High Andean Steppes of Southern Chile Contain Little-Explored Peltigera Lichen Symbionts
Abstract
1. Introduction
2. Materials and Methods
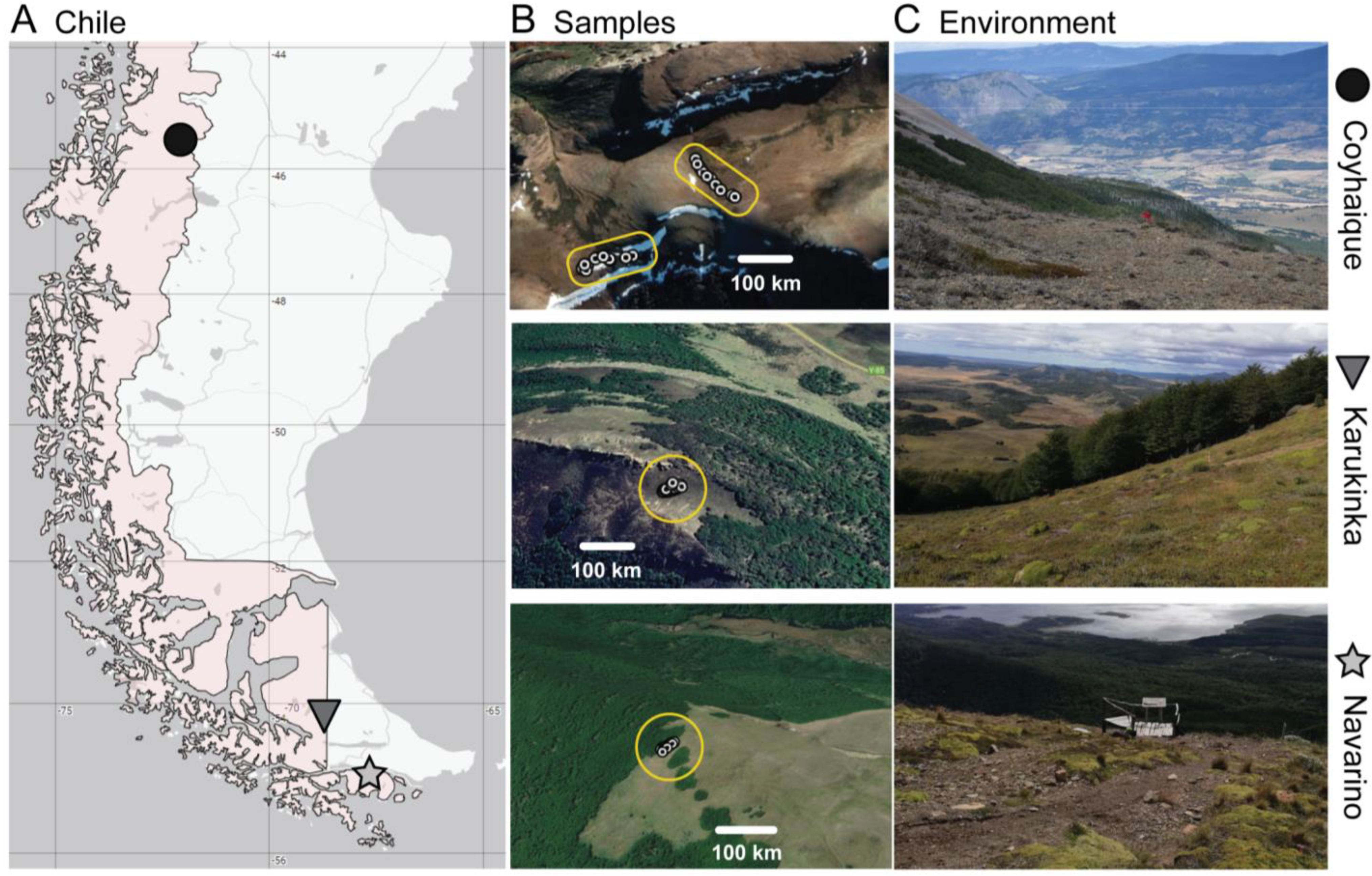
2.1. Study Sites and Sampling
2.2. Molecular Data
2.3. Mycobiont Identification
2.3.1. Phylogenetic Analyses
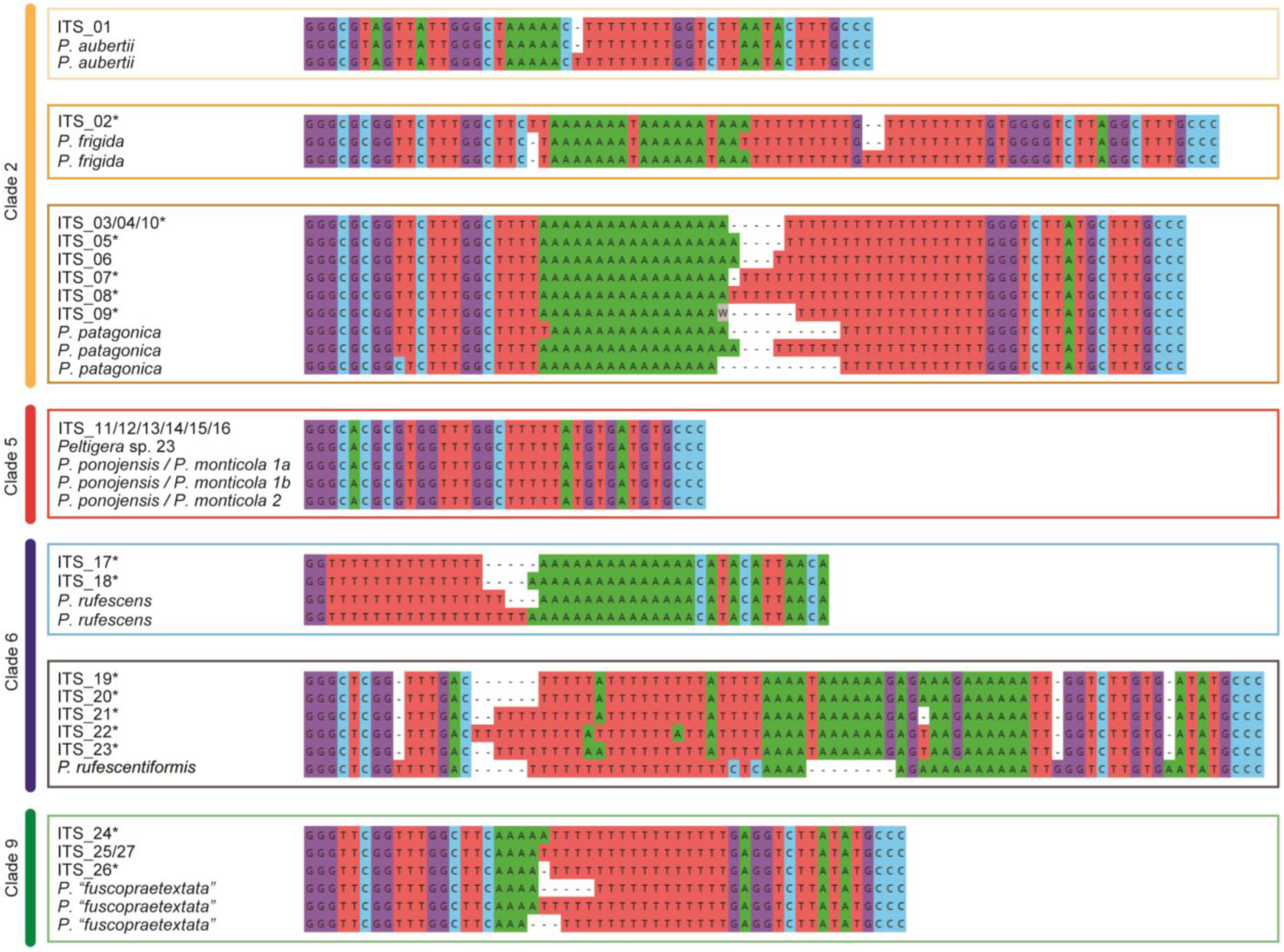
2.3.2. ITS1 Hypervariable Region Analyses
2.3.3. Haplotype Diversity
2.4. Cyanobiont Identification
3. Results
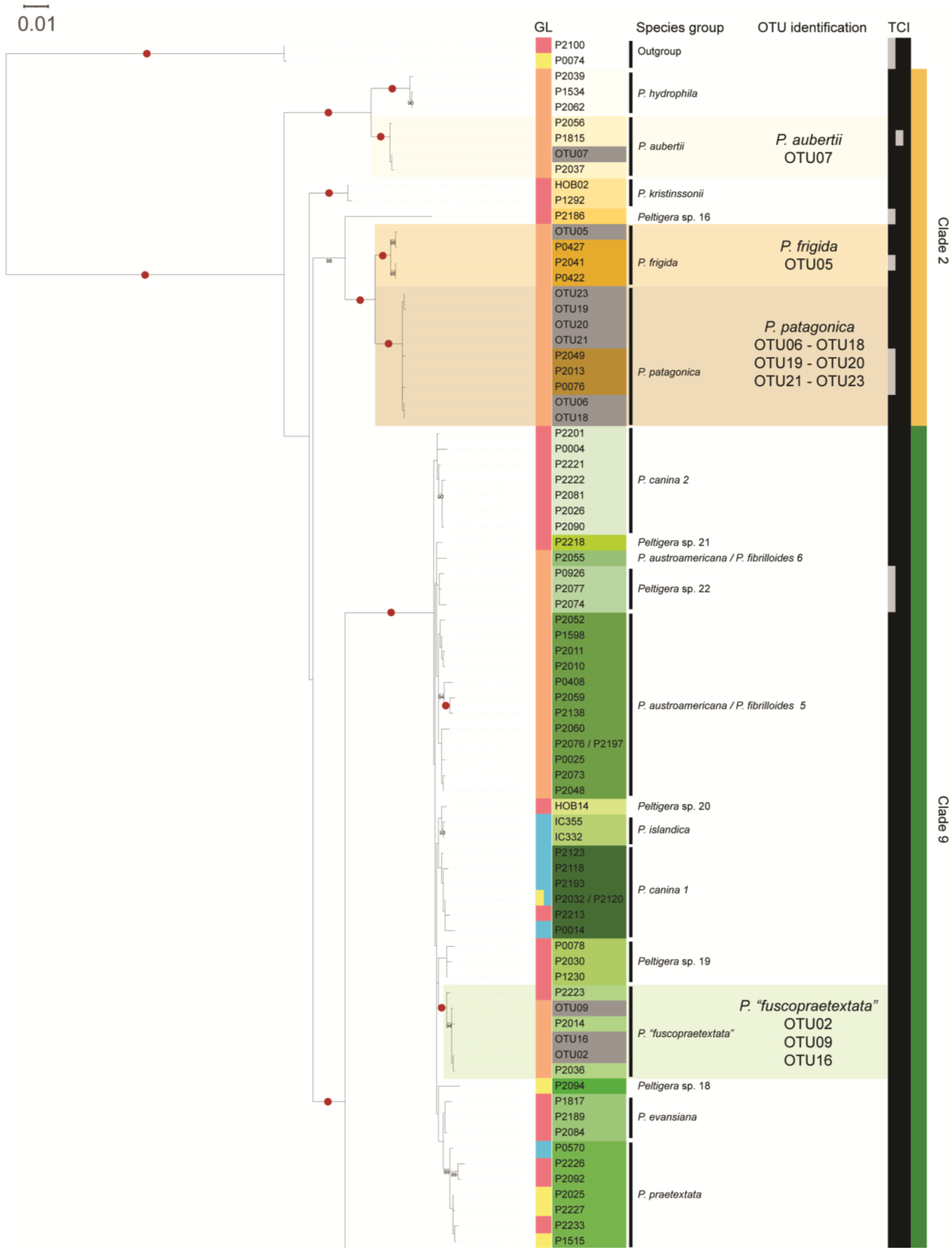
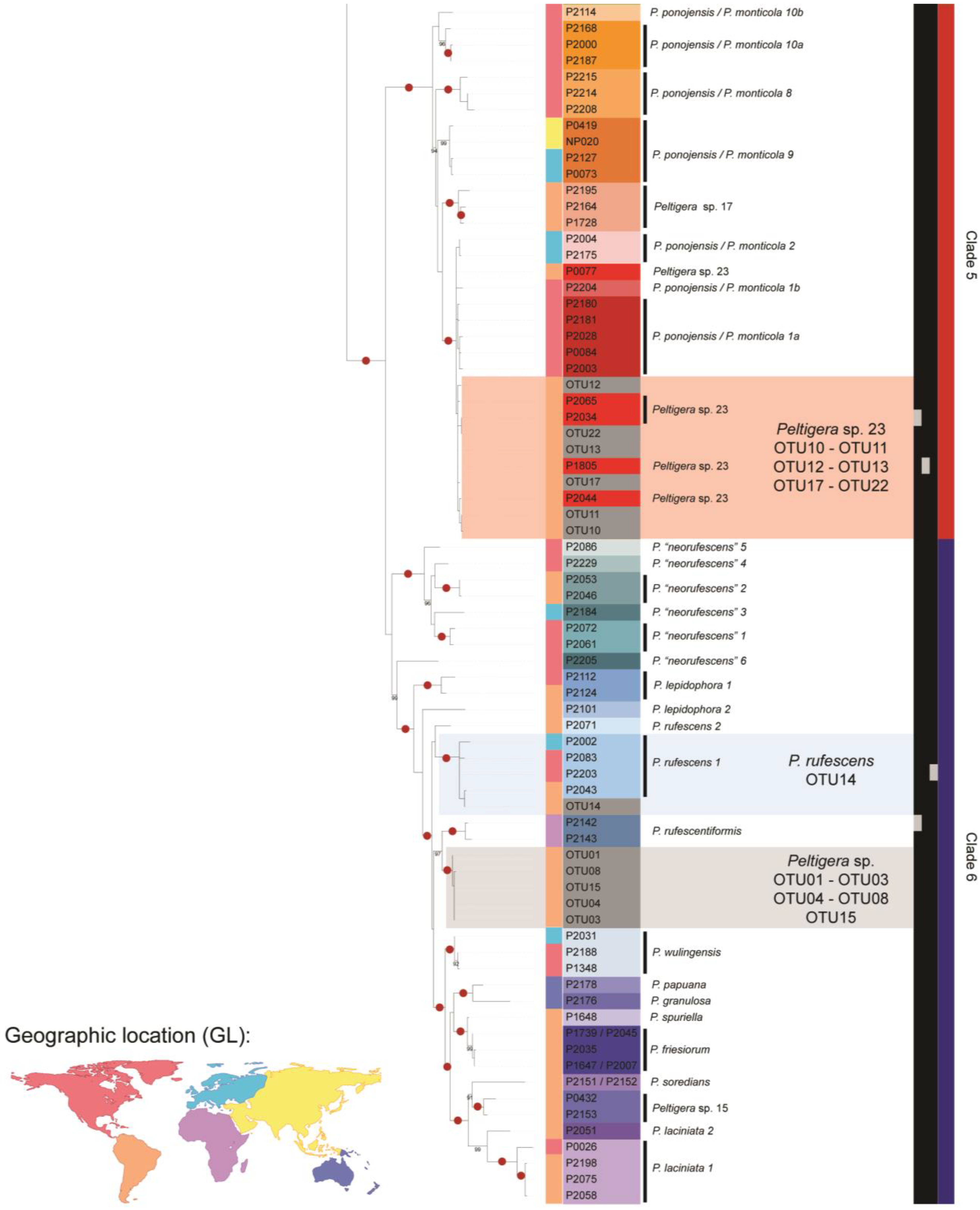
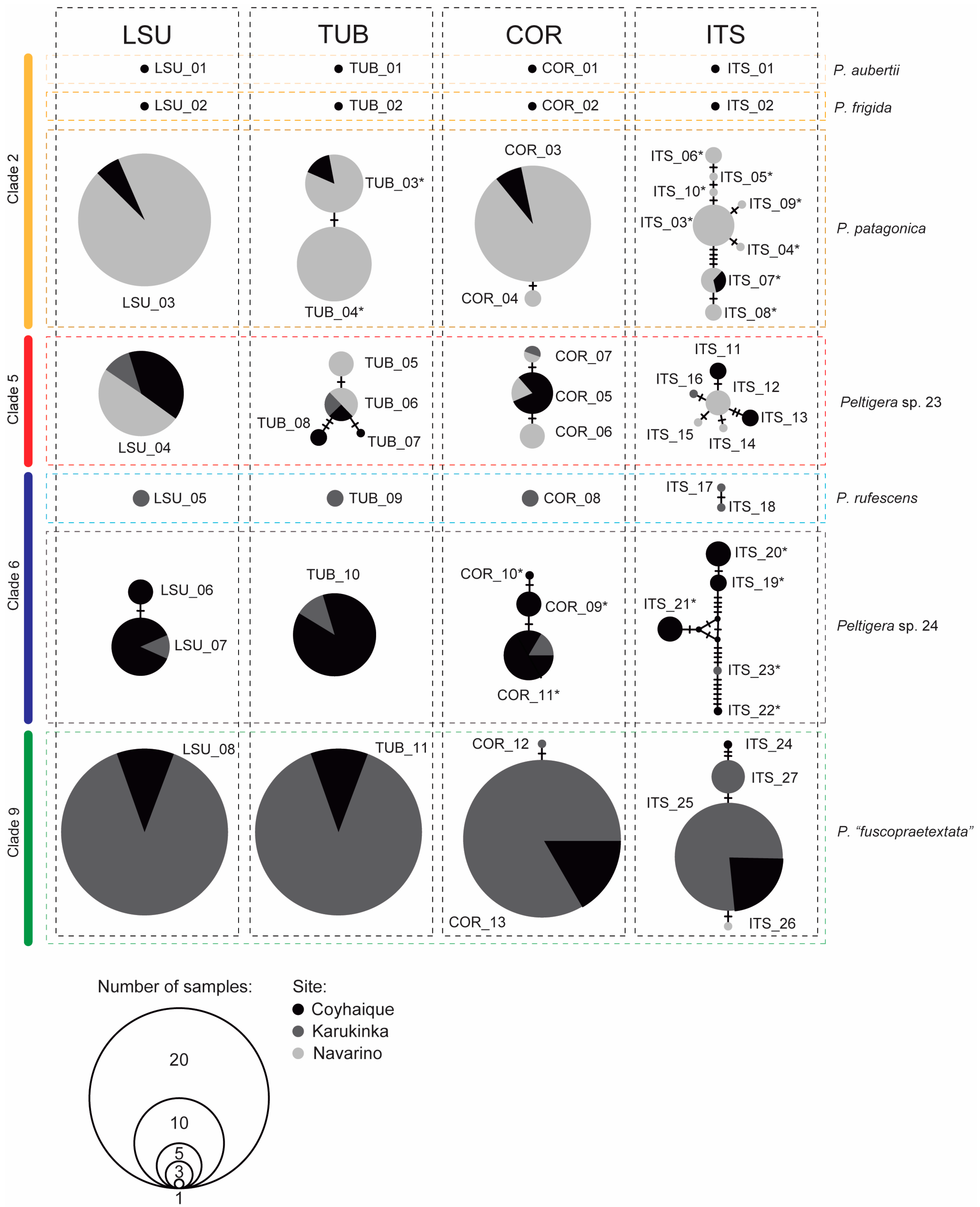
3.1. Mycobiont Identification
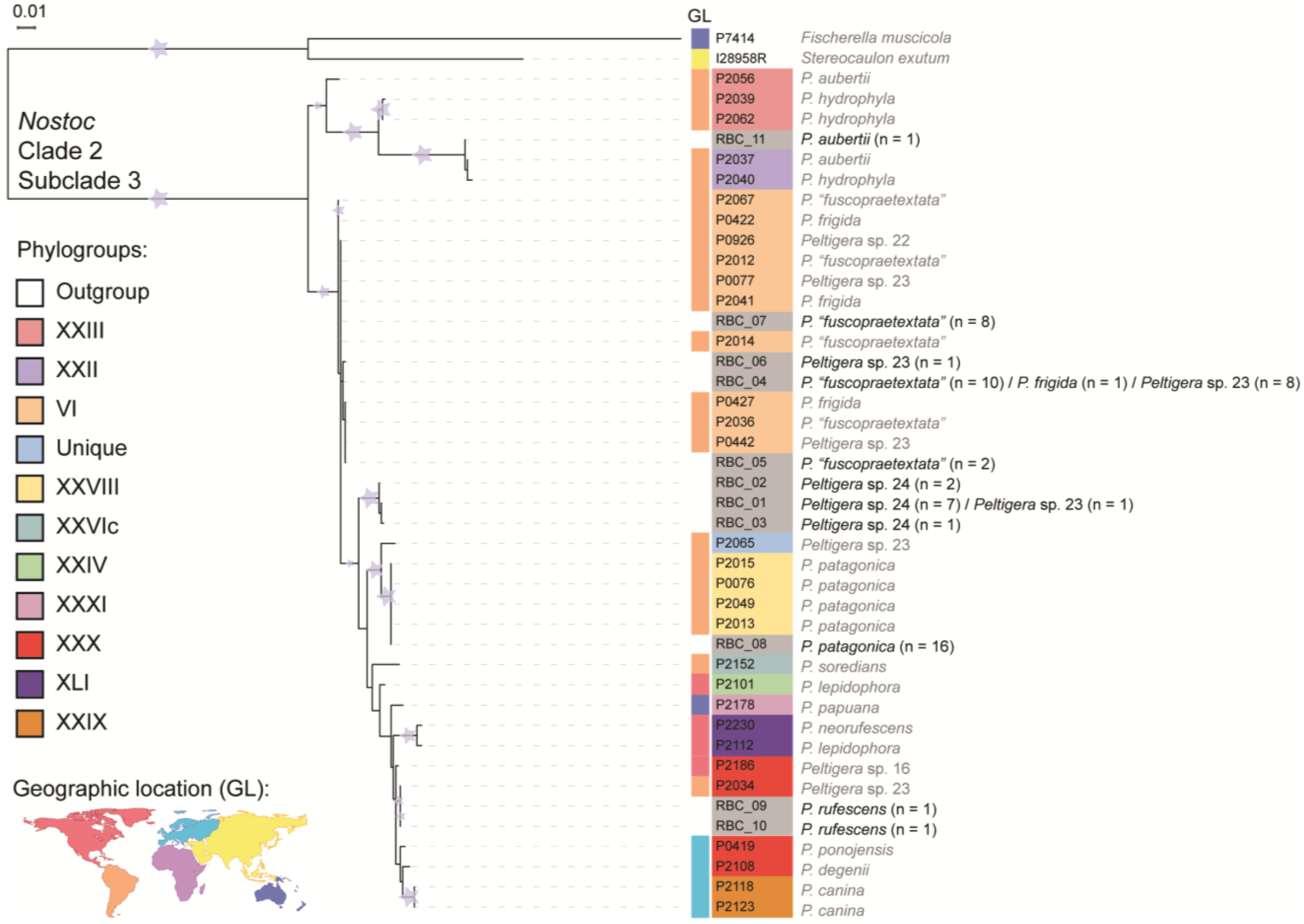
3.2. Cyanobiont Identification
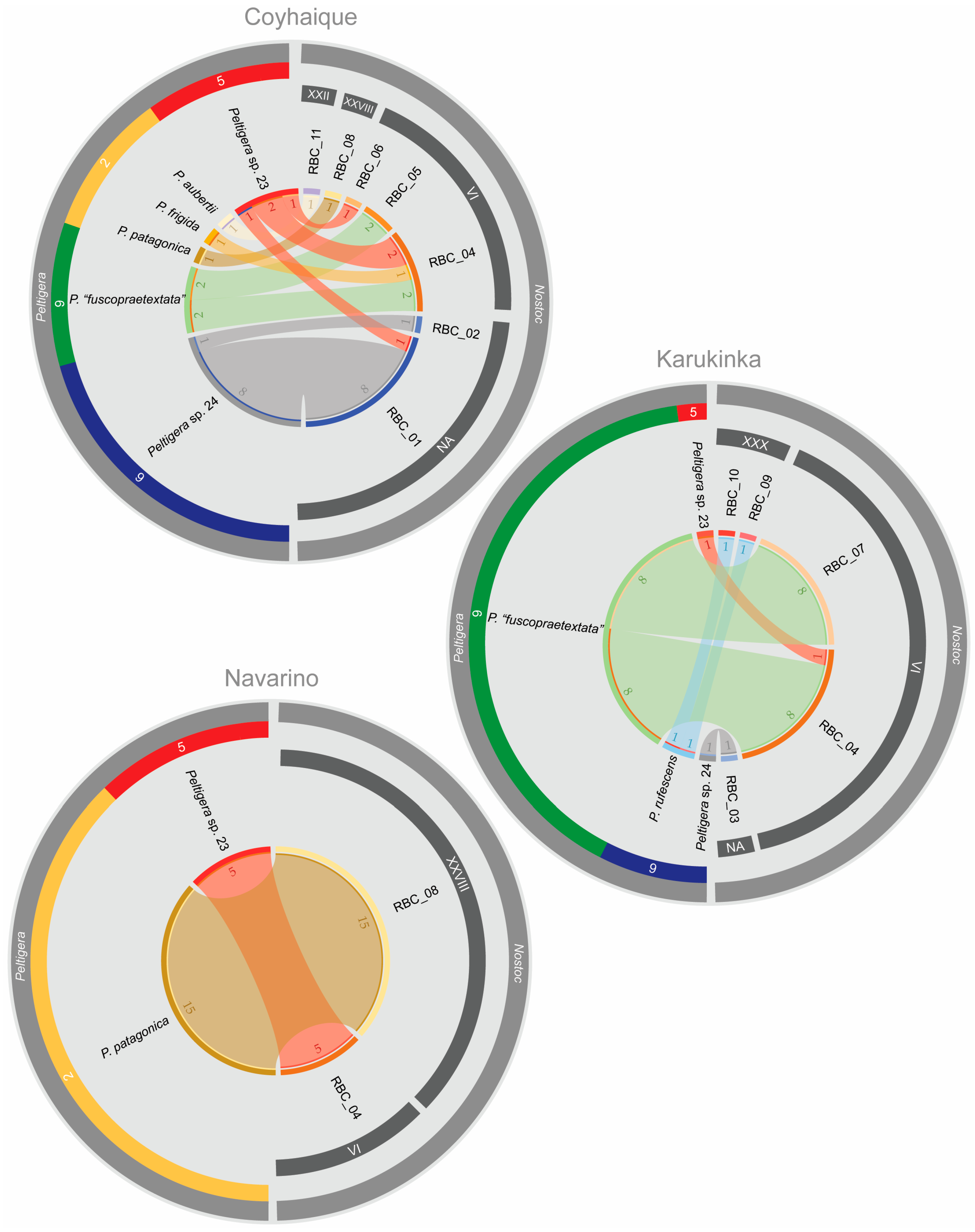
3.3. Peltigera Symbionts in the High Andean Steppes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arroyo, M.T.K.; Cavieres, L.A. High-Elevation Andean Ecosystems. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: London, UK, 2013; pp. 96–110. [Google Scholar]
- Grau, O.; Grytnes, J.A.; Birks, H.J.B. A Comparison of Altitudinal Species Richness Patterns of Bryophytes with Other Plant Groups in Nepal, Central Himalaya. J. Biogeogr. 2007, 34, 1907–1915. [Google Scholar] [CrossRef]
- Noroozi, J.; Talebi, A.; Doostmohammadi, M.; Rumpf, S.B.; Linder, H.P.; Schneeweiss, G.M. Hotspots within a Global Biodiversity Hotspot-Areas of Endemism Are Associated with High Mountain Ranges. Sci. Rep. 2018, 8, 10345. [Google Scholar] [CrossRef] [PubMed]
