First Description of Non-Enzymatic Radical-Generating Mechanisms Adopted by Fomitiporia mediterranea: An Unexplored Pathway of the White Rot Agent of the Esca Complex of Diseases
Abstract
1. Introduction
- CAZymes, laccases, class II-PODs (manganese peroxidases [MnPs] and generic peroxidases [GPs]), and other AAs have been reported in genomic, proteomic, and enzymological studies of Fmed, while genes encoding for versatile peroxidases (VPs) and lignin peroxidases (LiP) have been demonstrated to be absent in the Fmed genome [20,33,49,50,51];
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. Glassware Preparation
2.3. Liquid Culture Experimental Design, Sampling, and Measurement of Fungal Growth
2.4. LMWC Extraction
2.5. Determination of Total Phenols
2.6. LMW Fraction Analysis
2.7. Determination of Ferric Iron Reduction Activity (FeRA) in the LMW Fraction
2.8. Determination of Hydrogen Peroxide Levels by Ferrous Oxidation in the Presence of Xylenol Orange (FOX Assay)
2.9. Determination of Hydroxyl Radical Activity by Electro Paramagnetic Resonance (EPR)
2.10. Carbohydrate Oxidative Degradation Assays
2.11. Statistical Analysis
3. Results
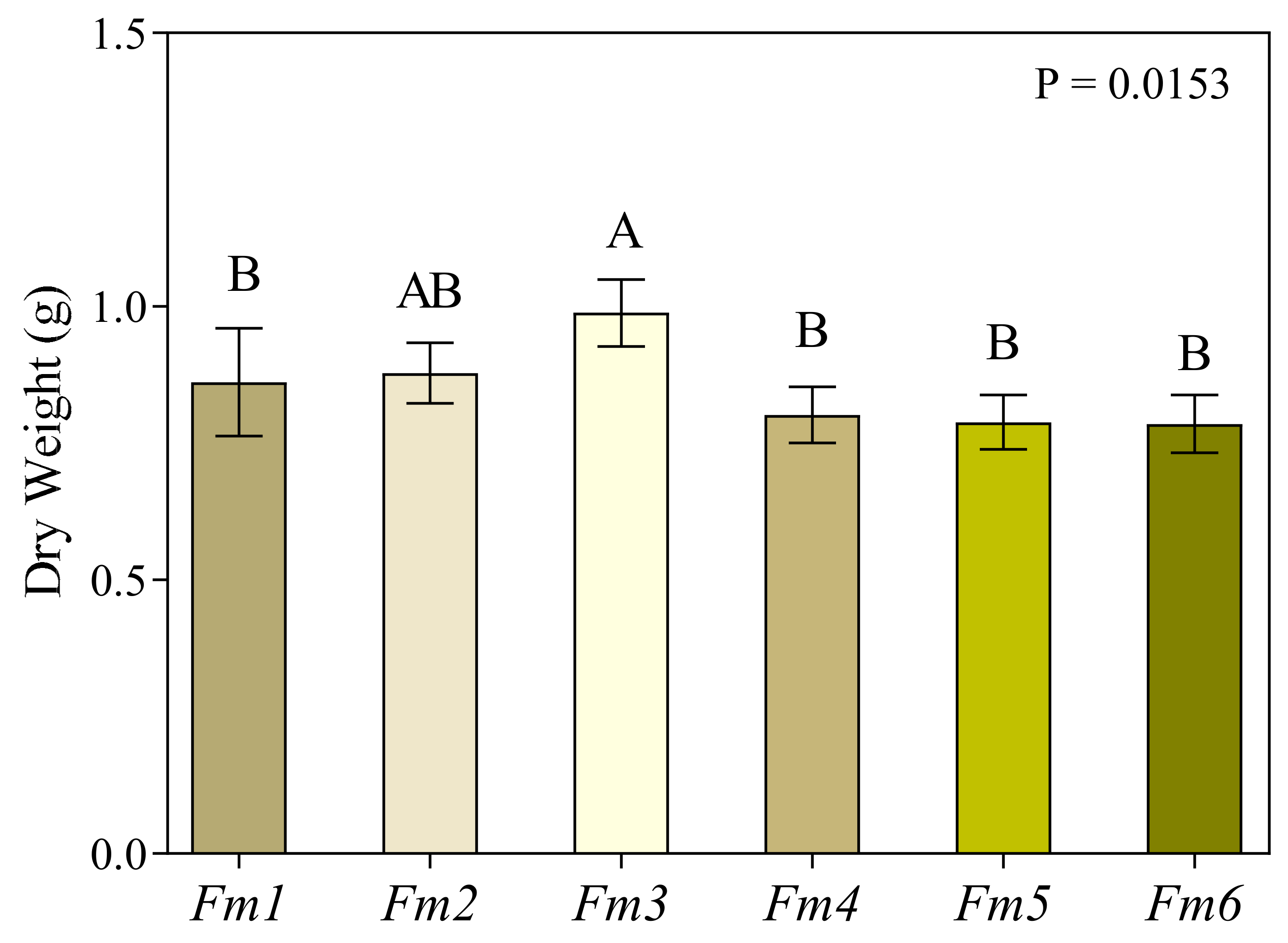
3.1. Fungal Biomass
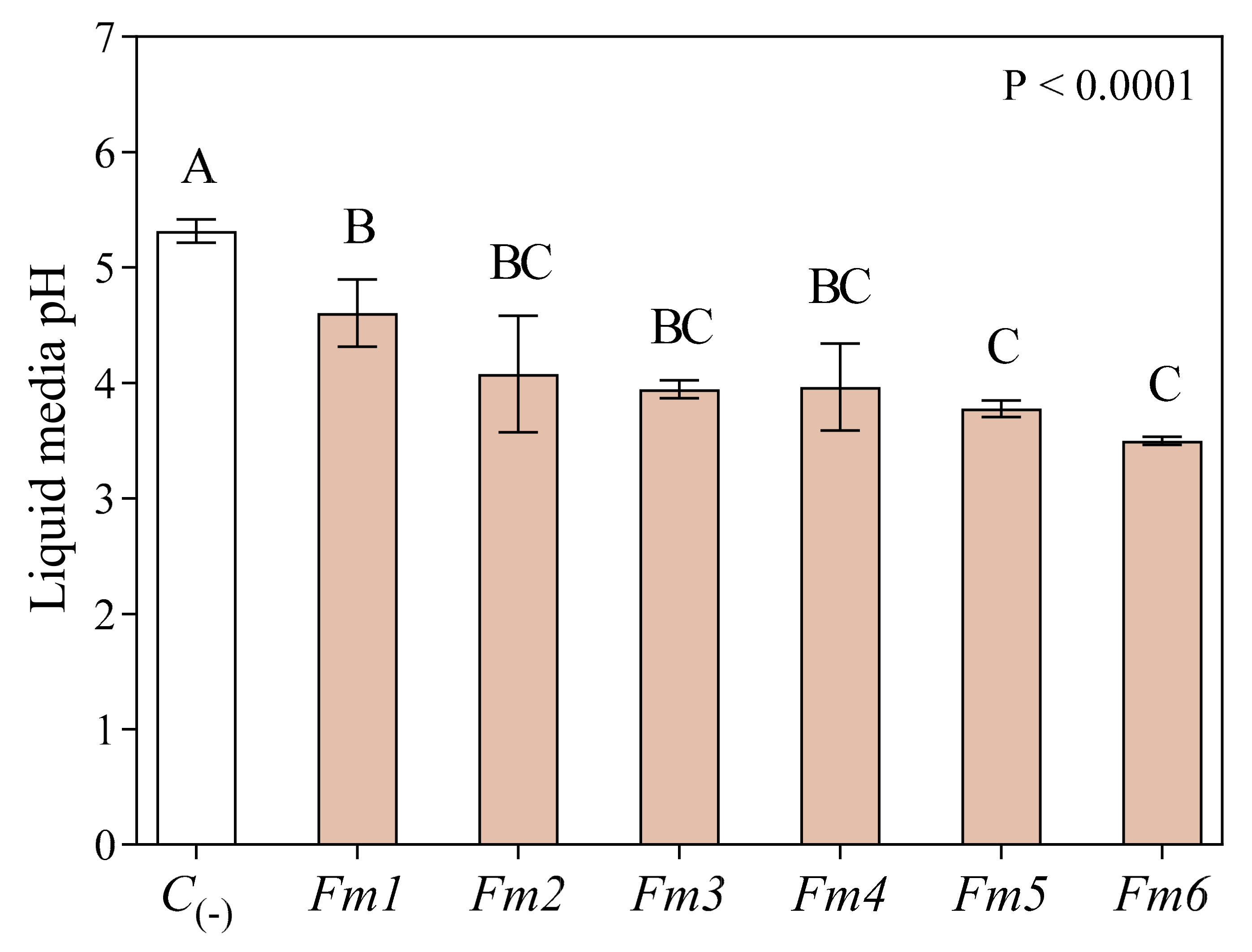
3.2. Culture Media Acidification
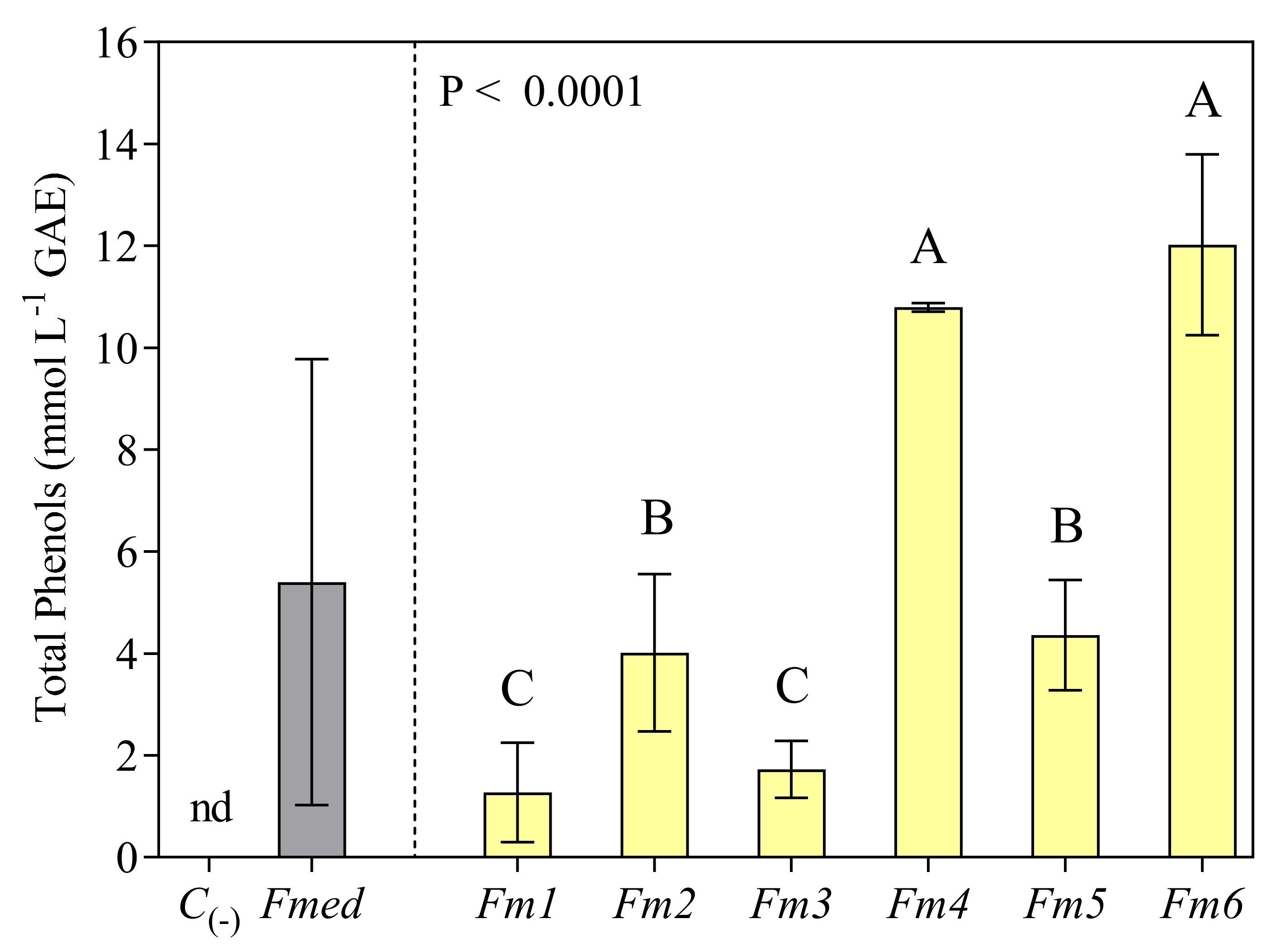
3.3. Total Phenols Analysis
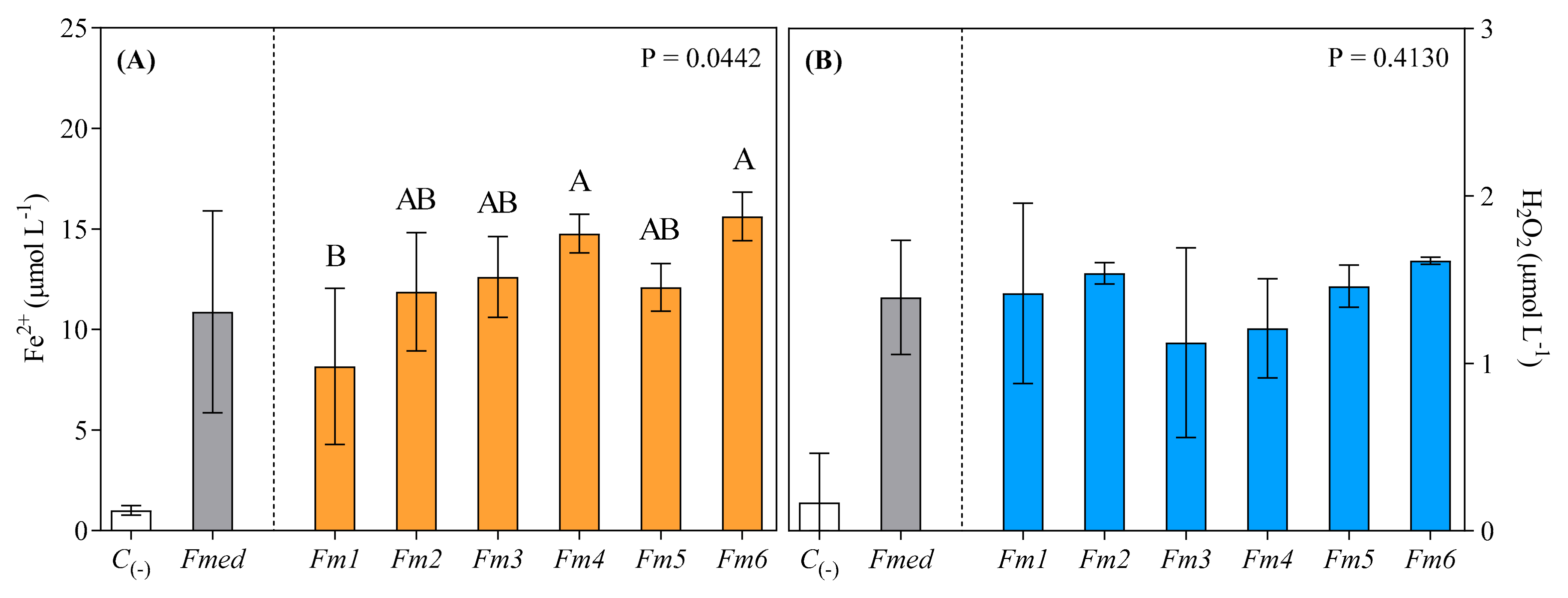
3.4. FeRA and Hydrogen Peroxide Generation from LMWC Redox Cycle
3.5. LMWC Fraction Characterization
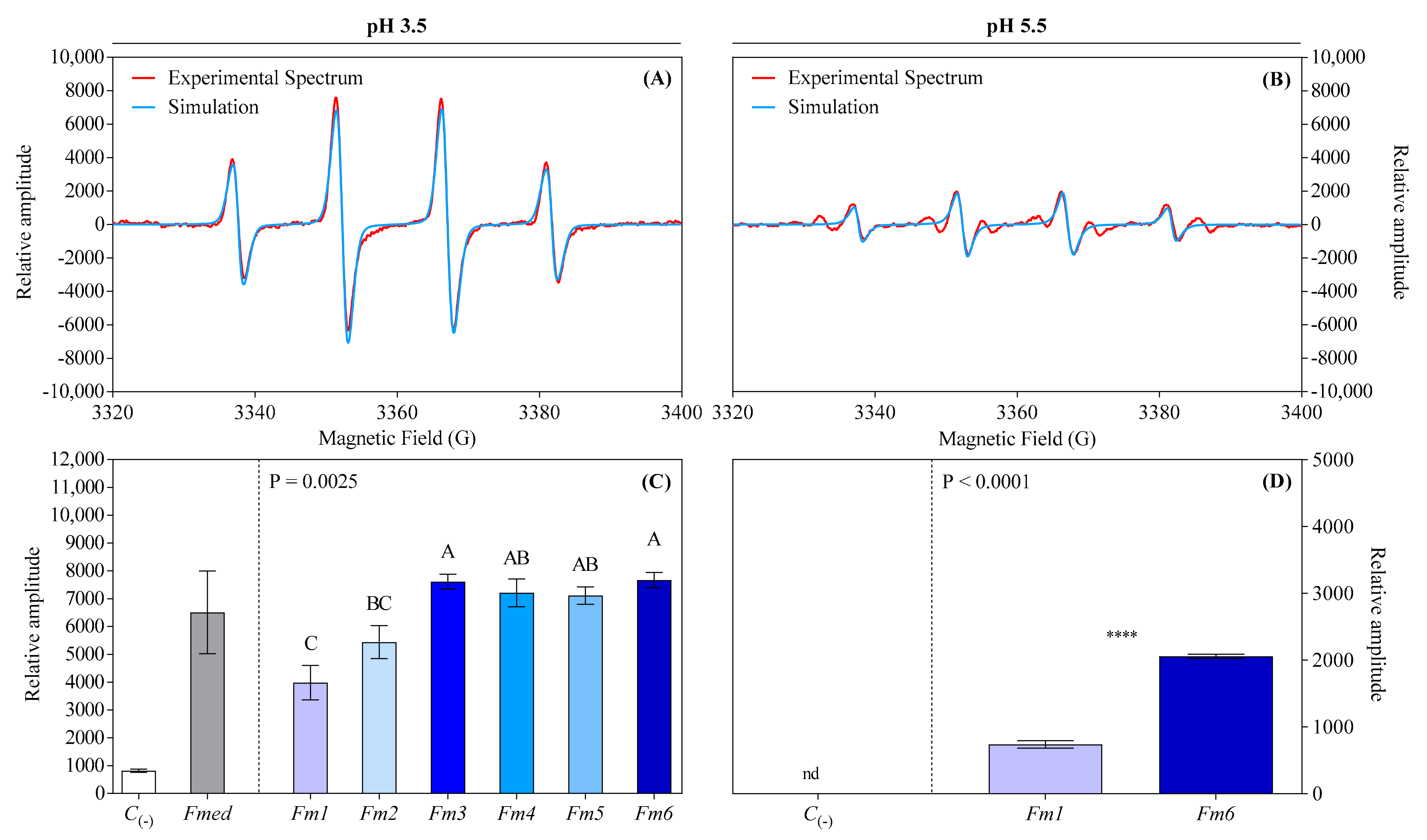
3.6. Determination of Hydroxyl Radical Activity by Electron Paramagnetic Resonance (EPR)
3.7. Carbohydrate Oxidative Degradation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| m/z Fragment (mDa) | Calculated Formula | Loss | Proposed Structure in Yellow for the Fragment |
|---|---|---|---|
| 445.092 | C25H17O8- | CO2 |  |
| 403.082 | C23H15O7- | C3H2O3 | 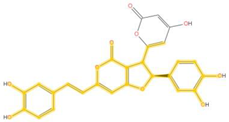 |
| 335.055 | C19H11O6- | C7H6O4 | 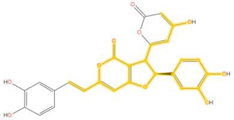 |
| 309.040 | C17H9O6- | C9H8O4 |  |
| 283.062 | C16H11O5- | C10H6O5 | 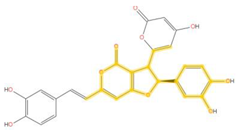 |
| 241.050 | C14H9O4- | C12H8O6 |  |
| 199.040 | C12H7O3- | C14H10O7 |  |
| 159.045 | C10H7O2- | C16H10O8 | 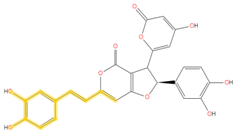 |
| 135.045 | C8H7O2- | C18H10O8 |  |
| RT [min] | m/z | M | Compound Name |
|---|---|---|---|
| 8.52 | 121.029 | 122.037 | (a) 4-Hydroxybenzaldehyde |
| 11.07 | 121.029 | 122.037 | (b) Benzoic acid |
| 8.58 | 167.037 | 168.042 | (c) 4-Hydroxy-3-methoxybenzoic acid (vanillic acid) |
| 9.74 | 163.040 | 164.047 | (d) 4-Hydroxycinnamic acid (p-coumaric acid) |
| 11.35 | 137.024 | 138.032 | (e) 2-Hydroxybenzoic acid (salicylic acid) |
| 9.30 | 135.045 | 136.052 | (f) 3-Methoxybenzaldehyde |
| 7.54 | 194.046 | 195.053 | (g) N-Acetyl-5-aminosalicylic acid |
| 9.17 | 151.040 | 152.047 | (h) 4-Hydroxy-3- methoxybenzaldehyde (vanillin) |
| 9.27 | 190.051 | 191.058 | (i) 5-Hydroxyindole-3-acetic acid |
| 10.98 | 151.040 | 152.047 | (j) Methyl-4-hydroxybenzoate |
| 11.80 | 489.082 | 490.090 | (k) Hypholomine B (isomer a) |
| 12.24 | 489.083 | 490.090 | (l) Hypholomine B (isomer b) |
References
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine Trunk Diseases: A Review of Fifteen Years of Trials for Their Control with Chemicals and Biocontrol Agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed]
- Guérin-Dubrana, L.; Fontaine, F.; Mugnai, L. Grapevine Trunk Disease in European and Mediterranean Vineyards: Occurrence, Distribution and Associated Disease-Affecting Cultural Factors. Phytopathol. Mediterr. 2019, 58, 49–71. [Google Scholar] [CrossRef]
- Kenfaoui, J.; Radouane, N.; Mennani, M.; Tahiri, A.; El Ghadraoui, L.; Belabess, Z.; Fontaine, F.; El Hamss, H.; Amiri, S.; Lahlali, R.; et al. A Panoramic View on Grapevine Trunk Diseases Threats: Case of Eutypa Dieback, Botryosphaeria Dieback, and Esca Disease. J. Fungi 2022, 8, 595. [Google Scholar] [CrossRef]
