Going over Fungal Allergy: Alternaria alternata and Its Allergens
Abstract
1. Classification, Morphology and Distribution of Alternaria alternata
2. Ecology of Alternaria alternata
3. Allergy, Aerobiology and Prevalence of Sensitisation of Alternaria alternata and Other Fungi
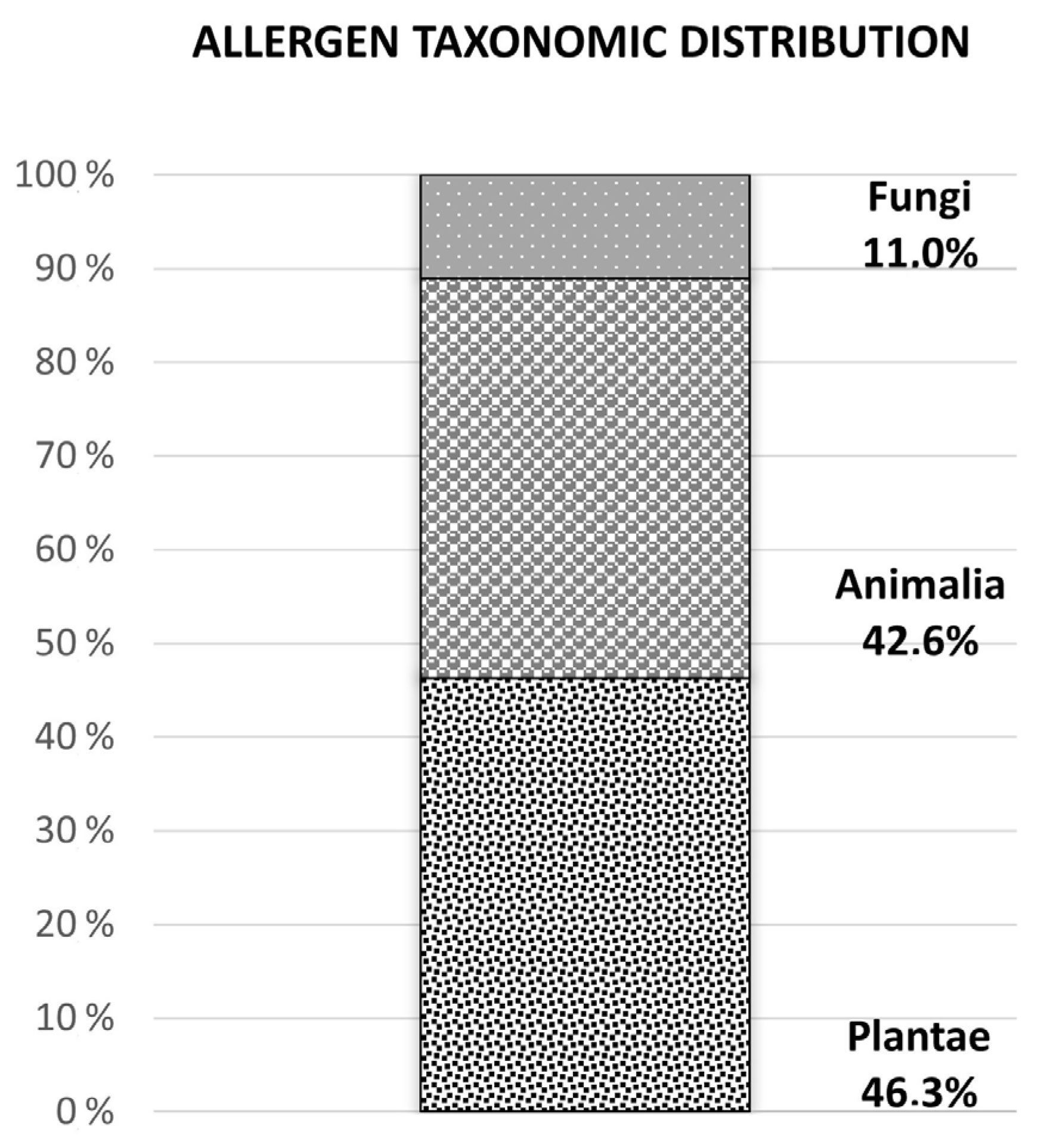
4. Allergen Distribution and Alternaria alternata Allergen Relevance
5. Diagnosis of Alternaria alternata Allergy and Its Immunotherapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hawksworth, D.L. The magnitude of fungal diversity: The 1.5 million species estimate revisited. Mycol. Res. 2001, 105, 1422–1432. [Google Scholar] [CrossRef]
- Kennedy, J.L.; Heymann, P.W.; Platts-Mills, T.A. The role of allergy in severe asthma. Clinical and experimental allergy. J. Br. Soc. Allergy Clin. Immunol. 2012, 42, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Kurup, V.P. Fungal allergens. Curr. Allergy Asthma Rep. 2003, 3, 416. [Google Scholar] [CrossRef] [PubMed]
- Simon-Nobbe, B.; Denk, U.; Poll, V.; Rid, R.; Breitenbach, M. The spectrum of fungal allergy. Int. Arch. Allergy Immunol. 2008, 145, 58–86. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.F.; Postigo, I.; Tomaz, C.T.; Martinez, J. Alternaria alternata allergens: Markers of exposure phylogeny and risk of fungi-induced respiratory allergy. Environ. Int. 2016, 89–90, 71–80. [Google Scholar] [CrossRef]
- Woudenberg, J.H.; Groenewald, J.Z.; Binder, M.; Crous, P.W. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef]
- Zabawski, J.B.E. Characteristics of Frequent Fungal Pathogens and Opportunistic Fungi with Subclasses: Zygomycotina Ascomycotina and Deuteromycotina; Wroclaw Medical University: Wrocław, Poland, 1998. [Google Scholar]
- Rotem, J. The Genus Alternaria: Biology Epidemiology and Pathogenicity; American Phytopathological Society: St Paul, MN, USA, 1994. [Google Scholar]
- Esch, R.E.; Codina, R. Fungal raw materials used to produce allergen extracts. Ann. Allergy Asthma. Immunol. 2017, 118, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kosuke, T. Fungal allergy—Fungal ecology in dwelling environments. Nippon. Ishinkin Gakkai Zasshi 2001, 42, 113–117. [Google Scholar]
- Bush, R.K.; Prochnau, J.J. Alternaria-induced asthma. J. Allergy Clin. Immunol. 2004, 113, 227–234. [Google Scholar] [CrossRef]
- Ren, P.; Ahearn, D. Mycotoxins of Alternaria alternata produced on ceiling tiles. J. Ind. Microbiol. Biotechnol. 1998, 20, 53–54. [Google Scholar] [CrossRef]
- Pose, G.; Patriarca, A.; Kyanko, V.; Pardo, A.; Fernández Pinto, V. Effect of water activity and temperature on growth of Alternaria alternata on a synthetic tomato medium. Int. J. Food Microbiol. 2009, 135, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Pose, G.; Patriarca, A.; Kyanko, V.; Pardo, A.; Fernández Pinto, V. Water activity and temperature effects on mycotoxin production by Alternaria alternata on a synthetic tomato medium. Int. J. Food Microbiol. 2010, 142, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, A.L.R. Aerobiología de las Esporas de Pleosoporales en Ambientes Intra y Extradomiciliarios en Barcelona: Aplicación en Población Alérgica. Ph.D. Thesis, Universitat Autònoma de Barcelona, Bellaterra, Spain, 2013. [Google Scholar]
- Bartra, J.; Belmonte, J.; Torres-Rodriguez, J.M.; Cistero-Bahima, A. Sensitization to Alternaria in patients with respiratory allergy. Front. Biosci. 2009, 14, 3372–3379. [Google Scholar] [CrossRef] [PubMed]
- Weber, R. Alternaria alternata. Ann. Allergy Asthma Immunol. 2001, 87, A-4. [Google Scholar]
- Kasprzyk, I.; Sulborska, A.; Nowak, M.; Szymańska, A.; Kaczmarek, J.; Haratym, W.; Weryszko-Chmielewska, E.; Jędryczka, M. Fluctuation range of the concentration of airborne Alternaria conidiospores sampled at different geographical locations in Poland (2010–2011). Acta Agrobot. 2013, 66, 65–76. [Google Scholar] [CrossRef]
- Krysińska-Traczyk, E. Microflora of the farming work environment as an occupational risk factor. Med. Pr. 2000, 51, 351–355. [Google Scholar]
- Prester, L. Indoor exposure to mould allergens. Arh. Hig. Rada Toksikol. 2011, 62, 371–379. [Google Scholar] [CrossRef]
- Peters, J.L.; Muilenberg, M.L.; Rogers, C.A.; Burge, H.A.; Spengler, J.D. Alternaria measures in inner-city low-income housing by immunoassay and culture-based analysis. Ann. Allergy Asthma Immunol. 2008, 100, 364–369. [Google Scholar] [CrossRef]
- Saijo, Y.; Sata, F.; Mizuno, S.; Yamaguchi, K.; Sunagawa, H.; Kishi, R. Indoor airborne mold spores in newly built dwellings. Environ. Health Prev. Med. 2005, 10, 157–161. [Google Scholar] [CrossRef]