- Vallese, C.; Nascimbene, J.; Giordani, P.; Benesperi, R.; Casazza, G. Modelling Range Dynamics of Terricolous Lichens of the Genus Peltigera in the Alps under a Climate Change Scenario. Fungal Ecol. 2021, 49, 101014. [Google Scholar] [CrossRef]
- Sierra, M.A.; Danko, D.C.; Sandoval, T.A.; Pishchany, G.; Moncada, B.; Kolter, R.; Mason, C.E.; Zambrano, M.M. The Microbiomes of Seven Lichen Genera Reveal Host Specificity, a Reduced Core Community and Potential as Source of Antimicrobials. Front. Microbiol. 2020, 11, 398. [Google Scholar] [CrossRef]
- Beckett, R.P.; Minibayeva, F.; Solhaug, K.A.; Roach, T. Photoprotection in Lichens: Adaptations of Photobionts to High Light. Lichenologist 2021, 53, 21–33. [Google Scholar] [CrossRef]
- Porada, P.; Weber, B.; Elbert, W.; Pöschl, U.; Kleidon, A. Estimating Impacts of Lichens and Bryophytes on Global Biogeochemical Cycles. Glob. Biogeochem. Cycles 2014, 28, 71–85. [Google Scholar] [CrossRef]
- Almendras, K.; Leiva, D.; Carú, M.; Orlando, J. Carbon Consumption Patterns of Microbial Communities Associated with Peltigera Lichens from a Chilean Temperate Forest. Molecules 2018, 23, 2746. [Google Scholar] [CrossRef]
- Liu, Y.R.; Delgado-Baquerizo, M.; Trivedi, P.; He, J.Z.; Singh, B.K. Species Identity of Biocrust-Forming Lichens Drives the Response of Soil Nitrogen Cycle to Altered Precipitation Frequency and Nitrogen Amendment. Soil Biol. Biochem. 2016, 96, 128–136. [Google Scholar] [CrossRef]
