Survey of Edible Amanita in Northern Thailand and Their Nutritional Value, Total Phenolic Content, Antioxidant and α-Glucosidase Inhibitory Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Identification of the Edible Amanita
2.2.1. Morphological Observations
2.2.2. DNA Extraction, Amplification, Sequencing, and Phylogenetic Analyses
2.3. Nutritional Analysis
2.4. Preparation of Mushroom Extracts
2.5. Determination of Total Phenolic Content
2.6. Antioxidant Assay
2.6.1. ABTS Scavenging Assay
2.6.2. DPPH Scavenging Assay
2.6.3. FRAP Assay
2.7. Determination of α-Glucosidase Inhibitory Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Sample Collection and Morphological Observations
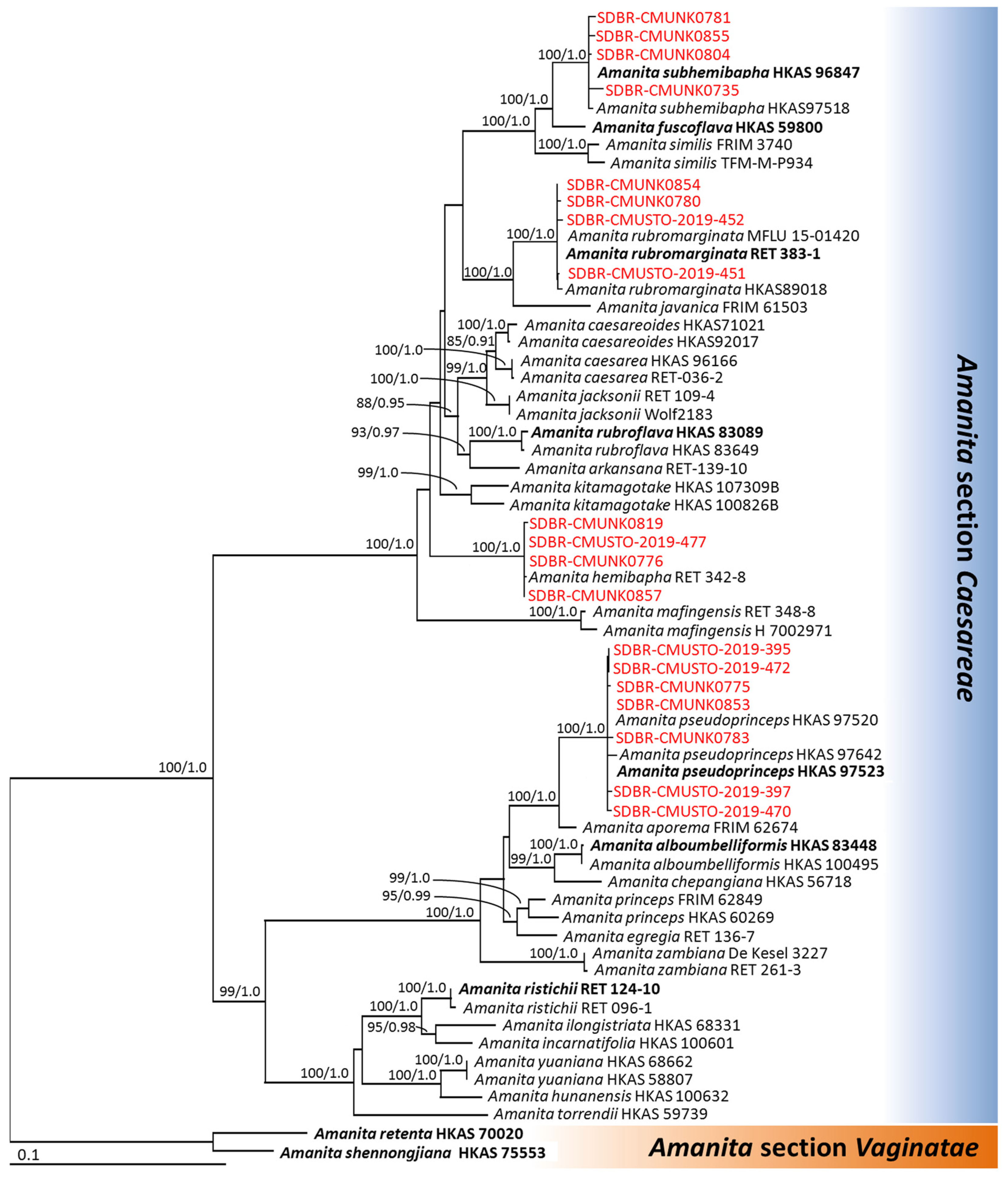
3.2. Phylogenetic Analyses
3.3. Morphological Descriptions
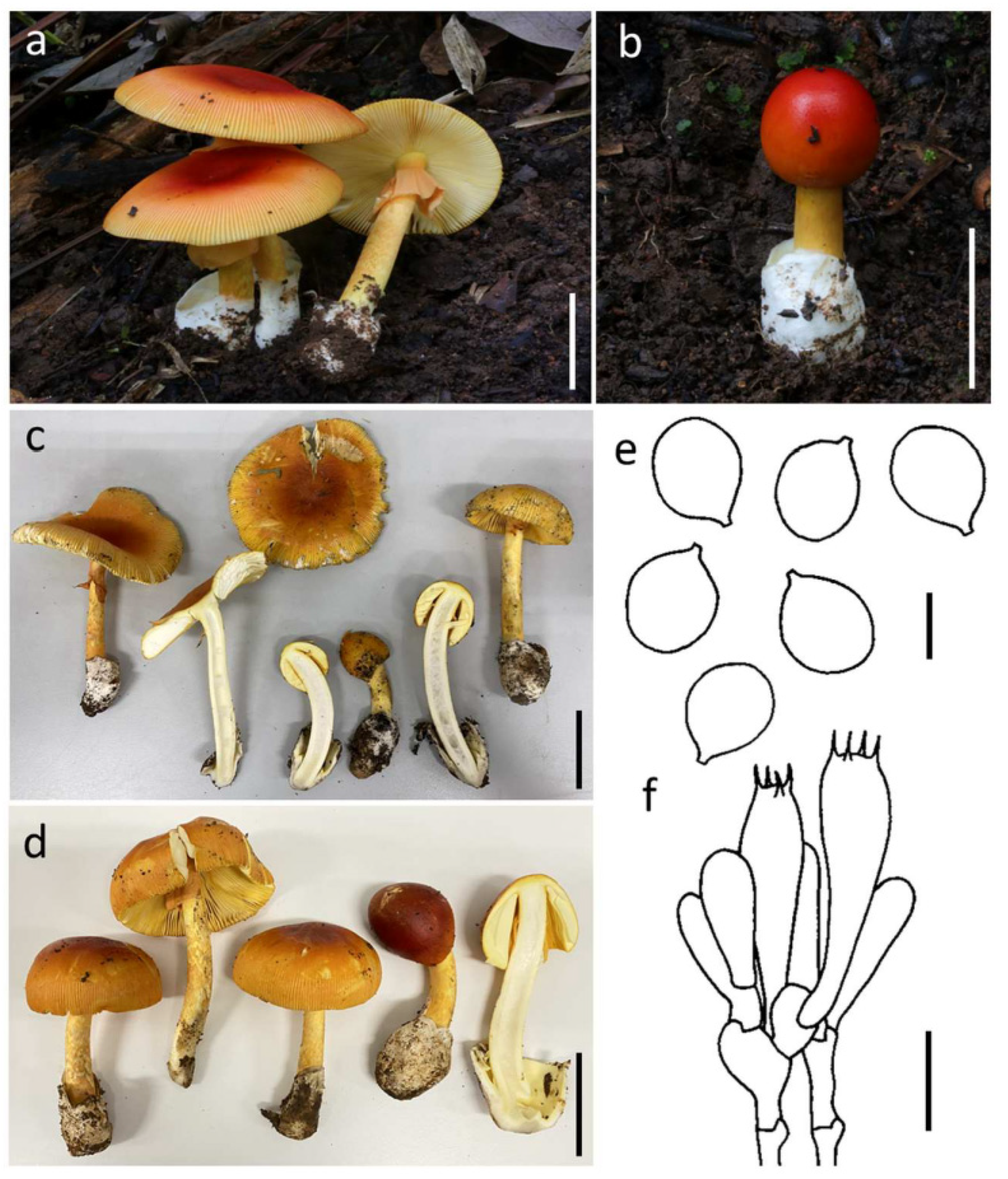
3.3.1. Amanita hemibapha (Berk. & Broome) Sacc., Syll. Fung. (Abellini) 5: 13 (1887) (Figure 3)

3.3.2. Amanita pseudoprinceps Y.Y. Cui, Q. Cai & Zhu L. Yang, Fungal Divers. 91: 59 (2018) (Figure 4)
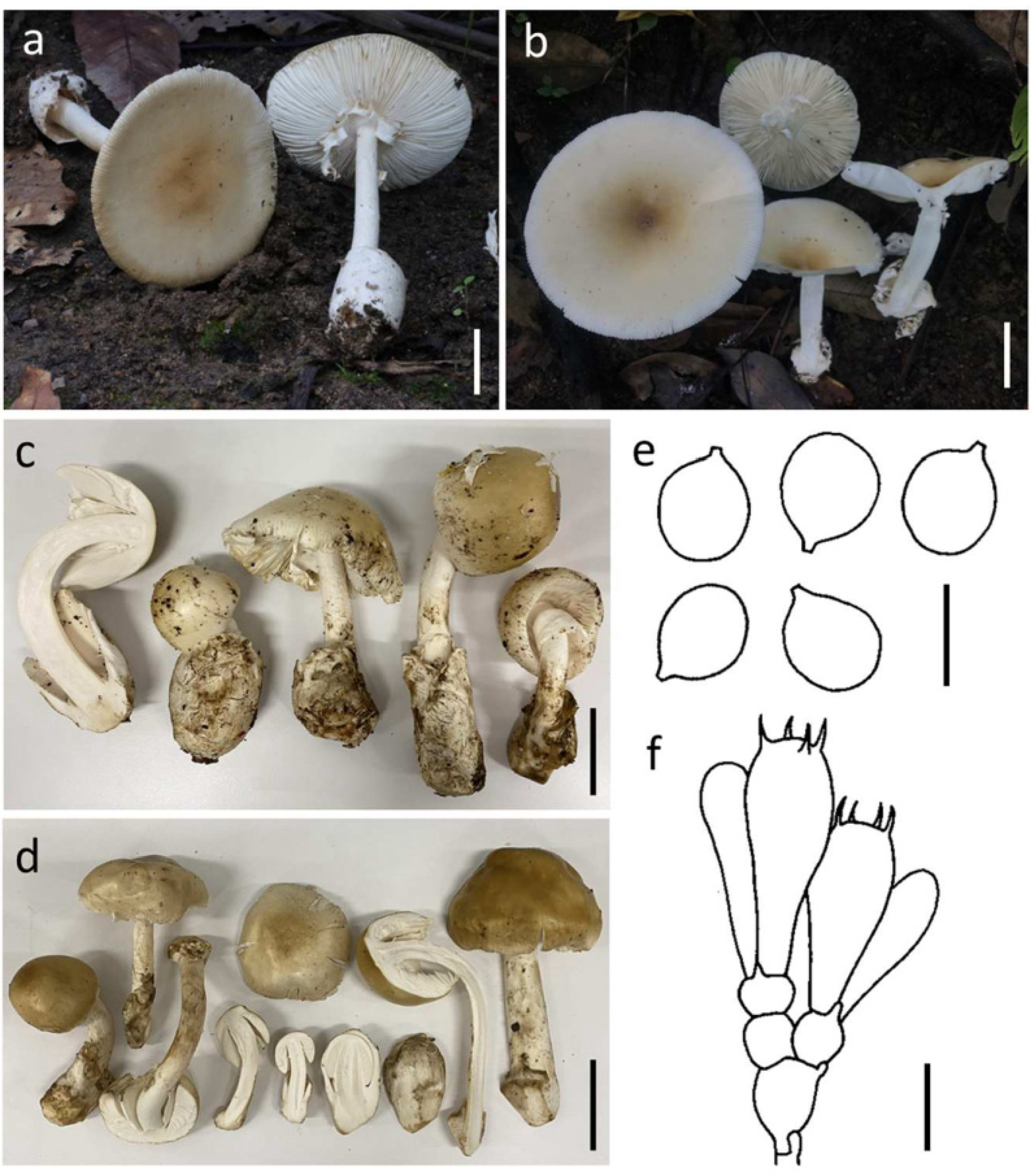
3.3.3. Amanita rubromarginata Har. Takah., Mycoscience 45: 372 (2004) (Figure 5)
3.3.4. Amanita subhemibapha Zhu L. Yang, Y.Y. Cui & Q. Cai, Fungal Divers. 91: 65 (2018) (Figure 6)

3.4. Nutritional Analysis
3.5. Determination of Total Phenolic Content
3.6. Antioxidant Assay
3.7. Determination of α-Glucosidase Inhibitory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Persoon, C.H. Tentamen Dispositionis Methodicae Fungorum; Apud Petrum Philippum Wolf: Leipzig, Germany, 1797. [Google Scholar]
- Bresinsky, A.; Besl, H. A Colour Atlas of Poisonous Fungi: A Handbook for Pharmacists, Doctors, and Biologists; CRC Press: London, UK, 1990. [Google Scholar]
- Yang, Z.L. Atlas of the Chinese Species of Amanitaceae; Science Press: Beijing, China, 2015; pp. 1–213. [Google Scholar]
- Cui, Y.Y.; Cai, Q.; Tang, L.P.; Liu, J.W.; Yang, Z.L. The family Amanitaceae: Molecular phylogeny, higher-rank taxonomy and the species in China. Fungal Divers. 2018, 91, 5–230. [Google Scholar] [CrossRef]
- Sanmee, R.; Tulloss, R.E.; Lumyong, P.; Dell, B.; Lumyong, S. Studies on Amanita (Basidiomycetes: Amanitaceae) in Northern Thailand. Fungal Divers. 2008, 3, 97–123. [Google Scholar]
- Tulloss, R.E. Amanita-distribution in the Americas, with comparison to eastern and southern Asia and notes on spore character variation with latitude and ecology. Mycotaxon 2005, 93, 189–231. [Google Scholar]
- Yang, Z.L.; Oberwinkler, F. Die Fruchtköperentwicklung von Amanita muscaria (Basidiomycetes). Nova Hedwig. 1999, 68, 441–468. [Google Scholar] [CrossRef]
- Yang, Z.L. Die Amanita-Arten von Südwestchina. Bibl. Mycol. 1997, 170, 1–240. [Google Scholar]
- Zhang, L.F.; Yang, J.B.; Yang, Z.L. Molecular phylogeny of eastern Asian species of Amanita (Agaricales, Basidiomycota): Taxonomic and biogeographic implications. Fungal Divers. 2004, 17, 219–238. [Google Scholar]
- Yang, Z.L. Amanitaceae. Flora Fungorum Sinicorum 27; Science Press: Beijing, China, 2005. [Google Scholar]
- Wood, A. Studies in the genus Amanita (Agaricales) in Australia. Aust. Syst. Bot. 1997, 10, 723–854. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, L.P.; Cai, Q.; Xu, J.P. A review on the diversity, phylogeography and population genetics of Amanita mushrooms. Mycology 2015, 6, 86–93. [Google Scholar] [CrossRef]
- Gilbert, E.J. Notules sur les Amanites III–XI. Bull. Trimest. Soc. Mycol. Fr. 1925, 41, 287–309. [Google Scholar]
- Tulloss, R.E.; Kuijper, T.W.M.; Vellinga, E.C.; Yang, Z.L.; Halling, R.E.; Geml, J.; Sánchez-Ramírez, S.; Gonçalves, S.C.; Hess, J.; Pringle, A. The genus Amanita should not be split. Amanitaceae 2016, 1, 1–16. [Google Scholar]
