez, L.E.; Gsaller, F.; Decristoforo, C.; Osherov, N.; Haas, H. Uptake of the siderophore triacetylfusarinine C, but not fusarinine C, is crucial for virulence of Aspergillus fumigatus. mBio 2022, 13, e0219222. [Google Scholar] [CrossRef]
- Finegold, A.A.; Shatwell, K.P.; Segal, A.W.; Klausner, R.D.; Dancis, A. Intramembrane bis-heme motif for transmembrane electron transport conserved in a yeast iron reductase and the human NADPH oxidase. J. Biol. Chem. 1996, 271, 31021–31024. [Google Scholar] [CrossRef]
- Dancis, A.; Klausner, R.D.; Hinnebusch, A.G.; Barriocanal, J.G. Genetic evidence that ferric reductase is required for iron uptake in Saccharomyces cerevisiae. Mol. Cell. Biol. 1990, 10, 2294–2301. [Google Scholar] [CrossRef]
- Georgatsou, E.; Alexandraki, D. Two distinctly regulated genes are required for ferric reduction, the first step of iron uptake in Saccharomyces cerevisiae. Mol. Cell. Biol. 1994, 14, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Yamada-Okabe, T.; Shimmi, O.; Doi, R.; Mizumoto, K.; Arisawa, M.; Yamada-Okabe, H. Isolation of the mRNA-capping enzyme and ferric-reductase-related genes from Candida albicans. Microbiology 1996, 142 Pt 9, 2515–2523. [Google Scholar] [CrossRef]
- Hammacott, J.E.; Williams, P.H.; Cashmore, A.M. Candida albicans CFL1 encodes a functional ferric reductase activity that can rescue a Saccharomyces cerevisiae fre1 mutant. Microbiology 2000, 146 Pt 4, 869–876. [Google Scholar] [CrossRef]
- Blatzer, M.; Binder, U.; Haas, H. The metalloreductase FreB is involved in adaptation of Aspergillus fumigatus to iron starvation. Fungal Genet. Biol. 2011, 48, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Saikia, S.; Oliveira, D.; Hu, G.; Kronstad, J. Role of ferric reductases in iron acquisition and virulence in the fungal pathogen Cryptococcus neoformans. Infect. Immun. 2014, 82, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.U.; Li, M.; Davis, D.A. Candida albicans ferric reductases are differentially regulated in response to distinct forms of iron limitation by the Rim101 and CBF transcription factors. Eukaryot. Cell 2008, 7, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Georgatsou, E.; Alexandraki, D. Regulated expression of the Saccharomyces cerevisiae Fre1p/Fre2p Fe/Cu reductase related genes. Yeast 1999, 15, 573–584. [Google Scholar] [CrossRef]