- Concostrina-Zubiri, L.; Valencia, E.; Ochoa, V.; Gozalo, B.; Mendoza, B.J.; Maestre, F.T. Biocrust-Forming Lichens Increase Soil Available Phosphorus under Simulated Climate Change. Eur. J. Soil Sci. 2022, 73, e13284. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Grube, M. Lichens Redefined as Complex Ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef]
- Nash, T.H. Lichen Biology, 2nd ed.; Cambridge University Press: London, UK, 2008; pp. 1–8. [Google Scholar]
- Magain, N.; Truong, C.; Goward, T.; Niu, D.; Goffinet, B.; Sérusiaux, E.; Vitikainen, O.; Lutzoni, F.; Miadlikowska, J. Species Delimitation at a Global Scale Reveals High Species Richness with Complex Biogeography and Patterns of Symbiont Association in Peltigera Section Peltigera (Lichenized Ascomycota: Lecanoromycetes). Taxon 2018, 67, 836–870. [Google Scholar] [CrossRef]
- Miadlikowska, J.; Magain, N.; Buck, W.R.; Vargas Castillo, R.; Barlow, G.T.; Pardo-De la Hoz, C.J.; LaGreca, S.; Lutzoni, F. Peltigera hydrophila (Lecanoromycetes, Ascomycota), a New Semi-Aquatic Cyanolichen Species from Chile. Plant Fungal Syst. 2020, 65, 210–218. [Google Scholar] [CrossRef]
- Orlando, J.; Almendras, K.; Veas-Mattheos, K.; Pezoa, M.; Carú, M. Peltigera Cyanolichens from Southern Chile. GBIF 2021. [Google Scholar] [CrossRef]