- Calzarano, F.; Osti, F.; D’Agostino, V.; Pepe, A.; Di Marco, S. Mixture of Calcium, Magnesium and Seaweed Affects Leaf Phytoalexin Contents and Grape Ripening on Vines with Grapevine Leaf Stripe Disease. Phytopathol. Mediterr. 2017, 56, 445–457. [Google Scholar] [CrossRef]
- Lorrain, B.; Ky, I.; Pasquier, G.; Jourdes, M.; Dubrana, L.G.; Gény, L.; Rey, P.; Donèche, B.; Teissedre, P.L. Effect of Esca Disease on the Phenolic and Sensory Attributes of Cabernet Sauvignon Grapes, Musts and Wines. Aust. J. Grape Wine Res. 2012, 18, 64–72. [Google Scholar] [CrossRef]
- Fontaine, F.; Gramaje, D.; Armengol, J.; Smart, R.; Nagy, Z.A.; Borgo, M.; Rego, C.; Corio-Costet, M.F. Grapevine Trunk Diseases. A Review; OIV Publications: Paris, France, 2016; p. 25. [Google Scholar]
- Surico, G. Towards a Redefinition of the Diseases within the Esca Complex of Grapevine. Phytopathol. Mediterr. 2009, 48, 5–10. [Google Scholar] [CrossRef]
- Camele, I.; Mang, S.M. First Report of Seimatosporium vitis Associated with Grapevine Trunk Diseases on Vitis vinifera in Italy. Plant Dis. 2019, 103, 771. [Google Scholar] [CrossRef]
- Del Frari, G.; Gobbi, A.; Aggerbeck, M.R.; Oliveira, H.; Hansen, L.H.; Ferreira, R.B. Characterization of the Wood Mycobiome of Vitis vinifera in a Vineyard Affected by Esca. Spatial Distribution of Fungal Communities and Their Putative Relation with Leaf Symptoms. Front. Plant Sci. 2019, 10, 910. [Google Scholar] [CrossRef]
- Niem, J.M.; Billones-Baaijens, R.; Stodart, B.; Savocchia, S. Diversity Profiling of Grapevine Microbial Endosphere and Antagonistic Potential of Endophytic Pseudomonas Against Grapevine Trunk Diseases. Front. Microbiol. 2020, 11, 477. [Google Scholar] [CrossRef]
- Larignon, P.; Dubos, B. Fungi Associated with Esca Disease in Grapevine. Eur. J. Plant Pathol. 1997, 103, 147–157. [Google Scholar] [CrossRef]
- Crous, P.W.; Gams, W. Phaeomoniella chlamydospora Gen. et Comb. Nov., a Causal Organism of Petri Grapevine Decline and Esca. Phytopathol. Mediterr. 2000, 39, 112–118. [Google Scholar] [CrossRef]
- Gramaje, D.; Mostert, L.; Groenewald, J.Z.; Crous, P.W. Phaeoacremonium: From Esca Disease to Phaeohyphomycosis. Fungal Biol. 2015, 119, 759–783. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M. Biodiversity and Geographic Distribution of Basidiomycetes Causing Esca-Associated White Rot in Grapevine: A Worldwide Perspective. Phytopathol. Mediterr. 2006, 45, 30–42. [Google Scholar] [CrossRef]
- Fischer, M. A New Wood-Decaying Basidiomycete Species Associated with Esca of Grapevine: Fomitiporia mediterranea (Hymenochaetales). Mycol. Prog. 2002, 1, 315–324. [Google Scholar] [CrossRef]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (Black Measles) and Brown Wood-Streaking: Two Old and Elusive Diseases of Grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef]
- Ouadi, L.; Bruez, E.; Bastien, S.; Vallance, J.; Lecomte, P.; Domec, J.-C.; Rey, P. Ecophysiological Impacts of Esca, a Devastating Grapevine Trunk Disease, on Vitis vinifera L. PLoS ONE 2019, 14, e0222586. [Google Scholar] [CrossRef]
- Moretti, S.; Pacetti, A.; Pierron, R.; Kassemeyer, H.-H.; Fischer, M.; Péros, J.-P.; Perez-Gonzalez, G.; Bieler, E.; Schilling, M.; Di Marco, S.; et al. Fomitiporia mediterranea M. Fisch., the Historical Esca Agent: A Comprehensive Review on the Main Grapevine Wood Rot Agent in Europe. Phytopathol. Mediterr. 2021, 60, 351–379. [Google Scholar] [CrossRef]
- Pacetti, A.; Moretti, S.; Perrin, C.; Gelhaye, E.; Bieler, E.; Kassemeyer, H.-H.; Mugnai, L.; Farine, S.; Bertsch, C. Grapevine Wood-Degrading Activity of Fomitiporia mediterranea M. Fisch.: A Focus on the Enzymatic Pathway Regulation. Front. Microbiol. 2022, 13, 844264. [Google Scholar] [CrossRef]