- Freitas, J.M.; Kim, J.H.; Poynton, H.; Su, T.; Wintz, H.; Fox, T.; Holman, P.; Loguinov, A.; Keles, S.; van der Laan, M.; et al. Exploratory and confirmatory gene expression profiling of mac1Delta. J. Biol. Chem. 2004, 279, 4450–4458. [Google Scholar] [CrossRef]
- Severance, S.; Chakraborty, S.; Kosman, D.J. The Ftr1p iron permease in the yeast plasma membrane: Orientation, topology and structure-function relationships. Biochem. J. 2004, 380 Pt 2, 487–496. [Google Scholar] [CrossRef]
- Ziegler, L.; Terzulli, A.; Gaur, R.; McCarthy, R.; Kosman, D.J. Functional characterization of the ferroxidase, permease high-affinity iron transport complex from Candida albicans. Mol. Microbiol. 2011, 81, 473–485. [Google Scholar] [CrossRef]
- Hassett, R.F.; Yuan, D.S.; Kosman, D.J. Spectral and kinetic properties of the Fet3 protein from Saccharomyces cerevisiae, a multinuclear copper ferroxidase enzyme. J. Biol. Chem. 1998, 273, 23274–23282. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.M.; Askwith, C.C.; Eide, D.; Kaplan, J. The FET3 gene product required for high affinity iron transport in yeast is a cell surface ferroxidase. J. Biol. Chem. 1995, 270, 1098–1101. [Google Scholar] [CrossRef]
- Taylor, A.B.; Stoj, C.S.; Ziegler, L.; Kosman, D.J.; Hart, P.J. The copper-iron connection in biology: Structure of the metallo-oxidase Fet3p. Proc. Natl. Acad. Sci. USA 2005, 102, 15459–15464. [Google Scholar] [CrossRef] [PubMed]
- di Patti, M.C.B.; Felice, M.R.; Camuti, A.P.; Lania, A.; Musci, G. The essential role of Glu-185 and Tyr-354 residues in the ferroxidase activity of Saccharomyces cerevisiae Fet3. FEBS Lett. 2000, 472, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.P.; Quintanar, L.; Severance, S.; Solomon, E.I.; Kosman, D.J. Targeted suppression of the ferroxidase and iron trafficking activities of the multicopper oxidase Fet3p from Saccharomyces cerevisiae. J. Biol. Inorg. Chem. 2003, 8, 611–620. [Google Scholar] [CrossRef]
