Succession of Fungal Community during Outdoor Deterioration of Round Bamboo
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Bamboo Sampling
2.2. Fungal Community Analysis
2.2.1. DNA Extraction and Sequencing
2.2.2. Sequence Data Processing
2.2.3. Statistical Analysis
2.3. Characteristics of Bamboo
2.3.1. Chemical Composition Determination
2.3.2. XPS Analysis
2.3.3. SEM Observation
2.3.4. Contact Angle Measurements
3. Results and Discussion
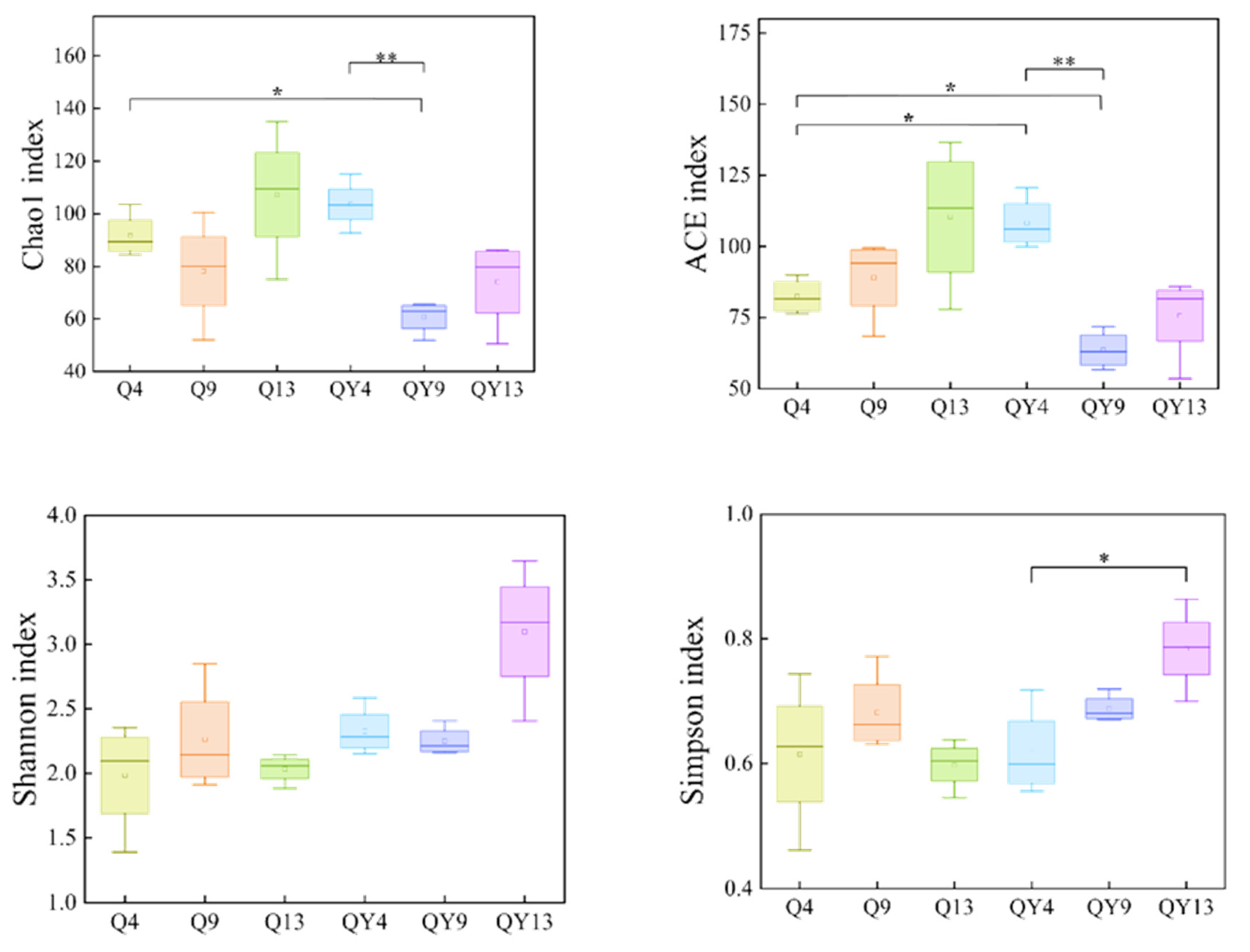
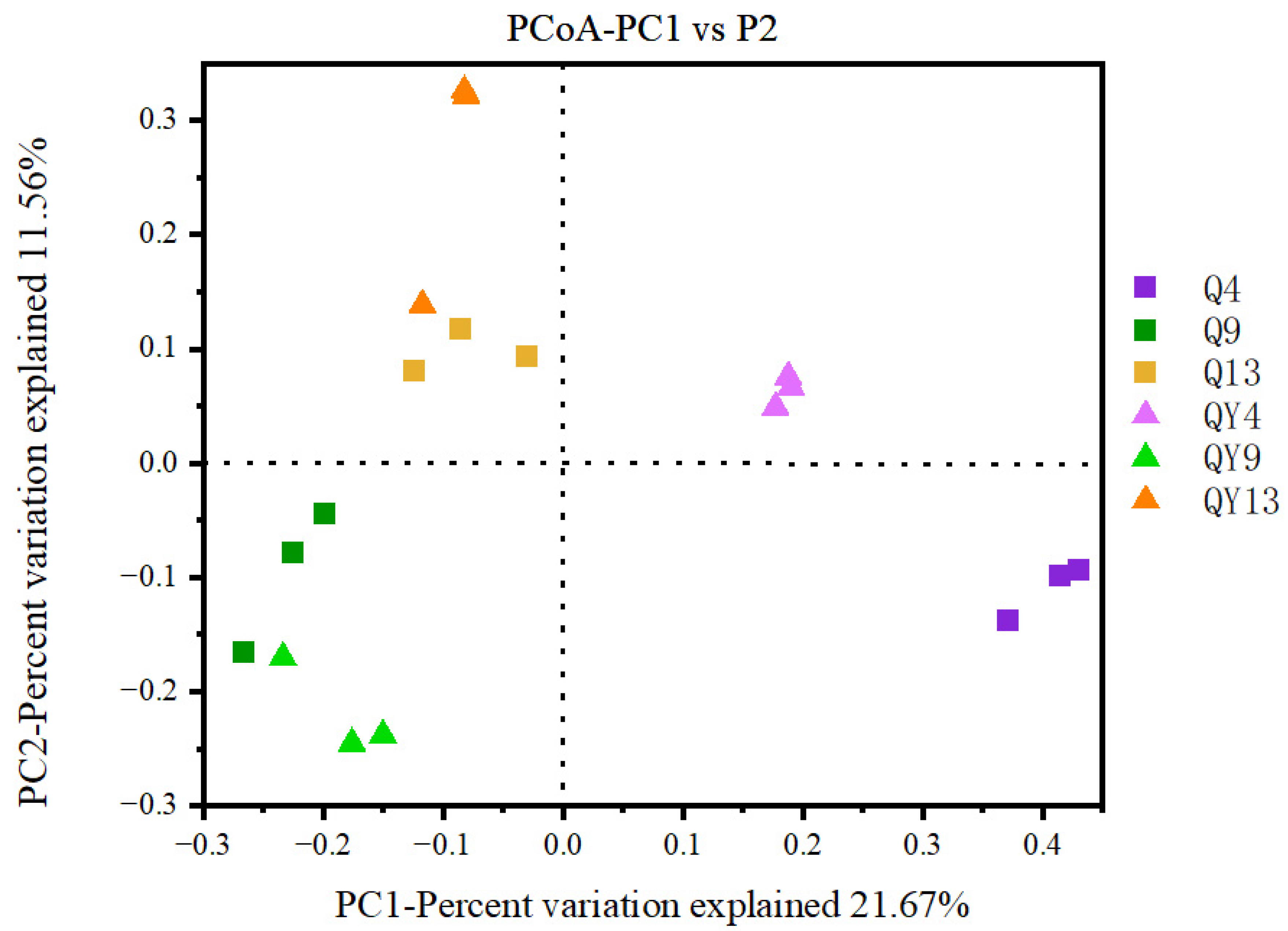
3.1. Richness and Diversity Assessment
3.1.1. Alpha Diversity Analysis
3.1.2. Beta Diversity Analysis
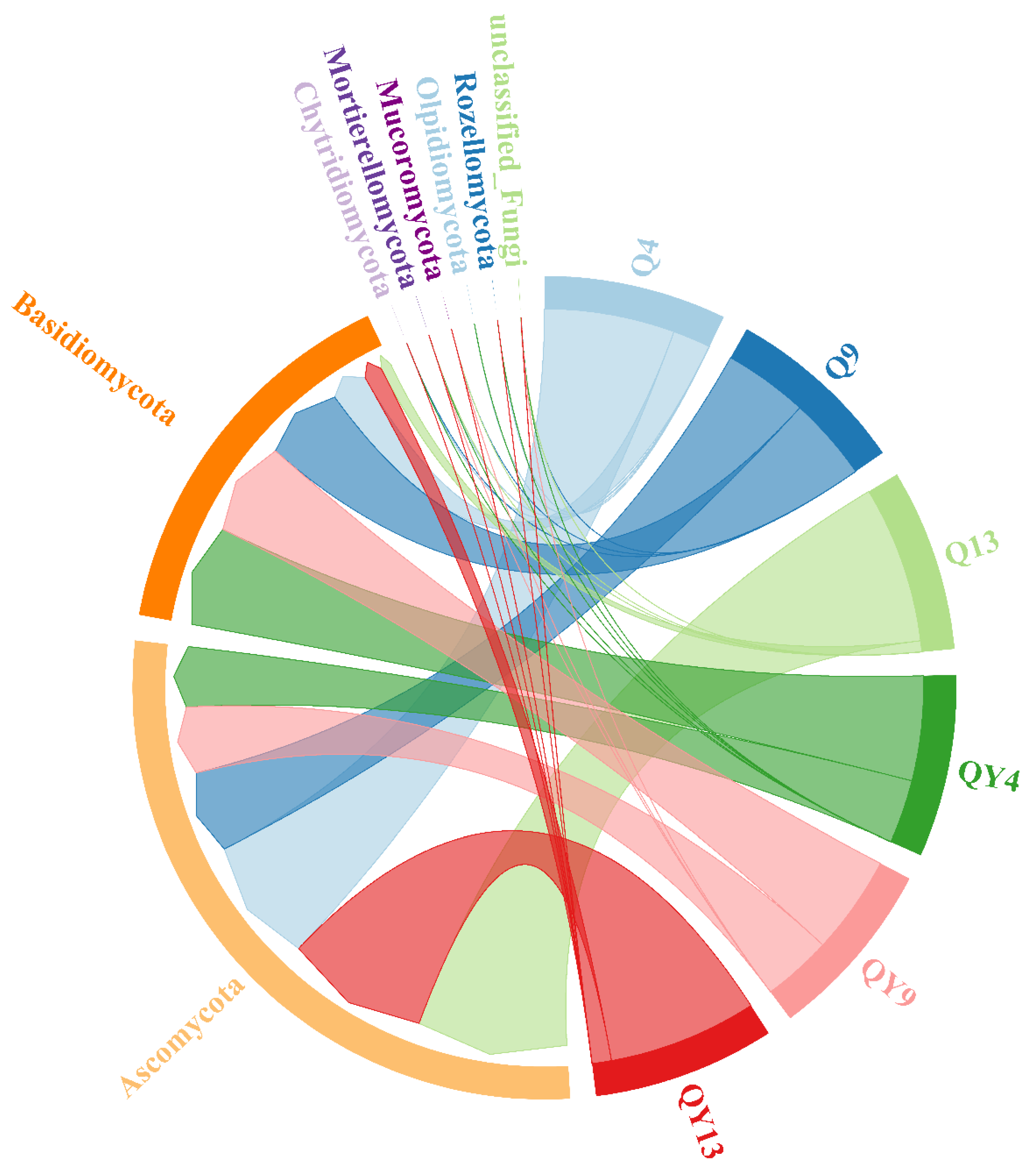
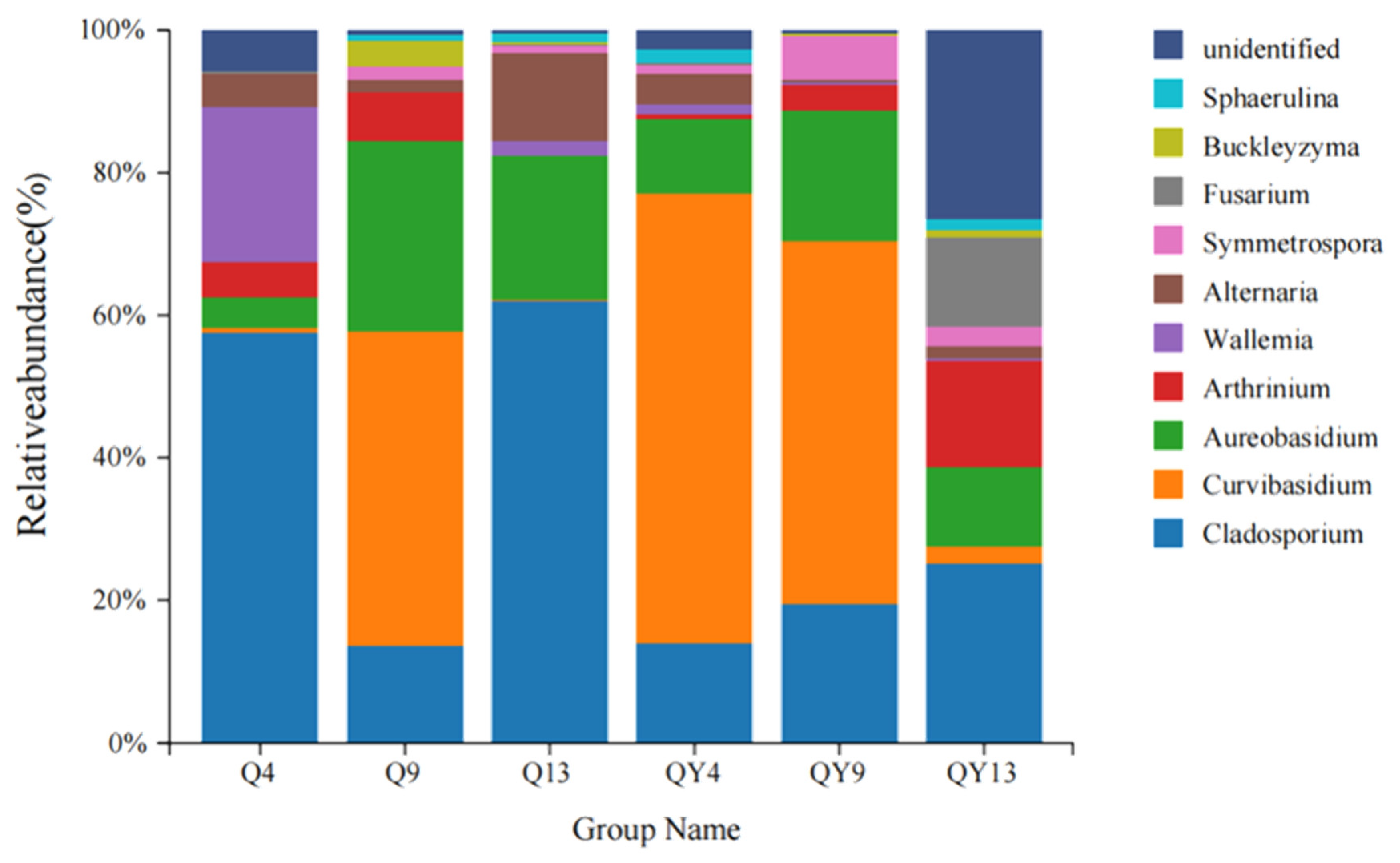
3.2. Microbial Community Composition
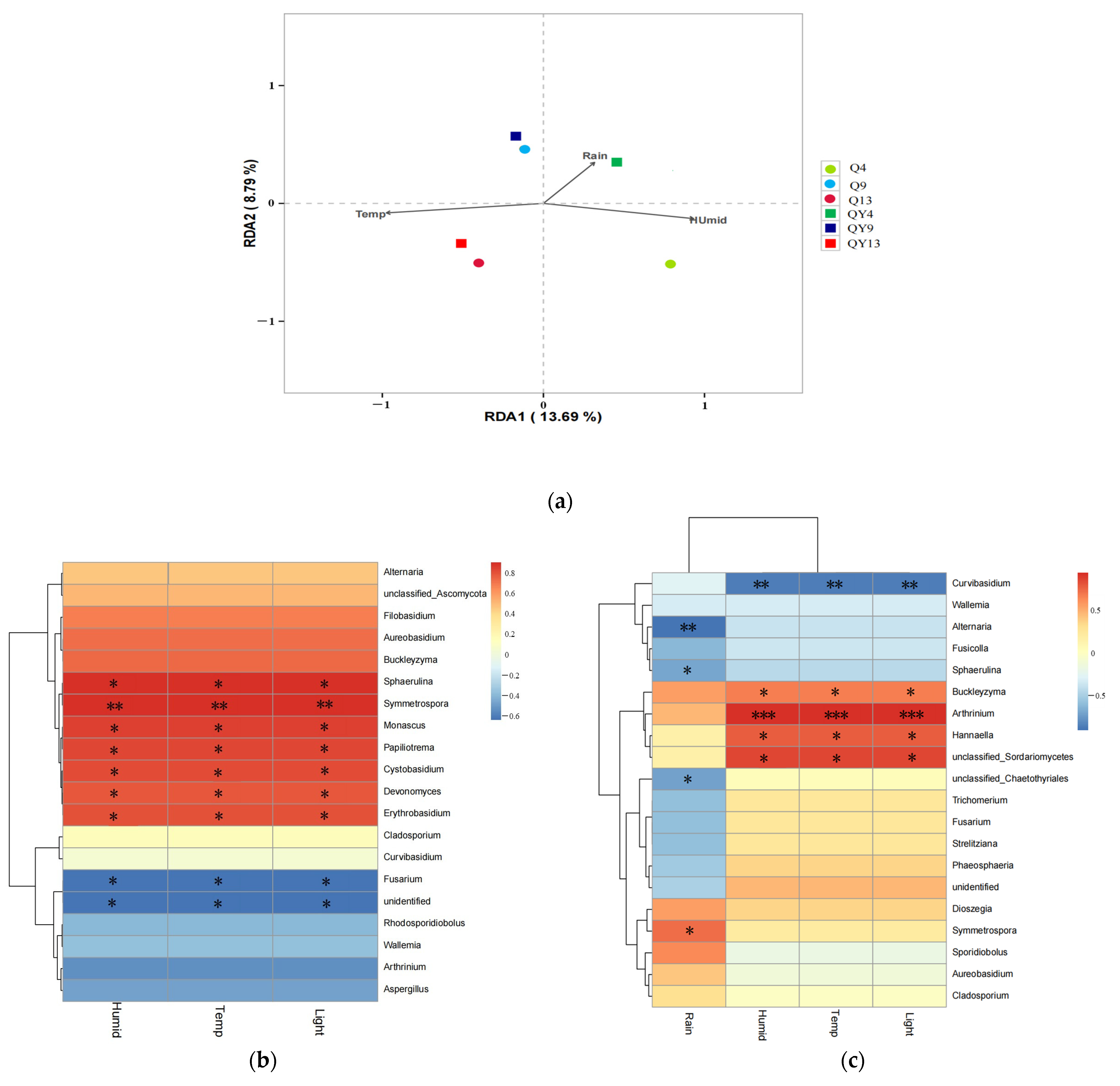
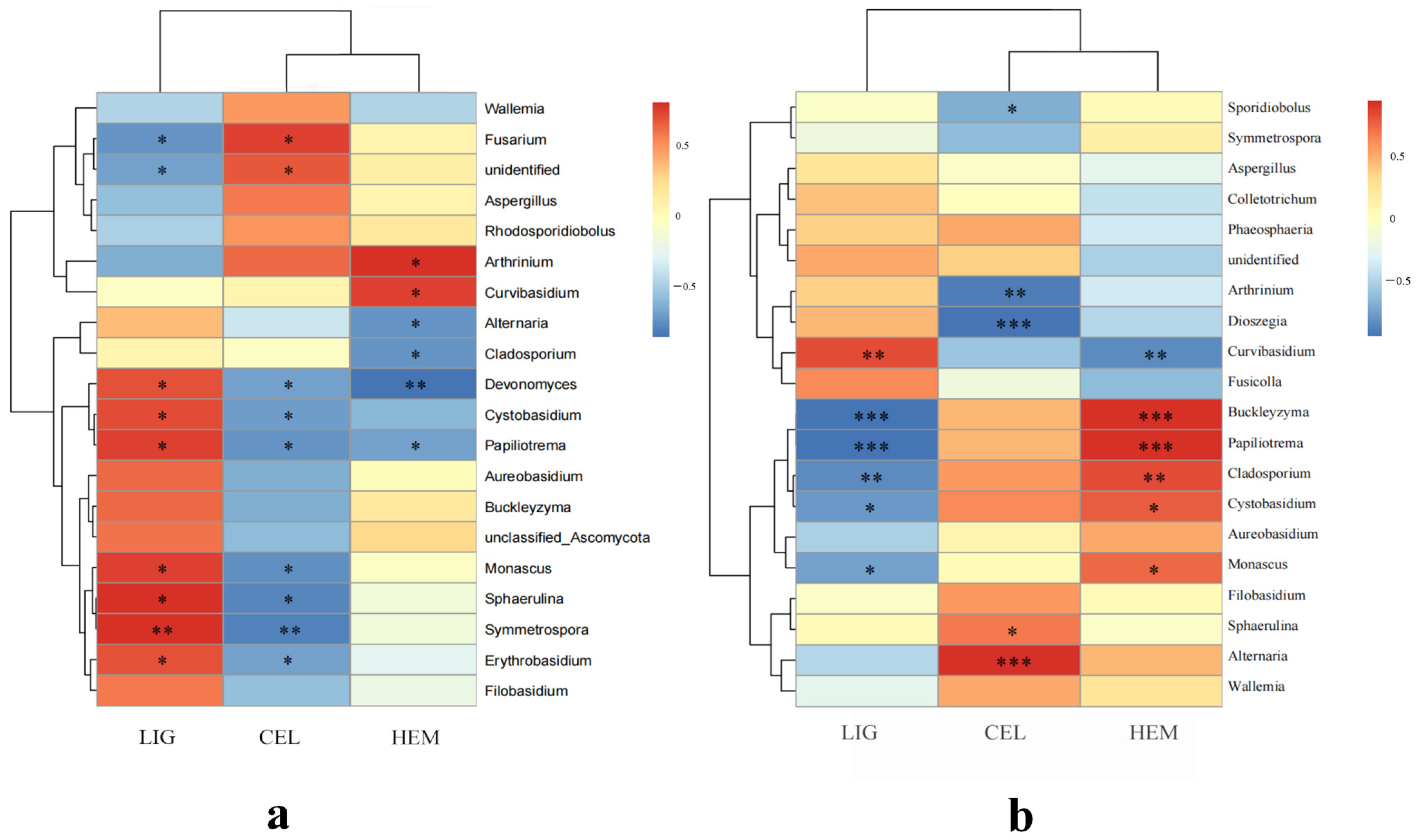
3.3. The Correlation between Microbial Community Structures and Environmental Factors
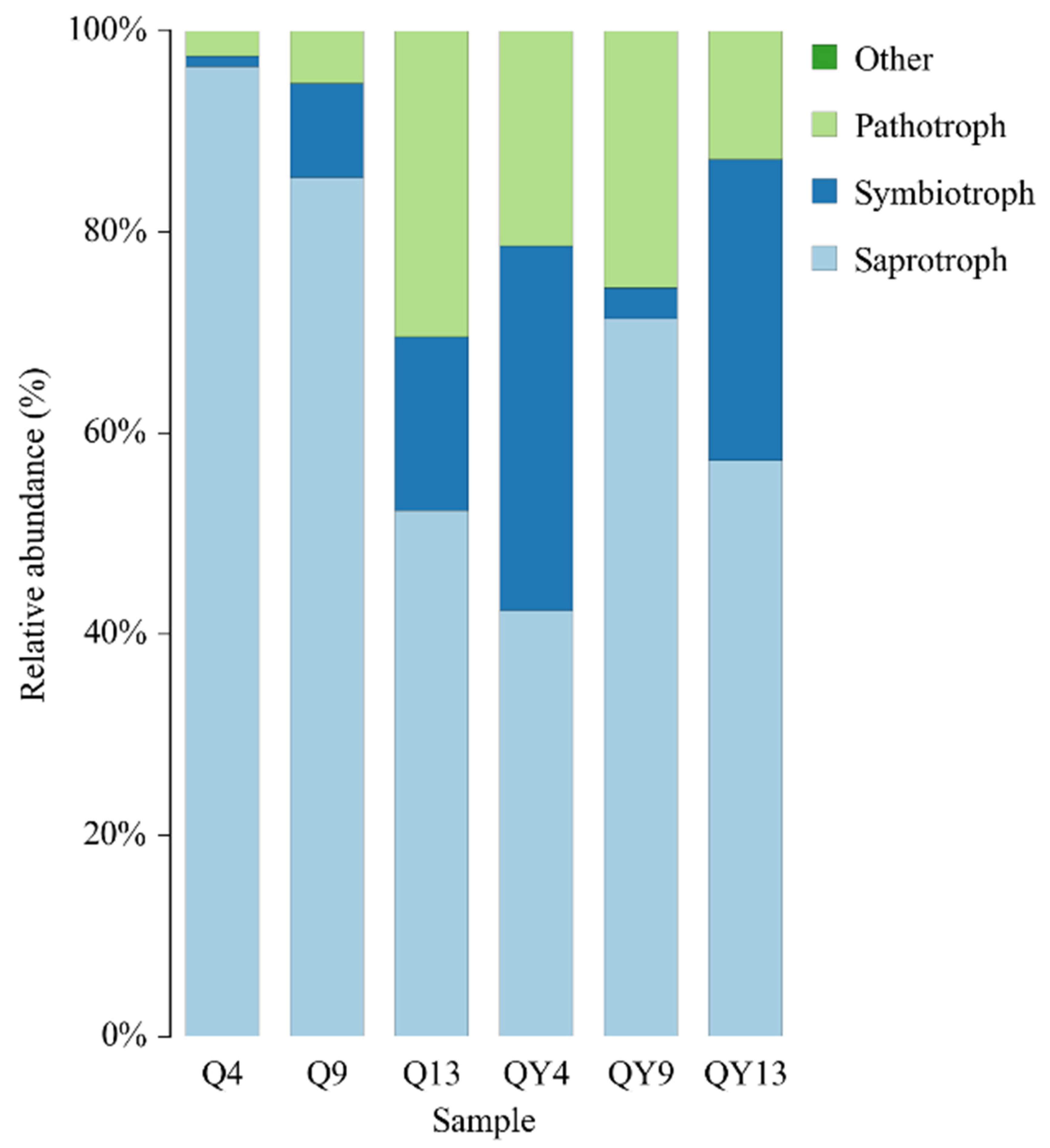
3.4. Function of Fungi
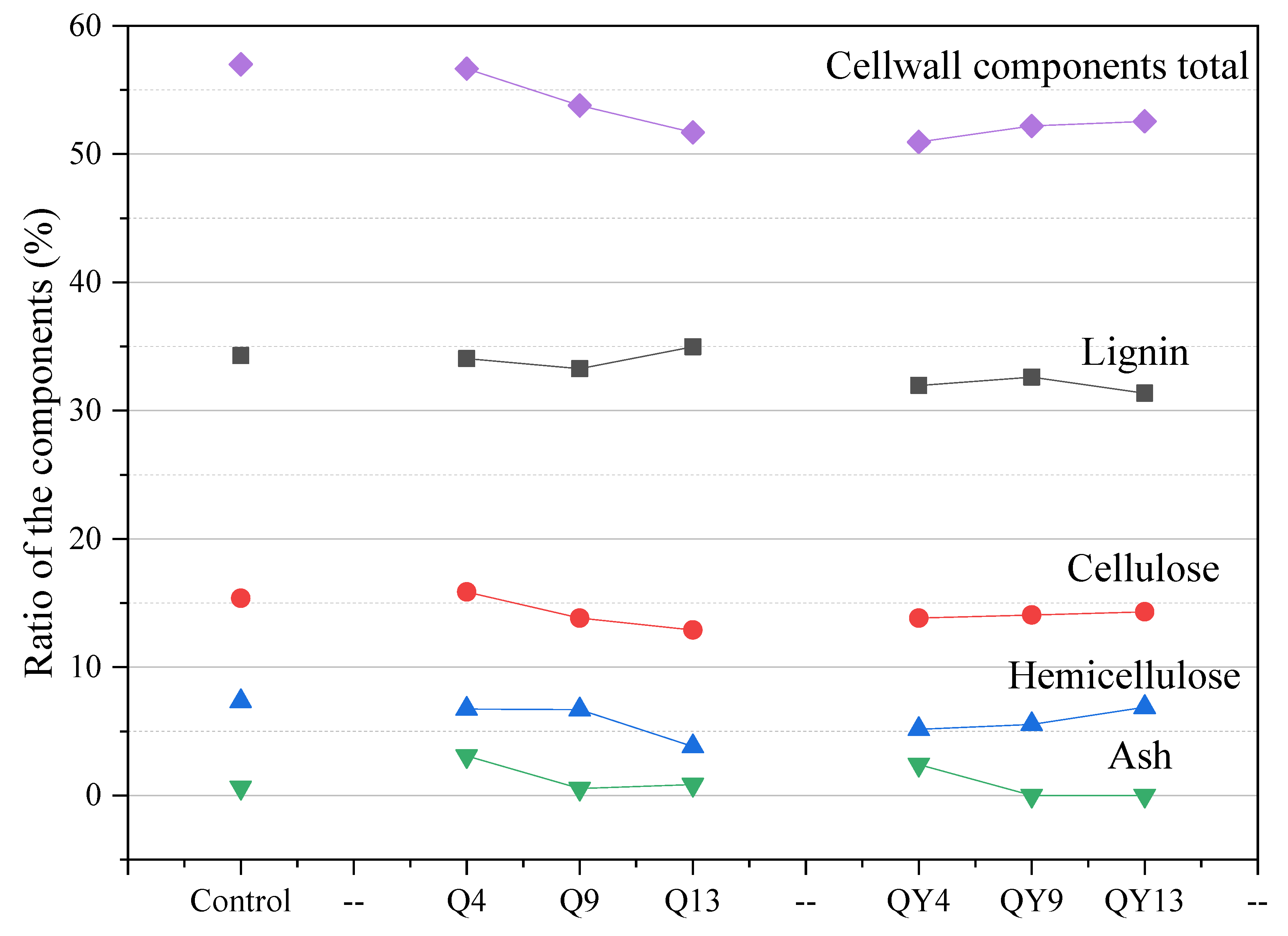
3.5. Chemical Composition Determination
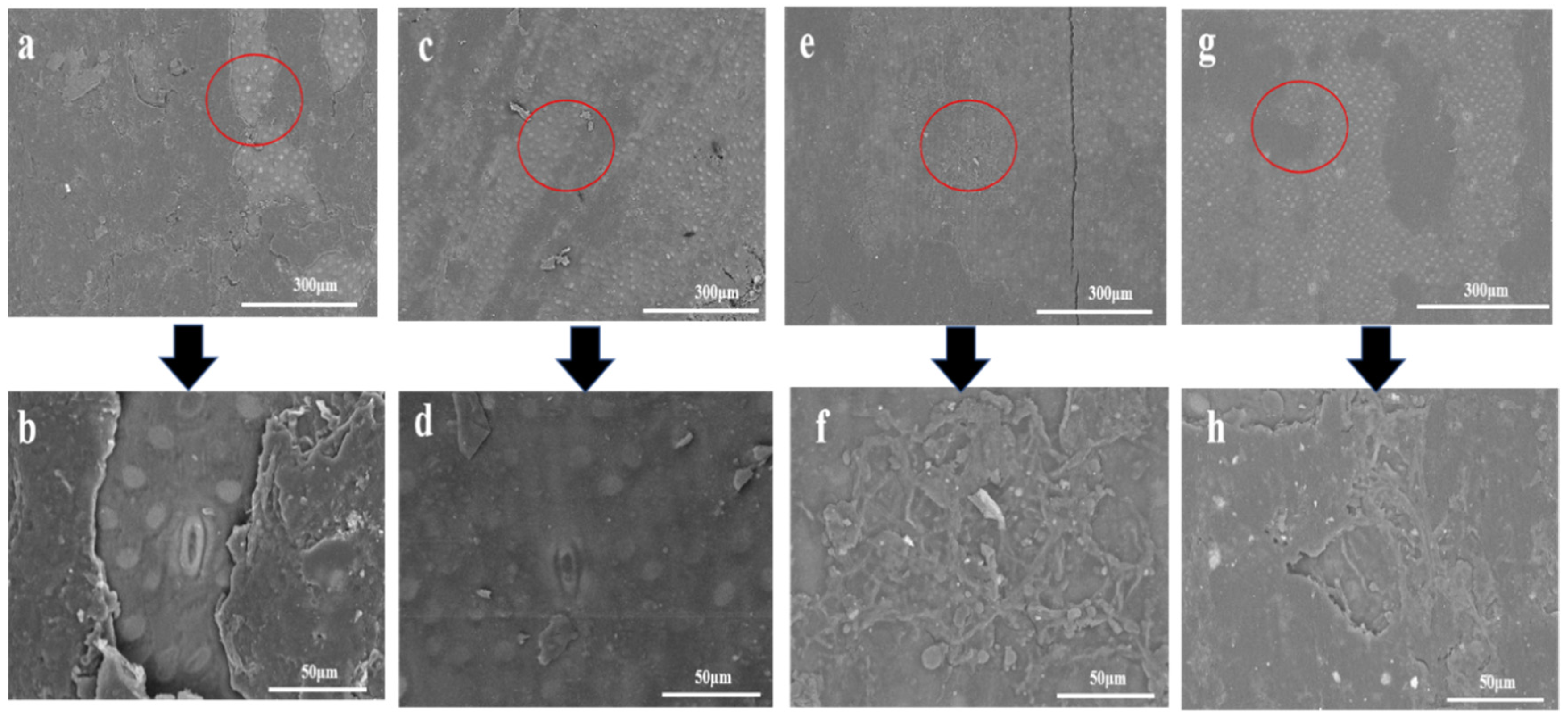
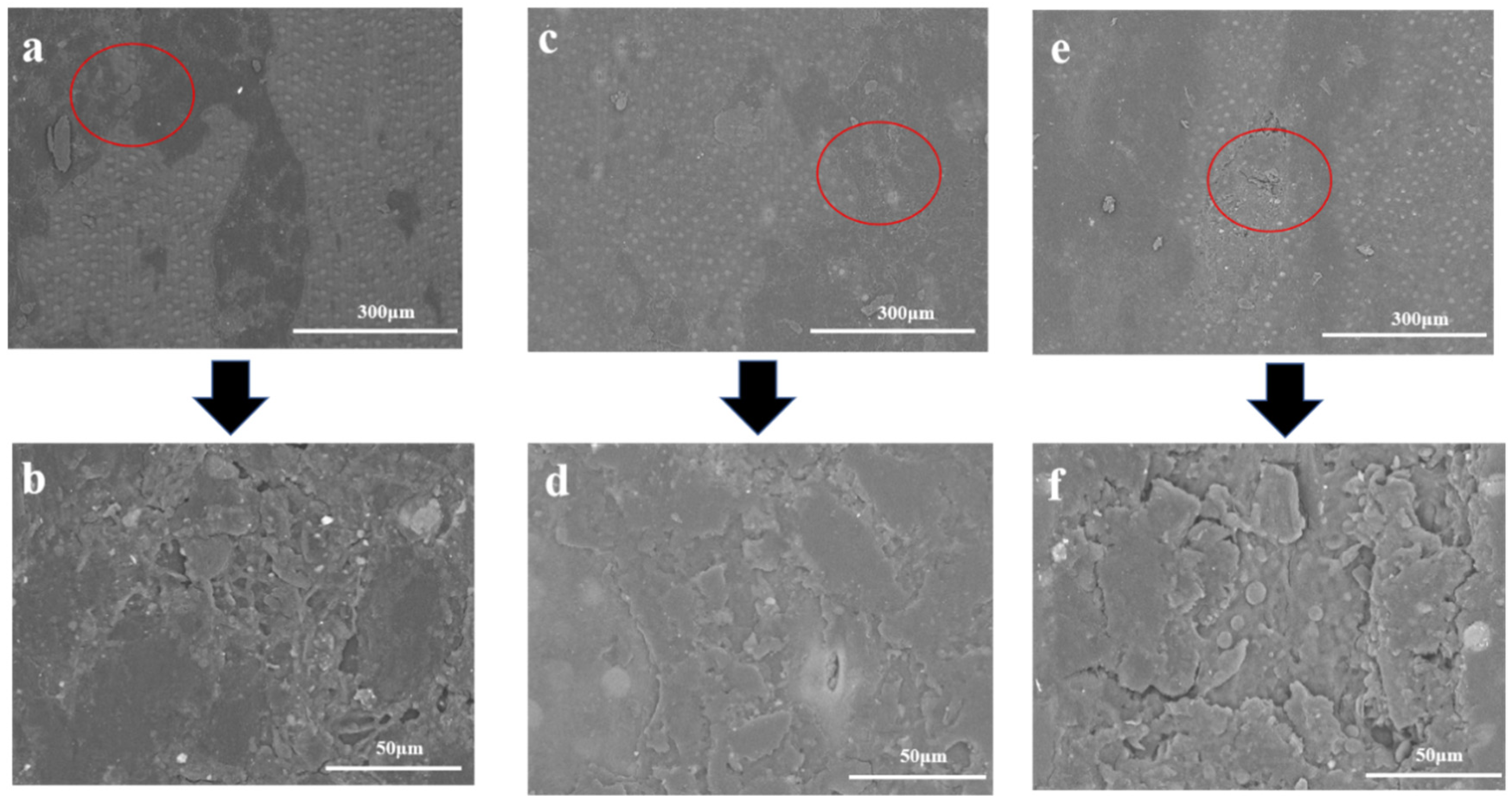
3.6. SEM Analysis
3.7. Contact Angle
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stephenson, J.; Newman, K.; Mayhew, S. Population dynamics and climate change: What are the links? J. Public Health 2010, 32, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Gajić, D.; Peulić, S.; Mavrič, S.; Sandak, A.; Tavzes, C.; Malešević, M.; Slijepčević, M. Energy Retrofitting Opportunities Using Renewable. Sustainability 2021, 13, 603. [Google Scholar] [CrossRef]
- Qi, S.; Song, B.; Liu, C.; Gong, P.; Luo, J.; Zhang, M.; Xiong, T. Bamboo Forest Mapping in China Using the Dense Landsat 8 Image Archive and Google Earth Engine. Remote Sens. 2022, 14, 762. [Google Scholar] [CrossRef]
- Liu, M.; Li, W.; Guo, F.; Wang, H.; Zhang, X.; Yu, Y. Dimensionally stable and highly durable bamboo material prepared through a simple surface furfurylation. Constr. Build. Mater. 2021, 276, 122156. [Google Scholar] [CrossRef]
- Li, Y.; Feng, P. Bamboo resources in china based on the ninth national forest inventory data. World Bamboo Ratt. 2019, 17, 45–48. [Google Scholar]
- Wang, X.-Q.; Ren, H.-Q. Surface deterioration of moso bamboo (Phyllostachys pubescens) induced by exposure to artificial sunlight. Jpn. Wood Res. Soc. 2008, 55, 47–52. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Yang, X.; Ji, H.; Zhong, T.; Wang, G. A comparative study of the microstructure and water permeability between flattened bamboo and bamboo culm. J. Wood Sci. 2019, 65, 64. [Google Scholar] [CrossRef]
- Lou, Z.; Han, X.; Liu, J.; Ma, Q.; Yan, H.; Yuan, C.; Yang, L.; Han, H.; Weng, F.; Li, Y. Nano-Fe3O4/bamboo bundles/phenolic resin oriented recombination ternary composite with enhanced multiple functions. Compos. Part B Eng. 2021, 226, 109335. [Google Scholar] [CrossRef]