- Zúñiga, C.; Leiva, D.; Ramírez-Fernández, L.; Carú, M.; Yahr, R.; Orlando, J. Phylogenetic Diversity of Peltigera Cyanolichens and Their Photobionts in Southern Chile and Antarctica. Microbes Environ. 2015, 30, 172–179. [Google Scholar] [CrossRef]
- Martínez, I.; Burgaz, A.; Vitikainen, O.; Escudero, A. Distribution patterns in the Genus Peltigera Wild. Lichenologist 2003, 35, 301–323. [Google Scholar] [CrossRef]
- Ramírez-Fernández, L.; Zúñiga, C.; Méndez, M.A.; Carú, M.; Orlando, J. Genetic Diversity of Terricolous Peltigera Cyanolichen Communities in Different Conservation States of Native Forest from Southern Chile. Int. Microbiol. 2013, 16, 243–252. [Google Scholar] [PubMed]
- Quilhot, W.; Cuellar, M.; Díaz, R.; Riquelme, F.; Rubio, C. Lichens of Aisen, Southern Chile. Gayana Bot. 2012, 69, 57–87. [Google Scholar] [CrossRef]
- Muster, C.; Leiva, D.; Morales, C.; Grafe, M.; Schloter, M.; Carú, M.; Orlando, J. Peltigera frigida Lichens and Their Substrates Reduce the Influence of Forest Cover Change on Phosphate Solubilizing Bacteria. Front. Microbiol. 2022, 13, 843490. [Google Scholar] [CrossRef]
- Miadlikowska, J.; Lutzoni, F. Phylogenetic Revision of the Genus Peltigera (Lichen—Forming Ascomycota) Based on Morphological, Chemical, and Large Subunit Nuclear Ribosomal DNA Data. Int. J. Plant Sci. 2000, 161, 925–958. [Google Scholar] [CrossRef]
