Cytochalasans from the Endophytic Fungus Aspergillus sp. LE2: Their Structures, Antibacterial and NO Production Inhibitory Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Fungal Material
2.3. Fermentation, Extraction and Isolation
2.4. Antibacterial Assay
2.5. Nitric Oxide Production in RAW 264.7 Macrophages
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, J.; Okyere, S.K.; Wang, S.; Wang, J.; Xie, L.; Ran, Y.; Hu, Y. Endophytic fungi: An effective alternative source of plant-derived bioactive compounds for pharmacological studies. J. Fungi 2022, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Flora Reipblicae Popularis Sinicae; China Science Publishing & Media Ltd.: Beijing, China, 1988; Volume 72, p. 236.
- Shang, X.F.; Pan, H.; Li, M.X.; Miao, X.L.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Jung, K.Y.; Chang, H.W.; Kim, H.P.; Kang, S.S. Triterpenoid saponins from the aerial parts of Lonicera japonica. Phytochemistry 1994, 35, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Tomassini, L.; Cometa, M.F.; Serafini, M.; Nicoletti, M. Isolation of secoiridoid artifacts from Lonicera japonica. J. Nat. Prod. 1995, 58, 1756–1758. [Google Scholar] [CrossRef]
- Machida, K.; Sasaki, H.; Iijima, T.; Kikuchi, M. Studies on the constituents of Lonicera species. XVII. New iridoid glycosides of the stems and leaves of Lonicera japonica THUNB. Chem. Pharm. Bull. 2002, 50, 1041–1044. [Google Scholar] [CrossRef]
- Lin, L.M.; Zhang, X.G.; Zhu, J.J.; Gao, H.M.; Wang, Z.M.; Wang, W.H. Two new triterpenoid saponins from the flowers and buds of Lonicera japonica. J. Asian Nat. Prod. Res. 2008, 10, 925–929. [Google Scholar] [CrossRef]
- Ge, L.L.; Li, J.M.; Wan, H.Q.; Zhang, K.D.; Wu, W.G.; Zou, X.T.; Wu, S.P.; Zhou, B.P.; Tian, J.; Zeng, X.B. Novel flavonoids from Lonicera japonica flower buds and validation of their anti-hepatoma and hepatoprotective activity in vitro studies. Ind. Crops Prod. 2018, 125, 114–122. [Google Scholar] [CrossRef]