- Liu, Y.S.; Kumla, J.; Suwannarach, N.; Sysouphanthong, P.; Lumyong, L. Three species of Amanita section Lepidella (Amanitaceae, Agaricales) from northern Thailand. Phytotaxa 2022, 570, 16–028. [Google Scholar] [CrossRef]
- Roberts, D.M.; Hall, M.J.; Falkland, M.M.; Strasser, S.I.; Buckley, N.A. Amanita phalloides poisoning and treatment with silibinin in the Australian Capital Territory and New South Wales. Med. J. Aust. 2013, 198, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Pegler, D.N. Useful fungi of the world: Caesar’s mushroom and the Christmas mushroom. Mycologist 2002, 16, 140–141. [Google Scholar] [CrossRef]
- Greeshma, A.A.; Sridhar, K.R.; Pavithra, M. Nutritional perspectives of an ectomycorrhizal edible mushroom Amanita of the southwestern India. Curr. Res. Environ. Appl. Mycol. 2018, 8, 54–68. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, S.; Tulloss, R.E.; Amalfi, M.; Moncalvo, J.M. Palaeotropical origins, boreotropical distribution and increased rates of diversification in a clade of edible ectomycorrhizal mushrooms (Amanita section Caesareae). J. Biogeogr. 2015, 42, 351–363. [Google Scholar] [CrossRef]
- Li, H.; Tian, Y.; Menolli, N.; Ye, L.; Karunarathna, S.C.; Perez-Moreno, J.; Rahman, M.M.; Rashid, M.H.; Phengsintham, P.; Rizal, L.; et al. Reviewing the world’s edible mushroom species: A new evidence-based classification system. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1982–2014. [Google Scholar] [CrossRef]
- Cheung, P.C. Nutritional value and health benefits of mushrooms. Mushrooms Funct. Foods 2008, 2, 71–109. [Google Scholar]
- Anusiya, G.; Gowthama, P.U.; Yamini, N.V.; Sivarajasekar, N.; Rambabu, K.; Bharath, G.; Banat, F. A review of the therapeutic and biological effects of edible and wild mushrooms. Bioengineered 2021, 12, 11239–11268. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible mushrooms: A comprehensive review on bioactive compounds with health benefits and processing aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Panda, M.K.; Tayung, K. Documentation and ethnomedicinal knowledge on wild edible mushrooms among ethnic tribes of Northern Odisha, India. Asian J. Pharm. Clin. Res. 2015, 8, 139–143. [Google Scholar]
- Atri, N.S.; Mridu, C. Mushrooms-Some ethnomycological and sociobiological aspects. Kavaka 2018, 51, 11–19. [Google Scholar]
- Sanmee, R.; Dell, B.; Lumyong, P.; Izumori, K.; Lumyong, S. Nutritive value of popular wild edible mushroom from Northen Thailand. Food Chem. 2003, 82, 527–532. [Google Scholar] [CrossRef]
- Watling, R. Foray in Thailand. Fungi 2013, 6, 45–46. [Google Scholar]
- Ruksawong, P.; Flegel, T.W. Thai Mushrooms and other Fungi; National Center for Genetic Engineering and Biotechnology (BIOTEC), National Science and Technology Development Agency: Bangkok, Thailand, 2001. [Google Scholar]
- Suwannarach, N.; Kumla, J.; Khuna, S.; Wannathes, N.; Thongklang, N.; Sysouphanthong, P.; Luangharn, T.; Wongkanoun, S.; Karunarathna, S.C.; Liu, Y.S.; et al. History of Thai mycology and resolution of taxonomy for Thai macrofungi confused with Europe and American names. Chiang Mai J. Sci. 2022, 49, 654–683. [Google Scholar] [CrossRef]
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour, 3rd ed.; Eyre Methuen: London, UK, 1978; pp. 1–243. [Google Scholar]
- Largent, D.L.; Johnson, D.; Watling, R. How to Identify Mushrooms to Genus III: Microscopic Features; Mad River Press Inc.: Eureka, CA, USA, 1977; pp. 1–148. [Google Scholar]
