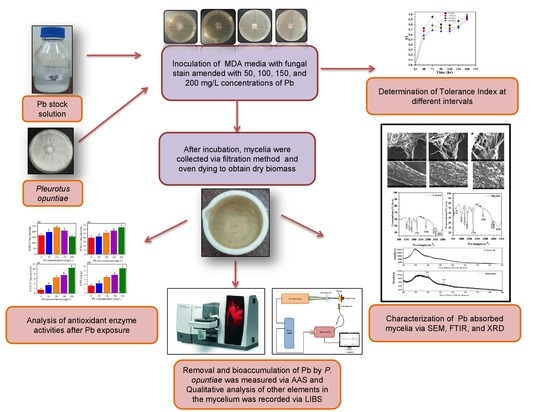
An Approach to Evaluate Pb Tolerance and Its Removal Mechanisms by Pleurotus opuntiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of Fungal Culture
2.2. Screening for Pb Tolerance Characteristics of P. opuntiae
2.3. Experimental Design for Growth, Removal, and Bioaccumulation Studies
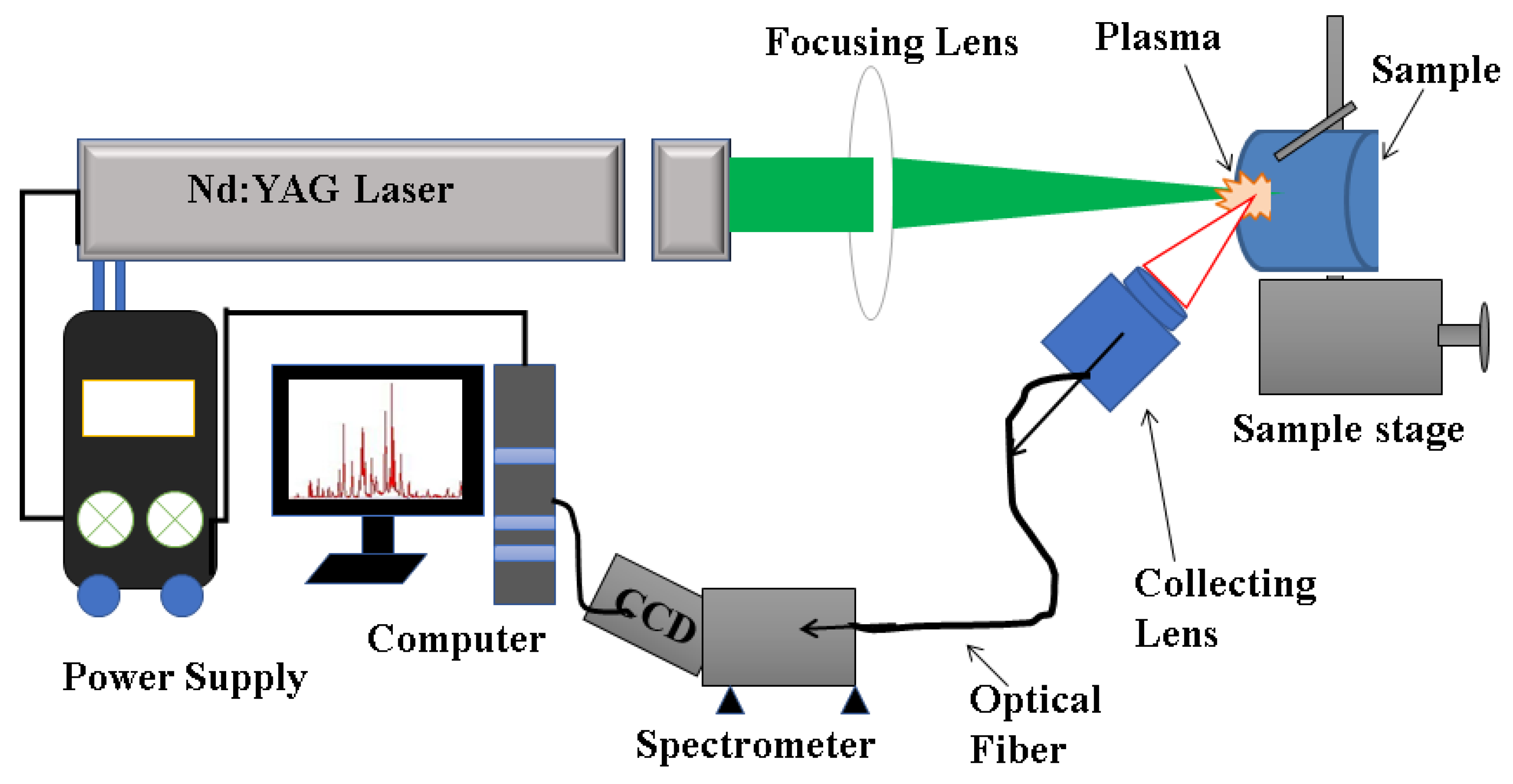
2.4. LIBS Analysis
2.5. SEM, FTIR, and XRD Analysis
2.6. Analysis of Proline Content and Malonaldehyde (MDA)
2.7. Analysis of Enzymatic Antioxidants in Pb Containing Mycelia
2.8. Statistical Analysis
3. Results and Discussion
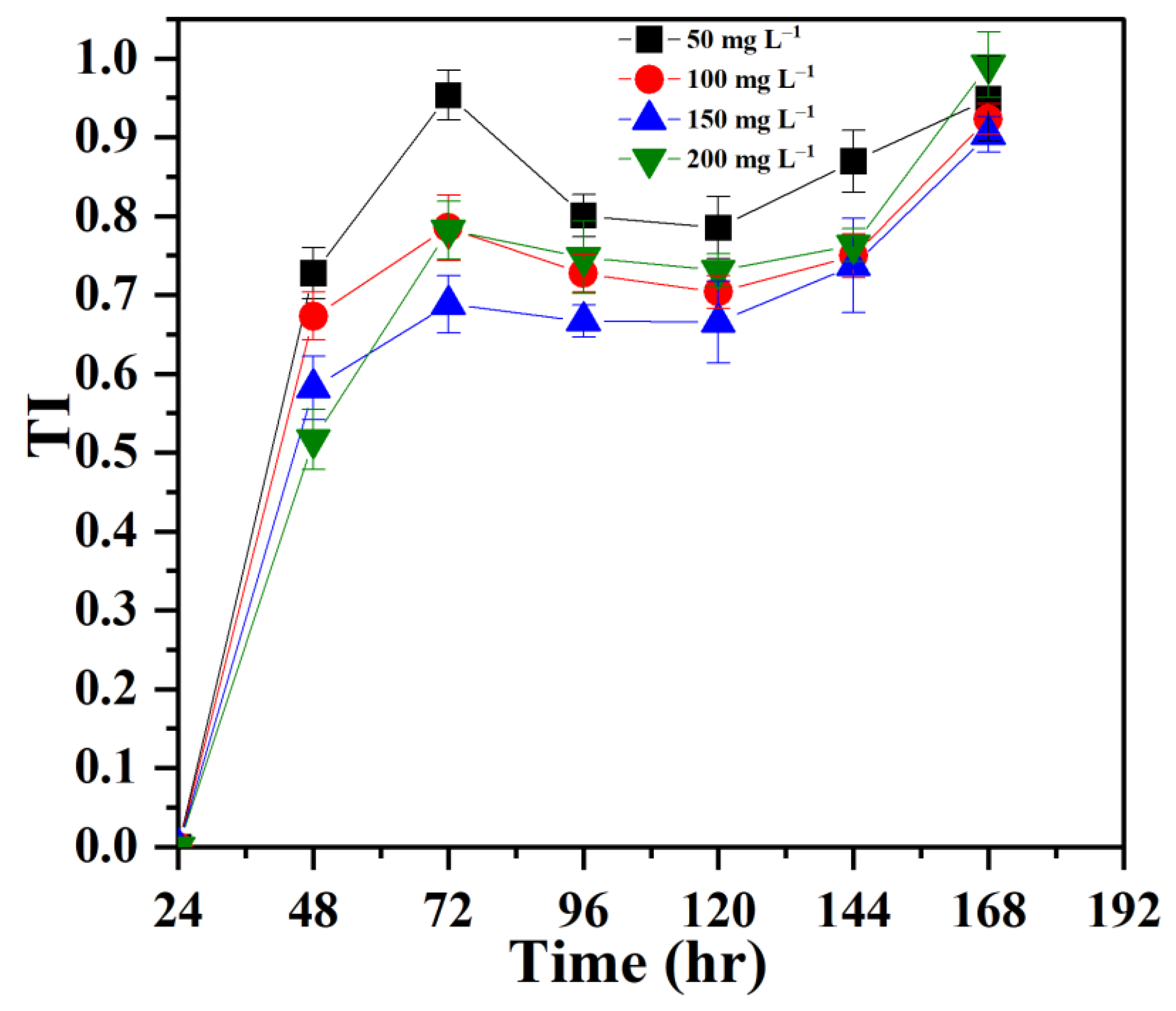
3.1. Establishing Tolerance of P. opuntiae to Pb
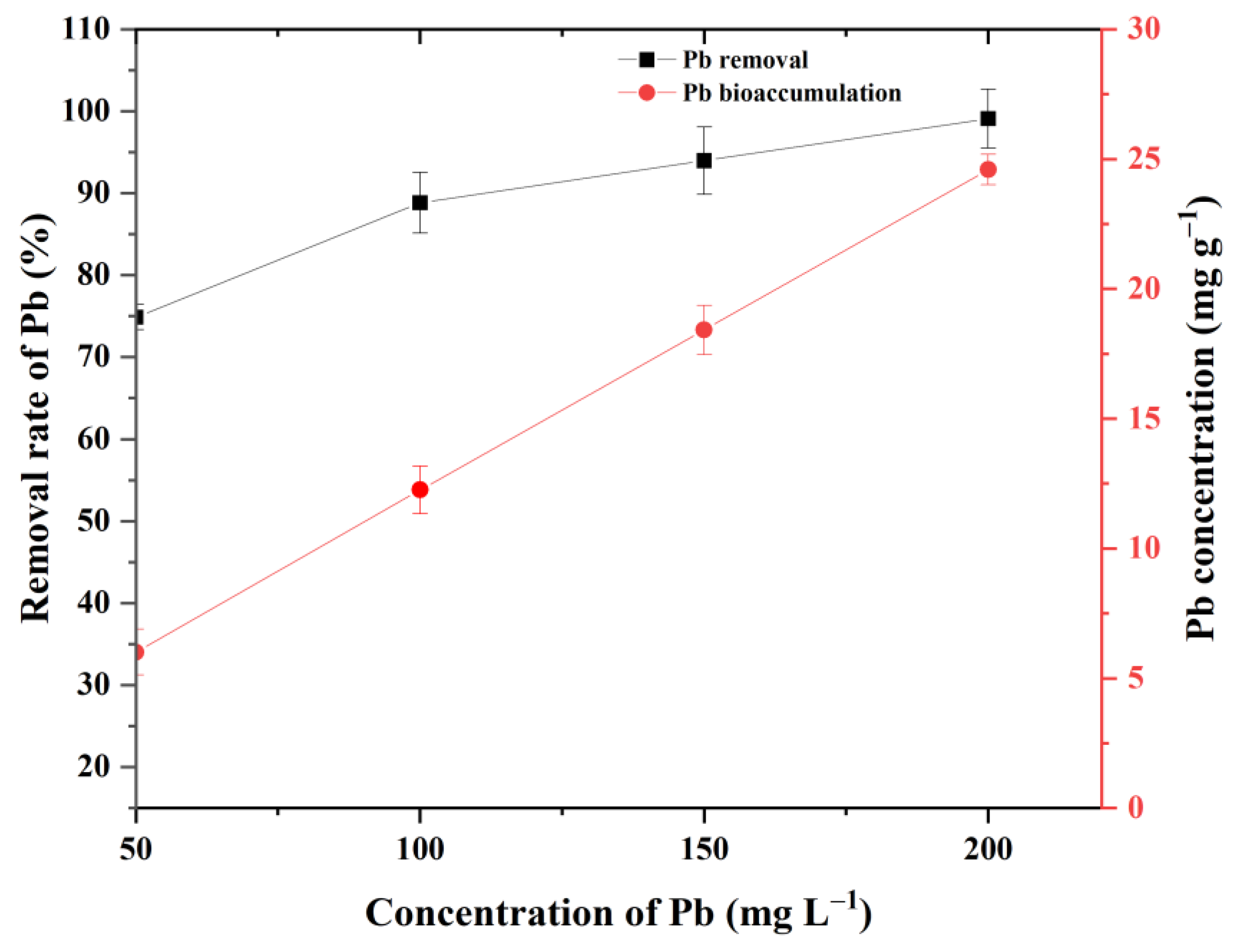
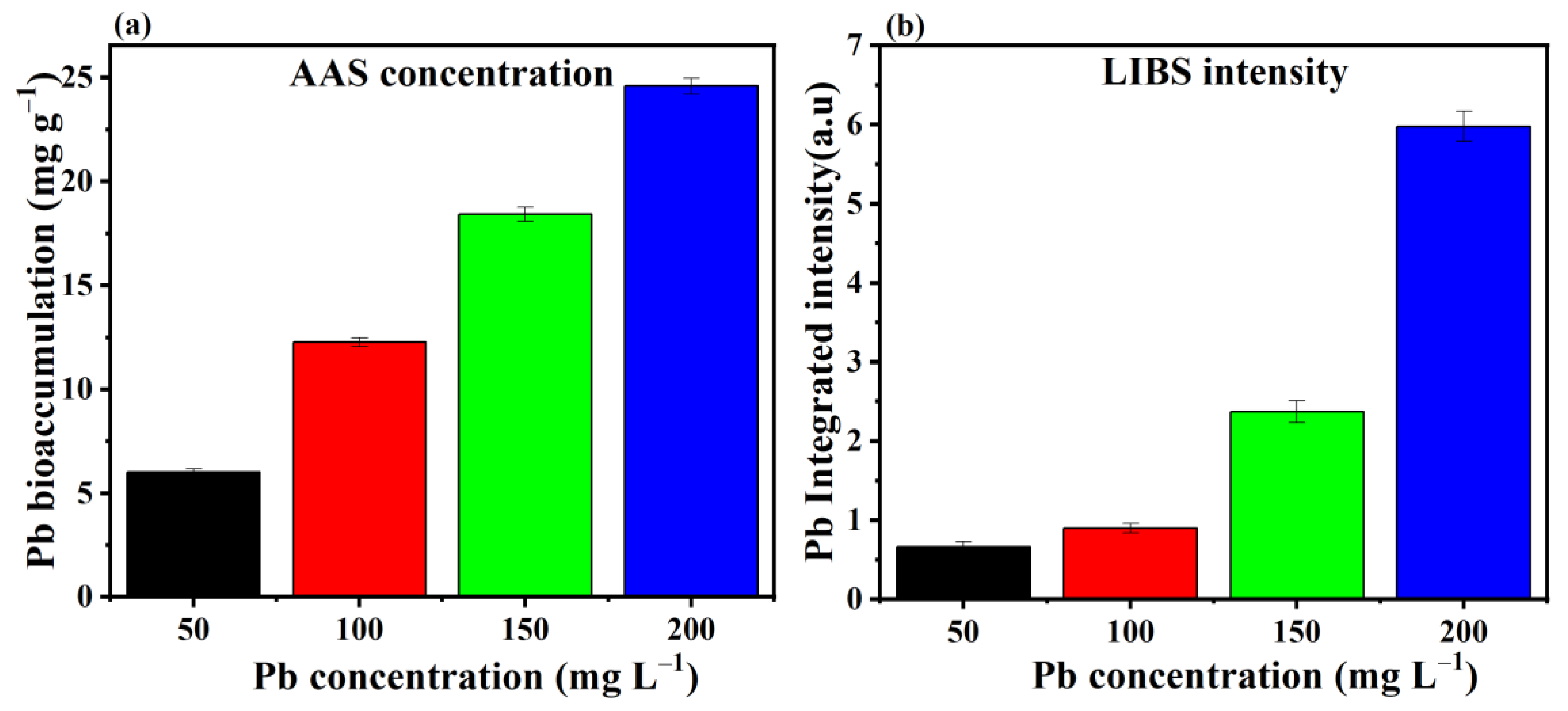
3.2. Removal and Bioaccumulation Efficiency of Pb by P. opuntiae
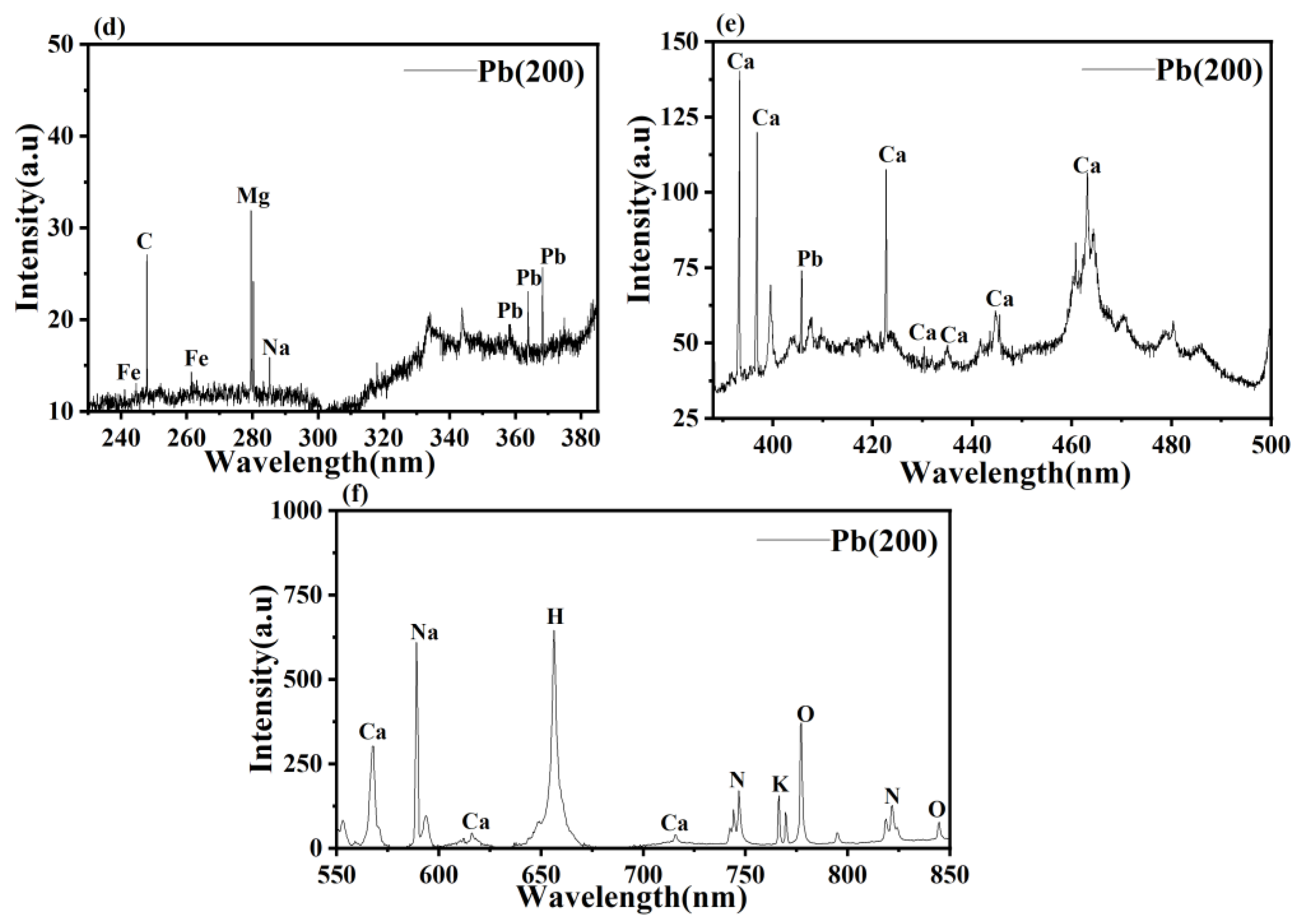
3.3. LIBS Analysis

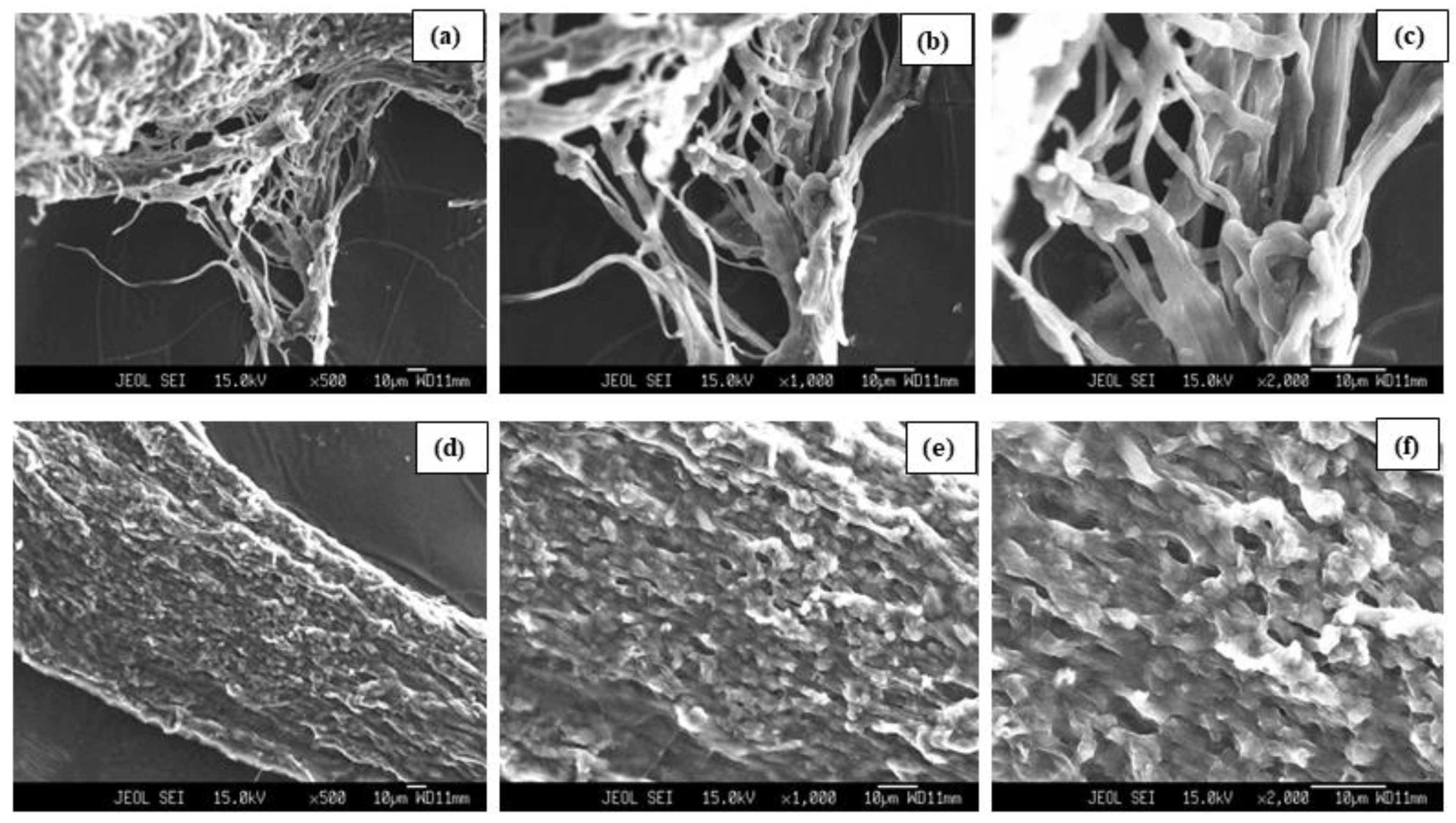
3.4. SEM Analysis
3.5. FTIR Analysis
| Control | Pb (200) mg L−1 | Functional Groups |
|---|---|---|
| 3292 | 3289 | O–H and N–H stretching |
| 2356 | 2352 | C–O stretch of carbonyl compound |
| 1737 | 1735 | C=O group of ester |
| 1374 | 1364 | Amide III group |
| 1217 | 1216 | Phosphate group |
| 1024 | 1015 | C–C, C=C, C–O–C, C–O–P of saccharides |
| - | 673 | C=O in amides |
| - | 527 | Nitro compounds and disulfide groups |

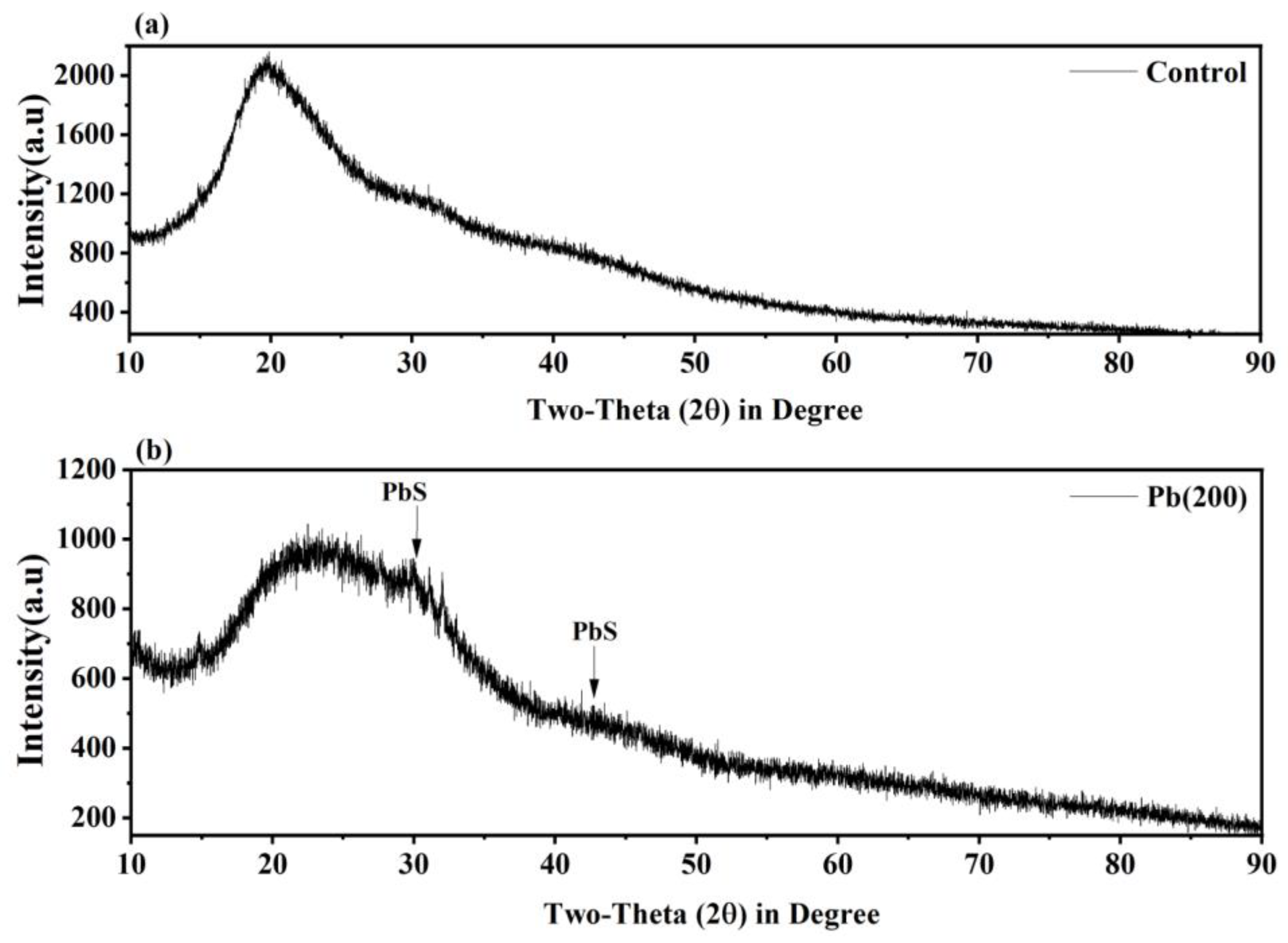
3.6. XRD Analysis
3.7. Effect of Pb Stress on Proline Content and MDA

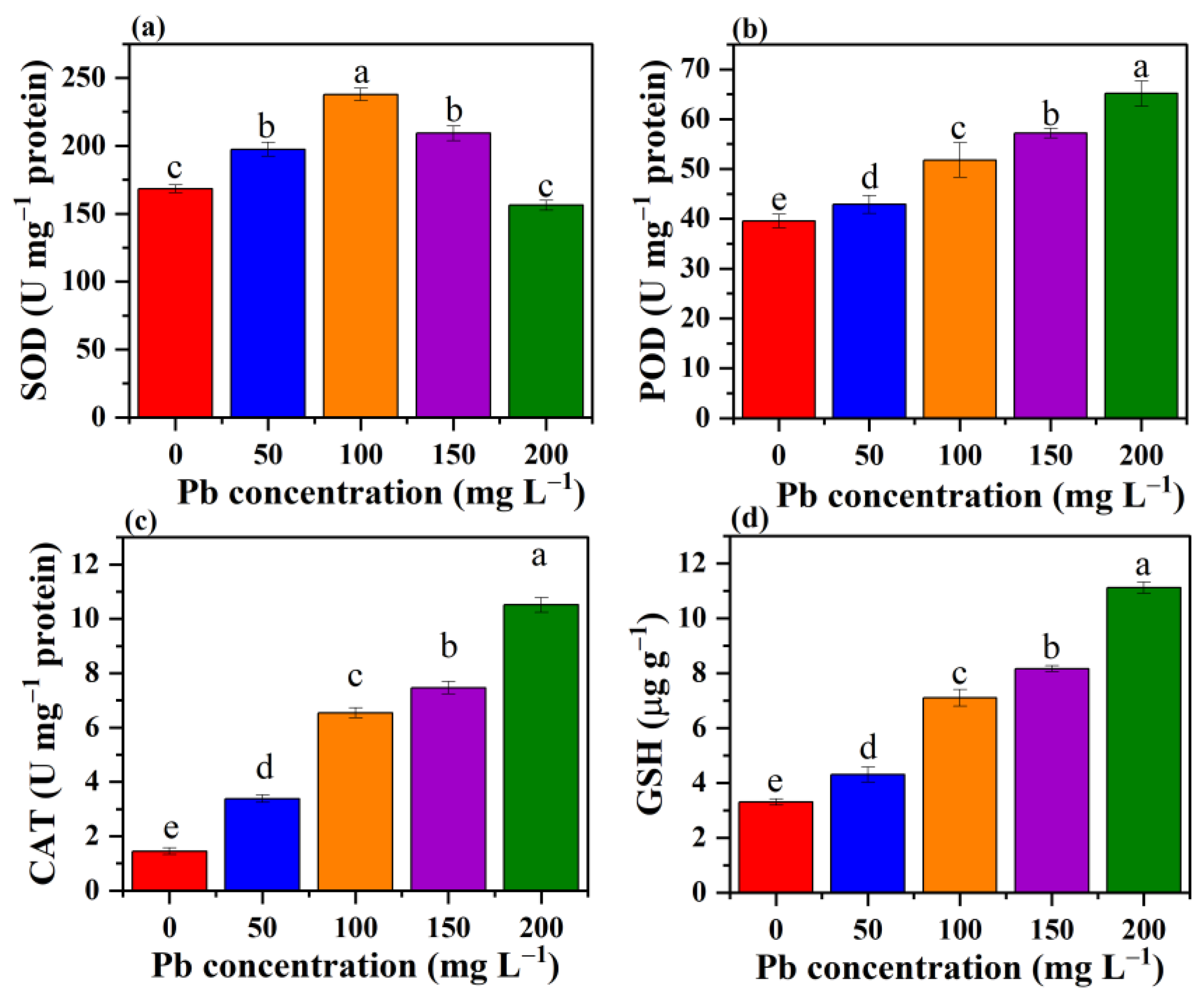
3.8. Effect of Pb Stress on the Antioxidant System of P. opuntiae