- Schrettl, M.; Kim, H.S.; Eisendle, M.; Kragl, C.; Nierman, W.C.; Heinekamp, T.; Werner, E.R.; Jacobsen, I.; Illmer, P.; Yi, H.; et al. SreA-mediated iron regulation in Aspergillus fumigatus. Mol. Microbiol. 2008, 70, 27–43. [Google Scholar] [CrossRef]
- Schrettl, M.; Beckmann, N.; Varga, J.; Heinekamp, T.; Jacobsen, I.D.; Jochl, C.; Moussa, T.A.; Wang, S.; Gsaller, F.; Blatzer, M.; et al. HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus. PLoS Pathog. 2010, 6, e1001124. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.H.; Mayfield, J.A.; Rine, J.; Sil, A. Histoplasma requires SID1, a member of an iron-regulated siderophore gene cluster, for host colonization. PLoS Pathog. 2008, 4, e1000044. [Google Scholar] [CrossRef] [PubMed]
- Hortschansky, P.; Eisendle, M.; Al-Abdallah, Q.; Schmidt, A.D.; Bergmann, S.; Thon, M.; Kniemeyer, O.; Abt, B.; Seeber, B.; Werner, E.R.; et al. Interaction of HapX with the CCAAT-binding complex—A novel mechanism of gene regulation by iron. EMBO J. 2007, 26, 3157–3168. [Google Scholar] [CrossRef]
- Grundlinger, M.; Yasmin, S.; Lechner, B.E.; Geley, S.; Schrettl, M.; Hynes, M.; Haas, H. Fungal siderophore biosynthesis is partially localized in peroxisomes. Mol. Microbiol. 2013, 88, 862–875. [Google Scholar] [CrossRef]
- Kuan, C.S.; Cham, C.Y.; Singh, G.; Yew, S.M.; Tan, Y.C.; Chong, P.S.; Toh, Y.F.; Atiya, N.; Na, S.L.; Lee, K.W.; et al. Genomic analyses of Cladophialophora bantiana, a major cause of cerebral phaeohyphomycosis provides insight into its lifestyle, virulence and adaption in host. PLoS ONE 2016, 11, e0161008. [Google Scholar] [CrossRef] [PubMed]
- Marty, A.J.; Broman, A.T.; Zarnowski, R.; Dwyer, T.G.; Bond, L.M.; Lounes-Hadj Sahraoui, A.; Fontaine, J.; Ntambi, J.M.; Keles, S.; Kendziorski, C.; et al. Fungal morphology, iron homeostasis, and lipid metabolism regulated by a GATA transcription factor in Blastomyces dermatitidis. PLoS Pathog. 2015, 11, e1004959. [Google Scholar] [CrossRef]