- Bas, C. Morphology and subdivision of Amanita and a monograph of its section Lepidella. Persoonia 1969, 5, 285–579. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; Volume 38, pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-a sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Cai, Q.; Tulloss, R.E.; Tang, L.P.; Tolgor, B.; Zhang, P.; Chen, Z.H.; Yang, Z.L. Multi-locus phylogeny of lethal amanitas: Implications for species diversity and historical biogeography. BMC Evol. Biol. 2014, 14, 143. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. Raxml-vi-hpc: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence intervals on phylogenetics: An approach using bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Hoehna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree Tree Figure Drawing Tool Version 131, Institute of Evolutionary 623 Biology, University of Edinburgh. Available online: http://treebioedacuk/software/figtree/ (accessed on 25 December 2022).
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Gaithersburg, MD, USA, 1996. [Google Scholar]
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Lumyong, S. Phenolic profile of various wild edible mushroom extracts from Thailand and their antioxidant properties, anti-tyrosinase and hyperglycaemic inhibitory activities. J. Funct. Foods 2016, 27, 352–364. [Google Scholar] [CrossRef]
- Thitilertdecha, N.; Teerawutgulrag, A.; Rakariyatham, N. Antioxidant and antimicrobial activities of Nephelium lappacium L. extracts. LWT Food Sci. Technol. 2008, 41, 2029–2035. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gülçın, İ.; Oktay, M.; Kıreçcı, E.; Küfrevıoǧlu, Ö.İ. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003, 83, 371–382. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Oki, T.; Matsui, T.; Osajima, Y. Inhibitory effect of α-glucosidase inhibitors varies according to its origin. J. Agric. Food Chem. 1999, 47, 550–553. [Google Scholar] [CrossRef]
- Tanruean, K.; Kaewnarin, K.; Suwannarach, N.; Lumyong, S. Comparative evaluation of phytochemicals, and antidiabetic and antioxidant activities of Cuscuta reflexa grown on different hosts in northern Thailand. Nat. Prod. Commun. 2017, 12, 51–54. [Google Scholar] [CrossRef]
- Thongbai, B.; Tulloss, R.E.; Miller, S.L.; Hyde, K.D.; Chen, J.; Zhao, R.L.; Raspé, O. A new species and four new records of Amanita (Amanitaceae; Basidiomycota) from Northern Thailand. Phytotaxa 2016, 286, 211–231. [Google Scholar] [CrossRef]
- Mu, M.; Huang, H.Y.; Zhang, W.H.; Tang, L.P. A new species with pink lamellae of Amanita section Caesareae from China. Phytotaxa 2021, 478, 141–150. [Google Scholar] [CrossRef]
- Tang, L.P.; Lee, S.S.; Zeng, N.K.; Cai, Q.; Zhang, P.; Yang, Z.L. Notes on Amanita section Caesareae from Malaysia. Mycologia 2017, 109, 557–567. [Google Scholar] [CrossRef]
- Vrinda, K.B.; Pradeep, C.K.; Kumar, S.S. Occurrence of a lesser known edible Amanita in the Western Ghats of Kerala. Mushroom Res. 2005, 14, 5–8. [Google Scholar]
- Upadhyay, R.; Semwal, K.; Tripathi, A.; Kumari, D. Three taxa of Amanita hemibapha from North-Western Himalaya (India). Mushroom Res. 2008, 17, 1–7. [Google Scholar]
- Pegler, D.N. Agaric flora of Sri Lanka. Kew Bull. Add. Ser. 1986, 12, 1–519. [Google Scholar]
- Endo, N.; Fangfuk, W.; Kodaira, M.; Sakuma, D.; Hadano, E.; Hadano, A.; Murakami, Y.; Phosri, C.; Matsushita, N.; Fukuda, M.; et al. Re-evaluation of Japanese Amanita section Caesareae species with yellow and brown pileus with descriptions of Amanita kitamagotake and A. chatamagotake spp. nov. Mycoscience 2017, 58, 457–471. [Google Scholar] [CrossRef]