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahmoud, M.E.; Nabil, G.M.; Mahmoud, S.M. High performance nano-zirconium silicate adsorbent for efficient removal of copper(II), cadmium(II) and lead(II). J. Environ. Chem. Eng. 2015, 3, 1320–1328. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, J.; Du, B.; Liu, H.; Zhang, W.; Liang, J.; Zhang, W.; You, L.; Zhou, J. Health risks to local residents from the exposure of heavy metals around the largest copper smelter in China. Ecotoxicol. Environ. Saf. 2019, 171, 329–336. [Google Scholar] [CrossRef]
- Shukla, A.; Srivastava, S.; D’Souza, S.F. An integrative approach toward biosensing and bioremediation of metals and metalloids. Int. J. Environ. Sci. Technol. 2018, 15, 2701–2712. [Google Scholar] [CrossRef]
- Simonescu, C.M.; Ferdes, M. Fungal biomass for Cu(II) uptake from aqueous systems. Pol. J. Environ. Stud. 2012, 21, 1831–1839. [Google Scholar]
- Miransari, M. Hyperaccumulators, arbuscular mycorrhizal fungi and stress of heavy metals. Biotechnol. Adv. 2011, 29, 645–653. [Google Scholar] [CrossRef]
- Tang, X.; Li, Q.; Wu, M.; Lin, L.; Scholz, M. Review of remediation practices regarding cadmium-enriched farmland soil with particular reference to China. J. Environ. Manag. 2016, 181, 646–662. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Rai, S.N.; Mishra, V.; Singh, M.P. Mycoremediation of environmental pollutants: A review with special emphasis on mushrooms. Environ. Sustain. 2021, 4, 605–618. [Google Scholar] [CrossRef]
- Birch, L.; Bachofen, R. Complexing agents from microorganisms. Experientia 1990, 46, 827–834. [Google Scholar] [CrossRef]