- Ishibashi, M.; Tonori, H.; Miki, T.; Miyajima, E.; Kudo, Y.; Tsunoda, M.; Sakabe, K.; Aizawa, Y. Classification of patients complaining of sick house syndrome and/or multiple chemical sensitivity. Tohoku J. Exp. Med. 2007, 211, 223–233. [Google Scholar] [CrossRef]
- O’Connor, G.T.; Walter, M.; Mitchell, H.; Kattan, M.; Morgan, W.J.; Gruchalla, R.S.; Pongracic, J.A.; Smartt, E.; Stout, J.W.; Iii, R.E. Airborne fungi in the homes of children with asthma in low-income urban communities: The Inner-City Asthma Study. J. Allergy Clin. Immunol. 2004, 114, 599–606. [Google Scholar] [CrossRef]
- Barnes, C.; Portnoy, J.; Sever, M.; Arbes, S., Jr.; Vaughn, B.; Zeldin, D.C. Comparison of enzyme immunoassay-based assays for environmental Alternaria alternata. Ann. Allergy Asthma Immunol. 2006, 97, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Schell, W.A. Unusual fungal pathogens in fungal rhinosinusitis. Otolaryngol. Clin. N. Am. 2000, 33, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Fujimura, M.; Amaike, S.; Matsumoto, Y.; Kitagawa, M.; Matsuda, T. Eosinophilic pneumonia caused by Alternaria alternata. Allergy 1997, 52, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, Z.; Kang, S.; Sivalingam, J.; Rapuano, C.J.; Cohen, E.J.; Hammersmith, K.M. Voriconazole in the management of Alternaria keratitis. Cornea 2006, 25, 242–244. [Google Scholar] [CrossRef]
- Romano, C.; Paccagnini, E.; Difonzo, E.M. Onychomycosis caused by Alternaria spp. in Tuscany Italy from 1985 to 1999. Mycoses 2001, 44, 73–76. [Google Scholar] [CrossRef]
- Mayser, P.; Nilles, M.; de Hoog, G.S. Case report. Cutaneous phaeohyphomycosis due to Alternaria alternata. Mycoses 2002, 45, 338–340. [Google Scholar] [CrossRef]
- Singh, B.; Denning, D.W. Allergic bronchopulmonary mycosis due to Alternaria: Case report and review. Med. Mycol. Case Rep. 2012, 1, 20–23. [Google Scholar] [CrossRef]
- Chowdhary, A.; Agarwal, K.; Randhawa, H.S.; Kathuria, S.; Gaur, S.N.; Najafzadeh, M.J.; Roy, P.; Arora, N.; Khanna, G.; Meis, J.F. A rare case of allergic bronchopulmonary mycosis caused by Alternaria alternata. Med. Mycol. 2012, 50, 890–896. [Google Scholar] [CrossRef]
- Airola, K.; Petman, L.; Makinen-Kiljunen, S. Clustered sensitivity to fungi: Anaphylactic reactions caused by ingestive allergy to yeasts. Ann. Allergy Asthma Immunol. 2006, 97, 294–297. [Google Scholar] [CrossRef]
- Neukirch, C.; Henry, C.; Leynaert, B.; Liard, R.; Bousquet, J.; Neukirch, F. Is sensitization to Alternaria alternata a risk factor for severe asthma? A population-based study. J. Allergy Clin. Immunol. 1999, 103, 709–711. [Google Scholar] [CrossRef]
- Black, P.N.; Udy, A.A.; Brodie, S.M. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy 2000, 55, 501–504. [Google Scholar] [CrossRef]
- Targonski, P.V.; Persky, V.W.; Ramekrishnan, V. Effect of environmental molds on risk of death from asthma during the pollen season. J. Allergy Clin. Immunol. 1995, 95 Pt 1, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Twaroch, T.E.; Curin, M.; Valenta, R.; Swoboda, I. Mold allergens in respiratory allergy: From structure to therapy. Allergy Asthma Immunol. Res. 2015, 7, 205–220. [Google Scholar] [CrossRef]
- Ricci, S.; Bruni, M.; Meriggi, A.; Corsico, R. Aerobiological monitoring of Alternaria fungal spores: A comparison between surveys in 1992 and 1993 and local meteorological conditions. Aerobiologia 1995, 11, 195–199. [Google Scholar] [CrossRef]
- Gravesen, S. On the connection between the occurrence of airborne microfungi and allergy symptoms. Grana 1981, 20, 225–227. [Google Scholar] [CrossRef]
- Mitakakis, T.Z.; Barnes, C.; Tovey, E.R. Spore germination increases allergen release from Alternaria. J. Allergy Clin. Immunol. 2001, 107, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Feo Brito, F.; Alonso, A.M.; Carnés, J.; Martín-Martín, R.; Fernández-Caldas, E.; Galindo, P.A.; Alfaya, T.; Amo-Salas, M. Correlation between, Alt a 1 levels and clinical symptoms in Alternaria alternata-monosensitized patients. J. Investig. Allergol. Clin. Immunol. 2012, 22, 154–159. [Google Scholar] [PubMed]
- Halonen, M.; Stern, D.A.; Wright, A.L.; Taussig, L.M.; Martinez, F.D. Alternaria as a major allergen for asthma in children raised in a desert environment. Am. J. Respir. Crit. Care Med. 1997, 155, 1356–1361. [Google Scholar] [CrossRef]
- Peat, J.K.; Tovey, E.; Mellis, C.M.; Leeder, S.R.; Woolcock, A.J. Importance of house dust mite and Alternaria allergens in childhood asthma: An epidemiological study in two climatic regions of Australia. Clin. Exp. Allergy 1993, 23, 812–820. [Google Scholar] [CrossRef]
- Lehrer, S.B.; Aukrust, L.; Salvaggio, J.E. Respiratory allergy induced by fungi. Clin. Chest Med. 1983, 4, 23–41. [Google Scholar] [CrossRef]
- Horner, W.E.; Helbling, A.; Salvaggio, J.E.; Lehrer, S.B. Fungal allergens. Clin. Microbiol. Rev. 1995, 8, 161–179. [Google Scholar] [CrossRef]