- Lv, H.; Chen, X.; Liu, X.; Fang, C.; Liu, H.; Zhang, B.; Fei, B. The vacuum-assisted microwave drying of round bamboos: Drying kinetics, color and mechanical property. Mater. Lett. 2018, 223, 159–162. [Google Scholar] [CrossRef]
- Su, N.; Fang, C.; Yu, Z.; Zhou, H.; Wang, X.; Tang, T.; Zhang, S.; Fei, B. Effects of rosin treatment on hygroscopicity, dimensional stability, and pore structure of round bamboo culm. Constr. Build. Mater. 2021, 287, 123037. [Google Scholar] [CrossRef]
- Su, N.; Zhou, H.; Tang, T.; Zhang, S.; Yu, Z.; Fang, C.; Fei, B. Penetration and distribution of rosin along the longitudinal axis of round bamboo culm. Constr. Build. Mater. 2021, 305, 124781. [Google Scholar] [CrossRef]
- Rao, J.; Jiang, J.; Prosper, N.K.; Yang, X.; Liu, T.; Cai, W.; Wang, H.; Sun, F. Combination of polyethylene glycol impregnation and paraffin heat treatment to protect round bamboo from cracking. R. Soc. Open Sci. 2019, 6, 190105. [Google Scholar] [CrossRef]
- Mild, R.M.G. Protection of Moso bamboo (Phyllostachys pubescens) materials against fungal decay and discolouration by treatment with wood preservatives. Eur. J. Wood Wood Prod. 2019, 77, 139–145. [Google Scholar]
- Xiao, Y.; Li, Z.; Liu, K.W. (Eds.) Modern Engineered Bamboo Structures. In Proceedings of the Third International Conference on Modern Bamboo Structures (ICBS 2018), Beijing, China, 25–27 June 2018; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Gent, J.F.; Ren, P.; Belanger, K.; Triche, E.; Bracken, M.B.; Holford, T.R.; Leaderer, B.P. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ. Health Perspect. 2002, 110, A781–A786. [Google Scholar] [CrossRef]
- Pestka, J.J.; Yike, I.; Dearborn, D.G.; Ward, M.D.; Harkema, J.R. Stachybotrys chartarum, trichothecene mycotoxins, and damp building–related Illness: New insights into a public health enigma. Toxicol. Sci. 2008, 104, 4–26. [Google Scholar] [CrossRef]
- Stokland, J.N.; Siitonen, J.; Jonsson, B. Biodiversity in Dead Wood; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Wei, D. Bamboo Inhabiting Fungi and Their Damage to the Substrate; Staats-und Universitätsbibliothek Hamburg Carl von Ossietzky: Hamburg, Germany, 2014. [Google Scholar]
- Kim, J.-J.; Lee, S.-S.; Ra, J.-B.; Lee, H.; Huh, N.; Kim, G.-H. Fungi associated with bamboo and their decay capabilities. Holzforschung. 2011, 65, 271–275. [Google Scholar] [CrossRef]
- Kjøller, A.H.; Struwe, S. Fungal communities, succession, enzymes, and decomposition. Enzym. Environ. Act. Ecol. Appl. 2002, 1, 267–284. [Google Scholar]
- Moitinho, M.A.; Chiaramonte, J.B.; Bononi, L.; Gumiere, T.; Melo, I.S.; Taketani, R.G. Fungal succession on the decomposition of three plant species from a Brazilian mangrove. Sci. Rep. 2022, 12, 14547. [Google Scholar] [CrossRef]
- Roy-Bolduc, A.; Laliberté, E.; Boudreau, S.; Hijri, M. Strong linkage between plant and soil fungal communities along a successional coastal dune system. FEMS Microbiol. Ecol. 2016, 92, 156. [Google Scholar] [CrossRef]