- Goodell, B.; Qian, Y.; Jellison, J. Fungal Decay of Wood: Soft Rot-Brown Rot-White Rot. In Development of Commercial Wood Preservatives; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2008; Volume 982, pp. 9–31. [Google Scholar] [CrossRef]
- Daniel, G. Fungal and Bacterial Biodegradation: White Rots, Brown Rots, Soft Rots, and Bacteria. In Deterioration and Protection of Sustainable Biomaterials; ACS Symposium Series; Nicholas Darrel, D., Goodell, B., Schultz Tor, P., Eds.; American Chemical Society: Washington, DC, USA, 2014; Volume 1158, pp. 23–58. [Google Scholar] [CrossRef]
- Haidar, R.; Yacoub, A.; Vallance, J.; Compant, S.; Antonielli, L.; Saad, A.; Habenstein, B.; Kauffmann, B.; Grélard, A.; Loquet, A.; et al. Bacteria Associated with Wood Tissues of Esca-Diseased Grapevines: Functional Diversity and Synergy with Fomitiporia mediterranea to Degrade Wood Components. Environ. Microbiol. 2021, 23, 6104–6121. [Google Scholar] [CrossRef]
- Embacher, J.; Zeilinger, S.; Kirchmair, M.; Rodriguez-R, L.M.; Neuhauser, S. Wood decay fungi and their bacterial interaction partners in the built environment—A systematic review on fungal bacteria interactions in dead wood and timber. Fungal Biol. Rev. 2023, 45, 100305. [Google Scholar] [CrossRef]
- Hartig, R. Die Zersetzungserscheinungen des Holzes der Nadelholzbäume und der Eiche in Forstlicher, Botanischer und Chemischer Richtung; Springer: Berlin/Heidelberg, Germany, 1878; pp. 1–214. [Google Scholar]
- Blanchette, R.A. Delignification by Wood-Decay Fungi. Annu. Rev. Phytopathol. 1991, 29, 381–403. [Google Scholar] [CrossRef]
- Levasseur, A.; Drula, E.; Lombard, V.; Coutinho, P.M.; Henrissat, B. Expansion of the Enzymatic Repertoire of the CAZy Database to Integrate Auxiliary Redox Enzymes. Biotechnol. Biofuels 2013, 6, 41. [Google Scholar] [CrossRef]
- Manavalan, T.; Manavalan, A.; Heese, K. Characterization of Lignocellulolytic Enzymes from White-Rot Fungi. Curr. Microbiol. 2015, 70, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Goodell, B. Fungi Involved in the Biodeterioration and Bioconversion of Lignocellulose Substrates. Genetics and Biotechnology. In The Mycota: A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research; Benz, J.P., Schipper, K., Eds.; Springer: Cham, Switzerland, 2020; Volume 2, pp. 369–397. [Google Scholar] [CrossRef]
- Rani Singhania, R.; Dixit, P.; Kumar Patel, A.; Shekher Giri, B.; Kuo, C.-H.; Chen, C.-W.; Di Dong, C. Role and Significance of Lytic Polysaccharide Monooxygenases (LPMOs) in Lignocellulose Deconstruction. Bioresour. Technol. 2021, 335, 125261. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Merényi, Z.; Bálint, B.; Kiss, B.; Sipos, G.; Owens, R.A.; Nagy, L.G. Hallmarks of Basidiomycete Soft- and White-Rot in Wood-Decay -Omics Data of Two Armillaria Species. Microorganisms 2021, 9, 149. [Google Scholar] [CrossRef]
- Drula, E.; Garron, M.-L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The Carbohydrate-Active Enzyme Database: Functions and Literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef]
- Floudas, D.; Binder, M.; Riley, R.; Barry, K.; Blanchette, R.A.; Henrissat, B.; Martínez, A.T.; Otillar, R.; Spatafora, J.W.; Yadav, J.S.; et al. The Paleozoic Origin of Enzymatic Lignin Decomposition Reconstructed from 31 Fungal Genomes. Science 2012, 336, 1715–1719. [Google Scholar] [CrossRef]
- Zhu, Y.; Plaza, N.; Kojima, Y.; Yoshida, M.; Zhang, J.; Jellison, J.; Pingali, S.V.; O’Neill, H.; Goodell, B. Nanostructural Analysis of Enzymatic and Non-Enzymatic Brown Rot Fungal Deconstruction of the Lignocellulose Cell Wall †. Front. Microbiol. 2020, 11, 1389. [Google Scholar] [CrossRef]
- Eastwood, D.C.; Floudas, D.; Binder, M.; Majcherczyk, A.; Schneider, P.; Aerts, A.; Asiegbu, F.O.; Baker, S.E.; Barry, K.; Bendiksby, M.; et al. The Plant Cell Wall-Decomposing Machinery Underlies the Functional Diversity of Forest Fungi. Science 2011, 333, 762–765. [Google Scholar] [CrossRef]