- Miadlikowska, J.; Lutzoni, F. Phylogenetic Classification of Peltigeralean Fungi (Peltigerales, Ascomycota) Based on Ribosomal RNA Small and Large Subunits. Am. J. Bot. 2004, 91, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Magain, N.; Miadlikowska, J.; Goffinet, B.; Sérusiaux, E.; Lutzoni, F. Macroevolution of Specificity in Cyanolichens of the Genus Peltigera Section Polydactylon (Lecanoromycetes, Ascomycota). Syst. Biol. 2017, 66, 74–99. [Google Scholar] [PubMed]
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes—Application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid Genetic Identification and Mapping of Several Cryptococcus Species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Cigelnik, E. Two Divergent Intragenomic rDNA ITS2 Types within a Monophyletic Lineage of the Fungus Fusarium are nonorthologous. Mol. Phylogenet Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- O’Brien, H.E.; Miadlikowska, J.; Lutzoni, F. Assessing Reproductive Isolation in Highly Diverse Communities of the Lichen-Forming Fungal Genus Peltigera. Evolution 2009, 63, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.L.; Donaldson, G.C. Development of Primer Sets Designed for Use with the PCR to Amplify Conserved Genes from Filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications, 1st ed.; Innis, M., Gelfand, D., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Carbone, I.; White, J.; Miadlikowska, J.; Arnold, E.; Miller, M.; Magain, N.; U’Ren, J.; Lutzoni, F. T-BAS Version 2.1: Tree-Based Alignment Selector Toolkit for Evolutionary Placement of DNA Sequences and Viewing Alignments and Specimen Metadata on Curated and Custom Trees. Microbiol. Resour. Announc. 2019, 8, e00328-19. [Google Scholar] [CrossRef]
