Entomopathogenicity of Ascomycete Fungus Cordyceps militaris on the Cotton Bollworm, Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Source and Rearing
2.2. Identity of Fungal Isolate
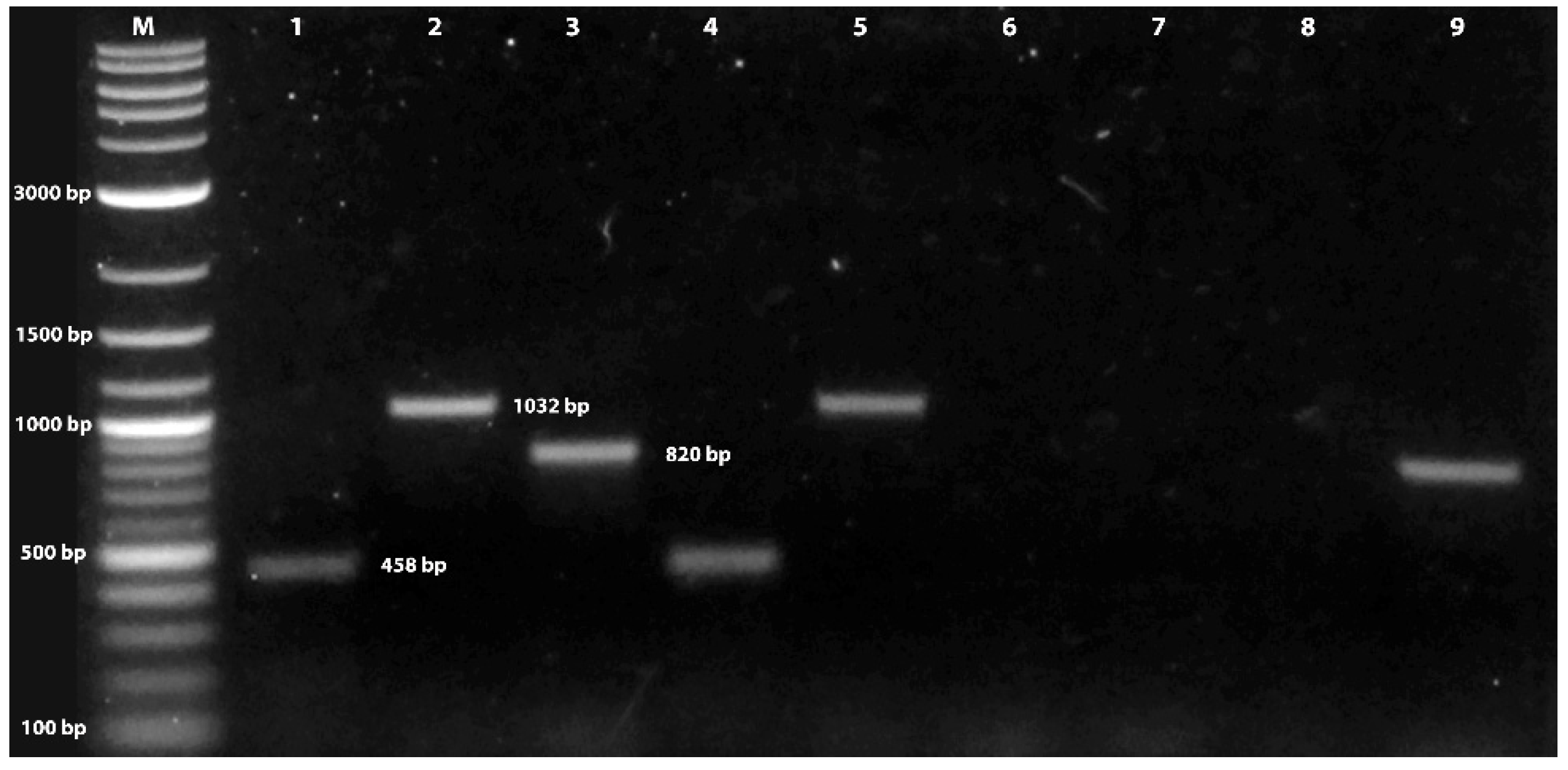
Mating Type Determination
2.3. Spore Cultivation
2.4. Exposure Protocol
2.4.1. Direct Exposure: Spores Applied Directly to Insect Cuticle
2.4.2. Contact Exposure: Treated Corn Leaf Tissue
2.5. Statistical Analysis
3. Results
3.1. Verification of Fungal Isolate Identity
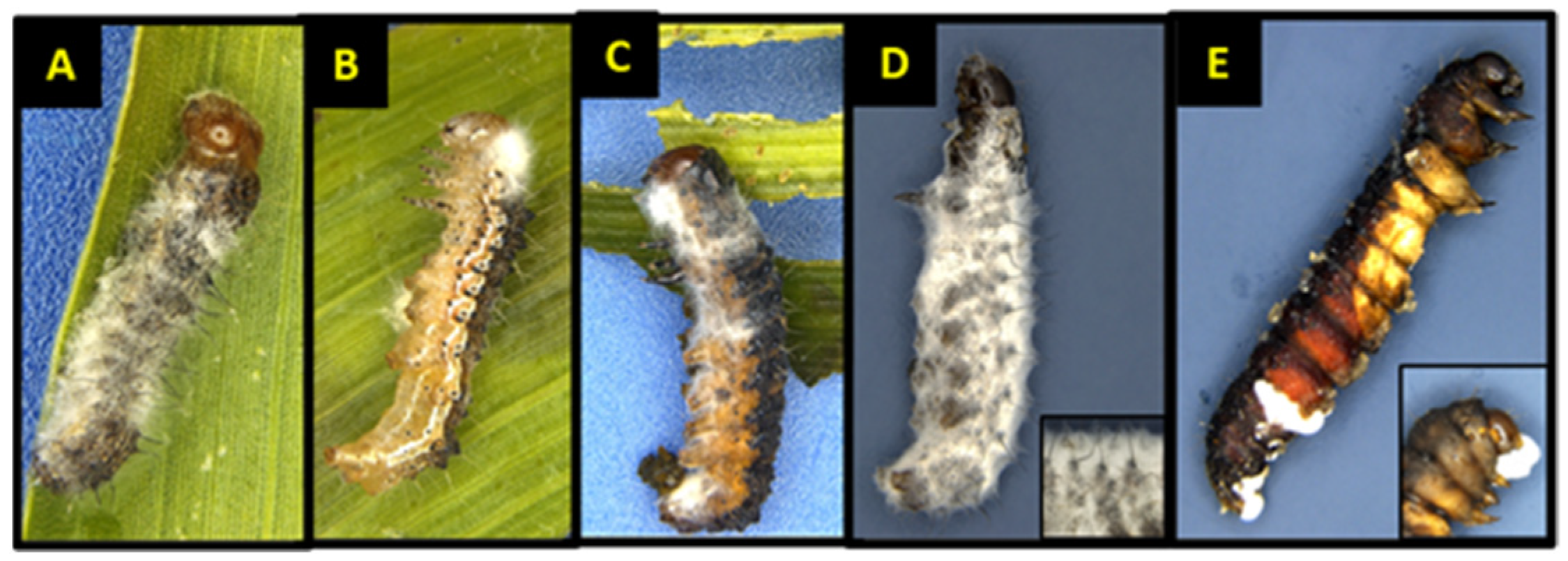
3.2. Larval, Pupal, and Adult Mortality
3.3. Larval Mortality and Sporulation
3.3.1. First and Second Instars Cotton Bollworm
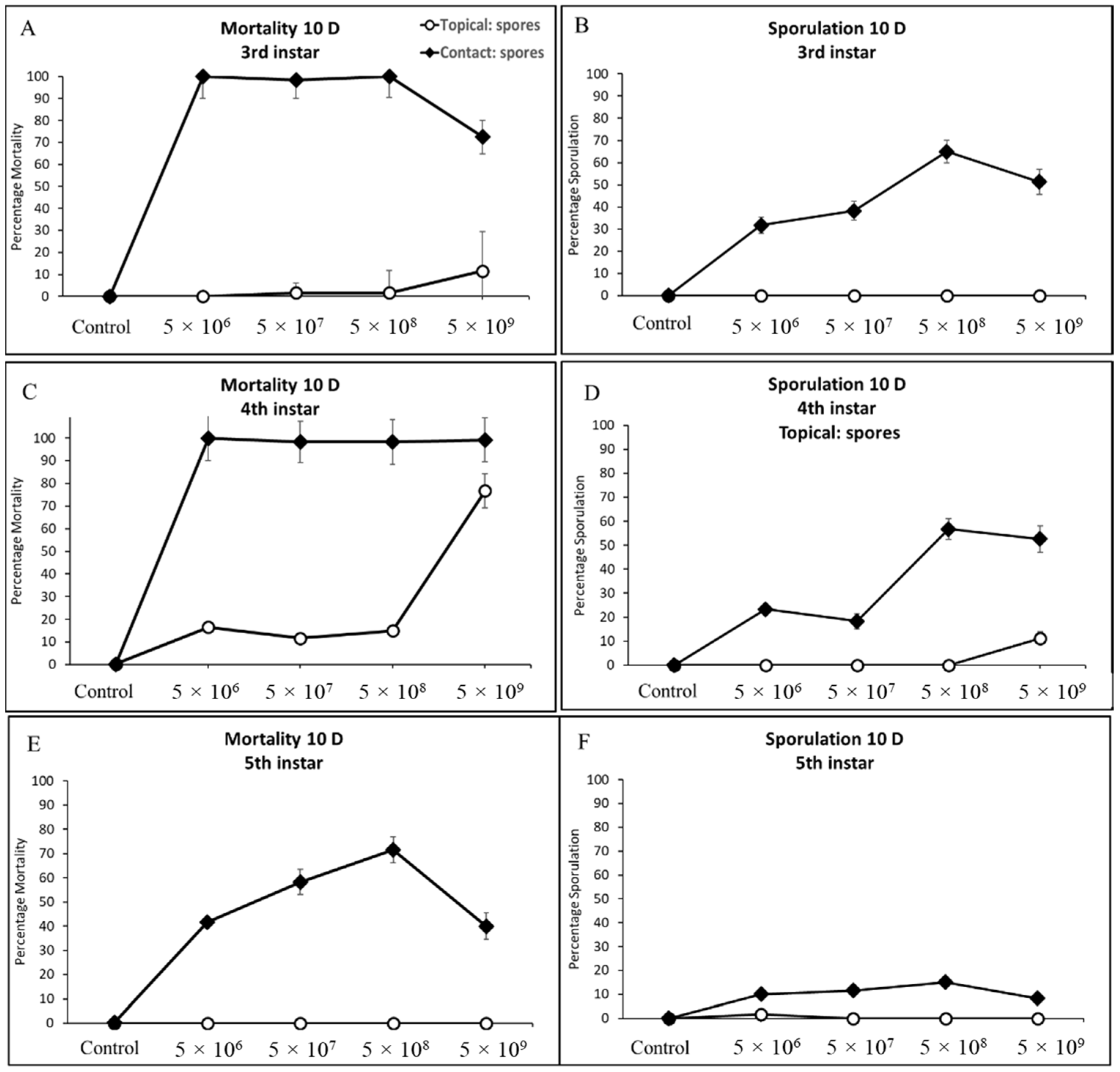
3.3.2. Third, Fourth, and Fifth Instar Cotton Bollworm
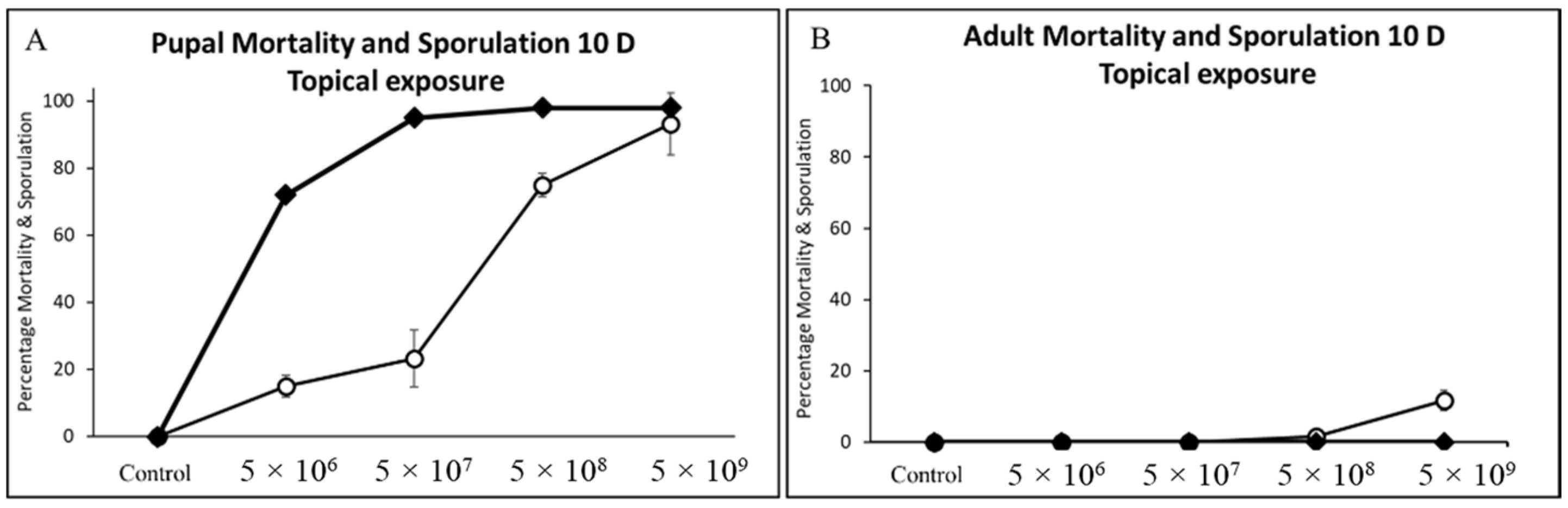
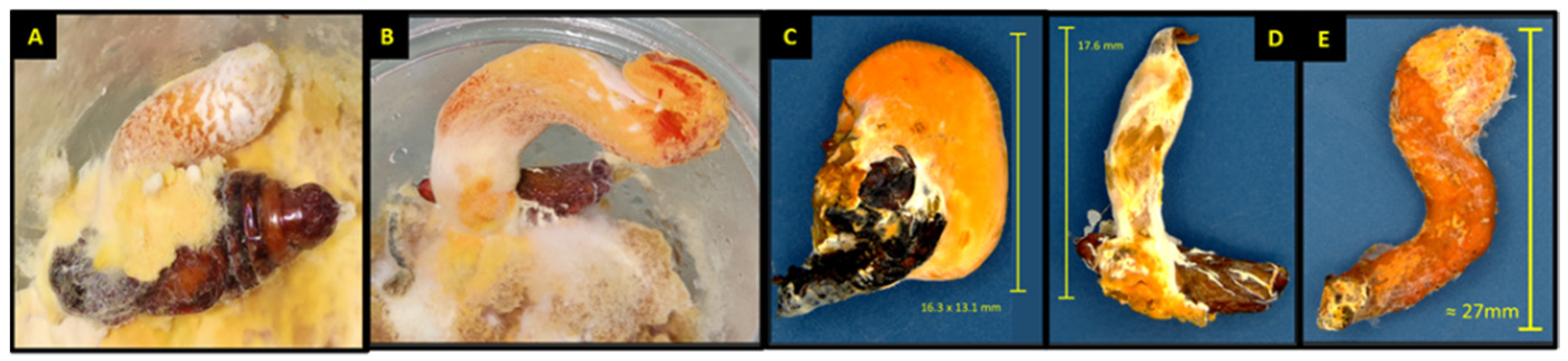
3.4. Pupal and Adult Mortality and Sporulation
3.5. Lethal Morality and Response of Cotton Bollworm Instars
3.6. Cordyceps militaris Concentration-Sporulation Response (LS50) on Cotton Bollworm Instars
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Chaneiko, S.M.; de Brida, A.L.; Bassa, P.G.; Telles, M.H.; dos Santos, L.A.; Pereira, D.I.; Pereira, R.M.; Garcia, F.R. Pathogenicity of Beauveria bassiana and Metarhizium anisopliae to Anastrepha fraterculus (Diptera: Tephritidae) and effects on adult longevity. J. Agric. Sci. 2019, 11, 132. [Google Scholar] [CrossRef]
- Jackson, M.A.; Dunlap, C.A.; Jaronski, S.T. Ecological considerations in producing and formulating fungal entomopathogens for use in insect biocontrol. BioControl 2010, 55, 129–145. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jaronski, S.T. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 2016, 32, 177. [Google Scholar] [CrossRef]