- Winkelmann, G. Siderophore transport in fungi. In Microbial Transport Systems; Winkelmann, G., Ed.; Wiley-VCH: Weinheim, Germany, 2001; pp. 463–479. [Google Scholar]
- Aguiar, M.; Orasch, T.; Misslinger, M.; Dietl, A.M.; Gsaller, F.; Haas, H. The siderophore transporters Sit1 and Sit2 are essential for utilization of ferrichrome-, ferrioxamine- and coprogen-type siderophores in Aspergillus fumigatus. J. Fungi 2021, 7, 768. [Google Scholar] [CrossRef]
- Haas, H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 2014, 31, 1266–1276. [Google Scholar] [CrossRef]
- Powell, P.E.; Szaniszlo, P.J.; Reid, C.P. Confirmation of occurrence of hydroxamate siderophores in soil by a novel Escherichia coli bioassay. Appl. Environ. Microbiol. 1983, 46, 1080–1083. [Google Scholar] [CrossRef]
- Reiss, E.; Shadomy, H.J.; Lyon, G.M. Chromoblastomycosis. In Fundamental Medical Mycology, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 479–491. [Google Scholar]
- De Hoog, G.S.; Queiroz-Telles, F.; Haase, G.; Fernandez-Zeppenfeldt, G.; Attili Angelis, D.; Gerrits Van Den Ende, A.H.; Matos, T.; Peltroche-Llacsahuanga, H.; Pizzirani-Kleiner, A.A.; Rainer, J.; et al. Black fungi: Clinical and pathogenic approaches. Med. Mycol. 2000, 38 (Suppl. S1), 243–250. [Google Scholar] [CrossRef]
- Fonseca-Garcia, C.; Coleman-Derr, D.; Garrido, E.; Visel, A.; Tringe, S.G.; Partida-Martinez, L.P. The cacti microbiome: Interplay between habitat-filtering and host-specificity. Front. Microbiol. 2016, 7, 150. [Google Scholar] [CrossRef]
- Bonifaz, A.; Carrasco-Gerard, E.; Saul, A. Chromoblastomycosis: Clinical and mycologic experience of 51 cases. Mycoses 2001, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Georgatsou, E.; Mavrogiannis, L.A.; Fragiadakis, G.S.; Alexandraki, D. The yeast Fre1p/Fre2p cupric reductases facilitate copper uptake and are regulated by the copper-modulated Mac1p activator. J. Biol. Chem. 1997, 272, 13786–13792. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.J.; Jensen, L.T.; Simon, J.R.; Keller, G.L.; Winge, D.R. Metalloregulation of FRE1 and FRE2 homologs in Saccharomyces cerevisiae. J. Biol. Chem. 1998, 273, 23716–23721. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Bauler, M.; Moore, R.E.; Klebba, P.E.; Philpott, C.C. The role of the FRE family of plasma membrane reductases in the uptake of siderophore-iron in Saccharomyces cerevisiae. J. Biol. Chem. 2001, 276, 10218–10223. [Google Scholar] [CrossRef] [PubMed]