- Vimala, R.; Das, N. Biosorption of cadmium(II) and lead(II) from aqueous solutions using mushrooms: A comparative study. J. Hazard. Mater. 2009, 168, 376–382. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Parhi, P.K.; Pandey, S.; Bindhani, B.K.; Thatoi, H.; Panda, C.R. Active and passive biosorption of Pb(II)using live and dead biomass of marine bacterium Bacillus xiamenensis PbRPSD202: Kinetics and isotherm studies. J. Environ. Manag. 2019, 247, 121–134. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Bao, H.; Wu, T.; Zhao, Y.; Liu, D.; Li, X.; Yang, T.; Yu, H. A Novel high biosorbent of Pb-resistant bacterium isolate for the removal of hazardous lead from alkaline soil and water: Biosorption isotherms In vivo and bioremediation strategy. Geomicrobiol. J. 2018, 35, 174–185. [Google Scholar] [CrossRef]
- Ezzouhri, L.; Castro, E.; Moya, M.; Espinola, F.; Lairini, K. Heavy metal tolerance of filamentous fungi isolated from polluted sites in Tangier, Morocco. Afr. J. Microbiol. Res. 2009, 3, 35–48. [Google Scholar]
- Singh, M.P. Mushroom Biotechnology: The rise of the fallen. Smart Biomed. Physiol. Sens. Technol. XVI 2019, 11020, 1102003. [Google Scholar]
- Iskandar, N.L.; Zainudin, N.A.I.M.; Tan, S.G. Tolerance and biosorption of copper (Cu) and lead (Pb) by filamentous fungi isolated from a freshwater ecosystem. J. Environ. Sci. 2011, 23, 824–830. [Google Scholar] [CrossRef]
- Budzyńska, S.; Siwulski, M.; Budka, A.; Kalač, P.; Niedzielski, P.; Gąsecka, M.; Mleczek, M. Mycoremediation of Flotation Tailings with Agaricus bisporus. J. Fungi 2022, 8, 883. [Google Scholar] [CrossRef] [PubMed]
