Antifungal Susceptibility of Oral Candida Isolates from Mother-Infant Dyads to Nystatin, Fluconazole, and Caspofungin
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Sample
2.3. Clinical Sample Collection and Isolate Verification
2.4. DNA Extraction and Library Preparation for Whole Genome Sequencing
2.5. Laboratory Preparation of Antifungal Drugs
2.6. In Vitro Antifungal Drug Susceptibility Testing
2.7. Spectrophotometric Measurement and Determination of Minimum Inhibitory Concentration (MIC) of Antifungal Drugs
2.8. Bioinformatic Analysis of Antifungal Medication Resistance Genes
2.9. Data and Statistical Analysis
3. Results
3.1. MIC of Clinical Candida Isolates
3.2. Isolate Susceptibilities to Antifungal Drugs
3.3. Candida MIC Distribution among Mother-Child Dyads
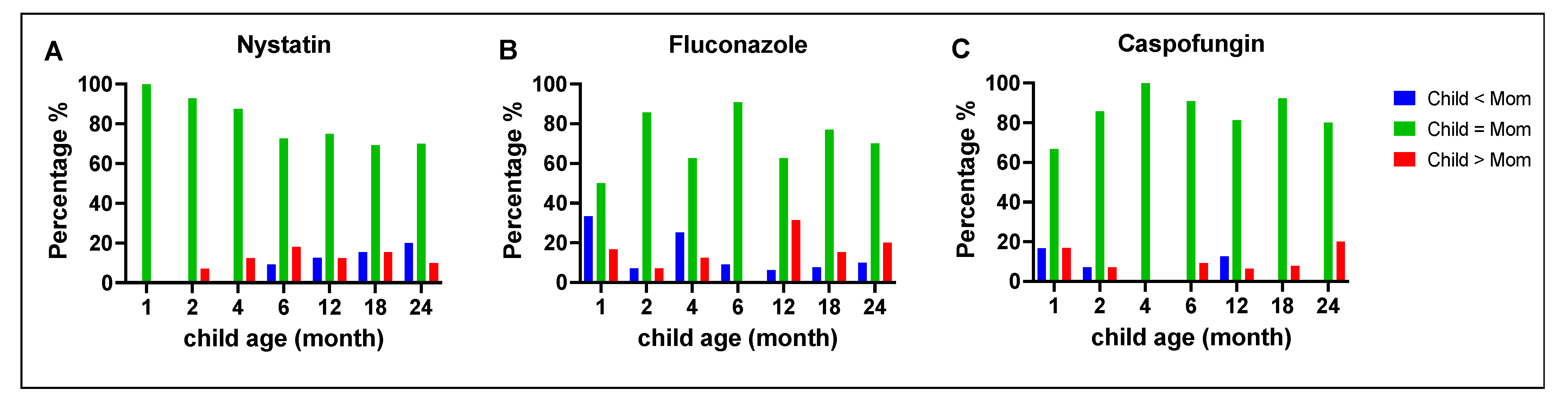
3.4. Comparison of Antifungal Susceptibility between Mothers and Children
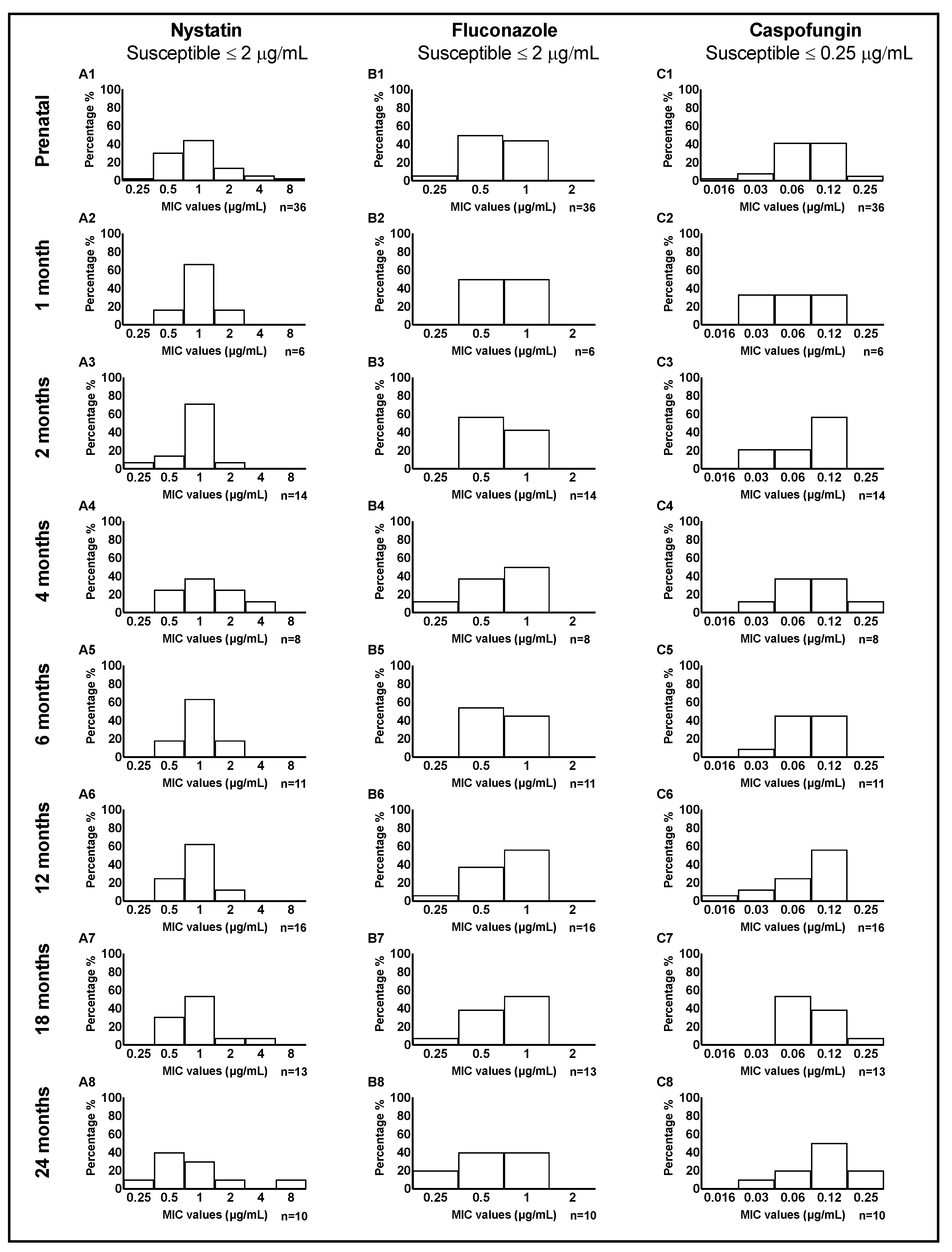
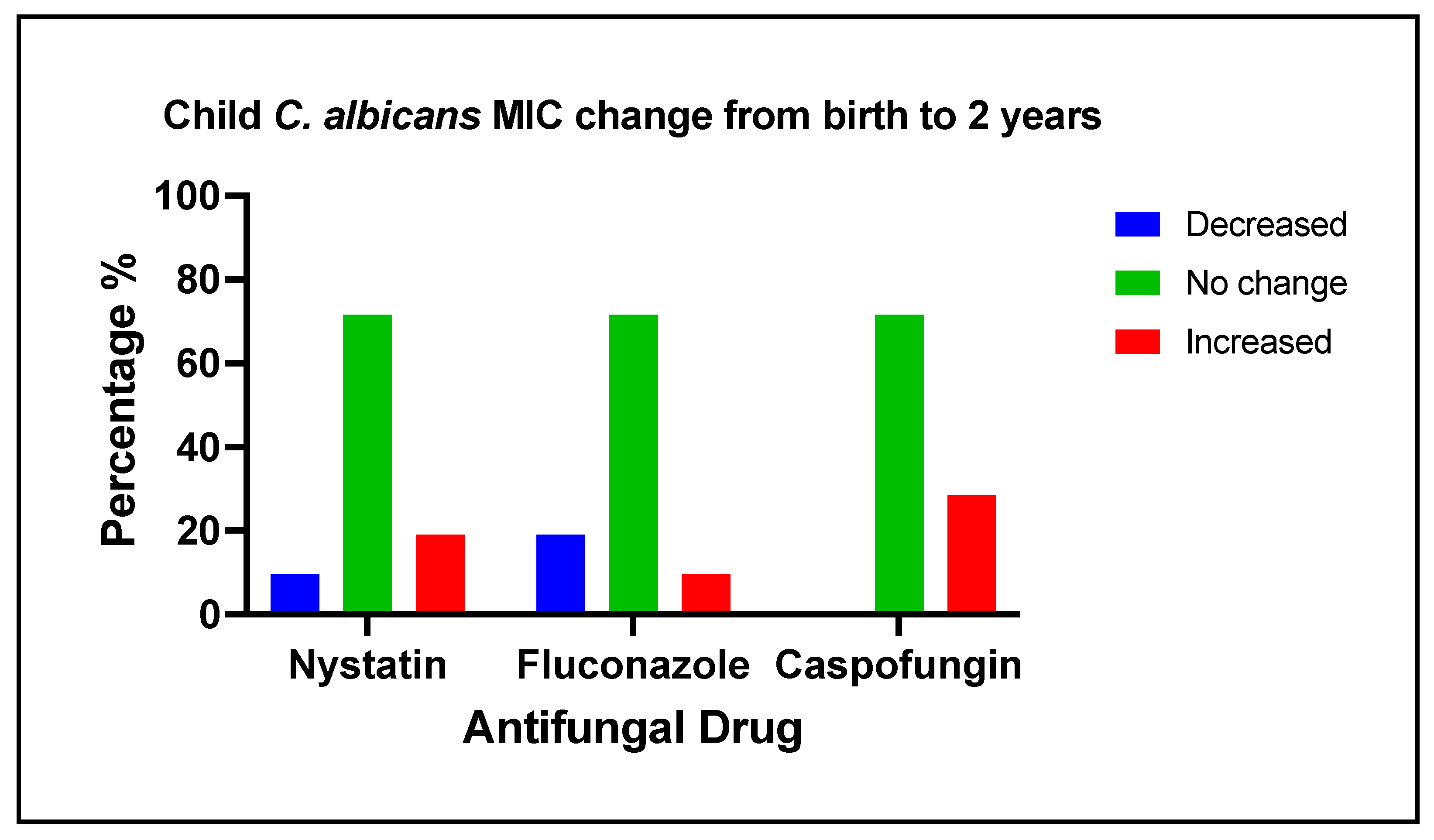
3.5. Changes of Antifungal Medication Susceptibility in Early Life
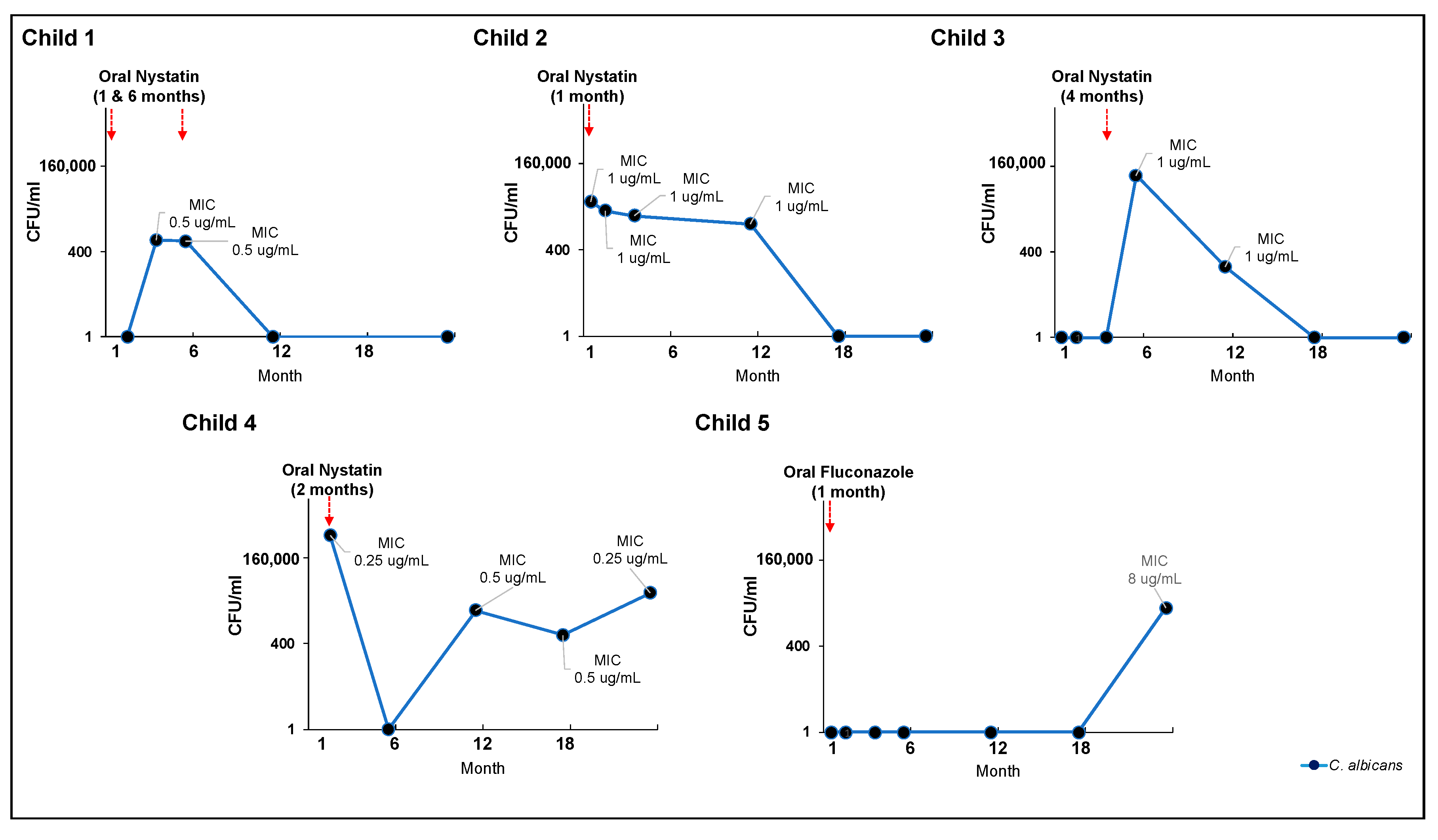
3.6. Effectiveness of Clinical Oral Consumption of Antifungal Medication in Early Life
3.7. Point Mutations in Antifungal Resistance Genes
4. Discussion
5. Limitations and Alternative Approach
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slavkin, H.C. Streptococcus mutans, early childhood caries and new opportunities. J. Am. Dent. Assoc. 1999, 130, 1787–1792. [Google Scholar] [CrossRef]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Front. Microbiol. 2015, 6, 1391. [Google Scholar] [CrossRef]
- Barnett, J.A. A history of research on yeasts 12: Medical yeasts part 1, Candida albicans. Yeast 2008, 25, 385–417. [Google Scholar] [CrossRef]
- Kadir, T.; Uygun, B.; Akyuz, S. Prevalence of Candida species in Turkish children: Relationship between dietary intake and carriage. Arch. Oral. Biol. 2005, 50, 33–37. [Google Scholar] [CrossRef]
- Cannon, R.D.; Holmes, A.R.; Mason, A.B.; Monk, B.C. Oral Candida: Clearance, colonization, or candidiasis? J. Dent. Res. 1995, 74, 1152–1161. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, L.; Lei, Y.L.; Ren, B.; Zhou, X. The Interactions Between Candida albicans and Mucosal Immunity. Front. Microbiol. 2021, 12, 652725. [Google Scholar] [CrossRef]