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 50–59. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, A.R.A. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Portilla, M.; Abbas, H.K.; Accinelli, C.; Luttrell, R. Laboratory and field investigation on compatibility of Beauveria bassiana (Hypocreales: Clavicipotaceae) spores with a sprayable bioplastic formulation for application in the biocontrol of tarnished plant bug in cotton. J. Econ. Entomol. 2018, 112, 549–557. [Google Scholar] [CrossRef] [PubMed]
- De Faria, M.R.; Wraight, S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. A Review of Biopesticides and Their Mode of Action against Insect Pests. In Environmental Sustainability: Role of Green Technologies; Springer: New Delhi, India, 2015; pp. 49–63. [Google Scholar]
- Sullivan, C.F.; Parker, B.L.; Skinner, M. A Review of Commercial Metarhizium- and Beauveria-Based Biopesticides for the Biological Control of Ticks in the USA. Insects 2022, 13, 260. [Google Scholar] [CrossRef]
- Medo, J.; Medová, J.; Michalko, J.; Cagáň, Ľ. Variability in virulence of Beauveria spp. soil isolates against Ostrinia nubilalis. J. Appl. Entomol. 2021, 145, 92–103. [Google Scholar] [CrossRef]
- Singh, H.B.; Keswani, C.; Ray, S.; Yadav, S.K.; Singh, S.P.; Singh, S.; Sarma, B.K. Beauveria bassiana: Biocontrol Beyond Lepidopteran Pests. Biocontrol Lepid. Pests 2015, 43, 219–235. [Google Scholar] [CrossRef]
- Kryukov, V.Y.; Kryukova, N.A.; Tomilova, O.G.; Vorontsova, Y.; Chertkova, E.; Pervushin, A.L.; Slepneva, I.; Glupov, V.V.; Yaroslavtseva, O.N. Comparative analysis of the immune response of the wax moth Galleria mellonella after infection with the fungi Cordyceps militaris and Metarhizium robertsii. Microb. Pathog. 