- Sim, C.S.F.; Tan, W.S.; Ting, A.S.Y. Endophytes from Phragmites for metal removal: Evaluating their metal tolerance, adaptive tolerance behaviour and biosorption efficacy. Desalin. Water Treat. 2016, 57, 6959–6966. [Google Scholar] [CrossRef]
- Mishra, A.; Malik, A. Simultaneous bioaccumulation of multiple metals from electroplating effluent using Aspergillus lentulus. Water Res. 2012, 46, 4991–4998. [Google Scholar] [CrossRef]
- Singh, M.P.; Pandey, V.K.; Srivastava, A.K.; Vishwakarma, S. Enzyme technology and mycoremediation by white rot fungi. In Recent Trends in Biotechnology; Singh, M., Agrawal, A., Sharma, B., Eds.; Nova Science Publishers: New York, NY, USA, 2011; Volume 2, pp. 157–163. [Google Scholar]
- Javaid, A.; Bajwa, R.; Shafique, U.; Anwar, J. Removal of heavy metals by adsorption on Pleurotus ostreatus. Biomass Bioenergy 2011, 35, 1675–1682. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Chang, C.; Yuan, Z.; Wang, T.; Zhao, Y.; Yang, X.; Zhang, Y.; La, G.; Wu, K.; et al. Improvement of tolerance to lead by filamentous fungus Pleurotus ostreatus HAU-2 and its oxidative responses. Chemosphere 2016, 150, 33–39. [Google Scholar] [CrossRef]
- Coreño-Alonso, A.; Solé, A.; Diestra, E.; Esteve, I.; Gutiérrez-Corona, J.F.; López, G.R.; Fernández, F.J.; Tomasini, A. Mechanisms of interaction of chromium with Aspergillus niger var tubingensis strain Ed8. Bioresour. Technol. 2014, 158, 188–192. [Google Scholar] [CrossRef]