- Molero, G.; Díez-Orejas, R.; Navarro-García, F.; Monteoliva, L.; Pla, J.; Gil, C.; Sánchez-Pérez, M.; Nombela, C. Candida albicans: Genetics, dimorphism and pathogenicity. Int. Microbiol. 1998, 1, 95–106. [Google Scholar]
- Stecksen-Blicks, C.; Granstrom, E.; Silfverdal, S.A.; West, C.E. Prevalence of oral Candida in the first year of life. Mycoses 2015, 58, 550–556. [Google Scholar] [CrossRef]
- Vainionpaa, A.; Tuomi, J.; Kantola, S.; Anttonen, V. Neonatal thrush of newborns: Oral candidiasis? Clin. Exp. Dent. Res. 2019, 5, 580–582. [Google Scholar] [CrossRef]
- Flynn, P.M.; Cunningham, C.K.; Kerkering, T.; San Jorge, A.R.; Peters, V.B.; Pitel, P.A.; Harris, J.; Gilbert, G.; Castagnaro, L.; Robinson, P. Oropharyngeal candidiasis in immunocompromised children: A randomized, multicenter study of orally administered fiuconazole suspension versus nystatin. J. Pediatr. 1995, 127, 322–328. [Google Scholar] [CrossRef]
- Harris, L.J.; Pritzker, H.G.; Eisen, A.; Steiner, J.W.; Shack, L. The effect of nystatin (mycostatin) on neonatal candidiasis (thrush): A method of eradicating thrush from hospital nurseries. Can. Med. Assoc. J. 1958, 79, 891–896. [Google Scholar]
- Klinke, T.; Urban, M.; Luck, C.; Hannig, C.; Kuhn, M.; Kramer, N. Changes in Candida spp., mutans streptococci and lactobacilli following treatment of early childhood caries: A 1-year follow-up. Caries Res. 2014, 48, 24–31. [Google Scholar] [CrossRef]
- Qiu, R.; Li, W.; Lin, Y.; Yu, D.; Zhao, W. Genotypic diversity and cariogenicity of Candida albicans from children with early childhood caries and caries-free children. BMC Oral Health 2015, 15, 144. [Google Scholar] [CrossRef]
- Hodson, J.J.; Craig, G.T. The incidence of Candida albicans in the plaques of teeth of children. Dent. Pract. Dent. Rec. 1972, 22, 296–301. [Google Scholar]
- Xiao, J.; Huang, X.; Alkhers, N.; Alzamil, H.; Alzoubi, S.; Wu, T.T.; Castillo, D.A.; Campbell, F.; Davis, J.; Herzog, K.; et al. Candida albicans and Early Childhood Caries: A Systematic Review and Meta-Analysis. Caries Res. 2018, 52, 102–112. [Google Scholar] [CrossRef]
- Samaranayake, L.P.; Hughes, A.; Weetman, D.A.; MacFarlane, T.W. Growth and acid production of Candida species in human saliva supplemented with glucose. J. Oral. Pathol. 1986, 15, 251–254. [Google Scholar] [CrossRef]