- Stanford, F.A.; Matthies, N.; Cseresnyes, Z.; Figge, M.T.; Hassan, M.I.A.; Voigt, K. Expression patterns in reductive iron assimilation and functional consequences during phagocytosis of Lichtheimia corymbifera, an emerging cause of mucormycosis. J. Fungi 2021, 7, 272. [Google Scholar] [CrossRef]
- Stearman, R.; Yuan, D.S.; Yamaguchi-Iwai, Y.; Klausner, R.D.; Dancis, A. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science 1996, 271, 1552–1557. [Google Scholar] [CrossRef]
- Zhou, L.W.; Haas, H.; Marzluf, G.A. Isolation and characterization of a new gene, sre, which encodes a GATA-type regulatory protein that controls iron transport in Neurospora crassa. Mol. Gen. Genet. 1998, 259, 532–540. [Google Scholar] [CrossRef]
- Labbe, S.; Pelletier, B.; Mercier, A. Iron homeostasis in the fission yeast Schizosaccharomyces pombe. Biometals 2007, 20, 523–537. [Google Scholar] [CrossRef]
- Zhang, N.; MohdZainudin, N.A.; Scher, K.; Condon, B.J.; Horwitz, B.A.; Turgeon, B.G. Iron, oxidative stress, and virulence: Roles of iron-sensitive transcription factor Sre1 and the redox sensor ChAp1 in the maize pathogen Cochliobolus heterostrophus. Mol. Plant Microbe Interact. 2013, 26, 1473–1485. [Google Scholar] [CrossRef]
- Hwang, L.H.; Seth, E.; Gilmore, S.A.; Sil, A. SRE1 regulates iron-dependent and -independent pathways in the fungal pathogen Histoplasma capsulatum. Eukaryot. Cell 2012, 11, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Berges, M.S.; Capilla, J.; Turra, D.; Schafferer, L.; Matthijs, S.; Jochl, C.; Cornelis, P.; Guarro, J.; Haas, H.; Di Pietro, A. HapX-mediated iron homeostasis is essential for rhizosphere competence and virulence of the soilborne pathogen Fusarium oxysporum. Plant Cell 2012, 24, 3805–3822. [Google Scholar] [CrossRef] [PubMed]