- Corner, E.J.H.; Bas, C. The genus Amanita in Singapore and Malaya. Persoonia 1962, 2, 241–304. [Google Scholar]
- Boedijn, K.B. Notes on Indonesian fungi: The genus Amanita. Sydowia 1951, 5, 317–327. [Google Scholar]
- Imazeki, R.; Otani, Y.; Hongo, T. Fungi of Japan (Revisied and Enlarged Edition); Yama-kei Publishers Co.: Tokyo, Japan, 2011. [Google Scholar]
- Takahashi, H. Two new species of Agaricales from southwestern islands of Japan. Mycoscience 2004, 45, 372–376. [Google Scholar] [CrossRef]
- Parnmen, S.; Sikaphan, S.; Leudang, S.; Boonpratuang, T.; Rangsiruji, A.; Naksuwankul, K. Molecular identification of poisonous mushroom using nuclear ITS region and peptide toxins: A retrospective study on fetal cases in Thailand. J. Toxical. Sci. 2016, 41, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Tafere, D.A. Chemical composition and uses of wild edible mushrooms—A review. EC Nutr. 2022, 17, 8. [Google Scholar]
- Boa, E. Wild Edible Fungi: A Global Overview of Their Use and Importance to People; The Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Dimopoulou, M.; Kolonas, A.; Mourtakos, S.; Androutsos, O.; Gortzi, O. Nutritional composition and biological properties of sixteen edible mushroom species. Appl. Sci. 2022, 12, 8074. [Google Scholar] [CrossRef]
- Giri, A.; Rana, P. Ethnomycological knowledge and nutritional analysis of some wild edible mushrooms of sagarmatha national park (snp), Nepal. J. Nat. Hist. Mus. 2008, 23, 65–77. [Google Scholar] [CrossRef]
- Kalač, P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Kissanga, R.; Liberal, Â.; Diniz, I.; Rodrigues, A.S.B.; Baptista-Ferreira, J.L.; Batista, D.; Ivanov, M.; Sokovi’c, M.; Ferreira, I.C.F.R.; Fernandes, Â.; et al. Biochemical and molecular profiling of wild edible mushrooms from Huila, Angola. Foods 2022, 11, 3240. [Google Scholar] [CrossRef]
- León-Guzmón, M.F.; Silva, I.; López, M.G. Proximate chemical composition, free amino acid contents, and free fatty acid contents of some wild edible mushrooms from Querétaro, Mexico. J. Agric. Food Chem. 1997, 45, 4329–4332. [Google Scholar] [CrossRef]
- Raphael, O.; Izuchukwu, D.U.; Sandra, U.I. Proximate composition and mineral profiles of selected edible mushroom consumed in northern part of Nigeria. Acade. J. Sci. Res. 2017, 5, 349–364. [Google Scholar]
- Ravikrishnan, V.; Sanjeev, G.; Rajashekhar, M. Compositional and nutritional studies on two wild mushrooms from Western Ghat forests of Karnataka, India. Int. Food Res. J. 2017, 24, 679–684. [Google Scholar]
- Reid, T.; Munyanyi, M.; Mduluza, T. Effect of cooking and preservation on nutritional and phytochemical composition of the mushroom Amanita zambiana. Food Sci. Nutr. 2017, 5, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Gautam, N. Chemical, bioactive, and antioxidant potential of twenty wild culinary mushroom species. BioMed Res. Int. 2015, 2015, 346508. [Google Scholar] [CrossRef] [PubMed]
- Srikram, A.; Supapvanich, S. Proximate compositions and bioactive compounds of edible wild and cultivated mushrooms from Northeast Thailand. Arg. Nat. Resour. 2016, 50, 432–436. [Google Scholar] [CrossRef]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Antibacterial, antiradical potential and phenolic compounds of thirty-one polish mushroom. PLoS ONE 2015, 10, e0140355. [Google Scholar] [CrossRef] [PubMed]
- Kosanić, M.; Ranković, B.; Dašić, M. Mushrooms as possible antioxidant and antimicrobial agents. Iran J. Pharm. Res. 2012, 11, 1095–1102. [Google Scholar] [PubMed]
- Rameah, C.; Pattar, M.G. Antimicribial properties, antioxidant activity and bioactive compounds from six wild edible mushrooms of western ghats of Karnataka, India. Pharm. Res. 2010, 2, 107–112. [Google Scholar]