- Maitra, P.; Zheng, Y.; Wang, Y.-L.; Mandal, D.; Lü, P.-P.; Gao, C.; Babalola, B.J.; Ji, N.-N.; Li, X.-C.; Guo, L.-D. Phosphorus fertilization rather than nitrogen fertilization, growing season and plant successional stage structures arbuscular mycorrhizal fungal community in a subtropical forest. Biol. Fertil. Soils 2021, 57, 685–697. [Google Scholar] [CrossRef]
- Song, Z.; Liu, G.; Zhang, C. Response of rhizosphere microbial communities to plant succession along a grassland chronosequence in a semiarid area. J. Soils Sediments 2019, 19, 2496–2508. [Google Scholar] [CrossRef]
- Latz, M.A.C.; Kerrn, M.H.; Sørensen, H.; Collinge, D.B.; Jensen, B.; Brown, J.K.M.; Madsen, A.M.; Jørgensen, H.J.L. Succession of the fungal endophytic microbiome of wheat is dependent on tissue-specific interactions between host genotype and environment. Sci. Total Environ. 2021, 759, 143804. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Hyde, K. Fungal Succession on Bamboo in Hong Kong. Fungal Divers. 2002, 10, 213–227. [Google Scholar]
- Nakasaki, K.; Hirai, H.; Mimoto, H.; Quyen, T.N.M.; Koyama, M.; Takeda, K. Succession of microbial community during vigorous organic matter degradation in the primary fermentation stage of food waste composting. Sci. Total Environ. 2019, 671, 1237–1244. [Google Scholar] [CrossRef]
- Zhu, L.; Li, T.; Xu, X.; Shi, X.; Wang, B. Succession of Fungal Communities at Different Developmental Stages of Cabernet Sauvignon Grapes from an Organic Vineyard in Xinjiang. Front. Microbiol. 2021, 12, 718261. [Google Scholar] [CrossRef]
- Wen, R.; Li, X.-a.; Han, G.; Chen, Q.; Kong, B. Fungal community succession and volatile compound dynamics in Harbin dry sausage during fermentation. Food Microbiol. 2021, 99, 103764. [Google Scholar] [CrossRef]
- Xie, G.; Kong, X.; Kang, J.; Su, N.; Fei, J.; Luo, G. Fungal community succession contributes to product maturity during the co-composting of chicken manure and crop residues. Bioresour. Technol. 2021, 328, 124845. [Google Scholar] [CrossRef]
- Douterelo, I.; Fish, K.; Boxall, J. Succession of bacterial and fungal communities within biofilms of a chlorinated drinking water distribution system. Water Res. 2018, 141, 74–85. [Google Scholar] [CrossRef]
- Matsuoka, S.; Suzuki, Y.; Hobara, S.; Osono, T. Fungal succession and decomposition of composted aquatic plants applied to soil. Fungal Ecol. 2018, 35, 34–41. [Google Scholar] [CrossRef]
- Dossa, G.G.O.; Yang, Y.-Q.; Hu, W.; Paudel, E.; Schaefer, D.; Yang, Y.-P.; Cao, K.-F.; Xu, J.-C.; Bushley, K.E.; Harrison, R.D. Fungal succession in decomposing woody debris across a tropical forest disturbance gradient. Soil Biol. Biochem. 2021, 155, 108142. [Google Scholar] [CrossRef]
- Yihui, D.; Chan, J.C.L. The East Asian summer monsoon: An overview. Meteorol. Atmos. Phys. 2005, 89, 117–142. [Google Scholar] [CrossRef]
- Wang, X.; Dong, X.; Deng, Y.; Cui, C.; Wan, R.; Cui, W. Contrasting pre-Mei-Yu and Mei-Yu extreme precipitation in the Yangtze River valley: Influencing systems and precipitation mechanisms. J. Hydrometeorol. 2019, 20, 1961–1980. [Google Scholar] [CrossRef]