- Karthik, C.; Barathi, S.; Pugazhendhi, A.; Ramkumar, V.S.; Thi, N.B.D.; Arulselvi, P.I. Evaluation of Cr(VI) reduction mechanism and removal by Cellulosimicrobium funkei strain AR8, a novel haloalkaliphilic bacterium. J. Hazard. Mater. 2017, 333, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Rafi, S.; Shoaib, A.; Awan, Z.A.; Rizvi, N.B.; Shafiq, M. Chromium tolerance, oxidative stress response, morphological characteristics, and FTIR studies of phytopathogenic fungus Sclerotium rolfsii. Folia Microbiol. 2017, 62, 207–219. [Google Scholar] [CrossRef]
- Yang, S.; Sun, X.; Shen, Y.; Chang, C.; Guo, E.; La, G.; Zhao, Y.; Li, X. Tolerance and removal mechanisms of heavy metals by fungus Pleurotus ostreatus Haas. Water Air Soil Pollut. 2017, 228, 1–9. [Google Scholar] [CrossRef]
- Valix, M.; Tang, J.Y.; Cheung, W.H. The effects of mineralogy on the biological leaching of nickel laterite ores. Miner. Eng. 2001, 14, 1629–1635. [Google Scholar] [CrossRef]
- Valix, M.; Loon, L.O. Adaptive tolerance behaviour of fungi in heavy metals. Miner. Eng. 2003, 16, 193–198. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Pan, Y.; Yu, H.; Zhang, X.; Shen, Y.; Jiao, S.; Wu, K.; La, G.; Yuan, Y.; et al. Mechanisms of Cd and Cr removal and tolerance by macrofungus Pleurotus ostreatus HAU-2. J. Hazard. Mater. 2017, 330, 1–8. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Chen, S.H.; Ng, S.L.; Cheow, Y.L.; Ting, A.S.Y. A novel study based on adaptive metal tolerance behavior in fungi and SEM-EDX analysis. J. Hazard. Mater. 2017, 334, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Gola, D.; Dey, P.; Bhattacharya, A.; Mishra, A.; Malik, A.; Namburath, M.; Ahammad, S.Z. Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Bioresour. Technol. 2016, 218, 388–396. [Google Scholar] [CrossRef]