- Doğan, H.H.; Akbas, G. Biological activity and fatty acid composition of Caesar’s mushroom. Pharm. Biol. 2013, 51, 863–871. [Google Scholar] [CrossRef]
- Butkhup, L.; Samappito, W.; Jorjong, S. Evaluation of bioactivities and phenolic contents of wild edible mushrooms from northeastern Thailand. Food Sci. Biotechnol. 2018, 27, 193–202. [Google Scholar] [CrossRef]
- Champatasi, L.; Chamnantap, N.; Saisong, A.; Naksuwankul, K. The evaluation of potentials of antioxidant activities, total phenolic, flavonoid, and tannin contents from selected species in Amanita crude extract. J. Thai Trad. Alt. Med. 2022, 20, 282–294. [Google Scholar]
- Doğan, H.H. Evaluation of phenolic compounds, antioxidant activities and fatty acid composition of Amanita ovoidea (Bull.) Link. in Turkey. J. Food Compost. Anal. 2013, 31, 87–93. [Google Scholar] [CrossRef]
- Smolskaitė, L.; Venskutonis, P.R.; Talou, T. Comprehensive evaluation of antioxidant and antimicrobial properties of different mushroom species. LWT Food Sci. Technol. 2015, 60, 462–471. [Google Scholar] [CrossRef]
- Abugria, D.A.; McElhenneyb, W.H. Extraction of total phenolic and flavonoids from edible wild and cultivated medicinal mushrooms as affected by different solvents. J. Nat. Prod. Plant Resour. 2013, 3, 37–42. [Google Scholar]
- Khatua, S.; Acharya, K. Exploring the chemical composition and bioactivity of ethanol extract from Russula pseudocyanoxantha (Agaricomycetes), a novel mushroom of Tribal preference in India. Int. J. Med. Mushrooms 2022, 24, 87–93. [Google Scholar] [CrossRef]
- Yildiz, O.; Can, Z.; Laghari, A.B.; Sahin, H.; Malkoc, M. Wild edible mushrooms as a natural source of phenolics and antioxidants. J. Food Biochem. 2015, 39, 148–154. [Google Scholar] [CrossRef]
- Vamanu, E.; Nita, S. Antioxidant capacity and the correlation with major phenolic compounds, anthocyanin, and tocopherol content in various extracts from the wild edible Boletus edulis mushroom. Biomed Res. Int. 2013, 2013, e313905. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velazquez, A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990. [Google Scholar] [CrossRef]
- Samara, M.; Nasser, A.; Mingelgrin, U. Critical examination of the suitability of the Folin-Ciocalteu reagent assay for quantitative analysis of polyphenols–the case of olive-mill wastewater. Am. J. Analyt. Chem. 2022, 13, 476–493. [Google Scholar] [CrossRef]
- Martins, G.R.; Monteiro, A.F.; do Amaral, F.R.L.; da Silva, A.S. A validated Folin-Ciocalteu method for total phenolics quantification of condensed tannin-rich açaí (Euterpe oleracea Mart.) seeds extract. J. Food Sci. Technol. 2021, 58, 4693–4702. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Barros, L.; Abreu, R.M.V. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ching, L.T.; Chen, J.; Cheung, P.C.K. Antioxidant and anti-angiogenic effects of mushroom phenolics-rich fractions. J. Funct. Foods 2015, 17, 802–815. [Google Scholar] [CrossRef]
- Joshi, S.R.; Standl, E.; Tong, N.; Shah, P.; Kalra, S.; Rathod, R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: An evidence-based review. Expert Opi. Pharmacother. 2015, 16, 1959–1981. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H. Pharmacology of alpha-glucosidase inhibition. Eur. J. Clin. Invest. 1994, 3, 3–10. [Google Scholar]
- Pongkunakorn, T.; Watcharachaisoponsiri, T.; Chupeerach, C.; On-nom, N.; Suttisansanee, U. Inhibitions of key enzymes relevant to obesity and diabetes of Thai local mushroom extracts. Curr. Appl. Sci. Technol. 2017, 17, 181–190. [Google Scholar]