- Fan, Z.L.; Li, L.; Bai, X.L.; Zhang, H.; Liu, Q.R.; Zhang, H.; Fu, Y.J.; Moyo, R. Extraction optimization, antioxidant activity, and tyrosinase inhibitory capacity of polyphenols from Lonicera japonica. Food Sci. Nutr. 2019, 7, 1786–1794. [Google Scholar] [CrossRef]
- Yang, R.; Hao, H.; Li, J.; Xuan, J.; Xia, M.F.; Zhang, Y.Q. Three new secoiridoid glycosides from the flower buds of Lonicera japonica. Chin. J. Nat. Med. 2020, 18, 70–74. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Omichi, Y.; Kurimoto, S.; Shibata, H.; Miyake, Y.; Kirimoto, T.; Takaishi, Y. Conjugates of a secoiridoid glucoside with a phenolic glucoside from the flower buds of Lonicera japonica Thunb. Phytochemistry 2013, 96, 423–429. [Google Scholar] [CrossRef]
- Sun, L.; Lu, Z.; Bie, X.; Sun, D.; Yang, S. Isolation and identification of an antimicrobial endophytic strain EJH-2. Zhongguo Weishengtaixue Zazhi 2006, 18, 23–26. [Google Scholar]
- Liu, Y.L.; Zhang, J.; Yang, Z.R.; Wang, C. Isolation and identification of a chlorogenic acid-producing endophytic bacterium from flos Lonicerae japonicae. Sichuan Daxue Xuebao Ziran Kexueban 2014, 51, 603–608. [Google Scholar]
- Zhao, L.; Xu, Y.; Lai, X.-H.; Shan, C.; Deng, Z.; Ji, Y. Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Braz. J. Microbiol. 2015, 46, 977–989. [Google Scholar] [CrossRef]
- Gupta, H.; Saini, R.V.; Pagadala, V.; Kumar, N.; Sharma, D.K.; Saini, A.K. Analysis of plant growth promoting potential of endophytes isolated from Echinacea purpurea and Lonicera japonica. J. Soil Sci. Plant Nutr. 2016, 16, 558–577. [Google Scholar] [CrossRef]
- Liu, Y.P.; Dai, Q.; Wang, W.X.; He, J.; Li, Z.H.; Feng, T.; Liu, J.K. Psathyrins: Antibacterial diterpenoids from Psathyrella candolleana. J. Nat. Prod. 2020, 83, 1725–1729. [Google Scholar] [CrossRef]
- Feng, T.; Cai, J.L.; Li, X.M.; Zhou, Z.Y.; Li, Z.H.; Liu, J.K. Chemical constituents and their bioactivities of mushroom Phellinus rhabarbarinus. J. Agric. Food Chem. 2016, 64, 1945–1949. [Google Scholar] [CrossRef]
- Evidente, A.; Andolfi, A.; Vurro, M.; Zonno, M.C.; Motta, A. Cytochalasins Z4, Z5, and Z6, three new 24-oxa[14]cytochalasans produced by Phoma exigua var. heteromorpha. J. Nat. Prod. 2003, 66, 1540–1544. [Google Scholar] [CrossRef]