- Klinke, T.; Kneist, S.; de Soet, J.J.; Kuhlisch, E.; Mauersberger, S.; Forster, A.; Klimm, W. Acid production by oral strains of Candida albicans and lactobacilli. Caries Res. 2009, 43, 83–91. [Google Scholar] [CrossRef]
- Choukri, F.; Benderdouche, M.; Sednaoui, P. In vitro susceptibility profile of 200 recent clinical isolates of Candida spp. to topical antifungal treatments of vulvovaginal candidiasis, the imidazoles and nystatin agents. J. Mycol. Med. 2014, 24, 303–307. [Google Scholar] [CrossRef]
- Khalandi, H.; Masoori, L.; Farahyar, S.; Delbandi, A.A.; Raiesi, O.; Farzanegan, A.; Khalandi, G.; Mahmoudi, S.; Erfanirad, T.; Falahati, M. Antifungal Activity of Capric Acid, Nystatin, and Fluconazole and Their In Vitro Interactions Against Candida Isolates from Neonatal Oral Thrush. Assay. Drug. Dev. Technol. 2020, 18, 195–201. [Google Scholar] [CrossRef]
- Tonon, C.C.; Francisconi, R.S.; Bordini, E.A.F.; Huacho, P.M.M.; Sardi, J.C.O.; Spolidorio, D.M.P. Interactions between Terpinen-4-ol and Nystatin on biofilm of Candida albicans and Candida tropicalis. Braz. Dent. J. 2018, 29, 359–367. [Google Scholar] [CrossRef]
- Ellepola, A.N.; Samaranayake, L.P. Oral candidal infections and antimycotics. Crit. Rev. Oral. Biol. Med. 2000, 11, 172–198. [Google Scholar] [CrossRef]
- Nenoff, P.; Krüger, C.; Neumeister, C.; Schwantes, U.; Koch, D. In vitro susceptibility testing of yeasts to nystatin—Low minimum inhibitory concentrations suggest no indication of in vitro resistance of Candida albicans, Candida species or non-Candida yeast species to nystatin. Clin. Med. Investig. 2016, 1, 71–76. [Google Scholar] [CrossRef]
- Mast, J.; Pina, E. Disappearance of Nystatin Resistance in Candida Mediated by Ergosterol. J. Gen. Microbiol. 1980, 117, 249–252. [Google Scholar]
- Martin, M.V. The use of fluconazole and itraconazole in the treatment of Candida albicans infections: A review. J. Antimicrob. Chemother. 1999, 44, 429–437. [Google Scholar] [CrossRef]
- Quindos, G.; Gil-Alonso, S.; Marcos-Arias, C.; Sevillano, E.; Mateo, E.; Jauregizar, N.; Eraso, E. Therapeutic tools for oral candidiasis: Current and new antifungal drugs. Med. Oral Patol. Oral. Cir. Bucal 2019, 24, e172–e180. [Google Scholar] [CrossRef]