- Krober, A.; Scherlach, K.; Hortschansky, P.; Shelest, E.; Staib, P.; Kniemeyer, O.; Brakhage, A.A. HapX mediates iron homeostasis in the pathogenic dermatophyte Arthroderma benhamiae but is dispensable for virulence. PLoS ONE 2016, 11, e0150701. [Google Scholar] [CrossRef]
- Chen, C.; Pande, K.; French, S.D.; Tuch, B.B.; Noble, S.M. An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe 2011, 10, 118–135. [Google Scholar] [CrossRef]
- Hissen, A.H.; Wan, A.N.; Warwas, M.L.; Pinto, L.J.; Moore, M.M. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding L-ornithine N5-oxygenase, is required for virulence. Infect. Immun. 2005, 73, 5493–5503. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, X.X.; Geng, Q.; Chi, Z.M. Role of a GATA-type transcriptional repressor Sre1 in regulation of siderophore biosynthesis in the marine-derived Aureobasidium pullulans HN6.2. Biometals 2013, 26, 955–967. [Google Scholar] [CrossRef]
- Poyntner, C.; Blasi, B.; Arcalis, E.; Mirastschijski, U.; Sterflinger, K.; Tafer, H. The transcriptome of Exophiala dermatitidis during ex-vivo skin model infection. Front. Cell. Infect. Microbiol. 2016, 6, 136. [Google Scholar] [CrossRef]
- Heymann, P.; Gerads, M.; Schaller, M.; Dromer, F.; Winkelmann, G.; Ernst, J.F. The siderophore iron transporter of Candida albicans (Sit1p/Arn1p) mediates uptake of ferrichrome-type siderophores and is required for epithelial invasion. Infect. Immun. 2002, 70, 5246–5255. [Google Scholar] [CrossRef] [PubMed]
- Vicente, V.A.; Weiss, V.A.; Bombassaro, A.; Moreno, L.F.; Costa, F.F.; Raittz, R.T.; Leao, A.C.; Gomes, R.R.; Bocca, A.L.; Fornari, G.; et al. Comparative genomics of sibling species of Fonsecaea associated with human chromoblastomycosis. Front. Microbiol. 2017, 8, 1924. [Google Scholar] [CrossRef]
- Eisendle, M.; Oberegger, H.; Zadra, I.; Haas, H. The siderophore system is essential for viability of Aspergillus nidulans: Functional analysis of two genes encoding l-ornithine N 5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC). Mol. Microbiol. 2003, 49, 359–375. [Google Scholar] [CrossRef]