- Kim, J.H.; Lee, E.J.; Seok, S.J. Fibrinolytic and α-glucosidase inhibition activities of wild mushroom methanol extracts. Korean J. Mycol. 2007, 35, 128–132. [Google Scholar]
- Nagarani, G.; Abirami, A.; Siddhuraju, P. A comparative study on antioxidant potentials, inhibitory activities against key enzymes related to metabolic syndrome, and anti-inflammatory activity of leaf extract from different Momordica species. Food Sci. Hum. Wellness 2014, 3, 36–46. [Google Scholar] [CrossRef]
- Shodehinde, S.A.; Oboh, G. In vitro antioxidant activities and inhibitory effects of aqueous extracts of unripe plantain pulp (Musa paradisiaca) on enzymes linked with type 2 diabetes and hypertension. J. Toxicol. Environ. Health Sci. 2012, 4, 65–75. [Google Scholar]

| Amanita Species | Strain/Voucher | Country | GenBank Accession Number | |||
|---|---|---|---|---|---|---|
| ITS | nrLSU | rpb2 | tef-1 | |||
| A. alboumbelliformis | HKAS 83448 T | China | – | MH486635 | MH486085 | MH508892 |
| A. alboumbelliformis | HKAS 100495 | China | – | MH486634 | MH486084 | MH508891 |
| A. aporema | FRIM 62674 | Malaysia | KU714575 | KU714551 | KU714593 | KU714538 |
| A. arkansana | RET-139-10 | USA | JX844675 | KF877195 | KF877036 | KP724416 |
| A. caesarea | HKAS 96166 | Italy | MH508283 | MH486418 | MH485898 | MH508705 |
| A. caesarea | RET-036-2 | Italy | JX844687 | KF877205 | KF877042 | KP724491 |
| A. caesareoides | HKAS92017 | China | MH508286 | MH486422 | MH485902 | MH508709 |
| A. caesareoides | HKAS71021 | Japan | MH508284 | MH486419 | MH485899 | MH508706 |
| A. chepangiana | HKAS 56718 | China | KU714569 | KU714545 | KU714588 | KU714534 |
| A. egregia | RET 136-7 | Australia | JX844707 | KF877227 | KF877052 | KF877119 |
| A. fuscoflava | HKAS 59800 T | China | MH508372 | MH486557 | MH486023 | MH508827 |
| A. hemibapha | RET 342-8 | India | – | KF877233 | KF877055 | KF877124 |
| A. hemibapha | SDBR-CMUNK0776 | Thailand | OQ199032 | OQ187796 | OQ200073 | OQ200092 |
| A. hemibapha | SDBR-CMUNK0819 | Thailand | OQ199033 | OQ187797 | OQ200074 | OQ200093 |
| A. hemibapha | SDBR-CMUNK0857 | Thailand | OQ199034 | OQ187798 | OQ200075 | OQ200094 |
| A. hemibapha | SDBR-CMUSTO-2019-477 | Thailand | OQ199035 | OQ187799 | OQ200076 | OQ200095 |
| A. hunanensis | HKAS 100632 | China | MH508396 | MH486588 | MH486050 | MH508856 |
| A. incarnatifolia | HKAS 100601 | China | MH508403 | MH486597 | MH486059 | MH508865 |
| A. jacksonii | RET 109-4 | USA | – | KF877247 | KF877064 | KP724551 |
| A. jacksonii | Wolf2183 | USA | – | MH486606 | MH486063 | MH508872 |
| A. javanica | FRIM 61503 | Malaysia | KU714572 | KU714548 | – | KU714536 |
| A. kitamagotake | HKAS 100826B | China | MW258868 | MW258920 | – | MW324494 |
| A. kitamagotake | HKAS 107309B | China | MW258874 | MW258921 | – | MW324495 |
| A. longistriata | HKAS 68331 | China | MH508428 | MH486631 | MH486081 | MH508888 |
| A. mafingensis | RET 348-8 | Zambia | JX844729 | KF877259 | – | KF877148 |
| A. mafingensis | H 7002971 | Tanzania | JF710834 | JF710802 | – | JF710822 |
| A. princeps | FRIM 62849 | Malaysia | KU714576 | KU714552 | KU714594 | KU714539 |
| A. princeps | HKAS 60269 | China | – | MH486766 | MH486184 | MH508993 |
| A. pseudoprinceps | HKAS 97523 T | China | MH508527 | MH486788 | MH486202 | – |
| A. pseudoprinceps | HKAS 97642 | China | – | MH486789 | MH486203 | MH509015 |
| A. pseudoprinceps | HKAS 97520 | China | MH508526 | MH486787 | MH486201 | – |
| A. pseudoprinceps | SDBR-CMUNK0775 | Thailand | OQ199036 | OQ187800 | OQ200077 | OQ200096 |
| A. pseudoprinceps | SDBR-CMUNK0783 | Thailand | OQ199037 | OQ187801 | OQ200078 | OQ200097 |