- Boros-Majewska, J.; Salewska, N.; Borowski, E.; Milewski, S.; Malic, S.; Wei, X.Q.; Hayes, A.J.; Wilson, M.J.; Williams, D.W. Novel Nystatin A(1) derivatives exhibiting low host cell toxicity and antifungal activity in an in vitro model of oral candidosis. Med. Microbiol. Immunol. 2014, 203, 341–355. [Google Scholar] [CrossRef]
- De-la-Torre, J.; Ortiz-Samperio, M.E.; Marcos-Arias, C.; Marichalar-Mendia, X.; Eraso, E.; Echebarria-Goicouria, M.A.; Aguirre-Urizar, J.M.; Quindos, G. In Vitro Antifungal Susceptibility of Oral Candida Isolates from Patients Suffering from Caries and Chronic Periodontitis. Mycopathologia 2017, 182, 471–485. [Google Scholar] [CrossRef]
- Goins, R.A.; Ascher, D.; Waecker, N.; Arnold, J.; Moorefield, E. Comparison of fluconazole and nystatin oral suspensions for treatment of oral candidiasis in infants. Pediatr. Infect. Dis. J. 2002, 21, 1165–1167. [Google Scholar] [CrossRef]
- Kalkanci, A.; Berk, E.; Aykan, B.; Caglar, K.; Hizel, K.; Arman, D.; Kustimur, S. Epidemiology and antifungal susceptibility of Candida species isolated from hospitalized patients. J. De Mycol. Médicale 2007, 17, 16–20. [Google Scholar] [CrossRef]
- Dismukes, W.E. Introduction to Antifungal Drugs. Clin. Infect. Dis. 2000, 30, 653–657. [Google Scholar] [CrossRef]
- Wanjare, S.; Gupta, R.; Mehta, P. Caspofungin MIC Distribution amongst Commonly Isolated Candida Species in a Tertiary Care Centre—An Indian Experience. J. Clin. Diagn. Res. 2016, 10, DC11–DC13. [Google Scholar] [CrossRef] [PubMed]
- Chryssanthou, E.; Cuenca-Estrella, M. Comparison of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antibiotic Susceptibility Testing proposed standard and the E-test with the NCCLS broth microdilution method for voriconazole and caspofungin susceptibility testing of yeast species. J. Clin. Microbiol. 2002, 40, 3841–3844. [Google Scholar] [CrossRef] [PubMed]
- Eschenauer, G.; DePestel, D.D.; Carver, P.L. Comparison of echinocandin antifungals. Ther. Clin. Risk Manag. 2007, 3, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Serefko, A.; Los, R.; Biernasiuk, A.; Malm, A. Comparison of microdilution method and E-test procedure in susceptibility testing of caspofungin against Candida non-albicans species. New. Microbiol. 2008, 31, 257–262. [Google Scholar] [PubMed]