- GB/T 27651-2011; Use Category and Specification for Preservative-Treated Wood. GB/T, Chinese National Standard. Chinese Standard Publishing House: Beijing, China, 2011. (In Chinese)
- Omasa, T. Gene Amplification and Its Application in Cell and Tissue Engineering. J. Biosci. Bioeng. 2002, 94, 600–605. [Google Scholar] [CrossRef]
- Clarke, L.J.; Czechowski, P.; Soubrier, J.; Stevens, M.I.; Cooper, A. Modular tagging of amplicons using a single PCR for high-throughput sequencing. Mol. Ecol. Resour. 2014, 14, 117–121. [Google Scholar] [CrossRef]
- Kong, N.; Ng, W.; Thao, K.; Agulto, R.; Weis, A.; Kim, K.S.; Korlach, J.; Hickey, L.; Kelly, L.; Lappin, S. Automation of PacBio SMRTbell NGS library preparation for bacterial genome sequencing. Stand. Genom. Sci. 2017, 12, 27. [Google Scholar] [CrossRef]
- Shokralla, S.; Porter, T.M.; Gibson, J.F.; Dobosz, R.; Janzen, D.H.; Hallwachs, W.; Golding, G.B.; Hajibabaei, M. Massively parallel multiplex DNA sequencing for specimen identification using an Illumina MiSeq platform. Sci. Rep. 2015, 5, 9687. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE database for molecular identification of fungi—Recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef]
- Rai, S.N.; Qian, C.; Pan, J.; Rai, J.P.; Song, M.; Bagaitkar, J.; Merchant, M.; Cave, M.; Egilmez, N.K.; McClain, C.J. McClain. Microbiome data analysis with applications to pre-clinical studies using QIIME2: Statistical considerations. Genes Dis. 2021, 8, 215–223. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of extractives in biomass laboratory analytical procedure. National Renewable Energy Laboratory. Natl. Renew. Energy Lab. 2005, 17, 3–8. [Google Scholar]
- Bouskill, N.J.; Lim, H.C.; Borglin, S.; Salve, R.; Wood, T.E.; Silver, W.L.; Brodie, E.L. Pre-exposure to drought increases the resistance of tropical forest soil bacterial communities to extended drought. ISME J. 2013, 7, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.J.; Satya, S.; Pant, K.K.; Naik, S.N. Eco-friendly preservation of bamboo species: Traditional to modern techniques. Bioresources 2016, 11, 10604–10624. [Google Scholar] [CrossRef]
- Niu, M.; Hu, W.; Cheng, B.; Wu, L.; Ren, L.; Deng, J.; Shen, F.; Fu, P. Influence of rainfall on fungal aerobiota in the urban atmosphere over Tianjin, China: A case study. Atmos. Environ. X 2021, 12, 100137. [Google Scholar] [CrossRef]
- Heo, K.J.; Kim, H.B.; Lee, B.U. Concentration of environmental fungal and bacterial bioaerosols during the monsoon season. J. Aerosol. Sci. 2014, 77, 31–37. [Google Scholar] [CrossRef]
- Itani, G.N.; Smith, C. Dust rains deliver diverse assemblages of microorganisms to the Eastern Mediterranean. Sci. Rep. 2016, 6, 22657. [Google Scholar] [CrossRef]
- Creamean, J.M.; Suski, K.J.; Rosenfeld, D.; Cazorla, A.; DeMott, P.J.; Sullivan, R.C.; White, A.B.; Ralph, F.M.; Minnis, P.; Comstock, J.M.; et al. Dust and biological aerosols from the Sahara and Asia influence precipitation in the western US. Science 2013, 339, 1572–1578. [Google Scholar] [CrossRef]