- Matzanke, B.F.; Bill, E.; Trautwein, A.X.; Winkelmann, G. Role of siderophores in iron storage in spores of Neurospora crassa and Aspergillus ochraceus. J. Bacteriol. 1987, 169, 5873–5876. [Google Scholar] [CrossRef] [PubMed]
- Wallner, A.; Blatzer, M.; Schrettl, M.; Sarg, B.; Lindner, H.; Haas, H. Ferricrocin, a siderophore involved in intra- and transcellular iron distribution in Aspergillus fumigatus. Appl. Environ. Microbiol. 2009, 75, 4194–4196. [Google Scholar] [CrossRef] [PubMed]
- Hof, C.; Eisfeld, K.; Welzel, K.; Antelo, L.; Foster, A.J.; Anke, H. Ferricrocin synthesis in Magnaporthe grisea and its role in pathogenicity in rice. Mol. Plant Pathol. 2007, 8, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Happacher, I.; Aguiar, M.; Alilou, M.; Abt, B.; Baltussen, T.J.H.; Decristoforo, C.; Melchers, W.J.G.; Haas, H. The siderophore ferricrocin mediates iron acquisition in Aspergillus fumigatus. Microbiol. Spectr. 2023, 11, e0049623. [Google Scholar] [CrossRef]
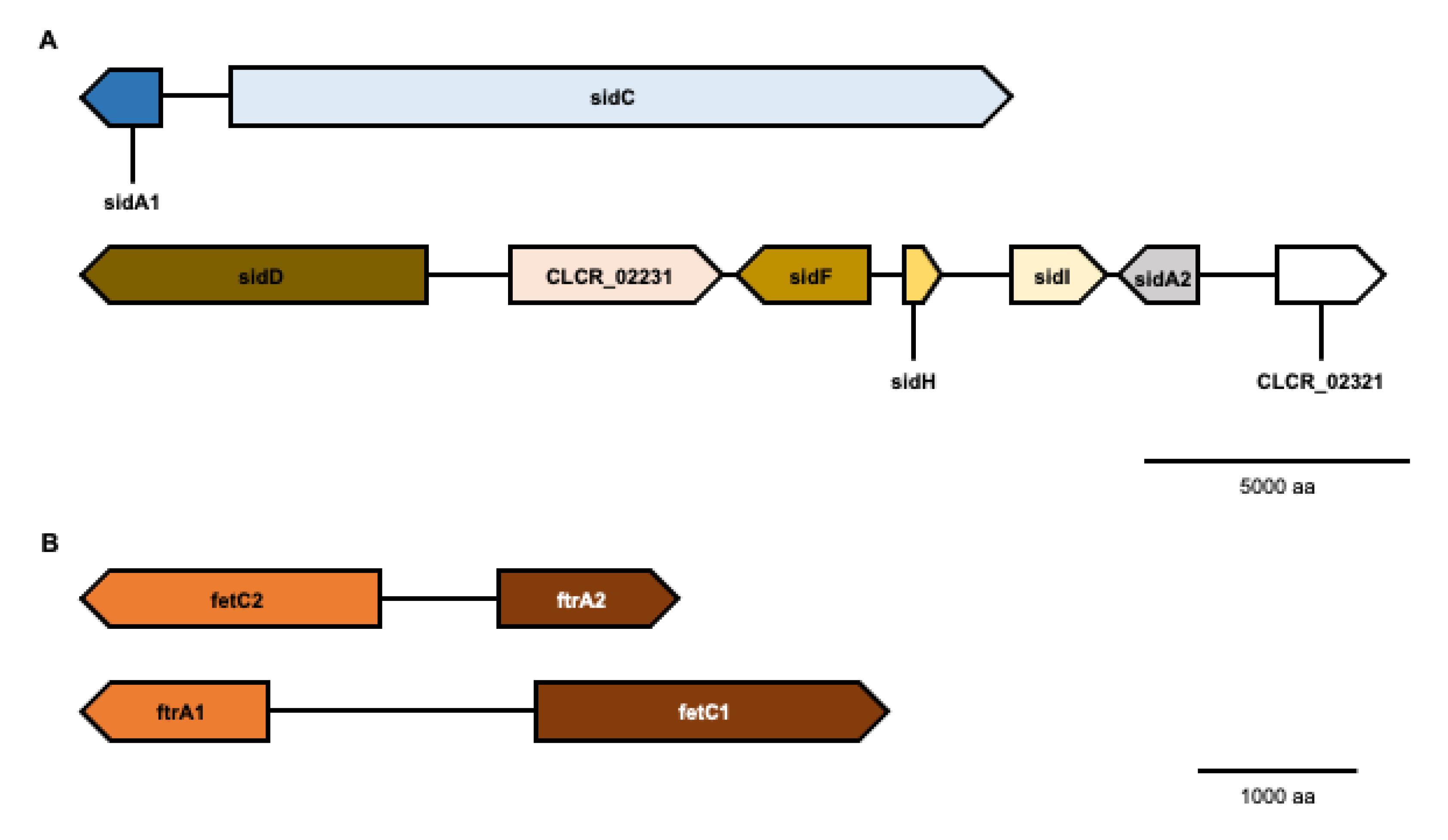

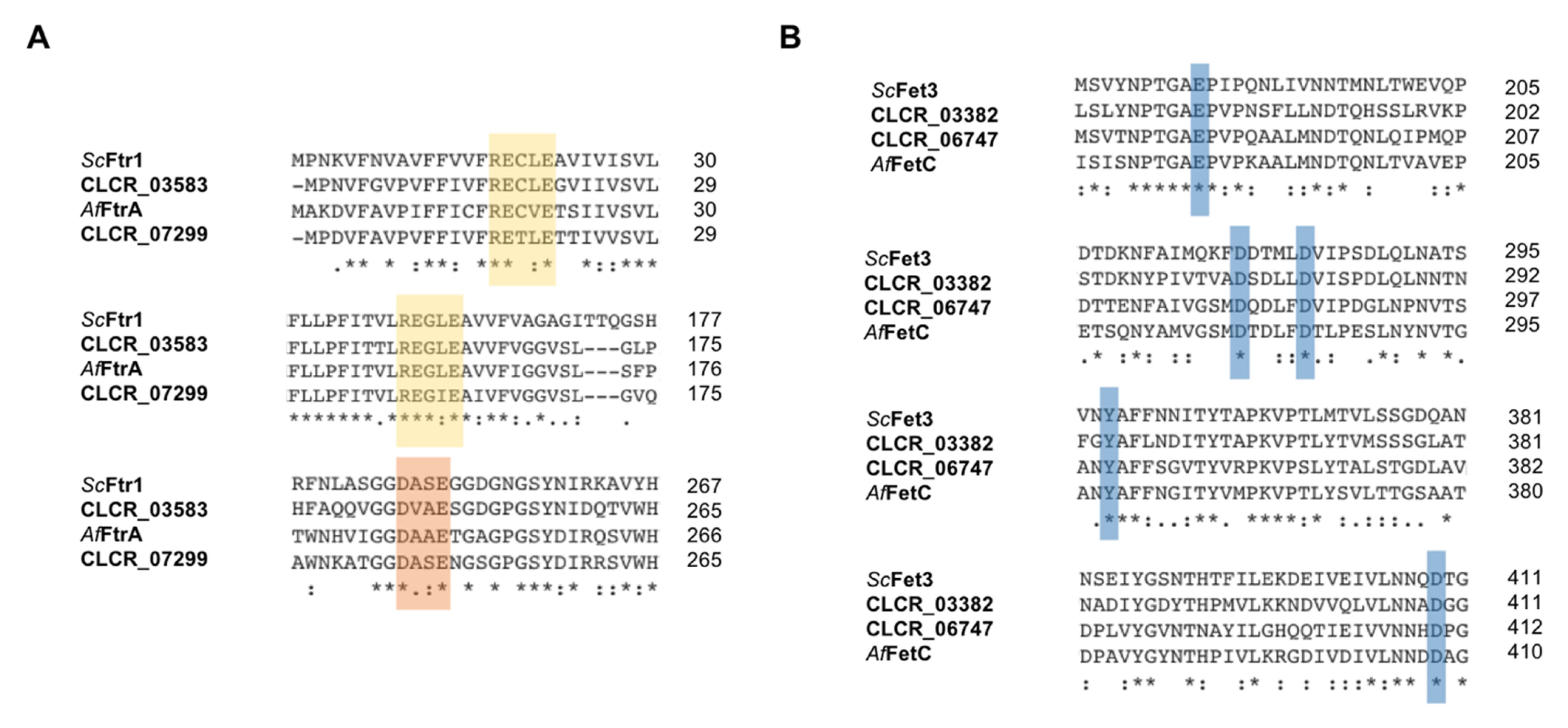
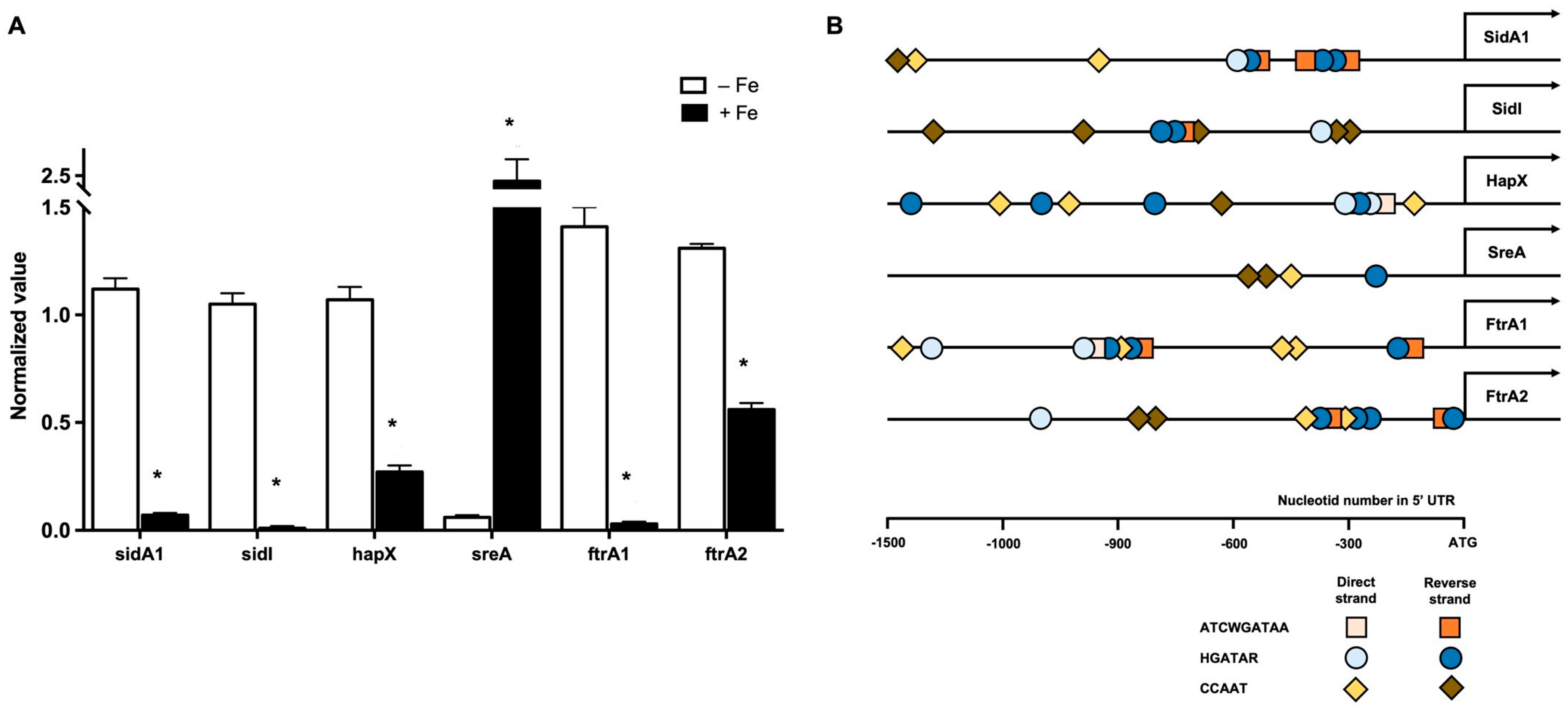
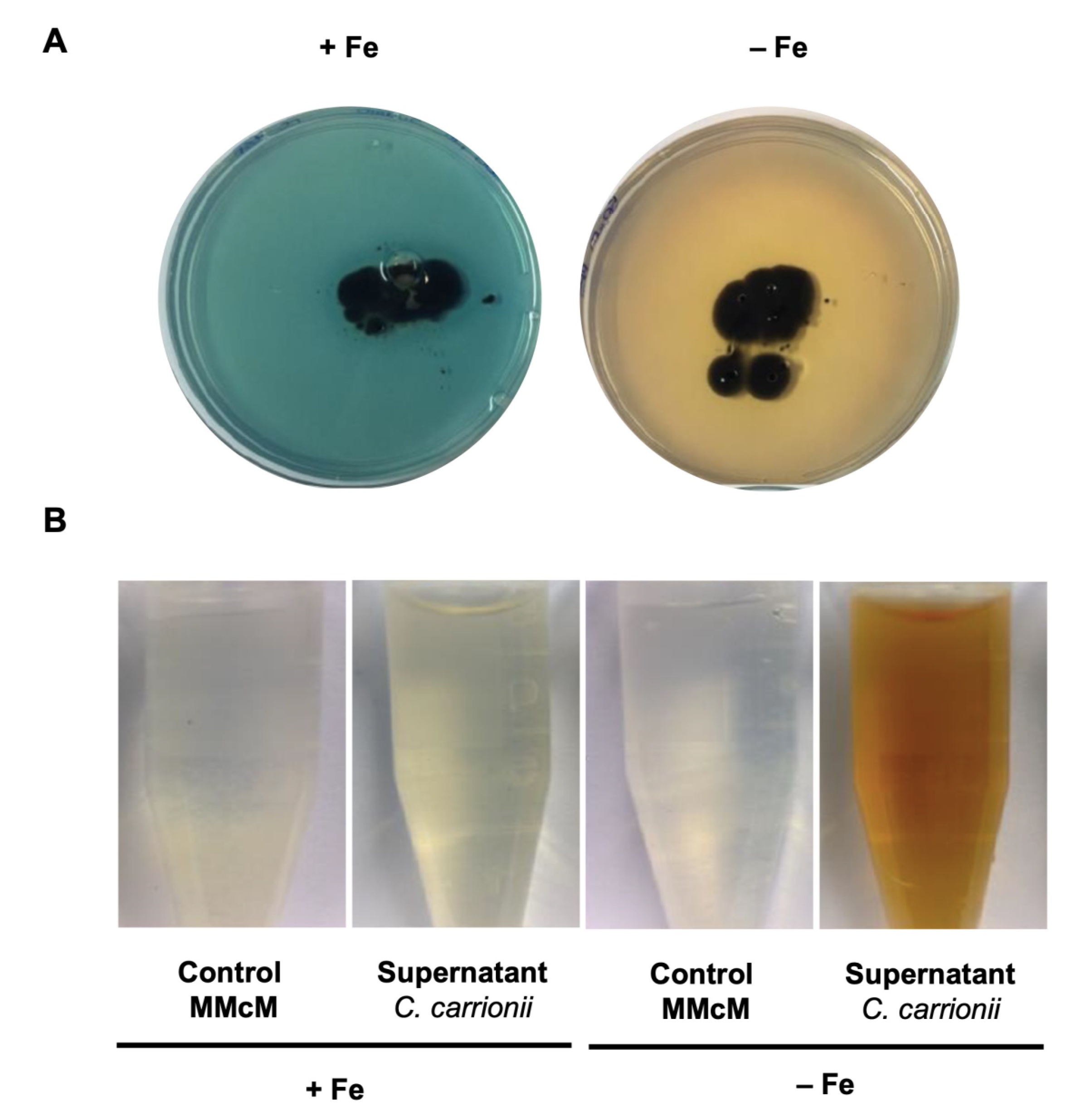
| Iron Related Pathway | Cladophialophora carrionii Genes * |
|---|---|
| Siderophore biosynthesis | CLCR_04832 (SidA1) |
| CLCR_02414 (SidA2) | |
| CLCR_02426 (SidF) | |
| CLCR_04490 (SidC) | |
| CLCR_02213 (SidD) | |
| CLCR_04002 (SidL) | |
| CLCR_02353 (SidI) | |
| CLCR_02613 (SidH) | |
| Siderophore transport | CLCR_02321 (MirD) |
| CLCR_03365 (MirB) | |
| CLCR_06890 (Sit2) | |
| CLCR_03414 (Sit2) | |
| CLCR_01135 (Sit1) | |
| CLCR_04614 | |
| Reductive iron acquisition (RIA) | CLCR_07299 (FtrA1) |
| CLCR_03583 (FtrA2) | |
| CLCR_06747 (FetC1) | |
| CLCR_03382 (FetC2) | |
| Regulation of iron metabolism | CLCR_05818 (SreA) |
| CLCR_01554 (HapX) |
| C. carrionii Sequence ID | Ortholog Group | Orthologs in Fungi * | Signal Peptide | TM Domains | FRD # | FAD-Binding Domain # | NAD-Binding Domain # |
|---|---|---|---|---|---|---|---|
| CLCR_00371 | OG6_114877 | FRE2Sc/CFL1Ca/FRE10Ca/FREBAf | MAGLRYLGPIILVLSTIALA | 7 | ✓ | ✓ | ✓ |
| CLCR_00599 | OG6_114877 | FRE2Sc/CFL1Ca/FRE10Ca/FREBAf | N/A | 7 | ✓ | ✓ | ✓ |