- Goodell, B.; Jellison, J.; Liu, J.; Daniel, G.; Paszczynski, A.; Fekete, F.; Krishnamurthy, S.; Jun, L.; Xu, G. Low Molecular Weight Chelators and Phenolic Compounds Isolated from Wood Decay Fungi and Their Role in the Fungal Biodegradation of Wood. J. Biotechnol. 1997, 53, 133–162. [Google Scholar] [CrossRef]
- Xu, G.; Goodell, B. Mechanisms of Wood Degradation by Brown-Rot Fungi: Chelator-Mediated Cellulose Degradation and Binding of Iron by Cellulose. J. Biotechnol. 2001, 87, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Milagres, A.M.F.; Filley, T.R.; Goodell, B. Lignocellulosic Polysaccharides and Lignin Degradation by Wood Decay Fungi: The Relevance of Nonenzymatic Fenton-Based Reactions. J. Ind. Microbiol. Biotechnol. 2011, 38, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mahaney, J.; Jellison, J.; Cao, J.; Gressler, J.; Hoffmeister, D.; Goodell, B. Fungal Variegatic Acid and Extracellular Polysaccharides Promote the Site-Specific Generation of Reactive Oxygen Species. J. Ind. Microbiol. Biotechnol. 2017, 44, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Goodell, B.; Zhu, Y.; Kim, S.; Kafle, K.; Eastwood, D.; Daniel, G.; Jellison, J.; Yoshida, M.; Groom, L.; Pingali, S.V.; et al. Modification of the Nanostructure of Lignocellulose Cell Walls via a Non-Enzymatic Lignocellulose Deconstruction System in Brown Rot Wood-Decay Fungi. Biotechnol. Biofuels 2017, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gonzalez, G.; Sebestyen, D.; Petit, E.; Jellison, J.; Mugnai, L.; Gelhaye, E.; Lee, N.; Farine, S.; Bertsch, C.; Goodell, B. Oxygen Radical-Generating Metabolites Secreted by Eutypa and Esca Fungal Consortia: Understanding the Mechanisms Behind Grapevine Wood Deterioration and Pathogenesis. Front. Plant Sci. 2022, 13, 921961. [Google Scholar] [CrossRef] [PubMed]
- Sebestyen, D.; Perez-Gonzalez, G.; Goodell, B. Antioxidants and Iron Chelators Inhibit Oxygen Radical Generation in Fungal Cultures of Plant Pathogenic Fungi. Fungal Biol. 2022, 126, 480–487. [Google Scholar] [CrossRef]
- Arantes, V.; Goodell, B. Current Understanding of Brown-Rot Fungal Biodegradation Mechanisms: A Review. In Deterioration and Protection of Sustainable Biomaterials; ACS Symposium Series; Schultz, T.P., Goodell, B., Nicholas, D.D., Eds.; American Chemical Society: Washington, DC, USA, 2014; Volume 1158, pp. 3–21. [Google Scholar] [CrossRef]
- Varela, E.; Tien, M. Effect of pH and Oxalate on Hydroquinone-Derived Hydroxyl Radical Formation during Brown Rot Wood Degradation. Appl. Environ. Microbiol. 2003, 69, 6025–6031. [Google Scholar] [CrossRef]
- Arantes, V.; Qian, Y.; Milagres, A.M.F.; Jellison, J.; Goodell, B. Effect of pH and Oxalic Acid on the Reduction of Fe3+ by a Biomimetic Chelator and on Fe3+ Desorption/Adsorption onto Wood: Implications for Brown-Rot Decay. Int. Biodeterior. Bbiodegrad. 2009, 63, 478–483. [Google Scholar] [CrossRef]
- Schwarze, F.W.M.R. Wood Decay under the Microscope. Fungal Biol. Rev. 2007, 21, 133–170. [Google Scholar] [CrossRef]
- Riley, R.; Salamov, A.A.; Brown, D.W.; Nagy, L.G.; Floudas, D.; Held, B.W.; Levasseur, A.; Lombard, V.; Morin, E.; Otillar, R.; et al. Extensive Sampling of Basidiomycete Genomes Demonstrates Inadequacy of the White-Rot/Brown-Rot Paradigm for Wood Decay Fungi. Proc. Natl. Acad. Sci. USA 2014, 111, 9923–9928. [Google Scholar] [CrossRef]
- Shabaev, A.V.; Moiseenko, K.V.; Glazunova, O.A.; Savinova, O.S.; Fedorova, T.V. Comparative Analysis of Peniophora lycii and Trametes hirsuta Exoproteomes Demonstrates “Shades of Gray” in the Concept of White-Rotting Fungi. Int. J. Mol. Sci. 2022, 23, 10322. [Google Scholar] [CrossRef] [PubMed]