- Canova, C.; Heinrich, J.; Anto, J.M.; Leynaert, B.; Smith, M.; Kuenzli, N.; Zock, J.-P.; Janson, C.; Cerveri, I.; De Marco, R.; et al. The influence of sensitisation to pollens and moulds on seasonal variations in asthma attacks. Eur. Respir. J. 2013, 42, 935–945. [Google Scholar] [CrossRef]
- Rodriguez-Rajo, F.J.; Iglesias, I.; Jato, V. Variation assessment of airborne Alternaria and Cladosporium spores at different bioclimatical conditions. Mycol. Res. 2005, 109 Pt 4, 497–507. [Google Scholar] [CrossRef] [PubMed]
- de Ana, S.G.; Torres-Rodriguez, J.M.; Ramirez, E.A.; Garcia, S.M.; Belmonte-Soler, J. Seasonal distribution of Alternaria, Aspergillus, Cladosporium and Penicillium species isolated in homes of fungal allergic patients. J. Investig. Allergol. Clin. Immunol. 2006, 16, 357–363. [Google Scholar] [PubMed]
- Chakrabarti, H.S.; Das, S.; Gupta-Bhattacharya, S. Outdoor airborne fungal spora load in a suburb of Kolkata India: Its variation meteorological determinants and health impact. Int. J. Environ. Health Res. 2012, 22, 37–50. [Google Scholar] [CrossRef]
- Kasprzyk, I. Aeromycology-main research fields of interest during the last 25 years. Ann. Agric. Environ. Med. 2008, 15, 1–7. [Google Scholar] [PubMed]
- Pulimood, T.B.; Corden, J.M.; Bryden, C.; Sharples, L.; Nasser, S.M. Epidemic asthma and the role of the fungal mold Alternaria alternata. J. Allergy Clin. Immunol. 2007, 120, 610–617. [Google Scholar] [CrossRef]
- Wolf, J.; O’Neill, N.R.; Rogers, C.A.; Muilenberg, M.L.; Ziska, L.H. Elevated atmospheric carbon dioxide concentrations amplify Alternaria alternata sporulation and total antigen production. Environ. Health Perspect. 2010, 118, 1223–1228. [Google Scholar] [CrossRef]
- Pang, C.; Bian, S.N.; Liu, C.H.; Guo, L.L.; Cui, Y.; Lin, F.; Yin, X.; Liu, C.; Guan, K. The characteristics and change of aeroallergens in children from 2015 to 2020 in a hospital of pediatric in Beijing. Zhonghua YuFang YiXue ZaZhi 2021, 55, 840–846. [Google Scholar]
- D’Amato, G.; Chatzigeorgiou, G.; Corsico, R.; Gioulekas, D.; Jager, L.; Jager, S.; Kontou-Fili, K.; Kouridakis, S.; Liccardi, G.; Meriggi, A.; et al. Evaluation of the prevalence of skin prick test positivity to Alternaria and Cladosporium in patients with suspected respiratory allergy. A European multicenter study promoted by the Subcommittee on Aerobiology and Environmental Aspects of Inhalant Allergens of the European Academy of Allergology and Clinical Immunology. Allergy 1997, 52, 711–716. [Google Scholar]
- Bousquet, P.J.; Hooper, R.; Kogevinas, M.; Jarvis, D.; Burney, P. Number of allergens to be tested to assess allergenic sensitization in epidemiologic studies: Results of the European Community Respiratory Health Survey I. Clin. Exp. Allergy 2007, 37, 780–787. [Google Scholar] [CrossRef]
- Mari, A.; Schneider, P.; Wally, V.; Breitenbach, M.; Simon-Nobbe, B. Sensitization to fungi: Epidemiology comparative skin tests and IgE reactivity of fungal extracts. Clin. Exp. Allergy 2003, 33, 1429–1438. [Google Scholar] [CrossRef]
- Crameri, R.; Garbani, M.; Rhyner, C.; Huitema, C. Fungi: The neglected allergenic sources. Allergy 2014, 69, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, E.; Nittner-Marszalska, M.; Fal, A.M.; Kuzniar, J.; Nikiel, E.; Malolepszy, J. Allergy to mould allergens as a risk factor for bronchial asthma in patients suffering from allergic rhinitis. Pol. Merkur. Lek. 2003, 14, 388–392. [Google Scholar]
- Heinzerling, L.; Frew, A.J.; Bindslev-Jensen, C.; Bonini, S.; Bousquet, J.; Bresciani, M.; Carlsen, K.-H.; van Cauwenberge, P.; Darsow, U.; Fokkens, W.J.; et al. Standard skin prick testing and sensitization to inhalant allergens across Europe-a survey from the G.A.LEN network. Allergy 2005, 60, 1287–1300. [Google Scholar] [CrossRef]
- Burbach, G.J.; Heinzerling, L.M.; Edenharter, G.; Bachert, C.; Bindslev-Jensen, C.; Bonini, S.; Bousquet, J.; Bousquet-Rouanet, L.; Bousquet, P.J.; Bresciani, M.; et al. GA(2)LEN skin test study, I.I.: Clinical relevance of inhalant allergen sensitizations in Europe. Allergy 2009, 64, 1507–1515. [Google Scholar] [CrossRef]
- Heinzerling, L.M.; Burbach, G.J.; Edenharter, G.; Bachert, C.; Bindslev-Jensen, C.; Bonini, S.; Bousquet, J.; Bousquet-Rouanet, L.; Bresciani, M.; Bruno, A.; et al. GA(2)LEN skin test study, I.: GA(2)LEN harmonization of skin prick testing: Novel sensitization patterns for inhalant allergens in Europe. Allergy 2009, 64, 1498–1506. [Google Scholar] [CrossRef]
- Alergológica Factores Epidemiológicos Clínicos y Socioeconómicos de las Enfermedades Alérgicas en España; Sociedad Española de Alergología e Inmunología Clínica AeI: Madrid, Spain, 1995.