- Zhao, Q.; Shen, W.; Chen, Q.; Helmisaari, H.-S.; Sun, Q.; Jian, S. Spring drying and intensified summer rainfall affected soil microbial community composition but not enzyme activity in a subtropical forest. Appl. Soil. Ecol. 2018, 130, 219–225. [Google Scholar] [CrossRef]
- Christiansen, C.T.; Haugwitz, M.S.; Priemé, A.; Nielsen, C.S.; Elberling, B.O.; Michelsen, A.; Grogan, P.; Blok, D. Enhanced summer warming reduces fungal decomposer diversity and litter mass loss more strongly in dry than in wet tundra. Glob. Chang. Biol. 2017, 23, 406–420. [Google Scholar] [CrossRef]
- Baldrian, P.; Valášková, V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef]
- Tudor, D.; Robinson, S.C.; Cooper, P.A. The influence of pH on pigment formation by Lignicolous fungi. Int. Biodeterior. Biodegrad. 2013, 80, 22–28. [Google Scholar] [CrossRef]
- James, T.Y.; Kauff, F.; Schoch, C.L.; Matheny, P.B.; Hofstetter, V.; Cox, C.J.; Celio, G.; Gueidan, C.; Fraker, E.; Miadlikowska, J.; et al. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 2006, 443, 818–822. [Google Scholar] [CrossRef]
- Blaalid, R.; Khomich, M. Current knowledge of Chytridiomycota diversity in Northern Europe and future research needs. Fungal Biol. Rev. 2021, 36, 42–51. [Google Scholar] [CrossRef]
- Guo, H.N.; Huang, Z.J.; Li, M.Q.; Min, W. Response of soil fungal community structure and diversity to saline water irrigation in alluiall grey desert suils. Appl. Ecol. Environ. Res. 2020, 18, 4969–4985. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, N.-F.; Zhang, Y.-Q.; Liu, H.-Y.; Yu, L.-Y. Diversity and Distribution of Aquatic Fungal Communities in the Ny-Ålesund Region, Svalbard (High Arctic) Aquatic Fungi in the Arctic. Microb. Ecol. 2016, 71, 543–554. [Google Scholar] [CrossRef]
- Xu, W.; Gong, L.-f.; Pang, K.-L.; Luo, Z.-H. Fungal diversity in deep-sea sediments of a hydrothermal vent system in the Southwest Indian Ridge. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2018, 131, 16–26. [Google Scholar] [CrossRef]
- Gleason, F.H.; Kagami, M.; Lefevre, E.; Sime-Ngando, T. The ecology of chytrids in aquatic ecosystems: Roles in food web dynamics. Fungal Biol. Rev. 2008, 22, 17–25. [Google Scholar] [CrossRef]
- Hanrahan-Tan, D.G.; Lilje, O.; Henderson, L. Chytrids in Soil Environments: Unique Adaptations and Distributions. Encyclopedia 2023, 3, 642–664. [Google Scholar] [CrossRef]
- Tedersoo, L.; Sánchez-Ramírez, S.; Koljalg, U.; Bahram, M.; Döring, M.; Schigel, D.; May, T.; Ryberg, M.; Abarenkov, K. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018, 90, 135–159. [Google Scholar] [CrossRef]
- Wu, X.; Liu, P.; Wegner, C.-E.; Luo, Y.; Xiao, K.-Q.; Cui, Z.; Zhang, F.; Liesack, W.; Peng, J. Deciphering microbial mechanisms underlying soil organic carbon storage in a wheat-maize rotation system. Sci. Total Environ. 2021, 788, 147798. [Google Scholar] [CrossRef]