- Alkhars, N.; Zeng, Y.; Alomeir, N.; Al Jallad, N.; Wu, T.T.; Aboelmagd, S.; Youssef, M.; Jang, H.; Fogarty, C.; Xiao, J. Oral Candida Predicts Streptococcus mutans Emergence in Underserved US Infants. J. Dent. Res. 2022, 101, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Moon, Y.; Li, L.; Rustchenko, E.; Wakabayashi, H.; Zhao, X.; Feng, C.; Gill, S.R.; McLaren, S.; Malmstrom, H.; et al. Candida albicans Carriage in Children with Severe Early Childhood Caries (S-ECC) and Maternal Relatedness. PLoS ONE 2016, 11, e0164242. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Fogarty, C.; Wu, T.T.; Alkhers, N.; Zeng, Y.; Thomas, M.; Youssef, M.; Wang, L.; Cowen, L.; Abdelsalam, H.; et al. Oral health and Candida carriage in socioeconomically disadvantaged US pregnant women. BMC Pregnancy Childbirth 2019, 19, 480. [Google Scholar] [CrossRef]
- Jordan, S.; Baker, B.; Dunn, A.; Edwards, S.; Ferranti, E.; Mutic, A.D.; Yang, I.; Rodriguez, J. Maternal-Child Microbiome: Specimen Collection, Storage, and Implications for Research and Practice. Nurs. Res. 2017, 66, 175–183. [Google Scholar] [CrossRef]
- M27-S3; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Third Informational Supplement. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2020, 18, E246–E247. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R.; Ostrosky-Zeichner, L. Antifungal Susceptibility Testing: Current Approaches. Clin. Microbiol. Rev. 2020, 33, e00069-19. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly. 2014, 6, 80–92. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Andes, D.; Diekema, D.J.; Espinel-Ingroff, A.; Sheehan, D.; Testing, C.S.f.A.S. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: Time for harmonization of CLSI and EUCAST broth microdilution methods. Drug. Resist. Updat. 2010, 13, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Markey, L.; Hooper, A.; Melon, L.C.; Baglot, S.; Hill, M.N.; Maguire, J.; Kumamoto, C.A. Colonization with the commensal fungus Candida albicans perturbs the gut-brain axis through dysregulation of endocannabinoid signaling. Psychoneuroendocrinology 2020, 121, 104808. [Google Scholar] [CrossRef]
- M60; Performance Standards for Antifungal Susceptibility Testing of Yeasts. 2nd ed. Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2020.