- López Couso, V.P.; Tortajada-Girbés, M.; Rodriguez Gil, D.; Martínez Quesada, J.; Palacios Pelaez, R. Fungi Sensitization in Spain: Importance of the Alternaria alternata Species and Its Major Allergen Alt a 1 in the Allergenicity. J. Fungi 2021, 7, 631. [Google Scholar] [CrossRef] [PubMed]
- Arbes, S.J., Jr.; Gergen, P.J.; Elliott, L.; Zeldin, D.C. Prevalences of positive skin test responses to 10 common allergens in the U.S. population: Results from the third National Health and Nutrition Examination Survey. J. Allergy Clin. Immunol. 2005, 116, 377–383. [Google Scholar] [CrossRef]
- D’Amato, G.; Spieksma, F.T. Aerobiologic and clinical aspects of mould allergy in Europe. Allergy 1995, 50, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Horst, M.; Hejjaoui, A.; Horst, V.; Michel, F.B.; Bousquet, J. Double-blind placebo-controlled rush immunotherapy with a standardized Alternaria extract. J. Allergy Clin. Immunol. 1990, 85, 460–472. [Google Scholar] [CrossRef]
- Randriamanantany, Z.A.; Annesi-Maesano, I.; Moreau, D.; Raherison, C.; Charpin, D.; Kopferschmitt, C.; Lavaud, F.; Taytard, A.; De Blay, F.; Caillaud, D. Alternaria sensitization and allergic rhinitis with or without asthma in the French Six Cities study. Allergy 2010, 65, 368–375. [Google Scholar] [CrossRef]
- Ciprandi, G.; Cirillo, I. Monosensitization and polysensitization in allergic rhinitis. Eur. J. Intern. Med. 2011, 22, e75–e79. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Valero, A.; Loureiro, C.; Dávila, I.; Martinez-Cócera, C.; Murio, C.; Rico, P.; Palomino, R. Iberian study of aeroallergens sensitisation in allergic rhinitis. Eur. Ann. Allergy Clin. Immunol. 2006, 38, 186–194. [Google Scholar]
- Cardona Villa, R.; Yépes Núñez, J.J.; Salgado Vélez, H.; Montoya Guarín, C.J. Aspectos básicos de las reacciones de hipersensibilidad y la alergia. In Alergia. Abordaje Clínico Diagnóstico y Tratamiento; Editorial Medica Panamericana Sa de: Madrid, Spain, 2010; p. 736. [Google Scholar]
- Radauer, C.; Bublin, M.; Wagner, S.; Mari, A.; Breiteneder, H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J. Allergy Clin. Immunol. 2008, 121, 847–852.e7. [Google Scholar] [CrossRef]
- Kobayashi, T.; Iijima, K.; Radhakrishnan, S.; Mehta, V.; Vassallo, R.; Lawrence, C.B.; Cyong, J.-C.; Pease, L.R.; Oguchi, K.; Kita, H. Asthma-related environmental fungus Alternaria activates dendritic cells and produces potent Th2 adjuvant activity. J. Immunol. 2009, 182, 2502–2510. [Google Scholar] [CrossRef]
- Borger, P.; Koeter, G.H.; Timmerman, J.A.; Vellenga, E.; Tomee, J.F.; Kauffman, H.F. Proteases from Aspergillus fumigatus induce interleukin (IL)-6 and I.L.-8 production in airway epithelial cell lines by transcriptional mechanisms. J. Infect. Dis. 1999, 180, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, H.F.; Tomee, J.F.C.; van de Riet, M.A.; Timmerman, A.J.B.; Borger, P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J. Allergy Clin. Immunol. 2000, 105, 1185–1193. [Google Scholar] [CrossRef]
- Tai, H.-Y.; Tam, M.F.; Chou, H.; Peng, H.-J.; Su, S.-N.; Perng, D.-W.; Shen, H.-D. Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. Allergy 2006, 61, 382–388. [Google Scholar] [CrossRef]
- Lamhamedi-Cherradi, S.-E.; Martin, R.E.; Ito, T.; Kheradmand, F.; Corry, D.B.; Liu, Y.-J.; Moyle, M. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of I.L.-12. J. Immunol. 2008, 180, 6000–6009. [Google Scholar] [CrossRef]
- Boitano, S.; Flynn, A.N.; Sherwood, C.L.; Schulz, S.M.; Hoffman, J.; Gruzinova, I.; Daines, M.O. Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via P.A.R2. American journal of physiology. Lung Cell. Mol. Physiol. 2011, 300, L605–L614. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Chuang, J.G.; Su, Y.Y.; Chiang, B.L.; Lin, Y.S.; Chow, L.P. The protease allergen Pen c 13 induces allergic airway inflammation and changes in epithelial barrier integrity and function in a murine model. J. Biol. Chem. 2011, 286, 26667–26679. [Google Scholar] [CrossRef]
- Snelgrove, R.J.; Gregory, L.G.; Peiró, T.; Akthar, S.; Campbell, G.A.; Walker, S.A.; Lloyd, C.M. Alternaria-derived serine protease activity drives I.L.-33-mediated asthma exacerbations. J. Allergy Clin. Immunol. 2014, 134, 583–592.e6. [Google Scholar] [CrossRef] [PubMed]
- Kustrzeba-Wojcicka, I.; Siwak, E.; Terlecki, G.; Wolanczyk-Medrala, A.; Medrala, W. Alternaria alternata and its allergens: A comprehensive review. Clin. Rev. Allergy Immunol. 2014, 47, 354–365. [Google Scholar] [CrossRef]
- Arroyo, L.; Puerta, L. Importancia de los epítopes de carbohidratos en los alérgenos. Rev. Salud UIS 2003, 35, 71–79. [Google Scholar]
- Altmann, F. The role of protein glycosylation in allergy. Int. Arch. Allergy Immunol. 2007, 142, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, J.; Olson, I.; Pacheco, F.; Barnes, C. Affinity purification of a major Alternaria allergen using a monoclonal antibody. Ann. Allergy 1990, 65, 109–114. [Google Scholar]
- Ponton, J. The fungal cell wall and the mechanism of action of anidulafungin. Rev. Iberoam. Micol. 2008, 25, 78–82. [Google Scholar]