- Gratão, P.L.; Monteiro, C.C.; Antunes, A.M.; Peres, L.E.P.; Azevedo, R.A. Acquired tolerance of tomato (Lycopersicon esculentum cv. Micro-Tom) plants to cadmium-induced stress. Ann. Appl. Biol. 2008, 153, 321–333. [Google Scholar] [CrossRef]
- Li, H.S.; Sun, Q.; Zhao, S.J.; Zhang, W.H. Principles and Techniques of Plant Physiological Biochemical Experiment; Higher Education Press: Beijing, China, 2000; pp. 195–197. [Google Scholar]
- Lowry, O.H.; Rosenbrough, N.G.; Farr, A.L.; Randall, R.G. Protein measurements with folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Das, K.; Samanta, L.; Chainy, G.B.N. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Ind. J. Biochem. Biophys. 2000, 37, 201–204. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–127. [Google Scholar]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lungs and liver. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Anahid, S.; Yaghmaei, S.; Ghobadinejad, Z. Heavy metal tolerance of fungi. Sci. Iran. 2011, 18, 502–508. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, B.; Sun, X.; Yu, L.; Wu, L.; Liu, W.; Wang, D.; Li, Y.; Jia, R.; Yu, H.; et al. Removal and tolerance mechanism of Pb by a filamentous fungus: A case study. Chemosphere 2019, 225, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.C.; Zalups, R.K. Molecular and ionic mimicry and the transport of toxic metals. Toxicol. Appl. Pharmacol. 2005, 204, 274–308. [Google Scholar] [CrossRef]
- Dwivedi, S.N.; Mishra, R.P.; Alava, S. Phytochemistry, Pharmacological studies and Traditional benefits of Trachyspermum ammi (Linn.) Sprague. Int. J. Pharm. Life Sci. 2012, 3, 1705–1709. [Google Scholar]