- Pfaller, M.A.; Diekema, D.J. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J. Clin. Microbiol. 2012, 50, 2846–2856. [Google Scholar] [CrossRef]
- Epstein, J.B.; Pearsall, N.N.; Truelove, E.L. Quantitative relationships between Candida albicans in saliva and the clinical status of human subjects. J. Clin. Microbiol. 1980, 12, 475–476. [Google Scholar] [CrossRef]
- Zida, A.; Yacouba, A.; Bamba, S.; Sangare, I.; Sawadogo, M.; Guiguemde, T.; Kone, S.; Traore, L.K.; Ouedraogo-Traore, R.; Guiguemde, R.T. In vitro susceptibility of Candida albicans clinical isolates to eight antifungal agents in Ouagadougou (Burkina Faso). J. Mycol. Med. 2017, 27, 469–475. [Google Scholar] [CrossRef]
- Su, C.W.; Gaskie, S.; Jamieson, B.; Triezenberg, D. Clinical inquiries. What is the best treatment for oral thrush in healthy infants? J. Fam. Pract. 2008, 57, 484–485. [Google Scholar]
- Jewtuchowicz, V.M.; Brusca, M.I.; Mujica, M.T.; Gliosca, L.A.; Finquelievich, J.L.; Lovannitti, C.A.; Rosa, A.C. Subgingival distribution of yeast and their antifungal susceptibility in immunocompetent subjects with and without dental devices. Acta Odontol. Latinoam. 2007, 20, 17–22. [Google Scholar]
- Brito, G.N.B.; Inocêncio, A.C.; Querido, S.M.R.; Jorge, A.O.C.; Koga-Ito, C.Y. In vitro antifungal susceptibility of Candida spp. oral isolates from HIV positive patients and control individuals. Braz. Oral. Res. 2010, 25, 28–33. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Fluconazole: Rationale for the EUCAST Clinical Breakpoints, version 3.0; European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2020. [Google Scholar]
- Khalaf, R.A.; Fattouh, N.; Medvecky, M.; Hrabak, J. Whole genome sequencing of a clinical drug resistant Candida albicans isolate reveals known and novel mutations in genes involved in resistance acquisition mechanisms. J. Med. Microbiol. 2021, 70, 001351. [Google Scholar] [CrossRef] [PubMed]
- Spettel, K.; Barousch, W.; Makristathis, A.; Zeller, I.; Nehr, M.; Selitsch, B.; Lackner, M.; Rath, P.M.; Steinmann, J.; Willinger, B. Analysis of antifungal resistance genes in Candida albicans and Candida glabrata using next generation sequencing. PLoS ONE 2019, 14, e0210397. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, T.; Williams, W.D.; Bagg, J.; Coulter, W.A.; Ready, D.; Lewis, M.A. In vitro susceptibility of oral Candida to seven antifungal agents. Oral Microbiol. Immunol. 2005, 20, 349–353. [Google Scholar] [CrossRef]
- Redding, S.; Smith, J.; Farinacci, G.; Rinaldi, M.; Fothergill, A.; Rhine-Chalberg, J.; Pfaller, M. Resistance of Candida albicans to fluconazole during treatment of oropharyngeal candidiasis in a patient with AIDS: Documentation by in vitro susceptibility testing and DNA subtype analysis. Clin. Infect. Dis. 1994, 18, 240–242. [Google Scholar] [CrossRef]
- Heald, A.E.; Cox, G.M.; Schell, W.A.; Bartlett, J.A.; Perfect, J.R. Oropharyngeal yeast flora and fluconazole resistance in HIV-infected patients receiving long-term continuous versus intermittent fluconazole therapy. Aids 1996, 10, 263–268. [Google Scholar] [CrossRef]
- Lyu, X.; Zhao, C.; Yan, Z.M.; Hua, H. Efficacy of nystatin for the treatment of oral candidiasis: A systematic review and meta-analysis. Drug. Des. Devel Ther. 2016, 10, 1161–1171. [Google Scholar] [CrossRef]