- Doweld, A.B. Nomenclatural novelties Olpidiales ord. nov., Olpidiomycetes class. nov., Olpidiomycota phyl. nov., Olpidiomycotina subphyl. nov. Index Fungorum 2013, 42, 1. [Google Scholar]
- Cannon, P.F.; Paul, M.K. (Eds.) Fungal Families of the World; CABI: Wallingford, UK, 2007. [Google Scholar]
- Wijayawardene, N.N.; Pawłowska, J.; Letcher, P.M.; Kirk, P.M.; Humber, R.A.; Schüßler, A.; Wrzosek, M.; Muszewska, A.; Okrasińska, A.; Istel, Ł.; et al. Notes for genera: Basal clades of Fungi (including Aphelidiomycota, Basidiobolomycota, Blastocladiomycota, Calcarisporiellomycota, Caulochytriomycota, Chytridiomycota, Entomophthoromycota, Glomeromycota, Kickxellomycota, Monoblepharomycota, Mortierellomycota, Mucoromycota, Neocallimastigomycota, Olpidiomycota, Rozellomycota and Zoopagomycota). Fungal Divers. 2018, 92, 43–129. [Google Scholar]
- Quandt, C.A.; Beaudet, D.; Corsaro, D.; Walochnik, J.; Michel, R.; Corradi, N.; James, T.Y. The genome of an intranuclear parasite, Paramicrosporidium saccamoebae, reveals alternative adaptations to obligate intracellular parasitism. Elife 2017, 6, e29594. [Google Scholar] [CrossRef] [PubMed]
- Grossart, H.-P.; Wurzbacher, C.; James, T.Y.; Kagami, M. Discovery of dark matter fungi in aquatic ecosystems demands a reappraisal of the phylogeny and ecology of zoosporic fungi. Fungal Ecol. 2016, 19, 28–38. [Google Scholar] [CrossRef]
- Costa, P.P.; Rosado, A.W.; Pereira, O.L. Six new species of Cladosporium associated with decayed leaves of native bamboo (Bambusoideae) in a fragment of Brazilian Atlantic Forest. Phytotaxa 2022, 560, 1–29. [Google Scholar] [CrossRef]
- Hyde, K.D.; Ho, W.H.; McKenzie, E.H.C.; Dalisay, T. Saprobic fungi on bamboo culms. Fungal Divers. 2001, 7, 35–48. [Google Scholar]
- Shen, X.Y.; Cheng, Y.L.; Cai, C.J.; Fan, L.; Gao, J.; Hou, C.L. Diversity and antimicrobial activity of culturable endophytic fungi isolated from moso bamboo seeds. PLoS ONE 2014, 9, e95838. [Google Scholar] [CrossRef]
- Sampaio, J.P.; Golubev, W.I.; Fell, J.W.; Gadanho, M.; Golubev, N.W. Curvibasidium cygneicollum gen. nov., sp. nov. and Curvibasidium pallidicorallinum sp. nov., novel taxa in the Microbotryomycetidae (Urediniomycetes), and their relationship with Rhodotorula fujisanensis and Rhodotorula nothofagi. Int. J. Syst. Evol. Microbiol. 2004, 54, 1401–1407. [Google Scholar] [CrossRef]
- Bourret, T.B.; Edwards, C.G.; Glawe, D.A. Curvibasidium rogersii, a new yeast species in the Microbotryomycetes. N. Am. Fungi 2012, 7, 1–8. [Google Scholar]
- Zhang, T.; Fei Wang, N.; Qin Zhang, Y.; Yu Liu, H.; Yan Yu, L. Diversity and distribution of fungal communities in the marine sediments of Kongsfjorden, Svalbard (High Arctic). Sci. Rep. 2015, 5, srep14524. [Google Scholar]
- Knapp, D.G.; Lázár, A.; Molnár, A.; Vajna, B.; Karácsony, Z.; Váczy, K.Z.; Kovacs, G.M. Above-ground parts of white grapevine Vitis vinifera cv. Furmint share core members of the fungal microbiome. Environ. Microbiol. Rep. 2021, 13, 509–520. [Google Scholar] [CrossRef]