- Wilmotte, A.; Van der Auwera, G.; De Wachter, R. Structure of the 16S Ribosomal RNA of the Thermophilic Cyanobacterium Chlorogloeopsis HTF (‘Mastigocladus laminosus HTF’) strain PCC75 18, and Phylogenetic Analysis. FEBS Lett. 1993, 317, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Rudi, K.; Skulberg, O.M.; Jakobsen, K.S. Evolution of Cyanobacteria by Exchange of Genetic Material among Phyletically Related Strains. J. Bacteriol. 1998, 180, 3453–3461. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Benson, D.; Karsch-Mizrachi, I.; Lipman, D.; Ostell, J.; Sayers, E. GenBank. Nucleic Acids Res. 2011, 39, D32–D37. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J.; Pennsylvania, T.; Park, U. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Manoharan-Basil, S.S.; Miadlikowska, J.; Goward, T.; Andrésson, Ó.S.; Miao, V.P.W. Peltigera islandica, a New Cyanolichen Species in Section Peltigera (‘P. Canina Group’). Lichenologist 2016, 48, 451–467. [Google Scholar] [CrossRef]
- Berger, S.A.; Stamatakis, A. Aligning Short Reads to Reference Alignments and Trees. Bioinformatics 2011, 27, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Miadlikowska, J.; (Duke University, Durham, NC, USA); Magain, N.; (University of Liège, Wallonia, Belgium). Personal communication, 2023.