2020, 141, 103995. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, V.Y.; Yaroslavtseva, O.N.; Dubovskiy, I.M.; Tyurin, M.V.; Kryukova, N.A.; Glupov, V.V. Insecticidal and immunosuppressive effect of ascomycete Cordyceps militaris on the larvae of the Colorado potato beetle Leptinotarsa decemlineata. Biol. Bull. 2014, 41, 276–283. [Google Scholar] [CrossRef]
- Gebremariam, A.; Chekol, Y.; Assefa, F. Extracellular enzyme activity of entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae and their pathogenicity potential as a bio-control agent against whitefly pests, Bemisia tabaci and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). BMC Res. Notes 2022, 15, 117. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E.; Elkinton, J.S.; Witcosky, J.J. Introduction and spread of the fungal pathogen Entomophaga maimaiga (Zygomycetes: Entomophthorales) along the leading edge of gypsy moth (Lepidoptera: Lymantriidae) spread. Environ. Entomol. 1996, 25, 1235–1247. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Shrestha, B.; Xu, J.; Wang, C.; Liu, X. Ophiocordyceps sinensis and Cordyceps militaris: Research advances, issues and perspectives. Mycosystema 2013, 32, 577–597. [Google Scholar]
- Agrawal, D.C.; Tsay, H.S.; Shyur, L.F.; Wu, Y.C.; Wang, S.Y. (Eds.) Medicinal Plants and Fungi: Recent Advances in Research and Development; Cordyceps: A Highly Coveted Medicinal Mushroom; Springer: Singapore, 2017; pp. 59–91. [Google Scholar]
- Lian, T.; Yang, T.; Sun, J.; Guo, S.; Yang, H.; Dong, C. Variations of SSU rDNA group I introns in different isolates of Cordyceps militaris and the loss of an intron during cross-mating. J. Microbiol. 2014, 52, 659–666. [Google Scholar] [CrossRef]