- Wang, X.; Schlatter, D.C.; Glawe, D.A.; Edwards, C.G.; Weller, D.M.; Paulitz, T.C.; Abatzoglou, J.T.; Okubara, P.A. Native yeast and non-yeast fungal communities of Cabernet Sauvignon berries from two Washington State vineyards, and persistence in spontaneous fermentation. Int. J. Food Microbiol. 2021, 350, 109225. [Google Scholar] [CrossRef]
- Zajc, J.; Gunde-Cimerman, N. The genus Wallemia—From contamination of food to health threat. Microorganisms. 2018, 6, 46. [Google Scholar] [CrossRef]
- Zalar, P.; Sybren de Hoog, G.; Schroers, H.-J.; Frank, J.M.; Gunde-Cimerman, N. Taxonomy and phylogeny of the xerophilic genus Wallemia (Wallemiomycetes and Wallemiales, cl. et ord. nov.). Antonie Van Leeuwenhoek. 2005, 87, 311–328. [Google Scholar] [CrossRef]
- Magan, N. Ecophysiology: Impact of environment on growth, synthesis of compatible solutes and enzyme production. Br. Mycol. Soc. Symp. Ser. 2008, 28, 63–78. [Google Scholar]
- Tokumasu, S. Fungal successions on pine needles fallen at different seasons: The succession of surface colonizers. Mycoscience 1998, 39, 417–423. [Google Scholar] [CrossRef]
- Abbasi, P.A.; Hildebrand, P.D.; Ali, S.; Moreau, D.L.; Renderos, W.E. Effect of RH. Effect of RH, Temperature, Light, and Plant Age on Infection of Lowbush Blueberry by Sphaerulina vaccinii. Plant Dis. 2022, 106, 297–303. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, N.; Zhou, G.; Dang, P.; Yang, X.; Qiu, L.; Huang, M.; Gong, Y.; Zhao, S.; Chen, J. Response of soil microbial community to plant composition changes in broad-leaved forests of the karst area in Mid-Subtropical China. PeerJ 2022, 10, e12739. [Google Scholar] [CrossRef]
- Yang, S.; Xu, W.; Gao, Y.; Chen, X.; Luo, Z.-H. Fungal diversity in deep-sea sediments from Magellan seamounts environment of the western Pacific revealed by high-throughput Illumina sequencing. J. Microbiol. 2020, 58, 841–852. [Google Scholar] [CrossRef]
- Yan, G.; Wang, Q.; Zhang, J.; Liu, G.; Wang, L.; Huang, B.; Wang, H.; Xing, Y. Long-term nitrogen addition and reduced precipitation restructure soil fungal community in a temperate forest. Scand. J. For. Res. 2021, 36, 105–116. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, G.; Polle, A.; Janz, D.; Euring, D.; Yue, X.; Zhao, H.; Fei, B.; Jiang, Z. Comparative characterization of ethanol organosolv lignin polymer from bamboo green, timber and yellow. Wood Sci. Technol. 2018, 52, 1331–1341. [Google Scholar] [CrossRef]
- Yu, H.-x.; Pan, X.; Xu, M.-p.; Yang, W.-m.; Wang, J.; Zhuang, X.-w. Surface chemical changes analysis of UV-light irradiated Moso bamboo (Phyllostachys pubescens Mazel). R. Soc. Open Sci. 2018, 5, 180110. [Google Scholar] [CrossRef] [PubMed]
- Afrin, T.; Tsuzuki, T.; Wang, X. UV absorption property of bamboo. J. Text. Inst. 2012, 103, 394–399. [Google Scholar] [CrossRef]