| CLCR_00776 | OG6_114877 | FRE2Sc/CFL1Ca/FRE10Ca/FREBAf | MAYLVAVWTFCLFLARVANA | 7 | ✓ | ✓ | ✓ |
| CLCR_01862 | OG6_137720 | FRE1Sc | N/A | 6 | ✓ | ✓ | ✓ |
| CLCR_04640 | OG6_210498 | - | N/A | 6 | ✓ | ✓ | N/A |
| CLCR_04694 | OG6_100242 | FRE2Cn | N/A | 6 | ✓ | ✓ | ✓ |
| CLCR_06584 | OG6_119573 | FRP1/FRP2Ca | N/A | 7 | ✓ | ✓ | ✓ |
| CLCR_06696 | OG6_114876 | FRE7Sc | N/A | 8 | ✓ | ✓ | ✓ |
| CLCR_06800 | OG6_151519 | - | N/A | 6 | ✓ | ✓ | ✓ |
| CLCR_06849 | OG6_142743 | FRE8Sc | N/A | 7 | ✓ | N/A | ✓ |
| CLCR_09538 | OG6_114877 | FRE2Sc/CFL1Ca/FRE10Ca/FREBAf | MKVLTILLTLSALSSA | 6 | ✓ | ✓ | ✓ |
| CLCR_11337 | OG6_109931 | - | N/A | 5 | ✓ | ✓ | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailão, A.M.; Silva, K.L.P.d.; Moraes, D.; Lechner, B.; Lindner, H.; Haas, H.; Soares, C.M.A.; Silva-Bailão, M.G. Iron Starvation Induces Ferricrocin Production and the Reductive Iron Acquisition System in the Chromoblastomycosis Agent Cladophialophora carrionii. J. Fungi 2023, 9, 727. https://doi.org/10.3390/jof9070727
Bailão AM, Silva KLPd, Moraes D, Lechner B, Lindner H, Haas H, Soares CMA, Silva-Bailão MG. Iron Starvation Induces Ferricrocin Production and the Reductive Iron Acquisition System in the Chromoblastomycosis Agent Cladophialophora carrionii. Journal of Fungi. 2023; 9(7):727. https://doi.org/10.3390/jof9070727
Chicago/Turabian StyleBailão, Alexandre Melo, Kassyo Lobato Potenciano da Silva, Dayane Moraes, Beatrix Lechner, Herbert Lindner, Hubertus Haas, Célia Maria Almeida Soares, and Mirelle Garcia Silva-Bailão. 2023. "Iron Starvation Induces Ferricrocin Production and the Reductive Iron Acquisition System in the Chromoblastomycosis Agent Cladophialophora carrionii" Journal of Fungi 9, no. 7: 727. https://doi.org/10.3390/jof9070727
APA StyleBailão, A. M., Silva, K. L. P. d., Moraes, D., Lechner, B., Lindner, H., Haas, H., Soares, C. M. A., & Silva-Bailão, M. G. (2023). Iron Starvation Induces Ferricrocin Production and the Reductive Iron Acquisition System in the Chromoblastomycosis Agent Cladophialophora carrionii. Journal of Fungi, 9(7), 727. https://doi.org/10.3390/jof9070727