- Portnoy, J.; Pacheco, F.; Ballam, Y.; Barnes, C. Separation of Alternaria into protein and carbohydrate fractions with phenyl sepharose. J. Allergy. Clin. Immunol. 1991, 87, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, J.; Pacheco, F.; Ballam, Y.; Barnes, C. The effect of time and extraction buffers on residual protein and allergen content of extracts derived from four strains of Alternaria. J. Allergy Clin. Immunol. 1993, 91, 930–938. [Google Scholar] [CrossRef]
- Esch, R.E. Manufacturing and standardizing fungal allergen products. J. Allergy Clin. Immunol. 2004, 113, 210–215. [Google Scholar] [CrossRef]
- Wills-Karp, M.; Nathan, A.; Page, K.; Karp, C.L. New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol. 2010, 3, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Shreffler, W.G.; Castro, R.R.; Kucuk, Z.Y.; Charlop-Powers, Z.; Grishina, G.; Yoo, S.; Burks, A.W.; Sampson, H.A. The major glycoprotein allergen from Arachis hypogaea Ara h 1, is a ligand of dendritic cell-specific I.C.AM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J. Immunol. 2006, 177, 3677–3685. [Google Scholar] [CrossRef] [PubMed]
- Thorn, J.; Rylander, R. Airways inflammation and glucan in a rowhouse area. Am. J. Respir. Crit. Care Med. 1998, 157 Pt 1, 1798–1803. [Google Scholar] [CrossRef]
- Rylander, R.; Lin, R.H. (1—>3)-beta-D-glucan—Relationship to indoor air-related symptoms allergy and asthma. Toxicology 2000, 152, 47–52. [Google Scholar] [CrossRef]
- Douwes, J. (1—>3)-Beta-D-glucans and respiratory health: A review of the scientific evidence. Indoor Air 2005, 15, 160–169. [Google Scholar] [CrossRef]
- Sánchez, P.; Vélez-Del-Burgo, A.; Suñén, E.; Martínez, J.; Postigo, I. Fungal Allergen and Mold Allergy Diagnosis: Role and Relevance of Alternaria alternata Alt a 1 Protein Family. J. Fungi 2022, 8, 277. [Google Scholar] [CrossRef]
- Vailes, L.; Perzanowski, M.; Wheatley, L.; Platts-Mills, T.; Chapman, M. IgE and IgG antibody responses to recombinant Alt a 1 as a marker of sensitization to Alternaria in asthma and atopic dermatitis. Clin. Exp. Allergy 2001, 31, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Asturias, J.A.; Ibarrola, I.; Ferrer, A.; Andreu, C.; López-Pascual, E.; Quiralte, J.; Florido, F.; Martínez, A. Diagnosis of Alternaria alternata sensitization with natural and recombinant Alt a 1 allergens. J. Allergy Clin. Immunol. 2005, 115, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Deards, M.J.; Montague, A.E. Purification and characterisation of a major allergen of Alternaria alternata. Mol. Immunol. 1991, 28, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Curran, I.H.; Young, N.M.; Burton, M.; Vijay, H.M. Purification and characterization of Alt a-29 from Alternaria alternata. Int. Arch. Allergy Immunol. 1993, 102, 267–275. [Google Scholar] [CrossRef]
- Paris, S.; Debeaupuis, J.P.; Prévost, M.C.; Casotto, M.; Latgé, J.P. The 31 kd major allergen Alt a I.1563, of Alternaria alternata. J. Allergy Clin. Immunol. 1991, 88, 902–908. [Google Scholar] [CrossRef]
- Postigo, I.; Gutiérrez-Rodríguez, A.; Fernández, J.; Guisantes, J.A.; Suñén, E.; Martínez, J. Diagnostic value of Alt a 1, fungal enolase and manganese-dependent superoxide dismutase in the component-resolved diagnosis of allergy to Pleosporaceae. Clin. Exp. Allergy 2011, 41, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Twaroch, T.E.; Arcalís, E.; Sterflinger, K.; Stöger, E.; Swoboda, I.; Valenta, R. Predominant localization of the major Alternaria allergen Alt a 1 in the cell wall of airborne spores. J. Allergy Clin. Immunol. 2012, 129, 1148–1149. [Google Scholar] [CrossRef] [PubMed]
- Green, B.J.; Zinovia Mitakakis, T.; Tovey, E.R. Allergen detection from 11 fungal species before and after germination. J. Allergy Clin. Immunol. 2003, 111, 285–289. [Google Scholar] [CrossRef]
- Gómez-Casado, C.; Murua-García, A.; Garrido-Arandia, M.; González-Melendi, P.; Sánchez-Monge, R.; Barber, D.; Pacios, L.F.; Díaz-Perales, A. Alt a 1 from Alternaria interacts with P.R.5 thaumatin-like proteins. FEBS Lett. 2014, 588, 1501–1508. [Google Scholar] [CrossRef]
- Shankar, J.; Singh, B.P.; Gaur, S.N.; Arora, N. Recombinant glutathione-S-transferase a major allergen from Alternaria alternata for clinical use in allergy patients. Mol. Immunol. 2006, 43, 1927–1932. [Google Scholar] [CrossRef]
- Schneider, P.B.; DU Breitenbach, M. Alternaria alternata N.A.DP-dependent mannitol dehydrogenase is an important fungal allergen. Clin. Exp. Allergy 2007, 36, 1513–1524. [Google Scholar] [CrossRef]
- Achatz, G.; Oberkofler, H.; Lechenauer, E.; Simon, B.; Unger, A.; Kandler, D.; Ebner, C.; Prillinger, H.; Kraft, D.; Breitenbach, M. Molecular cloning of major and minor allergens of Alternaria alternata and Cladosporium herbarum. Mol. Immunol. 1995, 32, 213–227. [Google Scholar] [CrossRef]
- Achatz, G.; Oberkofler, H.; Lechenauer, E.; Simon, B.; Unger, A.; Kandler, D.; Ebner, C.; Prillinger, H.; Kraft, D.; Breitenbach, M. Molecular characterization of Alternaria alternata and Cladosporium herbarum allergens. Adv. Exp. Med. Biol. 1996, 409, 157–161. [Google Scholar]
- Breitenbach, M.; Simon-Nobbe, B. The allergens of Cladosporium herbarum and Alternaria alternata. Chem. Immunol. 2002, 81, 48–72. [Google Scholar] [PubMed]