- Hoppe, J.E. Treatment of oropharyngeal candidiasis and candidal diaper dermatitis in neonates and infants: Review and reappraisal. Pediatr. Infect. Dis. J. 1997, 16, 885–894. [Google Scholar] [CrossRef]
- Rex, J.H.; Pfaller, M.A.; Walsh, T.J.; Chaturvedi, V.; Espinel-Ingroff, A.; Ghannoum, M.A.; Gosey, L.L.; Odds, F.C.; Rinaldi, M.G.; Sheehan, D.J.; et al. Antifungal susceptibility testing: Practical aspects and current challenges. Clin. Microbiol. Rev. 2001, 14, 643–658. [Google Scholar] [CrossRef]
- Fernandez, E.; Perez, R.; Hernandez, A.; Tejada, P.; Arteta, M.; Ramos, J.T. Factors and Mechanisms for Pharmacokinetic Differences between Pediatric Population and Adults. Pharmaceutics 2011, 3, 53–72. [Google Scholar] [CrossRef]
- Anderson, G.D.; Lynn, A.M. Optimizing pediatric dosing: A developmental pharmacologic approach. Pharmacotherapy 2009, 29, 680–690. [Google Scholar] [CrossRef] [PubMed]
| Categories | Mother n (%) | Child n (%) | |
|---|---|---|---|
| Demographic | |||
| Gender | Female | 41 (100) | 22 (53.7) |
| Race | Black | 28 (68.3) | 27 (65.9) |
| White | 9 (22.0) | 8 (19.5) | |
| Ethnicity | Non-Hispanic | 36 (87.8) | 36 (87.8) |
| Socioeconomic-behavior factors | |||
| Marital status | Married | 2 (4.9) | na |
| Employment | Employed | 24 (58.5) | na |
| Education | Middle school | 2 (4.9) | na |
| High school | 25 (61.0) | na | |
| Associate | 4 (9.8) | na | |
| ≥College | 10 (24.4) | na | |
| Medical and oral conditions (Y) | |||
| Smoking | 4 (9.8) | na | |
| Emotional condition | 21 (51.2) | 0 (0) | |
| Diabetes | 0 (0) | 0 (0) | |
| Hypertension | 5 (12.2) | 0 (0) | |
| Asthma | 2 (4.9) | 0 (0) | |
| History of yeast infection | 12 (29.3) | 16 (39.0) | |
| Diaper rash (Child) | na | 16 (39.0) | |
| Oral thrush (Child) | na | 8 (19.5) | |
| Caries | 32 (78.0) | 10 (33.3) # | |
| Medication use (Y) | |||
| Antifungal * (Prenatal) | 9 (21.9) | na | |
| Antibiotic * (Prenatal) | 18 (43.9) | na | |
| Antifungal * (6 months postpartum or child by 24 m) | 5 (12.2) | 16 (39.0) | |
| Antibiotic * (6 months postpartum or child by 24 m) | 11 (26.8) | 10 (24.4) | |
| Inhaler | 2 (4.9) | 1 (2.4) | |
| Oral hygiene practice | Brushing twice daily | 28 (68.3) | 16 (53.3) |
| Birth route | Vaginal | na | 32 (78.0) |
| Species (n) | Antifungal Agent | MIC (μg/mL) |
|---|---|---|
| Range | ||
| Candida albicans (n = 114) | Nystatin | 0.25–8 |
| Fluconazole | 0.25–1 | |
| Caspofungin | 0.016–0.25 | |
| Candida parapsilosis (n = 6) | Nystatin | 0.5–4 |
| Fluconazole | 2–4 | |
| Caspofungin | 0.25–0.5 | |
| Candida dubliniensis (n = 4) | Nystatin | 0.5 |
| Fluconazole | 0.5 | |
| Caspofungin | 0.06–0.12 | |
| Candida lusitaniae (n = 2) | Nystatin | 2 |
| Fluconazole | 1 | |
| Caspofungin | 0.03–0.12 |
| Species and Child Age at Isolation (Number of Isolates) | Susceptible (S) MIC ≤ 2 μg/mL * n (%) | Resistant (R) MIC > 2 μg/mL * n (%) |
|---|---|---|
| Candida albicans | ||
| Prenatal (36) | 33 (91.7) | 3 (8.3) |
| 1 month (6) | 6 (100) | 0 (0) |
| 2 months (14) | 14 (100) | 0 (0) |
| 4 months (8) | 7 (87.5) | 1 (12.5) |
| 6 months (11) | 11 (100) | 0 (0) |
| 12 months (16) | 16 (100) | 0 (0) |
| 18 months (13) | 12 (92.3) | 1 (7.7) |
| 24 months (10) | 9 (90) | 1 (10) |
| Candida parapsilosis | ||
| Prenatal (3) | 2 (66.7) | 1 (33.3) |
| 4 months (1) | 1 (100) | 0 (0) |
| 12 months (2) | 2 (100) | 0 (0) |