- Yokoyama, E.; Arakawa, M.; Yamagishi, K.; Hara, A. Phylogenetic and structural analyses of the mating-type loci in Clavicipitaceae. FEMS Microbiol. Lett. 2006, 264, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Kumar, A. A brief review on the medicinal uses of Cordyceps militaris. Pharmacol. Res.-Mod. Chin. Med. 2023, 7, 100228. [Google Scholar]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitoterapia 2010, 81, 961–968. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef]
- Fitt, G.P. The ecology of Heliothis species in relation to agroecosystems. Annu. Rev. Entomol. 1989, 34, 17–53. [Google Scholar] [CrossRef]
- Jackson, R.E.; Bradley, J.R.; Van Duyn, J.; Leonard, B.R.; Allen, K.C.; Luttrell, R.; Ruberson, J.; Adamczyk, J.; Gore, J.; Hardee, D.D.; et al. Regional assessment of Helicoverpa zea populations on cotton and non-cotton crop hosts. Entomol. Exp. Appl. 2008, 126, 89–106. [Google Scholar] [CrossRef]
- Williams, M.R. Cotton insect losses. In Proceedings of the Beltwide Cotton Conferences, Dallas, TX, USA, 4–6 January 2017; pp. 710–754. [Google Scholar]
- Dorman, S.J.; Hopperstad, K.A.; Reich, B.J.; Kennedy, G.; Huseth, A.S. Soybeans as a non-Bt refuge for Helicoverpa zea in maize-cotton agroecosystems. Agric. Ecosyst. Environ. 2021, 322, 107642. [Google Scholar] [CrossRef]
- Arends, B.R.; Reisig, D.D.; Gundry, S.; Greene, J.K.; Kennedy, G.G.; Reay-Jones, F.P.; Huseth, A.S. Helicoverpa zea (Lepidoptera: Noctuidae) feeding incidence and survival on Bt maize in relation to maize in the landscape. Pest Manag. Sci. 2022, 78, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. SAS 9.4 User’s Guide; SAS Institute: Cary, NC, USA, 2022. [Google Scholar]