- Lohman, K. Enzymatic transformation of phosphoglyceric acid into pyruvic and phosphoric acid. Biochem. Z. 1934, 273, 60–72. [Google Scholar]
- Breitenbach, M.; Simon, B.; Probst, G.; Oberkofler, H.; Ferreira, F.; Briza, P.; Achatz, G.; Unger, A.; Ebner, C.; Kraft, D.; et al. Enolases are highly conserved fungal allergens. Int. Arch. Allergy. Immunol. 1997, 113, 114–117. [Google Scholar] [CrossRef]
- Simon-Nobbe, B.; Probst, G.; Kajava, A.V.; Oberkofler, H.; Susani, M.; Crameri, R.; Ferreira, F.; Ebner, C.; Breitenbach, M. IgE-binding epitopes of enolases a class of highly conserved fungal allergens. J. Allergy Clin. Immunol. 2000, 106, 887–895. [Google Scholar] [CrossRef]
- Kustrzeba-Wójcicka, I.; Kmiecik, W. Studies on enolase-a principal allergen of moulds. Preparation and characteristics of enolase from Alternaria alternata. Mikol. Lek. 2001, 8, 141–146. [Google Scholar]
- De Vouge, M.W.; Thaker, A.J.; Zhang, L.; Muradia, G.; Rode, H.; Vijay, H.M. Molecular cloning of IgE–binding fragments of Alternaria alternata allergens. Int. Arch. Allergy Immunol. 1998, 116, 261–268. [Google Scholar] [CrossRef]
- Kiang, J.G.; Tsokos, G.C. Heat shock protein 70 kDa: Molecular biology biochemistry and physiology. Pharmacol. Ther. 1998, 80, 183–201. [Google Scholar] [CrossRef]
- Crameri, R. The problem of cross-reactivity in the diagnosis of fungal allergy. Clin. Exp. Allergy 2011, 41, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ishiguro, A.; Kanbe, T.; Tanaka, K.; Torii, S. Detection of, IgE antibody against Candida albicans enolase and its crossreactivity to Saccharomyces cerevisiae enolase. Clin. Exp. Allergy 1995, 25, 522–528. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Chou, H.; Tam, M.F.; Tang, R.-B.; Lai, H.-Y.; Shen, H.-D. Characterization of enolase allergen from Rhodotorula mucilaginosa. J. Biomed. Sci. 2002, 9, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Flückiger, S.; Scapozza, L.; Mayer, C.; Blaser, K.; Folkers, G.; Crameri, R. Immunological and structural analysis of IgE-mediated cross-reactivity between manganese superoxide dismutases. Int. Arch. Allergy Immunol. 2002, 128, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Horner, W.E.; Reese, G.; Lehrer, S.B. Identification of the allergen Psi c 2 from the basidiomycete Psilocybe cubensis as a fungal cyclophilin. Int. Arch. Allergy Immunol. 1995, 107, 298–300. [Google Scholar] [CrossRef]
- Flückiger, S.; Fijten, H.; Whitley, P.; Blaser, K.; Crameri, R. Cyclophilins a new family of cross-reactive allergens. Eur. J. Immunol. 2002, 32, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Glaser, A.G.; Limacher, A.; Fluckiger, S.; Scheynius, A.; Scapozza, L.; Crameri, R. Analysis of the cross-reactivity and of the 1.5 A crystal structure of the Malassezia sympodialis Mala s 6 allergen a member of the cyclophilin pan-allergen family. Biochem. J. 2006, 396, 41–49. [Google Scholar] [CrossRef]
- Shankar, J.; Gupta, P.; Sridhara, S.; Singh, B.P.; Gaur, S.N.; Arora, N. Immunobiochemical analysis of cross-reactive glutathione-S-transferase allergen from different fungal sources. Immunol. Investig. 2005, 34, 37–51. [Google Scholar] [CrossRef]
- Limacher, A.; Glaser, A.G.; Meier, C.; Schmid-Grendelmeier, P.; Zeller, S.; Scapozza, L.; Crameri, R. Cross-reactivity and 1.4-A crystal structure of Malassezia sympodialis thioredoxin (Mala s 13), a member of a new pan-allergen family. J. Immunol. 2006, 178, 389–396. [Google Scholar] [CrossRef]
- Glaser, A.G.; Menz, G.; Kirsch, A.I.; Zeller, S.; Crameri, R.; Rhyner, C. Auto- and cross-reactivity to thioredoxin allergens in allergic bronchopulmonary aspergillosis. Allergy 2008, 63, 1617–1623. [Google Scholar] [CrossRef]
- Chou, H.; Wu, K.-G.; Yeh, C.-C.; Tai, H.-Y.; Tam, M.F.; Chen, Y.-S.; Shen, H.-D. The transaldolase a novel allergen of Fusarium proliferatum demonstrates IgE cross-reactivity with its human analogue. PLoS ONE 2014, 9, e103488. [Google Scholar] [CrossRef]
- Gabriel, M.F.; Postigo, I.; Gutiérrez-Rodríguez, A.; Suñén, E.; Guisantes, J.A.; Fernández, J.; Tomaz, C.T.; Martínez, J. Alt a 15 is a new cross-reactive minor allergen of Alternaria alternata. Immunobiology 2016, 221, 153–160. [Google Scholar] [CrossRef]
- Lai, H.-Y.; Tam, M.F.; Tang, R.-B.; Chou, H.; Chang, C.-Y.; Tsai, J.-J.; Shen, H.-D. cDNA cloning and immunological characterization of a newly identified enolase allergen from Penicillium citrinum and Aspergillus fumigatus. Int. Arch. Allergy Immunol. 2002, 127, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Weichel, M.; Schmid-Grendelmeier, P.; Flückiger, S.; Breitenbach, M.; Blaser, K.; Crameri, R. Nuclear transport factor 2 represents a novel cross-reactive fungal allergen. Allergy 2003, 58, 198–206. [Google Scholar] [CrossRef]
- Gabriel, M.F.; Postigo, I.; Gutiérrez-Rodríguez, A.; Suñén, E.; Guisantes, J.; Tomaz, C.T.; Martínez, J. Characterisation of Alternaria alternata manganese-dependent superoxide dismutase a cross-reactive allergen homologue to Asp f 6. Immunobiology 2015, 220, 851–858. [Google Scholar] [CrossRef]
- Herrera-Mozo, I.; Ferrer, B.; Luis Rodriguez-Sanchez, J.; Juarez, C. Description of a novel panallergen of cross-reactivity between moulds and foods. Immunol. Investig. 2006, 35, 181–197. [Google Scholar] [CrossRef]