- Halaburgi, V.M.; Sharma, S.; Sinha, M.; Singh, T.P.; Karegoudar, T.B. Purification and characterization of a thermostable laccase from the ascomycetes Cladosporium cladosporioides and its applications. Process Biochem. 2011, 46, 1146–1152. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, H.; Huang, J.; Li, Z.; Pan, Y.; Du, Y. Carbonaceous nanomaterials stimulate extracellular enzyme release by the fungus Cladosporium sp. and enhance extracellular electron transfer to facilitate lignin biodegradation. Sci. Total Environ. 2019, 696, 134072. [Google Scholar] [CrossRef]
- De Wit, P.J.G.M.; Van Der Burgt, A.; Ökmen, B.; Stergiopoulos, I.; Abd-Elsalam, K.A.; Aerts, A.L.; Bahkali, A.H.; Beenen, H.G.; Chettri, P.; Cox, M.P.; et al. The genomes of the fungal plant pathogens Cladosporium fulvum and Dothistroma septosporum reveal adaptation to different hosts and lifestyles but also signatures of common ancestry. PLoS Genet. 2012, 8, e1003088. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Kim, Y.-H.; Jung, M.-H.; Jo, C.-W.; Choi, J.-E. Characterization of xylanase of Cladosporium cladosporioides H1 isolated from Janggyeong Panjeon in Haeinsa Temple. Mycobiology 2011, 39, 306–309. [Google Scholar] [CrossRef]
- Xu, R.; He, H.; Guo, H.; Zhu, F.; Wang, S.; Dai, C.; Zheng, X.; Xie, D.; Li, H.; Wang, C.; et al. Characteristics of silicon and phytolith distribution in bamboo (Ferrocalamus strictus): Variations between different organs and ages. Rev. Palaeobot. Palynol. 2023, 311, 104817. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, C.; Zhang, M.; Xu, J. Anti-condensation behavior of bamboo leaf surface (backside) and its bionic preparation. Mater. Res. Express 2021, 8, 055002. [Google Scholar] [CrossRef]
- Petridis, L.; Smith, J.C. Molecular-level driving forces in lignocellulosic biomass deconstruction for bioenergy. Nat. Rev. Chem. 2018, 2, 382–389. [Google Scholar] [CrossRef]
- Ma, F.; Huang, X.; Ke, M.; Shi, Q.; Chen, Q.; Shi, C.; Zhang, J.; Zhang, X.; Yu, H. Role of selective fungal delignification in overcoming the saccharification recalcitrance of bamboo culms. ACS Sustain. Chem. Eng. 2017, 5, 8884–8894. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, X.; Han, S.; Ren, X.; Sichone, J.; Fan, Z.; Wu, X.; Zhang, Y.; Wang, H.; Cai, W.; Sun, F. Succession of Fungal Community during Outdoor Deterioration of Round Bamboo. J. Fungi 2023, 9, 691. https://doi.org/10.3390/jof9060691
An X, Han S, Ren X, Sichone J, Fan Z, Wu X, Zhang Y, Wang H, Cai W, Sun F. Succession of Fungal Community during Outdoor Deterioration of Round Bamboo. Journal of Fungi. 2023; 9(6):691. https://doi.org/10.3390/jof9060691
Chicago/Turabian StyleAn, Xiaojiao, Shuaibo Han, Xin Ren, John Sichone, Zhiwei Fan, Xinxing Wu, Yan Zhang, Hui Wang, Wei Cai, and Fangli Sun. 2023. "Succession of Fungal Community during Outdoor Deterioration of Round Bamboo" Journal of Fungi 9, no. 6: 691. https://doi.org/10.3390/jof9060691
APA StyleAn, X., Han, S., Ren, X., Sichone, J., Fan, Z., Wu, X., Zhang, Y., Wang, H., Cai, W., & Sun, F. (2023). Succession of Fungal Community during Outdoor Deterioration of Round Bamboo. Journal of Fungi, 9(6), 691. https://doi.org/10.3390/jof9060691