- Abou-Mansour, E.; Polier, J.; Pezet, R.; Tabacchi, R. Purification and Partial Characterisation of a 60 KDa Laccase from Fomitiporia mediterranea. Phytopathol. Mediterr. 2009, 48, 447–453. [Google Scholar] [CrossRef]
- Morgenstern, I.; Robertson, D.L.; Hibbett, D.S. Characterization of Three Mnp Genes of Fomitiporia mediterranea and Report of Additional Class II Peroxidases in the Order Hymenochaetales. Appl. Environ. Microbiol. 2010, 76, 6431–6440. [Google Scholar] [CrossRef] [PubMed]
- Schilling, M.; Maia-Grondard, A.; Baltenweck, R.; Robert, E.; Hugueney, P.; Bertsch, C.; Farine, S.; Gelhaye, E. Wood Degradation by Fomitiporia mediterranea M. Fischer: Physiologic, Metabolomic and Proteomic Approaches. Front. Plant Sci. 2022, 13, 988709. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, K.; Niikura, M.; Kojima, Y.; Goodell, B.; Yoshida, M. Transcriptome Analysis of the Brown Rot Fungus Gloeophyllum trabeum during Lignocellulose Degradation. PLoS ONE 2020, 15, e0243984. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, S. Pan-Genome Analyses of 3 Strains of Inonotus obliquus and Prediction of Polysaccharide and Terpenoid Genes. Nat. Prod. Commun. 2021, 16, 1934578X211060922. [Google Scholar] [CrossRef]
- Tabacchi, R.; Dubin, G.-M.; Tabacchi, R. Phytotoxins from Fungi of Esca of Grapevine. Phytopathol. Mediterr. 2000, 39, 156–161. [Google Scholar] [CrossRef]
- Di Marco, S.; Mazzullo, A.; Cesari, A.; Osti, F. How Iron Could Be Involved in Esca Fungi Development. Phytopathol. Mediterr. 2001, 40, S449–S452. [Google Scholar] [CrossRef]
- Sparapano, L.; Bruno, G.; Campanella, A. Interactions between Three Fungi Associated with Esca of Grapevine, and Their Secondary Metabolites. Phytopathol. Mediterr. 2001, 40, S417–S422. [Google Scholar] [CrossRef]
- Bruno, G.; Sparapano, L. Effects of Three Esca-Associated Fungi on Vitis vinifera L.: I. Characterization of Secondary Metabolites in Culture Media and Host Responses to the Pathogens in Calli. Physiol. Mol. Plant Pathol. 2006, 69, 209–223. [Google Scholar] [CrossRef]
- Kassemeyer, H.-H.; Kluge, F.; Bieler, E.; Ulrich, M.; Grüner, J.; Fink, S.; Dürrenberger, M.; Fuchs, R. Trunk Anatomy of Asymptomatic and Symptomatic Grapevines Provides Insights into Degradation Patterns of Wood Tissues Caused by Esca-Associated Pathogens. Phytopathol. Mediterr. 2022, 61, 451–471. [Google Scholar] [CrossRef]
- Cowling, E.B.; Brown, W. Structural Features of Cellulosic Materials in Relation to Enzymatic Hydrolysis. In Cellulases and Their Applications; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1969; Volume 95, pp. 152–187. [Google Scholar] [CrossRef]
- Flournoy, D.S.; Kirk, T.K.; Highley, T.L. Wood Decay by Brown-Rot Fungi: Changes in Pore Structure and Cell Wall Volume. Holzforschung 1991, 45, 383–388. [Google Scholar] [CrossRef]
- Kleman-Leyer, K.; Agosin, E.; Conner, A.H.; Kirk, T.K. Changes in Molecular Size Distribution of Cellulose during Attack by White Rot and Brown Rot Fungi. Appl. Environ. Microbiol. 1992, 58, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Jellison, J.; Goodell, B. Peculiarities of Brown-Rot Fungi and Biochemical Fenton Reaction with Regard to Their Potential as a Model for Bioprocessing Biomass. Appl. Microbiol. Biotechnol. 2012, 94, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Gao, J.; Daniel, G. Cytochemical and Immunocytochemical Characterization of Wood Decayed by the White Rot Fungus Pycnoporus sanguineus I. Preferential Lignin Degradation Prior to Hemicelluloses in Norway Spruce Wood. Int. Biodeterior. Biodegrad. 2015, 105, 30–40. [Google Scholar] [CrossRef]
- Arantes, V.; Milagres, A.M.F. Evaluation of Different Carbon Sources for Production of Iron-Reducing Compounds by Wolfiporia cocos and Perenniporia medulla-panis. Process Biochem. 2006, 41, 887–891. [Google Scholar] [CrossRef]