- Tan, Q.; Cai, T.; Wei, J.; Feng, A.; Mao, W.; Bao, D. Molecular identification of mating type genes in asexual spores of Cordyceps militaris. In Mushroom Biology and Mushroom Products, Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products, Arcachon, France, 4–7 October 2011; Oral Presentations; Institut National de la Recherche Agronomique (INRA): Paris, France, 2011; Volume 1, pp. 52–56. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Zheng, P.; Xia, Y.; Xiao, G.; Xiong, C.; Hu, X.; Zhang, S.; Zheng, H.; Huang, Y.; Zhou, Y.; Wang, S. Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional Chinese medicine. Genome Biol. 2011, 12, R116. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, N.S.; Cho, S.Y.; Hwang, J.S.; Jin, B.R. Production of the wild entomopathogenic fungi, Cordyceps militaris, in the silkworm, Bombyx mori. Int. J. Ind. Entomol. 2001, 3, 105–108. [Google Scholar]
- Hong, I.P.; Kang, P.D.; Kim, K.Y.; Nam, S.H.; Lee, M.Y.; Choi, Y.S.; Kim, N.S.; Kim, H.K.; Lee, K.G.; Humber, R.A. Fruit body formation on silkworm by Cordyceps militaris. Mycobiology 2010, 38, 128–132. [Google Scholar] [CrossRef]



| Life Stages | Contact-Treated Leaf Tissue | Topical-Spray Application | ||||
|---|---|---|---|---|---|---|
| X2 | DF | p > X2 | X2 | DF | p > X2 | |
| 1st instar | 13.411 | 1 | 0.003 | 0.445 | 1 | 0.104 |
| 2nd instar | 10.045 | 1 | 0.002 | 19.000 | 1 | 0.001 |
| 3rd instar | 19.602 | 1 | 0.001 | 1.583 | 1 | 0.048 |
| 4th instar | 14.032 | 1 | 0.005 | 2.671 | 1 | 0.002 |
| 5th instar | 6.105 | 1 | 0.028 | 8.444 | 1 | 0.305 |
| Pupae | - | - | - | 16.333 | 1 | 0.001 |
| Adult | - | - | - | 11.314 | 1 | 0.428 |
| Larval Instars | Concentration Response (Spores/mm2) | ||||||
|---|---|---|---|---|---|---|---|
| n | Slope ± SE | LC50 (95% CI) | Probit Trend | ||||