- Lin, D.C.; Lin, S. High affinity binding of [3H]-dihydrocytochalasin B to peripheral membrane proteins related to the control of cell shape in the human red cell. J. Biol. Chem. 1978, 253, 1415–1419. [Google Scholar] [CrossRef]
- Evidente, A.; Andolfi, A.; Vurro, M.; Zonno, M.C.; Motta, A. Cytochalasins Z1, Z2 and Z3, three 24-oxa[14]cytochalasans produced by Pyrenophora semeniperda. Phytochemistry 2002, 60, 45–53. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Xue, Y.; Tong, Q.; Li, X.-N.; Chen, X.; Wang, J.; Yao, G.; Luo, Z.; Zhang, Y. Asperchalasine A, a cytochalasan dimer with an unprecedented decacyclic ring system, from Aspergillus flavipes. Angew. Chem. Int. Ed. 2015, 54, 13374–13378. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Tong, Q.; Li, X.-N.; Yang, J.; Xue, Y.; Luo, Z.; Wang, J.; Yao, G.; Zhang, Y. Epicochalasines A and B: Two bioactive merocytochalasans bearing caged epicoccine dimer units from Aspergillus flavipes. Angew. Chem. Int. Ed. 2016, 55, 3486–3490. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Tong, Q.; Yang, J.; Wei, G.; Xue, Y.; Wang, J.; Luo, Z.; Zhang, Y. Asperflavipine A: A cytochalasan heterotetramer uniquely defined by a highly complex tetradecacyclic ring system from Aspergillus flavipes QCS12. Angew. Chem. Int. Ed. 2017, 56, 5242–5246. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Li, Z.-H.; Feng, T.; Li, J.; Sun, H.; Huang, R.; Yuan, Q.-X.; Ai, H.-L.; Liu, J.-K. Curtachalasins A and B, two cytochalasans with a tetracyclic skeleton from the endophytic fungus Xylaria curta E10. Org. Lett. 2018, 20, 7758–7761. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Lei, X.; Ai, H.-L.; Bai, X.; Li, J.; He, J.; Li, Z.-H.; Zheng, Y.-S.; Feng, T.; Liu, J.-K. Cytochalasans from the endophytic fungus Xylaria cf. curta with resistance reversal activity against fluconazole-resistant Candida albicans. Org. Lett. 2019, 21, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Lei, X.; Yang, Y.-L.; Li, Z.-H.; Ai, H.-L.; Li, J.; Feng, T.; Liu, J.-K. Xylarichalasin A, a halogenated hexacyclic cytochalasan from the fungus Xylaria cf. curta. Org. Lett. 2019, 21, 6957–6960. [Google Scholar] [CrossRef]