| A. pseudoprinceps | SDBR-CMUNK0853 | Thailand | OQ199038 | OQ187802 | OQ200079 | OQ200098 |
| A. pseudoprinceps | SDBR-CMUSTO-2019-395 | Thailand | – | OQ187803 | OQ200080 | OQ200099 |
| A. pseudoprinceps | SDBR-CMUSTO-2019-397 | Thailand | OQ199039 | OQ187804 | OQ200081 | OQ200100 |
| A. pseudoprinceps | SDBR-CMUSTO-2019-470 | Thailand | OQ199040 | – | OQ200082 | OQ200101 |
| A. pseudoprinceps | SDBR-CMUSTO-2019-472 | Thailand | – | OQ187805 | OQ200083 | OQ200102 |
| A. ristichii | RET 124-10 T | USA | JX844737 | KF877277 | – | – |
| A. ristichii | RET 096-1 | Canada | JX844738 | JX844738 | KF877075 | KF877162 |
| A. rubroflava | HKAS 83089 T | China | MH508568 | MH486827 | MH486238 | MH509054 |
| A. rubroflava | HKAS 83649 | China | MH508569 | MH486828 | MH486239 | MH509055 |
| A. rubromarginata | RET 383-1 T | Japan | JX844739 | KF877279 | – | KF877164 |
| A. rubromarginata | MFLU 15-01420 | Thailand | KU904822 | KU877538 | – | – |
| A. rubromarginata | HKAS89018 | China | MH508573 | MH486832 | MH486243 | MH509059 |
| A. rubromarginata | SDBR-CMUNK0780 | Thailand | OQ199041 | OQ187806 | OQ200084 | OQ200103 |
| A. rubromarginata | SDBR-CMUNK0854 | Thailand | OQ199042 | OQ187807 | OQ200085 | OQ200104 |
| A. rubromarginata | SDBR-CMUSTO-2019-451 | Thailand | OQ199043 | OQ187808 | OQ200086 | OQ200105 |
| A. rubromarginata | SDBR-CMUSTO-2019-452 | Thailand | OQ199044 | OQ187809 | OQ200087 | OQ200106 |
| A. similis | FRIM 3740 | Malaysia | KU714566 | JF710796 | – | KU714531 |
| A. similis | TFM-M-P934 | Indonesia | KU714568 | JF710798 | – | KU714533 |
| A. subhemibapha | HKAS 96847 T | China | – | MH486906 | MH486307 | MH509127 |
| A. subhemibapha | HKAS97518 | China | MH508621 | MH486907 | MH486308 | – |
| A. subhemibapha | SDBR-CMUNK0735 | Thailand | OQ199045 | OQ187810 | OQ200088 | OQ200107 |
| A. subhemibapha | SDBR-CMUNK0781 | Thailand | OQ199046 | OQ187811 | OQ200089 | OQ200108 |
| A. subhemibapha | SDBR-CMUNK0804 | Thailand | OQ199047 | OQ187812 | OQ200090 | OQ200109 |
| A. subhemibapha | SDBR-CMUNK0855 | Thailand | OQ199048 | OQ187813 | OQ200091 | OQ200110 |
| A. torrendii | HKAS 59739 | Spain | KU714578 | KU714555 | KU714591 | KU714540 |
| A. yuaniana | HKAS 58807 | China | MH508653 | MH486954 | MH486347 | MH509174 |
| A. yuaniana | HKAS 68662 | China | MH508654 | MH486957 | MH486350 | MH509177 |
| A. zambiana | De Kesel 3227 | Benin | – | KF877307 | KF877093 | – |
| A. zambiana | RET 261-3 | Burundi | – | KF877311 | KF877096 | KF877193 |
| A. retenta | HKAS 70020 T | China | MH508543 | MH486802 | MH486215 | MH509028 |
| A. shennongjiana | HKAS 75553 T | China | MH508590 | MH486862 | MH486270 | MH509085 |
| Intitial Identification | Source | No. of Collection | Specimen Voucher SDBR-CMU |
|---|---|---|---|
| A. hemibapha | Natural forest | 2 | STO-2019-477 and NK0776 |
| Roadside market | 2 | NK0819 and NK0857 | |
| A. pseudoprinceps | Natural forest | 5 | STO-2019-395, STO-2019-397, STO-2019-470, STO-2019-472, and NK0775 |
| Roadside market | 2 | NK0783 and NK0853 | |
| A. rubromarginata | Natural forest | 2 | STO-2019-451 and STO-2019-452 |
| Roadside market | 2 | NK0780 and NK0854 | |
| A. subhemibapha | Natural forest | 1 | NK0781 |
| Roadside market | 3 | NK0735, NK0804, and NK0855 |
| Amanita Species | Pileus | Annulus | Basidia (μm) | Basidiospores (μm) |
|---|---|---|---|---|
| A. hemibapha | 6–12 cm diam., orange to yellow at center, and yellow to pale yellow at margin | Yellow | 32–50 × 8–12 | 8.0–12.0 × 5.5–7.0 |
| A. pseudoprinceps | 8.5–16 cm diam., brownish, yellow-brown to brown at center, and cream to white towards margin | White to cream | 36–53 × 12–18 | 9.0–13.0 × 8.0–12.5 |