| Test for Slope | Test for GoF 1 | ||||||
| X2 | p > X2 | X2 | p > X2 | ||||
| 1st instar | 324 | - | - | 0.77 | 0.389 | 2.565 | 0.0769 |
| 2nd instar | 324 | 5.08 ± 1.01 | 10.13 (9.54–11.47) | 29.52 | 0.0001 | 3.524 | 0.0295 |
| 3rd instar | 324 | 8.53 ± 2.15 | 9.80 (9.13–11.53) | 15.06 | 0.0001 | 5.026 | 0.0066 |
| 4th instar | 324 | - | - | 0.13 | 0.714 | 0.636 | 0.5292 |
| 5th instar | 324 | 3.91 ± 1.27 | 13.61 (11.19–16.53) | 8.21 | 0.0148 | 8.011 | 0.0003 |
| Stage | Concentration Response (Spores/mm2) | ||||||
|---|---|---|---|---|---|---|---|
| n | Slope ± SE | LC50 (95% CI) | Probit Trend | ||||
| Test for Slope | Test for GoF | ||||||
| X2 | p > X2 | X2 | p > X2 | ||||
| 1st instar | 324 | - | - | 0.16 | 0.6884 | 0.12 | 0.9406 |
| 2nd instar | 324 | - | - | 0.54 | 0.4602 | 5.53 | 0.0039 |
| 3rd instar | 324 | 3.27 ± 1.02 | 8.73 (8.35–9.89) | 14.23 | 0.0002 | 4.23 | 0.0146 |
| 4th instar | 324 | 6.16 ± 2.13 | 11.82 (10.05–22.07) | 11.02 | 0.0091 | 1.45 | 0.2333 |
| 5th instar | 324 | - | - | - | - | - | - |
| Pupae | 324 | 7.45 ± 1.47 | 4.01 (3.94–5.04) | 6.77 | 0.0093 | 7.52 | 0.0233 |
| Adult | 324 | - | - | 2.15 | 0.1426 | 9.49 | 0.0001 |
| Stage | Concentration Response (Spores/mm2) | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposure | n | Slope ± SE | LS50 (95% CI) | Probit Trend | ||||
| Test for Slope 1 | Test for GoF | |||||||
| X2 | p > X2 | X2 | p > X2 | |||||
| 1st Instar | Contact 2 | 324 | 3.14 ± 0.95 | 6.52 (4.54–11.73) | 13.16 | 0.0084 | 3.19 | 0.0225 |
| Topical 3 | 324 | - | - | 1.76 | 0.1944 | 4.24 | 0.0145 | |
| 2nd Instar | Contact | 324 | 1.33 ± 0.65 | 7.84 (5.42–16.75) | 25.22 | 0.0001 | 13.29 | 0.0001 |
| Topical | 324 | - | - | 1.72 | 0.1897 | 9.49 | 0.0001 | |