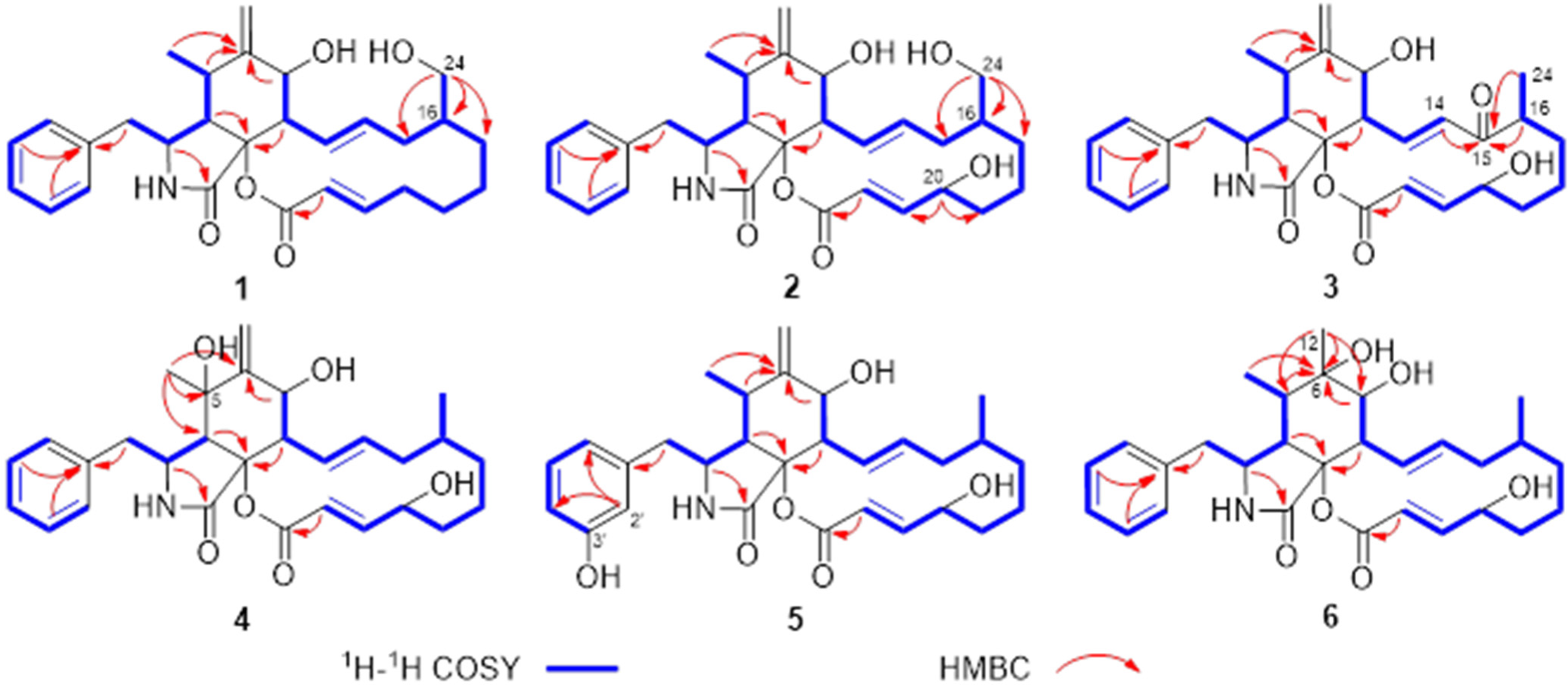

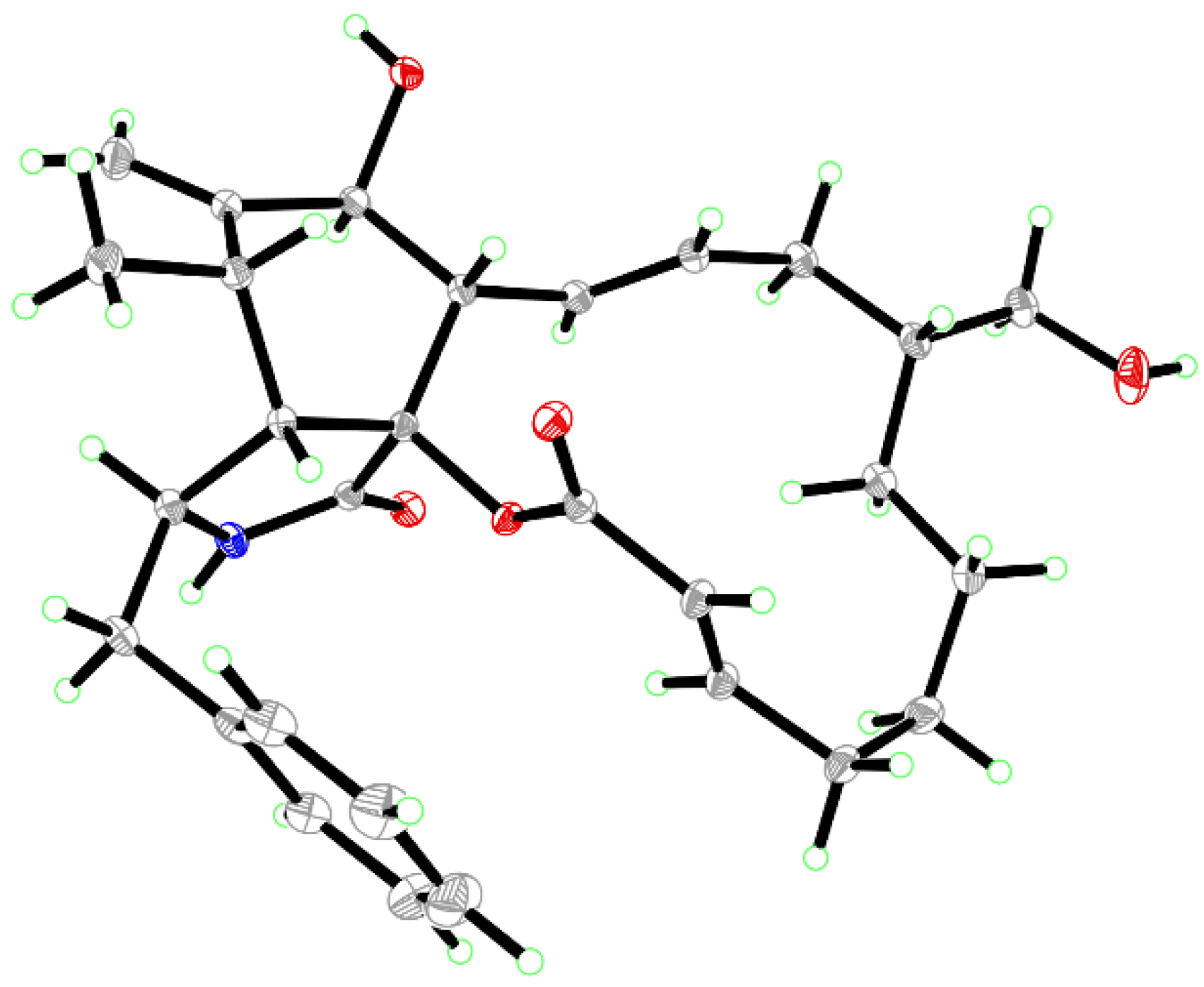
| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 173.9, C | 174.0, C | 173.0, C | |||
| 3 | 55.0, CH | 3.36, m | 54.8, CH | 3.40, m | 55.3, CH | 3.40, td (6.1, 2.8) |
| 4 | 48.9, CH | 2.80, m | 48.5, CH | 2.85, dd (5.0, 2.4) | 48.9, CH | 2.84, m |
| 5 | 32.9, CH | 3.15, m | 32.8, CH | 3.21, m | 33.1, CH | 3.17, m |
| 6 | 151.5, C | 151.4, C | 151.1, C | |||
| 7 | 71.1, CH | 3.80, d (11.1) | 71.5, CH | 3.80, d (10.8) | 70.8, CH | 4.02, d (11.1) |
| 8 | 49.9, CH | 3.30, dd (11.1, 10.1) | 49.2, CH | 3.40, dd (10.8, 10.1) | 49.0, CH | 3.47, dd (11.1, 9.9) |
| 9 | 84.9, C | 85.4, C | 86.1, C | |||
| 10 | 44.0, CH2 | 2.84, m 2.84, m | 44, CH2 | 2.80, m | 43.9, CH2 | 2.86, m |
| 11 | 14.3, CH3 | 0.83, d (6.7) | 14.1, CH3 | 0.85, d (6.7) | 14.3, CH3 | 0.88, d (6.7) |
| 12 | 114.3, CH2 | 5.08, s 5.30, s | 114.4, CH2 | 5.28, s; 5.09, s | 114.6, CH2 | 5.35, s; 5.13, s |
| 13 | 129.1, CH | 5.86, dd (15.0, 10.1) | 129.2, CH | 5.88, dd (14.7, 10.1) | 145, CH | 7.09, dd (15.3, 9.9) |
| 14 | 136.5, CH | 5.29, m | 136.5, CH | 5.24, m | 134.5, CH | 6.32, d (15.3) |
| 15 | 37.5, CH2 | 2.40, m; 1.69, m | 37.6, CH2 | 2.38, m; 1.66, m | 205.2, C | |
| 16 | 42.4, CH | 1.35, m | 42.6, CH | 1.29, m | 46.0, CH | 2.68, dd (12.7, 6.6) |
| 17 | 30.7, CH2 | 1.60, m; 0.86, m | 31.1, CH2 | 1.53, m; 0.84, m | 35.1, CH2 | 1.75, m; 1.29, m |
| 18 | 27.6, CH2 | 1.70, m; 1.07, m | 21.2, CH2 | 1.47, m; 1.29, m | 22.0, CH2 | 1.48, m; 1.13, m |
| 19 | 27.3, CH2 | 1.81, m; 1.35, m | 35.4, CH2 | 1.92, m; 1.53, m | 36.2, CH2 | 1.74, m; 1.66, m |
| 20 | 35.2, CH2 | 2.37, m; 2.18, m | 70.9, CH | 4.46, m | 71.5, CH | 4.25, m |
| 21 | 154.0, CH | 7.02, m | 154.4, CH | 6.94, dd (15.6, 4.3) | 153.1, CH | 6.91, dd (15.7, 6.6) |
| 22 | 122.0, CH | 5.63, d (15.5) | 119.6, CH | 5.79, dd (15.6) | 121.7, CH | 5.84, d (15.7) |
| 23 | 166.2, C | 166.3, C | 166.4, C | |||