- Arantes, V.; Milagres, A.M.F. The Synergistic Action of Ligninolytic Enzymes (MnP and Laccase) and Fe3+-Reducing Activity from White-Rot Fungi for Degradation of Azure B. Enzyme Microb. Technol. 2007, 42, 17–22. [Google Scholar] [CrossRef]
- Bruez, E.; Larignon, P.; Bertsch, C.; Robert-Siegwald, G.; Lebrun, M.-H.; Rey, P.; Fontaine, F. Impacts of Sodium Arsenite on Wood Microbiota of Esca-Diseased Grapevines. J. Fungi 2021, 7, 498. [Google Scholar] [CrossRef]
- Del Frari, G.; Oliveira, H.; Boavida Ferreira, R. White Rot Fungi (Hymenochaetales) and Esca of Grapevine: Insights from Recent Microbiome Studies. J. Fungi 2021, 7, 770. [Google Scholar] [CrossRef]
- Pacetti, A.; Moretti, S.; Pinto, C.; Compant, S.; Farine, S.; Bertsch, C.; Mugnai, L. Trunk Surgery as a Tool to Reduce Foliar Symptoms in Diseases of the Esca Complex and Its Influence on Vine Wood Microbiota. J. Fungi 2021, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- Jamaux-Despréaux, I.; Péros, J.P. Genetic Structure in Populations of the Fungus Fomitiporia punctata Associated with the Esca Syndrome in Grapevine. Vitis 2003, 42, 43–51. [Google Scholar] [CrossRef]
- Jellison, J.; Chandhoke, V.; Goodell, B.; Fekete, F.A. The Isolation and Immunolocalization of Iron-Binding Compounds Produced by Gloeophyllum trabeum. Appl. Microbiol. Biotechnol. 1991, 35, 805–809. [Google Scholar] [CrossRef]
- Highley, T.L. Influence of Carbon Source on Cellulase activity of white-rot and brown-rot fungi. Wood Fiber 1973, 5, 50–58. [Google Scholar]
- Cicco, N.; Lattanzio, V. The Influence of Initial Carbonate Concentration on the Folin-Ciocalteu Micro-Method for the Determination of Phenolics with Low Concentration in the Presence of Methanol: A Comparative Study of Real-Time Monitored Reactions. Am. J. Anal. Chem. 2011, 2, 840–848. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxi-dants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine—A New Spectrophotometric Reagent for Iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Rhee, S.G.; Chang, T.-S.; Jeong, W.; Kang, D. Methods for Detection and Measurement of Hydrogen Peroxide inside and Outside of Cells. Mol. Cells 2010, 29, 539–549. [Google Scholar] [CrossRef]
- Duling, D.R. Simulation of Multiple Isotropic Spin-Trap EPR Spectra. J. Magn. Reson. B 1994, 104, 105–110. [Google Scholar] [CrossRef]
- Arantes, V.; Milagres, A.M.F. Degradation of Cellulosic and Hemicellulosic Substrates Using a Chelator-Mediated Fenton Reaction. J. Chem. Technol. Biotechnol. 2006, 81, 413–419. [Google Scholar] [CrossRef]
- Sparapano, L. Effects on Plants of Metabolites Produced in Culture by Phaeoacremonium chlamydosporum, P. aleophilum and Fomitiporia punctata. Phytopathol. Mediterr. 2000, 39, 169–177. [Google Scholar] [CrossRef]
- Aguiar, A.; de Souza-Cruz, P.B.; Ferraz, A. Oxalic Acid, Fe3+-Reduction Activity and Oxidative Enzymes Detected in Culture Extracts Recovered from Pinus taeda Wood Chips Biotreated by Ceriporiopsis subvermispora. Enzym. Microb. Technol. 2006, 38, 873–878. [Google Scholar] [CrossRef]
- Schilling, J.S. Oxalate Production and Cation Translocation during Wood Biodegredation by Fungi. Electronic Theses and Dissertations. Ph.D. Thesis, The University of Maine, Orono, ME, USA, 2006. [Google Scholar]
- Arantes, V.; Milagres, A.M.F. Relevância de compostos de baixa massa molar produzidos por fungos e envolvidos na biodegradação da madeira. Quím. Nova 2009, 32, 1586–1595. [Google Scholar] [CrossRef]
- Fackler, K.; Gradinger, C.; Schmutzer, M.; Tavzes, C.; Burgert, I.; Schwanninger, M.; Hinterstoisser, B.; Watanabe, T.; Messner, K. Biotechnological Wood Modification with Selective White-Rot Fungi and Its Molecular Mechanisms. Food Technol. Biotechnol. 2007, 45, 269–276. [Google Scholar]
- Qin, X.; Sun, X.; Huang, H.; Bai, Y.; Wang, Y.; Luo, H.; Yao, B.; Zhang, X.; Su, X. Oxidation of a Non-Phenolic Lignin Model Compound by Two Irpex lacteus Manganese Peroxidases: Evidence for Implication of Carboxylate and Radicals. Biotechnol. Biofuels 2017, 10, 103. [Google Scholar] [CrossRef]