| Candida dubliniensis | ||
| Prenatal (2) | 2 (100) | 0 (0) |
| 24 months (2) | 2 (100) | 0 (0) |
| Candida lusitaniae | ||
| Prenatal (1) | 1 (100) | 0 (0) |
| 24 months (1) | 1 (100) | 0 (0) |
| Child Age (Number of Isolates) | Susceptible (S) MIC ≤ 2 μg/mL * n (%) | Susceptible Dose-Dependent (SDD) MIC = 4 μg/mL * n (%) | Resistant (R) MIC ≥ 8 μg/mL * n (%) |
|---|---|---|---|
| Candida albicans | |||
| Prenatal (36) | 36 (100) | 0 (0) | 0 (0) |
| 1 month (6) | 6 (100) | 0 (0) | 0 (0) |
| 2 months (14) | 14 (100) | 0 (0) | 0 (0) |
| 4 months (8) | 8 (100) | 0 (0) | 0 (0) |
| 6 months (11) | 11 (100) | 0 (0) | 0 (0) |
| 12 months (16) | 16 (100) | 0 (0) | 0 (0) |
| 18 months (13) | 13 (100) | 0 (0) | 0 (0) |
| 24 months (10) | 10 (100) | 0 (0) | 0 (0) |
| Candida parapsilosis | |||
| Prenatal (3) | 2 (66.7) | 1 (33.3) | 0 (0) |
| 4 months (1) | 1 (100) | 0 (0) | 0 (0) |
| 12 months (2) | 1 (50) | 1 (50) | 0 (0) |
| Species and Isolation Timepoint (Number of Candida albicans Isolates) | Susceptible (S) MIC ≤ 0.25 μg/mL * n (%) | Intermediate (I) MIC = 0.5 μg/mL * n (%) | Resistant MIC ≥ 1 μg/mL * n (%) |
|---|---|---|---|
| Prenatal (36) | 36 (100) | 0 (0) | 0 (0) |
| 1 month (6) | 6 (100) | 0 (0) | 0 (0) |
| 2 months (14) | 14 (100) | 0 (0) | 0 (0) |
| 4 months (8) | 8 (100) | 0 (0) | 0 (0) |
| 6 months (11) | 11 (100) | 0 (0) | 0 (0) |
| 12 months (16) | 16 (100) | 0 (0) | 0 (0) |
| 18 months (13) | 13 (100) | 0 (0) | 0 (0) |
| 24 months (10) | 10 (100) | 0 (0) | 0 (0) |
| Groups Based on Susceptibility | Gene | Gene Length | Gene Position | Nucleotide Substitution | Aminoacid Substituition | Allele |
|---|---|---|---|---|---|---|
| Nystatin resistance (MIC > 2 μg/mL) | CDR2 | 4500 | chr 3 | 2048G > A | Arg683Lys | homo/hetero |
| CDR2 | 4500 | chr 3 | 4011G > C | Leu1337Phe | homo/hetero | |
| Caspofungin reduced susceptibility (MIC = 0.25 μg/mL) | FKS1 | 5694 | chr 1 | 5657C > G | Ser1886Thr | homo/hetero |
| CDR2 | 4500 | chr 3 | 2048G > A | Arg683Lys | homo/hetero | |
| CDR2 | 4500 | chr 3 | 4011G > C | Leu1337Phe | homo/hetero | |
| ERG11 | 1587 | chr 5 | 348T > A | Glu116Asp | homo/hetero |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhars, N.; Gaca, A.; Zeng, Y.; Al-Jallad, N.; Rustchenko, E.; Wu, T.T.; Eliav, E.; Xiao, J. Antifungal Susceptibility of Oral Candida Isolates from Mother-Infant Dyads to Nystatin, Fluconazole, and Caspofungin. J. Fungi 2023, 9, 580. https://doi.org/10.3390/jof9050580
Alkhars N, Gaca A, Zeng Y, Al-Jallad N, Rustchenko E, Wu TT, Eliav E, Xiao J. Antifungal Susceptibility of Oral Candida Isolates from Mother-Infant Dyads to Nystatin, Fluconazole, and Caspofungin. Journal of Fungi. 2023; 9(5):580. https://doi.org/10.3390/jof9050580
Chicago/Turabian StyleAlkhars, Naemah, Anthony Gaca, Yan Zeng, Nisreen Al-Jallad, Elena Rustchenko, Tong Tong Wu, Eli Eliav, and Jin Xiao. 2023. "Antifungal Susceptibility of Oral Candida Isolates from Mother-Infant Dyads to Nystatin, Fluconazole, and Caspofungin" Journal of Fungi 9, no. 5: 580. https://doi.org/10.3390/jof9050580
APA StyleAlkhars, N., Gaca, A., Zeng, Y., Al-Jallad, N., Rustchenko, E., Wu, T. T., Eliav, E., & Xiao, J. (2023). Antifungal Susceptibility of Oral Candida Isolates from Mother-Infant Dyads to Nystatin, Fluconazole, and Caspofungin. Journal of Fungi, 9(5), 580. https://doi.org/10.3390/jof9050580