| 24 | 65.9, CH2 | 3.59, dd (10.8, 3.6) 3.27, dd (10.8, 7.5) | 65.9, CH2 | 3.60, dd (10.9, 3.4); 3.26, dd (10.9, 7.4) | 15.1, CH3 | 0.99, d (6.6) |
| 1′ | 138.5, C | 138.2, C | 138.4, C | |||
| 2′,6′ | 130.9, CH | 7.14, d (7.4) | 131.0, CH | 7.13, d (7.4) | 131.0, CH | 7.15, d (7.3) |
| 3′,5′ | 129.6, CH | 7.26, t (7.4) | 129.6, CH | 7.27, t (7.4) | 129.6, CH | 7.27, t (7.3) |
| 4′ | 127.8, CH | 7.18, t (7.4) | 127.9, CH | 7.19, t (7.4) | 127.9, CH | 7.23, t (7.3) |
| No. | 4 | 5 | 6 | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 173.3, C | 174.0, C | 173.7, C | |||
| 3 | 57.2, CH | 3.25, m | 54.8, CH | 3.32, m | 55.0, CH | 4.18, m |
| 4 | 55.6, CH | 2.86, d (4.2) | 48.6, CH | 2.81, dd (5.2, 2.6) | 50.1, CH | 2.58, m |
| 5 | 74.3, C | 32.9, CH | 3.16, m | 38.1, CH | 2.27, m | |
| 6 | 152.6, C | 151.5, C | 77.4, C | |||
| 7 | 69.2, CH | 3.94, d (12.2) | 71.5, CH | 3.76, d (10.9) | 76.3, CH | 3.45, d (12.3) |
| 8 | 51.3, CH | 3.83, dd (12.2, 10.2) | 49.3, CH | 3.34, dd (10.9, 9.8) | 49.4, CH | 2.86, dd (12.3, 10.2) |
| 9 | 85.2, C | 85.4, C | 86.7, C | |||
| 10 | 44.0, CH2 | 3.02, d (6.6) | 44.1, CH2 | 2.68, m | 45.0, CH2 | 2.79, dd (13.7, 7.9) 2.93, dd (13.7, 4.4) |
| 11 | 26.5, CH3 | 1.34, s | 14.1, CH3 | 0.84, d (6.5) | 13.0, CH3 | 0.99, d (7.1) |
| 12 | 114.4, CH2 | 5.51, s; 5.47, s | 114.3, CH2 | 5.24, s; 5.05, s | 22.3, CH3 | 1.18, s |
| 13 | 128.8, CH | 5.62, dd (14.2, 10.2) | 128.7, CH | 5.79, dd (15.0, 9.8) | 128.0, CH | 5.64, dd (14.4, 10.2) |
| 14 | 137.1, CH | 5.41, m | 136.7, CH | 5.20, m | 136.5, CH | 5.25, m |
| 15 | 42.7, CH2 | 2.19, m; 1.73, m | 43.2, CH2 | 2.06, m; 1.61, m | 42.8, CH2 | 2.14, m; 1.70, m |
| 16 | 33.9, CH | 1.46, m | 34.5, CH | 1.18, m | 34.3, CH | 1.35, m |
| 17 | 35.7, CH2 | 1.83, m; 0.65, m | 36.6, CH2 | 1.68, m; 0.53, m | 36.1, CH2 | 1.77, m; 0.62, m |
| 18 | 21.8, CH2 | 1.53, m; 1.42, m | 21.5, CH2 | 1.41, m; 1.23, m | 21.7, CH2 | 1.41, m |
| 19 | 35.3, CH2 | 1.85, m; 1.61, m | 35.4, CH2 | 1.87, m; 1.52, m | 35.4, CH2 | 1.86, m; 1.58, m |
| 20 | 72.8, CH | 4.29, m | 71.2, CH | 4.42, m | 72.0, CH | 4.34, m |