- Goodell, B.; Daniel, G.; Jellison, J.; Qian, Y. Iron-Reducing Capacity of Low-Molecular-Weight Compounds Produced in Wood by Fungi. Holzforschung 2006, 60, 630–636. [Google Scholar] [CrossRef]
- Tamaru, Y.; Yoshida, M.; Eltis, L.D.; Goodell, B. Multiple Iron Reduction by Methoxylated Phenolic Lignin Structures and the Generation of Reactive Oxygen Species by Lignocellulose Surfaces. Int. J. Biol. Macromol. 2019, 128, 340–346. [Google Scholar] [CrossRef]
- Niku-Paavola, M.-L.; Anke, H.; Poppius-Levlin, K.; Viikari, L. Siderophores as Natural Mediators in Laccase-Aided Degrada-tion of Lignin. In Applications of Enzymes to Lignocellulosics; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2003; Volume 855, pp. 176–190. [Google Scholar] [CrossRef]
- Kerem, Z.; Jensen, K.A.; Hammel, K.E. Biodegradative Mechanism of the Brown Rot Basidiomycete Gloeophyllum trabeum: Evidence for an Extracellular Hydroquinone-Driven Fenton Reaction. FEBS Lett. 1999, 446, 49–54. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-871748-5. [Google Scholar]
- Daniel, G.; Volc, J.; Filonova, L.; Plíhal, O.; Kubátová, E.; Halada, P. Characteristics of Gloeophyllum trabeum Alcohol Oxidase, an Extracellular Source of H2O2 in Brown Rot Decay of Wood. Appl. Environ. Microbiol. 2007, 73, 6241–6253. [Google Scholar] [CrossRef]
- Aguiar, A.; Ferraz, A. Fe3+- and Cu2+-Reduction by Phenol Derivatives Associated with Azure B Degradation in Fenton-like Reactions. Chemosphere 2007, 66, 947–954. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Lee, I.-K.; Seok, S.-J.; Lee, H.-J.; Kim, Y.-H.; Yun, B.-S. Antioxidant Polyphenols from the Mycelial Culture of the Medicinal Fungi Inonotus xeranticus and Phellinus linteus. J. Appl. Microbiol. 2008, 104, 1824–1832. [Google Scholar] [CrossRef]
- Smith, H.; Doyle, S.; Murphy, R. Target Directed Identification of Natural Bioactive Compounds from Filamentous Fungi. Food Chem. 2023, 405, 134743. [Google Scholar] [CrossRef] [PubMed]
- Paszczynski, A.; Crawford, R.; Funk, D.; Goodell, B. De Novo Synthesis of 4,5-Dimethoxycatechol and 2,5-Dimethoxyhydroquinone by the Brown Rot Fungus Gloeophyllum trabeum. Appl. Environ. Microbiol. 1999, 65, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Contreras, D.; Rodríguez, J.; Freer, J.; Schwederski, B.; Kaim, W. Enhanced Hydroxyl Radical Production by Dihydroxybenzene-Driven Fenton Reactions: Implications for Wood Biodegradation. JBIC J. Biol. Inorg. Chem. 2007, 12, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-K.; Han, M.-S.; Lee, M.-S.; Kim, Y.-S.; Yun, B.-S. Styrylpyrones from the Medicinal Fungus Phellinus baumii and Their Antioxidant Properties. Bioorg. Med. Chem. Lett. 2010, 20, 5459–5461. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Misra, R.; Mariam, A.; Yusuf, K.; Yusuf, L. Salicylic Acid Alters Antioxidant and Phenolics Metabolism in Catharanthus roseus Grown Under Salinity Stress. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 118–125. [Google Scholar] [CrossRef]
- Dhiman, S.; Kohli, S.K.; Bhardwaj, T.; Kapoor, D.; Srihindi, G.; Bhardwaj, R. ROS Regulation by Salicylic Acid Under Abiotic Stress. In Managing Plant Stress Using Salicylic Acid; Sharma, A., Bhardwaj, R., Kumar, V., Zheng, B., Tripathi, D.K., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 239–257. [Google Scholar] [CrossRef]
- Ng, T.B.; Liu, F.; Zhao, L. Antioxidative and Free Radical Scavenging Activities of Pineal Indoles. J. Neural Transm. 2000, 107, 1243–1251. [Google Scholar] [CrossRef]
- Grisham, M.B.; Ware, K.; Marshall, S.; Yamada, T.; Sandhu, I.S. Prooxidant Properties of 5-Aminosalicylic Acid. Possible Mechanism for Its Adverse Side Effects. Dig. Dis. Sci. 1992, 37, 1383–1389. [Google Scholar] [CrossRef]