- Flora, S.J.S.; Mittal, M.; Mehta, A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 2008, 128, 501–523. [Google Scholar] [PubMed]
- Kumar, V.; Singh, S.; Singh, G.; Dwivedi, S.K. Exploring the cadmium tolerance and removal capability of a filamentous fungus Fusarium solani. Geomicrobiol. J. 2019, 36, 782–791. [Google Scholar] [CrossRef]
- Xu, X.; Hao, R.; Xu, H.; Lu, A. Removal mechanism of Pb(II) by Penicillium polonicum: Immobilization, adsorption, and bioaccumulation. Sci. Rep. 2020, 10, 9079. [Google Scholar] [CrossRef]
- Sharma, K.R.; Giri, R.; Sharma, R.K. Lead, cadmium and nickel removal efficiency of white-rot fungus Phlebia brevispora. Lett. Appl. Microbiol. 2020, 71, 637–644. [Google Scholar] [CrossRef]
- Xu, P.; Liu, L.; Zeng, G.; Huang, D.; Lai, C.; Zhao, M.; Huang, C.; Li, N.; Wei, Z.; Wu, H.; et al. Heavy metal-induced glutathione accumulation and its role in heavy metal detoxification in Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2014, 98, 6409–6418. [Google Scholar] [CrossRef]
- Singh, M.P. Biotechnology in hazardous waste management. In Recent Trends in Biotechnology; Singh, M., Agrawal, A., Sharma, B., Eds.; Nova Science Publishers: New York, NY, USA, 2010; Volume 1, pp. 1–12. [Google Scholar]
- Zhu, C.; Li, Z.; Li, D.; Xin, Y. Pb tolerance and bioaccumulation by the mycelia of Flammulina velutipes in artificial enrichment medium. J. Microbiol. 2014, 52, 8–12. [Google Scholar] [CrossRef] [PubMed]
- NIST: National Institute of Standards and Technology USA, Electronic Database. Available online: http://physics.nist.gov/PhysRefData/ASD/linesform.html (accessed on 23 January 2023).