| 21 | 153.7, CH | 7.04, dd (15.6, 6.3) | 154.6, CH | 6.92, dd (15.7, 5.4) | 154.1, CH | 6.99, dd (15.6, 6.4) |
| 22 | 121.2, CH | 5.79, d (15.6) | 119.4, CH | 5.74, d (15.7) | 120.3, CH | 5.75, d (15.6) |
| 23 | 166.5, C | 166.4, C | 166.3, C | |||
| 24 | 20.7, CH3 | 0.90, d (6.5) | 20.7, CH3 | 0.84, d (6.5) | 20.7, CH3 | 0.88, d (6.6) |
| 1′ | 139.2, C | 139.7, C | 139.0, C | |||
| 2′ | 130.5, CH | 7.20, d (7.5) | 117.8, CH | 6.53, (1.8) | 130.7, CH | 7.19, d (7.4) |
| 3′ | 129.8, CH | 7.30, t (7.5) | 158.5, C | 129.6, CH | 7.27, t (7.4) | |
| 4′ | 127.9, CH | 7.22, t (7.5) | 114.9, CH | 6.57, dd (7.8, 1.8) | 127.7, CH | 7.19, t (7.4) |
| 5′ | 129.8, CH | 7.30, t (7.5) | 130.5, CH | 7.03, t (7.8) | 129.6, CH | 7.27, t (7.4) |
| 6′ | 130.5, CH | 7.20, d (7.5) | 122.2, CH | 6.55, d (7.8) | 130.7, CH | 7.19, d (7.4) |
| Entry | IC50 (μM) |
|---|---|
| 1 | 26.8 ± 2.69 |
| 2 | >40 |
| 3 | 17.1 ± 1.27 |
| 4 | >40 |
| 5 | 31.2 ± 1.02 |
| 6 | 12.7 ± 1.85 |
| SMS | 7.3 ± 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-C.; Yang, J.; Zhao, Y.-Z.; Ma, Q.-D.; Liu, B.-G.; Sun, J.-L. Cytochalasans from the Endophytic Fungus Aspergillus sp. LE2: Their Structures, Antibacterial and NO Production Inhibitory Activities. J. Fungi 2023, 9, 374. https://doi.org/10.3390/jof9030374
Li Y-C, Yang J, Zhao Y-Z, Ma Q-D, Liu B-G, Sun J-L. Cytochalasans from the Endophytic Fungus Aspergillus sp. LE2: Their Structures, Antibacterial and NO Production Inhibitory Activities. Journal of Fungi. 2023; 9(3):374. https://doi.org/10.3390/jof9030374
Chicago/Turabian StyleLi, Yong-Chao, Jing Yang, Yuan-Zeng Zhao, Qin-Di Ma, Ben-Guo Liu, and Jun-Liang Sun. 2023. "Cytochalasans from the Endophytic Fungus Aspergillus sp. LE2: Their Structures, Antibacterial and NO Production Inhibitory Activities" Journal of Fungi 9, no. 3: 374. https://doi.org/10.3390/jof9030374
APA StyleLi, Y.-C., Yang, J., Zhao, Y.-Z., Ma, Q.-D., Liu, B.-G., & Sun, J.-L. (2023). Cytochalasans from the Endophytic Fungus Aspergillus sp. LE2: Their Structures, Antibacterial and NO Production Inhibitory Activities. Journal of Fungi, 9(3), 374. https://doi.org/10.3390/jof9030374