- Rid, R.; Simon-Nobbe, B.; Langdon, J.; Holler, C.; Wally, V.; Pöll, V.; Ebner, C.; Hemmer, W.; Hawranek, T.; Lang, R.; et al. Cladosporium herbarum translationally controlled tumor protein (TCTP) is an IgE-binding antigen and is associated with disease severity. Mol. Immunol. 2008, 45, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Rid, R.; Önder, K.; MacDonald, S.; Lang, R.; Hawranek, T.; Ebner, C.; Hemmer, W.; Richter, K.; Simon-Nobbe, B.; Breitenbach, M. Alternaria alternata T.C.TP a novel cross-reactive ascomycete allergen. Mol. Immunol. 2009, 46, 3476–3487. [Google Scholar] [CrossRef] [PubMed]
- Popescu, F.D. Cross-reactivity between aeroallergens and food allergens. World J. Methodol. 2015, 5, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.F.; González-Delgado, P.; Postigo, I.; Fernández, J.; Soriano, V.; Cueva, B.; Martínez, J. From respiratory sensitization to food allergy: Anaphylactic reaction after ingestion of mushrooms (Agaricus bisporus). Med. Mycol. Case Rep. 2015, 8, 14–16. [Google Scholar] [CrossRef]
- Moreno, A.; Pineda, F.; Alcover, J.; Rodríguez, D.; Palacios, R.; Martínez-Naves, E. Orthologous Allergens and Diagnostic Utility of Major Allergen Alt a 1. Allergy Asthma Immunol. Res. 2016, 8, 428–437. [Google Scholar] [CrossRef]
- Dreborg, S.; Grimmer, O. Biological standardization of allergen extracts/preparations. Arb. Aus Paul-Ehrlich-Inst. Georg.-Speyer-Haus Ferdinand-Blum-Inst. Frankf. a.M 1983, 78, 77–82. [Google Scholar]
- Canonica, G.W.; Ansotegui, I.J.; Pawankar, R.; Schmid-Grendelmeier, P.; van Hage, M.; Baena-Cagnani, C.E.; Melioli, G.; Nunes, C.; Passalacqua, G.; Rosenwasser, L.; et al. A WAO-ARIA-GA²LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ. J. 2013, 6, 1–17. [Google Scholar] [CrossRef]
- Sastre, J. Molecular diagnosis in allergy. Clin. Exp. Allergy 2010, 40, 1442–1460. [Google Scholar] [CrossRef]
- Quan, P.L.; Sabaté-Brescó, M.; D’Amelio, C.M.; Pascal, M.; García, B.E.; Gastaminza, G.; Blanca-López, N.; Alvarado, M.I.; Fernández, J.; Moya, C.; et al. Validation of a commercial allergen microarray platform for specific immunoglobulin E. detection of respiratory and plant food allergens. Ann. Allergy Asthma Immunol. 2022, 128, 283–290.e4. [Google Scholar] [CrossRef]
- Valenta, R.; Lidholm, J.; Niederberger, V.; Hayek, B.; Kraft, D.; Grönlund, H. The recombinant allergen-based concept of component-resolved diagnostics and immunotherapy (CRD and C.R.IT). Clin. Exp. Allergy 1999, 29, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Lockey, R.; Malling, H.-J.; the WHO Panel Members. Allergen immunotherapy: Therapeutic vaccines for allergic diseases. J. Allergy Clin. Immunol. 1998, 53, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Criado Molina, A.; Guerra Pasadas, F.; Daza Munoz, J.C.; Moreno Aguilar, C.; Almeda Llamas, E.; Munoz Gomariz, E.; Font, U.P.; Alonso, D.C.; Germán, C.M.; Sánchez, G.P. Immunotherapy with an oral Alternaria extract in childhood asthma. Clinical safety and efficacy and effects on in vivo and in vitro parameters. Allergol. Immunopathol. 2002, 30, 319–330. [Google Scholar] [CrossRef]
- Dreborg, S.; Agrell, B.; Foucard, T.; Kjellman, N.I.; Koivikko, A.; Nilsson, S. A double-blind multicenter immunotherapy trial in children using a purified and standardized Cladosporium herbarum preparation. I. Clinical results. Allergy 1986, 41, 131–140. [Google Scholar] [CrossRef]
- Malling, H.J.; Dreborg, S.; Weeke, B. Diagnosis and immunotherapy of mould allergy. V. Clinical efficacy and side effects of immunotherapy with Cladosporium herbarum. Allergy 1986, 41, 507–519. [Google Scholar] [CrossRef]
- Karlsson, R.; Agrell, B.; Dreborg, S.; Foucard, T.; Kjellman, N.I.; Koivikko, A.; Einarsson, R. A double-blind multicenter immunotherapy trial in children using a purified and standardized Cladosporium herbarum preparation II. In vitro results. Allergy 1986, 41, 141–150. [Google Scholar] [CrossRef]
- Lizaso, M.T.; Martínez, A.; Asturias, J.; Algorta, J.; Madariaga, B.; Labarta, N.; Tabar, A.I. Biological standardization and maximum tolerated dose estimation of an Alternaria alternata allergenic extract. J. Investig. Allergol. Clin. Immunol. 2006, 16, 94–103. [Google Scholar] [PubMed]
- Tabar, A.I.; Lizaso, M.T.; García, B.E.; Gómez, B.; Echechipía, S.; Aldunate, M.T.; Madariaga, B.; Martínez, A. Double-blind placebo-controlled study of Alternaria alternata immunotherapy: Clinical efficacy and safety. Pediatr. Allergy Immunol. 2008, 19, 67–75. [Google Scholar] [CrossRef]
- Lizaso, M.T.; Tabar, A.I.; García, B.E.; Gómez, B.; Algorta, J.; Asturias, J.; Martínez, A. Double-blind placebo-controlled Alternaria alternata immunotherapy: In vivo and in vitro parameters. Pediatr. Allergy Immunol. 2008, 19, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Kuna, P.; Kaczmarek, J.; Kupczyk, M. Efficacy and safety of immunotherapy for allergies to Alternaria alternata in children. J. Allergy Clin. Immunol. 2011, 127, 502–508. [Google Scholar] [CrossRef]