| A. rubromarginata | 6–10 cm diam., red to orange-red at center, becoming reddish orange, orange-yellow to yellow towards margin | Reddish to orange-red | 32–46 × 8–13 | 7.0–10.0 × 6.0–8.0 |
| A. subhemibapha | 5–10 cm diam., orange at center, and yellow to yellowish at margin | Orange to yellow | 40–50 × 9–12 | 7.0–11.0 × 6.5–9.0 |
| Amanita Species/ Specimen Voucher SDBR | Nutritional Value (% Dry Weight) * | ||||
|---|---|---|---|---|---|
| Ash | Carbohydrate | Fat | Fiber | Protein | |
| A. hemibapha/CMUNK0776 | 14.39 ± 0.16 b | 34.67 ± 0.22 a | 9.94 ± 0.44 a | 10.03 ± 0.43 b | 23.30 ± 0.40 c |
| A. hemibapha/CMUNK0857 | 14.10 ± 0.22 b | 35.17 ± 0.38 a | 9.71 ± 0.28 a | 9.13 ± 0.25 c | 24.37 ± 0.52 c |
| A. pseudoprinceps/CMUNK0770 | 12.29 ± 0.23 c | 30.10 ± 0.26 d | 6.05 ± 0.42 c | 12.11 ± 0.50 a | 27.97 ± 0.43 a |
| A. pseudoprinceps/CMUNK0853 | 12.11 ± 0.61 c | 30.03 ± 0.64 d | 6.11 ± 0.06 c | 12.29 ± 0.38 a | 28.07 ± 0.59 a |
| A. rubromarginata/CMUNK0780 | 17.84 ± 0.65 a | 31.45 ± 0.26 c | 10.24 ± 0.81 a | 7.75 ± 0.13 e | 26.88 ± 0.19 b |
| A. subhemibapha/CMUNK0855 | 11.99 ± 0.44 c | 33.84 ± 0.16 b | 9.30 ± 0.08 b | 8.74 ± 0.51 d | 27.87 ± 0.67 a |
| Amanita Species/ Specimen Voucher SDBR | TPC (mg GAE/g dw) | DPPH Assay (mg TE/g dw) | ABTS Assay (mg TE/g dw) | FRAP Assay (mg TE/g dw) | AGI (% Inhibition) |
|---|---|---|---|---|---|
| A. hemibapha/CMUNK0776 | 1.03 ± 0.03 b | 0.69 ± 0.01 b | 0.87 ± 0.04 c | 0.45 ± 0.03 b | 20.37 ± 0.99 d |
| A. hemibapha/CMUNK0857 | 1.07 ± 0.02 b | 0.66 ± 0.04 b | 0.89 ± 0.02 c | 0.49 ± 0.03 b | 19.26 ± 0.34 d |
| A. pseudoprinceps/CMUNK0770 | 1.51 ± 0.03 a | 1.54 ± 0.01 a | 0.95 ± 0.04 b | 0.63 ± 0.02 a | 29.14 ± 0.71 b |
| A. pseudoprinceps/CMUNK0853 | 1.62 ± 0.10 a | 1.57 ± 0.05 a | 1.00 ± 0.03 a | 0.60 ± 0.04 a | 31.44 ± 0.71 b |
| A. rubromarginata/CMUNK0780 | 0.94 ± 0.02 c | 0.44 ± 0.12 d | 0.56 ± 0.01 e | 0.38 ± 0.03 c | 20.28 ± 0.23 d |
| A. subhemibapha/CMUNK0855 | 1.09 ± 0.08 b | 0.49 ± 0.02 c | 0.70 ± 0.05 d | 0.47 ± 0.01 b | 23.90 ± 1.10 c |
| Standard Compound: Acarbose | NT | NT | NT | NT | 44.06 ± 0.78 a |
| Parameter | Antioxidant Activity | AGI Activity | ||
|---|---|---|---|---|
| DPPH Activity | ABTS Activity | FRAP Activity | ||
| Total phenolic content | 0.975 * | 0.762 | 0.948 * | 0.959 * |
| p-value | p < 0.01 | p = 0.78 | p = 0.04 | p = 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumla, J.; Suwannarach, N.; Liu, Y.S.; Tanruean, K.; Lumyong, S. Survey of Edible Amanita in Northern Thailand and Their Nutritional Value, Total Phenolic Content, Antioxidant and α-Glucosidase Inhibitory Activities. J. Fungi 2023, 9, 343. https://doi.org/10.3390/jof9030343
Kumla J, Suwannarach N, Liu YS, Tanruean K, Lumyong S. Survey of Edible Amanita in Northern Thailand and Their Nutritional Value, Total Phenolic Content, Antioxidant and α-Glucosidase Inhibitory Activities. Journal of Fungi. 2023; 9(3):343. https://doi.org/10.3390/jof9030343
Chicago/Turabian StyleKumla, Jaturong, Nakarin Suwannarach, Yuan S. Liu, Keerati Tanruean, and Saisamorn Lumyong. 2023. "Survey of Edible Amanita in Northern Thailand and Their Nutritional Value, Total Phenolic Content, Antioxidant and α-Glucosidase Inhibitory Activities" Journal of Fungi 9, no. 3: 343. https://doi.org/10.3390/jof9030343
APA StyleKumla, J., Suwannarach, N., Liu, Y. S., Tanruean, K., & Lumyong, S. (2023). Survey of Edible Amanita in Northern Thailand and Their Nutritional Value, Total Phenolic Content, Antioxidant and α-Glucosidase Inhibitory Activities. Journal of Fungi, 9(3), 343. https://doi.org/10.3390/jof9030343