- Han, L.-F.; Zheng, T.-X.; Guo, S.-Y. A New Species in the Lichen Genus Peltigera from Northern China Based on Morphology and DNA Sequence Data. Bryologist 2015, 118, 46–53. [Google Scholar] [CrossRef]
- Sérusiaux, E.; Goffinet, B.; Miadlikowska, J.; Vitikainen, O. Taxonomy, Phylogeny and Biogeography of the Lichen Genus Peltigera in Papua New Guinea. Fungal Divers. 2009, 38, 185–224. [Google Scholar]
- Lutzoni, F.; Wagner, P.; Reeb, V.; Zoller, S. Integrating Ambiguously Aligned Regions of DNA Sequences in Phylogenetic Analyses without Violating Positional Homology. Syst. Biol. 2000, 49, 628–651. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author. Evolutionary Biology Centre Uppsala University, 2004. Available online: https://github.com/nylander/MrModeltest2/releases (accessed on 3 October 2022).
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-Joining Networks for Inferring Intraspecific Phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Leiva, D.; Clavero-León, C.; Carú, M.; Orlando, J. Intrinsic Factors of Peltigera Lichens Influence the Structure of the Associated Soil Bacterial Microbiota. FEMS Microbiol. Ecol. 2016, 92, fiw178. [Google Scholar] [CrossRef] [PubMed]
- Leiva, D.; Fernández-Mendoza, F.; Acevedo, J.; Carú, M.; Grube, M.; Orlando, J.; Orlando, J. The Bacterial Community of the Foliose Macro-Lichen Peltigera frigida Is More than a Mere Extension of the Microbiota of the Subjacent Substrate. Microb. Ecol. 2021, 81, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Deans, A.R. Bayesian Phylogenetics and Its Influence on Insect Systematics. Annu. Rev. Entomol. 2010, 55, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Miadlikowska, J.; Lutzoni, F.; Goward, T.; Zoller, S.; Posada, D. New Approach to an Old Problem: Incorporating Signal from Gap-Rich Regions of ITS and RDNA Large Subunit into Phylogenetic Analyses to Resolve the Peltigera canina Species Complex. Mycologia 2003, 95, 1181–1203. [Google Scholar] [CrossRef]
- Carstens, B.C.; Pelletier, T.A.; Reid, N.M.; Satler, J.D. How to Fail at Species Delimitation. Mol. Ecol. 2013, 22, 4369–4383. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium. Nuclear Ribosomal Internal Transcribed Spacer (ITS) Region as a Universal DNA Barcode Marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Noll, D.; Leon, F.; Brandt, D.; Pistorius, P.; Le Bohec, C.; Bonadonna, F.; Trathan, P.N.; Barbosa, A.; Rey, A.R.; Dantas, G.P.M.; et al. Positive Selection over the Mitochondrial Genome and Its Role in the Diversification of Gentoo Penguins in Response to Adaptation in Isolation. Sci. Rep. 2022, 12, 3767. [Google Scholar] [CrossRef]
- Delić, T.; Trontelj, P.; Rendoš, M.; Fišer, C. The Importance of Naming Cryptic Species and the Conservation of Endemic Subterranean Amphipods. Sci. Rep. 2017, 7, 3391. [Google Scholar] [CrossRef] [PubMed]
- Jüriado, I.; Kaasalainen, U.; Jylhä, M.; Rikkinen, J. Relationships between Mycobiont Identity, Photobiont Specificity and Ecological Preferences in the Lichen Genus Peltigera (Ascomycota) in Estonia (Northeastern Europe). Fungal Ecol. 2019, 39, 45–54. [Google Scholar] [CrossRef]
- Werth, S. Population Genetics of Lichen-Forming Fungi—A Review. Lichenologist 2010, 42, 499–519. [Google Scholar] [CrossRef]
- Magain, N. Integrating Photobiont Phylogenetic and Geographical Data in Macroevolutionary Studies of Lichens. Ph.D. Thesis, Université de Liége, Liége, Belgium, 2014. [Google Scholar]
- Almendras, K. Bacterias Solubilizadoras de Fosfato Asociadas a Talos y Sustratos de Cianolíquenes Peltigera Creciendo En Un Bosque y Una Pradera de La Reserva Nacional Coyhaique. Ph.D. Thesis, Universidad de Chile, Santiago de Chile, Chile, 2022. [Google Scholar]
- Garrido-Benavent, I.; Pérez-Ortega, S. Past, Present, and Future Research in Bipolar Lichen-Forming Fungi and Their Photobionts. Am. J. Bot. 2017, 104, 1660–1674. [Google Scholar] [CrossRef]
- Veas-Mattheos, K. Identificación de Cianolíquenes Del Género Peltigera En Dos Contextos Ambientales de La Reserva Nacional Coyhaique y Determinación de Las Fracciones de Fósforo En Sus Sustratos. Bachelor’s Thesis, Universidad de Chile, Santiago de Chile, Chile, 2019. [Google Scholar]