- Bruez, E.; Vallance, J.; Gautier, A.; Laval, V.; Compant, S.; Maurer, W.; Sessitsch, A.; Lebrun, M.-H.; Rey, P. Major Changes in Grapevine Wood Microbiota Are Associated with the Onset of Esca, a Devastating Trunk Disease. Environ. Microbiol. 2020, 22, 5189–5206. [Google Scholar] [CrossRef]
- Elena, K.; Fischer, M.; Dimou, D.; Dimou, D.M. Fomitiporia mediterranea Infecting Citrus Trees in Greece. Phytopathol. Mediterr. 2006, 45, 35–39. [Google Scholar] [CrossRef]
- Markakis, E.A.; Kavroulakis, N.; Ntougias, S.; Koubouris, G.C.; Sergentani, C.K.; Ligoxigakis, E.K. Characterization of Fungi Associated with Wood Decay of Tree Species and Grapevine in Greece. Plant Dis. 2017, 101, 1929–1940. [Google Scholar] [CrossRef]
- Wolf, S.; Schmidt, S.; Müller-Hannemann, M.; Neumann, S. In Silico Fragmentation for Computer Assisted Identification of Metabolite Mass Spectra. BMC Bioinform. 2010, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Shou, D.; Dong, Y.; Wang, N.; Li, H.; Zhang, Y.; Zhu, Y. Simultaneous Quantification of Antioxidant Compounds in Phellinus igniarius Using Ultra Performance Liquid Chromatography-Photodiode Array Detection-Electrospray Ionization Tandem Mass Spectrometry. PLoS ONE 2016, 11, e0163797. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Qiu, P.; Zhu, R.; Zhao, L.; Zhang, P.; Wang, Y.; Li, C.; Chai, K.; Shou, D.; Zhao, H. A Combined Phytochemistry and Network Pharmacology Approach to Reveal the Potential Antitumor Effective Substances and Mechanism of Phellinus igniarius. Front. Pharmacol. 2019, 10, 266. [Google Scholar] [CrossRef] [PubMed]
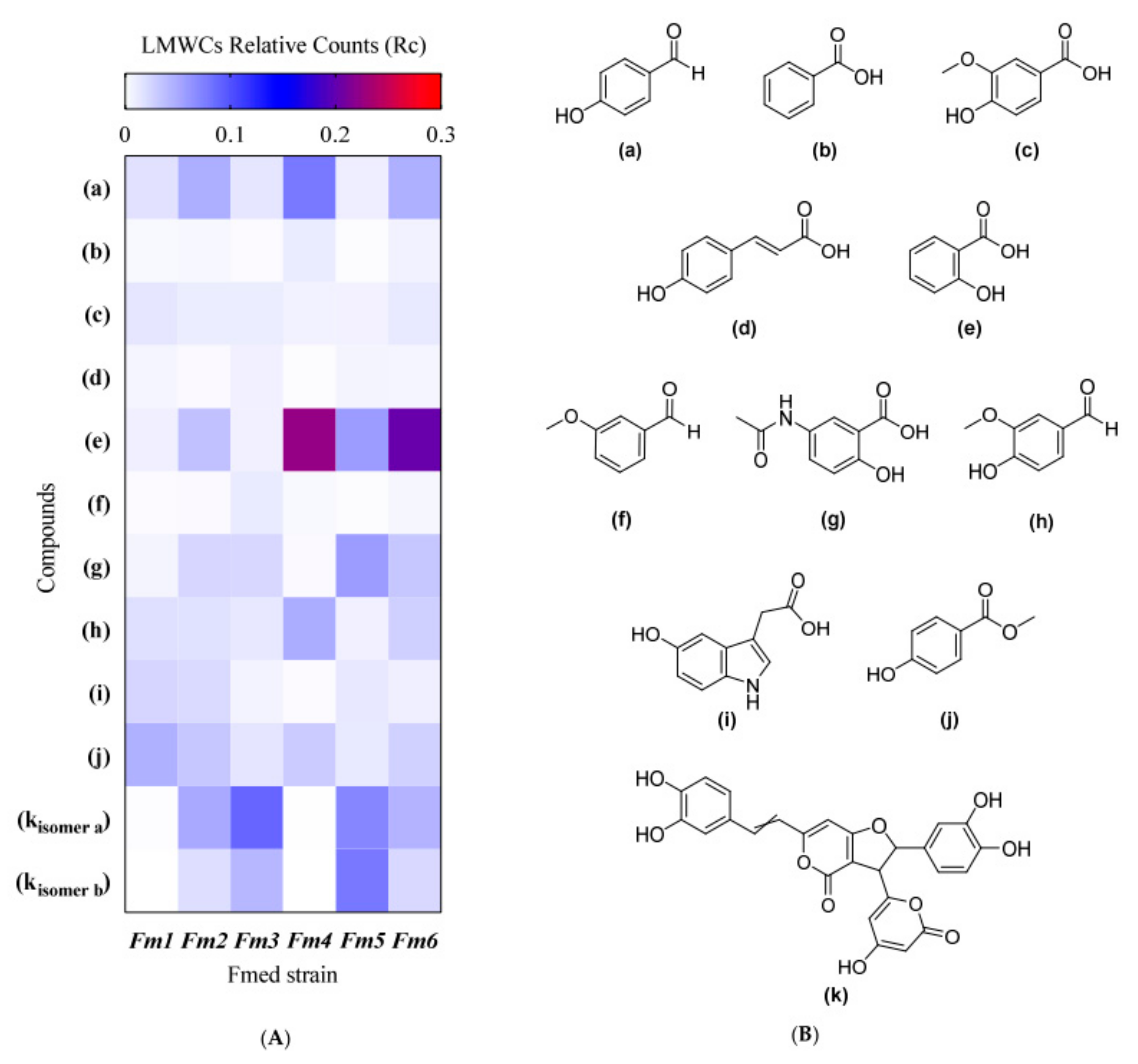

| Compound | FeRA | Type of Annotation |
|---|---|---|
| (a) 4-Hydroxybenzaldehyde | / | 1 |
| (b) Benzoic acid | / | 1 |
| (c) 4-Hydroxy-3-methoxybenzoic acid (vanillic acid) | † | 1 |
| (d) 4-Hydroxycinnamic acid (p-coumaric acid) | † | 1 |
| (e) 2-Hydroxybenzoic acid (salicylic acid) | † | 1 |
| (f) 3-Methoxybenzaldehyde | / | 1 |
| (g) N-acetyl-5-aminosalicylic acid | / | 2 |
| (h) 4-Hydroxy-3-methoxybenzaldehyde (vanillin) | † | 2 |
| (i) 5-Hydroxyindole-3-acetic acid | / | 2 |
| (j) Methyl-4-hydroxybenzoate | / | 2 |
| (k) Hypholomine B (isomers a and b) | / | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, S.; Goddard, M.-L.; Puca, A.; Lalevée, J.; Di Marco, S.; Mugnai, L.; Gelhaye, E.; Goodell, B.; Bertsch, C.; Farine, S. First Description of Non-Enzymatic Radical-Generating Mechanisms Adopted by Fomitiporia mediterranea: An Unexplored Pathway of the White Rot Agent of the Esca Complex of Diseases. J. Fungi 2023, 9, 498. https://doi.org/10.3390/jof9040498
Moretti S, Goddard M-L, Puca A, Lalevée J, Di Marco S, Mugnai L, Gelhaye E, Goodell B, Bertsch C, Farine S. First Description of Non-Enzymatic Radical-Generating Mechanisms Adopted by Fomitiporia mediterranea: An Unexplored Pathway of the White Rot Agent of the Esca Complex of Diseases. Journal of Fungi. 2023; 9(4):498. https://doi.org/10.3390/jof9040498
Chicago/Turabian StyleMoretti, Samuele, Mary-Lorène Goddard, Alessandro Puca, Jacques Lalevée, Stefano Di Marco, Laura Mugnai, Eric Gelhaye, Barry Goodell, Christophe Bertsch, and Sibylle Farine. 2023. "First Description of Non-Enzymatic Radical-Generating Mechanisms Adopted by Fomitiporia mediterranea: An Unexplored Pathway of the White Rot Agent of the Esca Complex of Diseases" Journal of Fungi 9, no. 4: 498. https://doi.org/10.3390/jof9040498
APA StyleMoretti, S., Goddard, M.-L., Puca, A., Lalevée, J., Di Marco, S., Mugnai, L., Gelhaye, E., Goodell, B., Bertsch, C., & Farine, S. (2023). First Description of Non-Enzymatic Radical-Generating Mechanisms Adopted by Fomitiporia mediterranea: An Unexplored Pathway of the White Rot Agent of the Esca Complex of Diseases. Journal of Fungi, 9(4), 498. https://doi.org/10.3390/jof9040498