- Kaad, P.H.; Ostergaard, P.A. The hazard of mould hyposensitization in children with asthma. Clin. Allergy 1982, 12, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, P.A.; Kaad, P.H.; Kristensen, T. A prospective study on the safety of immunotherapy in children with severe asthma. Allergy 1986, 41, 588–593. [Google Scholar] [CrossRef]
- Patterson, R.; Suszko, I.M.; Bacal, E.; Zeiss, C.R.; Kelly, J.F.; Pruzansky, J.J. Reduced allergenicity of high molecular weight ragweed polymers. J. Allergy Clin. Immunol. 1979, 63, 47–50. [Google Scholar] [CrossRef] [PubMed]
- de la Losa, F.P. Expresión y Purificación del Alérgeno, Alt a 1 de Alternaria alternata Implicación en la Hipersensibilidad Tipo I. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2005. [Google Scholar]
- Tabar, A.I.; Prieto, L.; Alba, P.; Nieto, A.; Rodríguez, M.; Torrecillas, M.; Huertas, B.; Gómez, E.; Fernández, F.J.; Blanca, M.; et al. Double-blind randomized placebo-controlled trial of allergen-specific immunotherapy with the major allergen Alt a 1. J. Allergy Clin. Immunol. 2019, 144, 216–223.e3. [Google Scholar] [CrossRef]
- Rodríguez, D.; Tabar, A.I.; Castillo, M.; Martínez-Gomariz, M.; Dobski, I.C.; Palacios, R. Changes in the Sensitization Pattern to Alternaria alternata Allergens in Patients Treated with Alt a 1 Immunotherapy. J. Fungi 2021, 7, 974. [Google Scholar] [CrossRef]
- Kustrzeba-Wójcicka, I.; Golczak, M. Enolase from Candida albicans—Purification and characterization. Comp. Biochem. Physiol. Part B. Biochem. Mol. Biol. 2000, 126, 109–120. [Google Scholar] [CrossRef]
| Allergen 1 | Protein Type | MW (kDa) | Relevance | Potential Cross-Reactive Allergens (Structural Database of Allergenic Proteins E Score) IUIS/Allergen.org/Allergome.org Data Bases |
|---|---|---|---|---|
| Alt a 1 | It has a unique, dimeric β-barrel structure that define a new protein family with unknown function found exclusively in fungi. | 16.4 and 15.3 bands | Major allergen | Alt b 1 Alternaria brasicola and other 138 Alternaria species Ulo c 1 Ulocladium chartarum Emb a 1 Embellisia allii and other 6 Embellisia species Nim c 1 Nimbya celosiae and other 4 Nimbya species Sin fu 1 Sinomyces fusoideus Ste b 1 Stemphylium botryosum and other 2 Stemphylium species Ulo c 1 Ulocladium chartarum and other 11 Ulocladium species |
| Alt a 3 | Heat shock protein 70 | 85 | Minor allergen | Pen c 19 Penicillium citrinum (1.9 × 10−27) Mala s 10 Malassezia sympodialis (1.2 × 10−3) |
| Alt a 4 | Disulfide isomerase | 57 | Minor allergen | |
| Alt a 5 | Ribosomal protein P2 | 11 | Minor allergen | Fus c 1 Fusarium culmorum (3.4 × 10−27) Cla h 5 Cladosporium herbarum (8.4 × 10−25) Asp f 8 Aspergillus fumigatus (2.0 × 10−22) |
| Alt a 6 | Enolase | 45 | Minor allergen | Cla h 6 Cladosporium herbarum (1.2 × 10−157) Asp f 22 Aspergillus fumigatus (1.9 × 10−154) Pen c 22 Penicillium citrinum (4.7 × 10−153) Cur l 2 Curvularia lunata (8.4 × 10−153) Rho m 1 Rhodotorula mucilaginosa (2.5 × 10−129) |
| Alt a 7 | Flavodoxin, YCP4 protein | 22 | Minor allergen | Cla h 7 Cladosporium herbarum (9.4 × 10−61) |
| Alt a 8 | Mannitol dehydrogenase | 29 | Minor allergen | Cla h 8 Cladosporium herbarum (6.6 × 10−91) |
| Alt a10 | Aldehyde dehydrogenase | 53 | Minor allergen | Cla h 10 Cladosporium herbarum (5.9 × 10−168) |
| Alt a 12 | Aid ribosomal protein P1 | 11 | Minor allergen | Cla h 12 Cladosporium herbarum (1.8 × 10−30) Pen cr 26 Penicillium crustosum (4.2 × 10−28) Pen b 26 Penicillium brevicompactum (6.8 × 10−28) |
| Alt a 13 | Glutathione-transferase | 26 | Minor allergen | |
| Alt a 14 | Manganese SO dismutase | 24 | Minor allergen | Asp f 6 Aspergillus fumigatus (5.5 × 10−48) Mala s 11 Malassezia sympodialis (4.9 × 10−36) |
| Alt a 15 | Vacuolar serine protease | 58 | Minor allergen | Cur l 4 Curvularia lunata (4.3 × 10−152) Cla h 9 Cladosporium herbarum (5.3 × 10−119) Pen o 18 Penicillium oxalicum (2.4 × 10−114) Asp f 18 Aspergillus fumigatus (4.8 × 10−111) Pen ch 18 Penicillium chrysogenum (7.9 × 10−111) Cla c 9 Cladosporium cladosporioides (1.2 × 10−99) Rho m 2 Rhodotorula mucilaginosa (1.6 × 10−73) Tri r 2 Trichophyton rubrum (1.6 × 10−37) Pen ch 13 Penicillium chrysogenum (1.1 × 10−23) Asp v 13 Aspergillus versicolor (1.5 × 10−20) Asp f 13 Aspergillus fumigatus (2.8 × 10−20) Asp o 13 Aspergillus oryzae (9.8 × 10−19) Asp fl 13 Aspergillus flavus (9.8 × 10−19) Pen c 13 Penicillium citrinum (5.3 × 10−15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abel-Fernández, E.; Martínez, M.J.; Galán, T.; Pineda, F. Going over Fungal Allergy: Alternaria alternata and Its Allergens. J. Fungi 2023, 9, 582. https://doi.org/10.3390/jof9050582
Abel-Fernández E, Martínez MJ, Galán T, Pineda F. Going over Fungal Allergy: Alternaria alternata and Its Allergens. Journal of Fungi. 2023; 9(5):582. https://doi.org/10.3390/jof9050582
Chicago/Turabian StyleAbel-Fernández, Eva, María José Martínez, Tania Galán, and Fernando Pineda. 2023. "Going over Fungal Allergy: Alternaria alternata and Its Allergens" Journal of Fungi 9, no. 5: 582. https://doi.org/10.3390/jof9050582
APA StyleAbel-Fernández, E., Martínez, M. J., Galán, T., & Pineda, F. (2023). Going over Fungal Allergy: Alternaria alternata and Its Allergens. Journal of Fungi, 9(5), 582. https://doi.org/10.3390/jof9050582