- Guimarães, P.R.; Pires, M.M.; Jordano, P.; Bascompte, J.; Thompson, J.N. Indirect Effects Drive Coevolution in Mutualistic Networks. Nature 2017, 550, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Kumari, R.; Patel, D.K.; Upreti, D.K. Characterization of the Diversity of Mycosporine-like Amino Acids in Lichens from High Altitude Region of Himalaya. Amino Acids 2016, 48, 129–136. [Google Scholar] [CrossRef]
- Allen, J.L.; Lendemer, J.C. Climate Change Impacts on Endemic, High-Elevation Lichens in a Biodiversity Hotspot. Biodivers. Conserv. 2016, 25, 555–568. [Google Scholar] [CrossRef]
- Costello, E.K.; Halloy, S.R.P.; Reed, S.C.; Sowell, P.; Schmidt, S.K. Fumarole-Supported Islands of Biodiversity within a Hyperarid, High-Elevation Landscape on Socompa Volcano, Puna de Atacama, Andes. Appl. Environ. Microbiol. 2009, 75, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Kosecka, M.; Kukwa, M.; Jabłońska, A.; Flakus, A.; Rodriguez-Flakus, P.; Ptach, L.; Guzow-Krzemińska, B. Phylogeny and Ecology of Trebouxia Photobionts From Bolivian Lichens. Front. Microbiol. 2022, 13, 779784. [Google Scholar] [CrossRef]
- Magain, N.; Goffinet, B.; Simon, A.; Seelan, J.S.S.; Medeiros, I.D.; Lutzoni, F.; Miadlikowska, J. Peltigera serusiauxii (Lecanoromycetes, Ascomycota), a New Species from Papua New Guinea and Malaysia. Plant Fungal Syst. 2020, 65, 139–146. [Google Scholar] [CrossRef]
- Ossowska, E.A.; Moncada, B.; Kukwa, M.; Flakus, A.; Rodriguez-Flakus, P.; Olszewska, S.; Lücking, R. New Species of Sticta (Lichenised Ascomycota, Lobarioid Peltigeraceae) from Bolivia Suggest a High Level of Endemism in the Central Andes. MycoKeys 2022, 92, 131–160. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.F.; Mccune, B.; Pardo-De La Hoz, C.J.; Magain, N.; Miadlikowska, J. Sinuicella denisonii, a New Genus and Species in the Peltigeraceae from Western North America. Lichenologist 2021, 53, 185–192. [Google Scholar] [CrossRef]
- Lu, J.; Magain, N.; Miadlikowska, J.; Coyle, J.R.; Truong, C.; Lutzoni, F. Bioclimatic Factors at an Intrabiome Scale Are More Limiting than Cyanobiont Availability for the Lichen-Forming Genus Peltigera. Am. J. Bot. 2018, 105, 1198–1211. [Google Scholar] [CrossRef]
- Singh, G.; Dal Grande, F.; Divakar, P.K.; Otte, J.; Crespo, A.; Schmitt, I. Fungal–Algal Association Patterns in Lichen Symbiosis Linked to Macroclimate. New Phytol. 2017, 214, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Baniya, C.B.; Solhøy, T.; Gauslaa, Y.; Palmer, M.W. The Elevation Gradient of Lichen Species Richness in Nepal. Lichenologist 2010, 42, 83–96. [Google Scholar] [CrossRef]
- Steinbauer, M.J.; Field, R.; Grytnes, J.A.; Trigas, P.; Ah-Peng, C.; Attorre, F.; Birks, H.J.B.; Borges, P.A.V.; Cardoso, P.; Chou, C.H.; et al. Topography-Driven Isolation, Speciation and a Global Increase of Endemism with Elevation. Glob. Ecol. Biogeogr. 2016, 25, 1097–1107. [Google Scholar] [CrossRef]
- Sipman, H.J.M. The Significance of the Northern Andes for Lichens. Bot. Rev. 2002, 68, 88–99. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veas-Mattheos, K.; Almendras, K.; Pezoa, M.; Muster, C.; Orlando, J. High Andean Steppes of Southern Chile Contain Little-Explored Peltigera Lichen Symbionts. J. Fungi 2023, 9, 372. https://doi.org/10.3390/jof9030372
Veas-Mattheos K, Almendras K, Pezoa M, Muster C, Orlando J. High Andean Steppes of Southern Chile Contain Little-Explored Peltigera Lichen Symbionts. Journal of Fungi. 2023; 9(3):372. https://doi.org/10.3390/jof9030372
Chicago/Turabian StyleVeas-Mattheos, Karla, Katerin Almendras, Matías Pezoa, Cecilia Muster, and Julieta Orlando. 2023. "High Andean Steppes of Southern Chile Contain Little-Explored Peltigera Lichen Symbionts" Journal of Fungi 9, no. 3: 372. https://doi.org/10.3390/jof9030372
APA StyleVeas-Mattheos, K., Almendras, K., Pezoa, M., Muster, C., & Orlando, J. (2023). High Andean Steppes of Southern Chile Contain Little-Explored Peltigera Lichen Symbionts. Journal of Fungi, 9(3), 372. https://doi.org/10.3390/jof9030372