| 3rd Instar | Contact | 324 | 1.41 ± 0.53 | 9.85 (3.14–11.25) | 6.43 | 0.0112 | 14.21 | 0.0002 |
| Topical | 324 | - | - | 1.61 | 0.2041 | 4.01 | 0.0183 | |
| 4th Instar | Contact | 324 | 2.36 ± 0.64 | 2.14 (1.74–2.26) | 12.23 | 0.0023 | 9.54 | 0.0005 |
| Topical | 324 | 4.45 ± 1.11 | 10.33 (9.42–13.17) | 17.01 | 0.0001 | 5.17 | 0.0057 | |
| 5th Instar | Contact | 324 | - | - | 0.56 | 0.4536 | 4.80 | 0.0082 |
| Topical | 324 | - | - | 0.51 | 0.9741 | 0.73 | 0.4823 | |
| Pupae | Topical | 324 | 4.07 ± 0.49 | 9.52 (8.18–11.19) | 11.44 | 0.0003 | 9.18 | 0.0035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glover, J.P.; Nufer, M.I.; Perera, O.P.; Portilla, M.; George, J. Entomopathogenicity of Ascomycete Fungus Cordyceps militaris on the Cotton Bollworm, Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae). J. Fungi 2023, 9, 614. https://doi.org/10.3390/jof9060614
Glover JP, Nufer MI, Perera OP, Portilla M, George J. Entomopathogenicity of Ascomycete Fungus Cordyceps militaris on the Cotton Bollworm, Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae). Journal of Fungi. 2023; 9(6):614. https://doi.org/10.3390/jof9060614
Chicago/Turabian StyleGlover, James P., Marissa I. Nufer, Omaththage P. Perera, Maribel Portilla, and Justin George. 2023. "Entomopathogenicity of Ascomycete Fungus Cordyceps militaris on the Cotton Bollworm, Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae)" Journal of Fungi 9, no. 6: 614. https://doi.org/10.3390/jof9060614
APA StyleGlover, J. P., Nufer, M. I., Perera, O. P., Portilla, M., & George, J. (2023). Entomopathogenicity of Ascomycete Fungus Cordyceps militaris on the Cotton Bollworm, Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae). Journal of Fungi, 9(6), 614. https://doi.org/10.3390/jof9060614