- Yang, H.; Huang, L.; Chen, T.; Rao, G.; Liu, M.; Chen, J.; Yao, M. Spectral filtering method for improvement of detection accuracy of lead in vegetables by laser induced breakdown spectroscopy. Chin. J. Anal. Chem. 2017, 45, 1123–1128. [Google Scholar]
- Peng, J.; He, Y.; Ye, L.; Shen, T.; Liu, F.; Kong, W.; Liu, X.; Zhao, Y. Moisture influence reducing method for heavy metals detection in plant materials using laser-induced breakdown spectroscopy: A case study for chromium content detection in rice leaves. Anal. Chem. 2017, 89, 7593–7600. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.; Rai, A.K.; Dwivedi, A.; Kumar, R.; Azam, M.; Singh, V.; Yadav, N.; Rai, A.K. Chemical Characterization for the Detection of Impurities in Tainted and Natural Curcuma longa from India Using LIBS Coupled with PCA. Atoms 2022, 10, 91. [Google Scholar] [CrossRef]
- Liu, Z.F.; Zeng, G.M.; Zhong, H.; Yuan, X.Z.; Jiang, L.L.; Fu, H.Y.; Ma, X.L.; Zhang, J.C. Effect of saponins on cell surface properties of Penicillium simplicissimum: Performance on adsorption of cadmium(II). Colloids Surf. B Biointerfaces 2011, 86, 364–369. [Google Scholar] [CrossRef]
- Chen, S.H.; Cheow, Y.L.; Ng, S.L.; Ting, A.S.Y. Mechanisms for metal removal established via electron microscopy and spectroscopy: A case study on metal tolerant fungi Penicillium simplicissimum. J. Hazard. Mater. 2019, 362, 394–402. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorption of heavy metals by Saccharomyces cerevisiae: A review. Biotechnol. Adv. 2006, 24, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cui, J.; Wei, Z. Effects of low molecular weight organic acids on the immobilization of aqueous Pb(II) using phosphate rock and different crystallized hydroxyapatite. Chemosphere 2014, 105, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, N.; Hwang, G.; Sathishkumar, M.; Choi, T.K.; Lee, K.J.; Oh, B.T.; Lee, Y.S. Isolation, identification, Pb(II) biosorption isotherms and kinetics of a lead adsorbing Penicillium sp. MRF-1 from South Korean mine soil. J. Environ. Sci. 2010, 22, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.Z.; Jiang, L.L.; Zeng, G.M.; Liu, Z.F.; Zhong, H.; Huang, H.J.; Zhou, M.F.; Cu, K.L. Effect of rhamnolipids on cadmium adsorption by Penicillium simplicissimum. J. Cent. South Univ. 2012, 19, 1073–1080. [Google Scholar] [CrossRef]
- Zhao, W.W.; Zhu, G.; Daugulis, A.J.; Chen, Q.; Ma, H.Y.; Zheng, P.; Liang, J.; Ma, X.K. Removal and biomineralization of Pb2+ in water by fungus Phanerochaete chrysoporium. J. Clean. Prod. 2020, 260, 120980. [Google Scholar] [CrossRef]
- Rhee, Y.J.; Hillier, S.; Pendlowski, H.; Gadd, G.M. Fungal transformation of metallic lead to pyromorphite in liquid medium. Chemosphere 2014, 113, 17–21. [Google Scholar] [CrossRef]
- Bačkor, M.; Fahselt, D.; Wu, C.T. Free proline content is positively correlated with copper tolerance of the lichen photobiont Trebouxia erici (Chlorophyta). Plant Sci. 2004, 167, 151–157. [Google Scholar] [CrossRef]
- Vasilaki, A.T.; McMillan, D.C. Lipid peroxidation. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Lazarova, N.; Krumova, E.; Stefanova, T.; Georgieva, N.; Angelova, M. The oxidative stress response of the filamentous yeast Trichosporon cutaneum R57 to copper, cadmium and chromium exposure. Biotechnol. Biotechnol. Equip. 2014, 28, 855–862. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.; Ge, Q.; Li, Y.; Sun, J.; Zhang, Y.; Liu, X. Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol. 2012, 196, 125–138. [Google Scholar] [CrossRef]
- Prasad, G.; Kumar, V.; Dwivedi, S.K. Antifungal activity of some selected medicinal plants against Fusarium solani causing wilt and rot in Pearl millet. AJBS 2018, 13, 21–27. [Google Scholar] [CrossRef]
- Meyer, A.J.; Hell, R. Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynth. Res. 2005, 86, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.B.; Chu, L.Y.; Lu, Z.H.; Kang, C.M. Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int. J. Biol. Sci. 2008, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Alcalá, G.; Gotor, C.; Meyer, A.J.; Fricker, M.; Vega, J.M.; Romero, L.C. Glutathione biosynthesis in Arabidopsis trichome cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11108–11113. [Google Scholar] [CrossRef]
- Sun, Q.; Ye, Z.H.; Wang, X.R.; Wong, M.H. Cadmium hyperaccumulation leads to an increase of glutathione rather than phytochelatins in the cadmium hyperaccumulator Sedum alfredii. J. Plant Physiol. 2007, 164, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, P.; Mishra, V.; Kumar, T.; Rai, A.K.; Gaur, A.; Singh, M.P. An Approach to Evaluate Pb Tolerance and Its Removal Mechanisms by Pleurotus opuntiae. J. Fungi 2023, 9, 405. https://doi.org/10.3390/jof9040405
Yadav P, Mishra V, Kumar T, Rai AK, Gaur A, Singh MP. An Approach to Evaluate Pb Tolerance and Its Removal Mechanisms by Pleurotus opuntiae. Journal of Fungi. 2023; 9(4):405. https://doi.org/10.3390/jof9040405
Chicago/Turabian StyleYadav, Priyanka, Vartika Mishra, Tejmani Kumar, Awadhesh Kumar Rai, Ayush Gaur, and Mohan Prasad Singh. 2023. "An Approach to Evaluate Pb Tolerance and Its Removal Mechanisms by Pleurotus opuntiae" Journal of Fungi 9, no. 4: 405. https://doi.org/10.3390/jof9040405
APA StyleYadav, P., Mishra, V., Kumar, T., Rai, A. K., Gaur, A., & Singh, M. P. (2023). An Approach to Evaluate Pb Tolerance and Its Removal Mechanisms by Pleurotus opuntiae. Journal of Fungi, 9(4), 405. https://doi.org/10.3390/jof9040405