Roles of the Fungal-Specific Lysine Biosynthetic Pathway in the Nematode-Trapping Fungus Arthrobotrys oligospora Identified through Metabolomics Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, and Culture Conditions
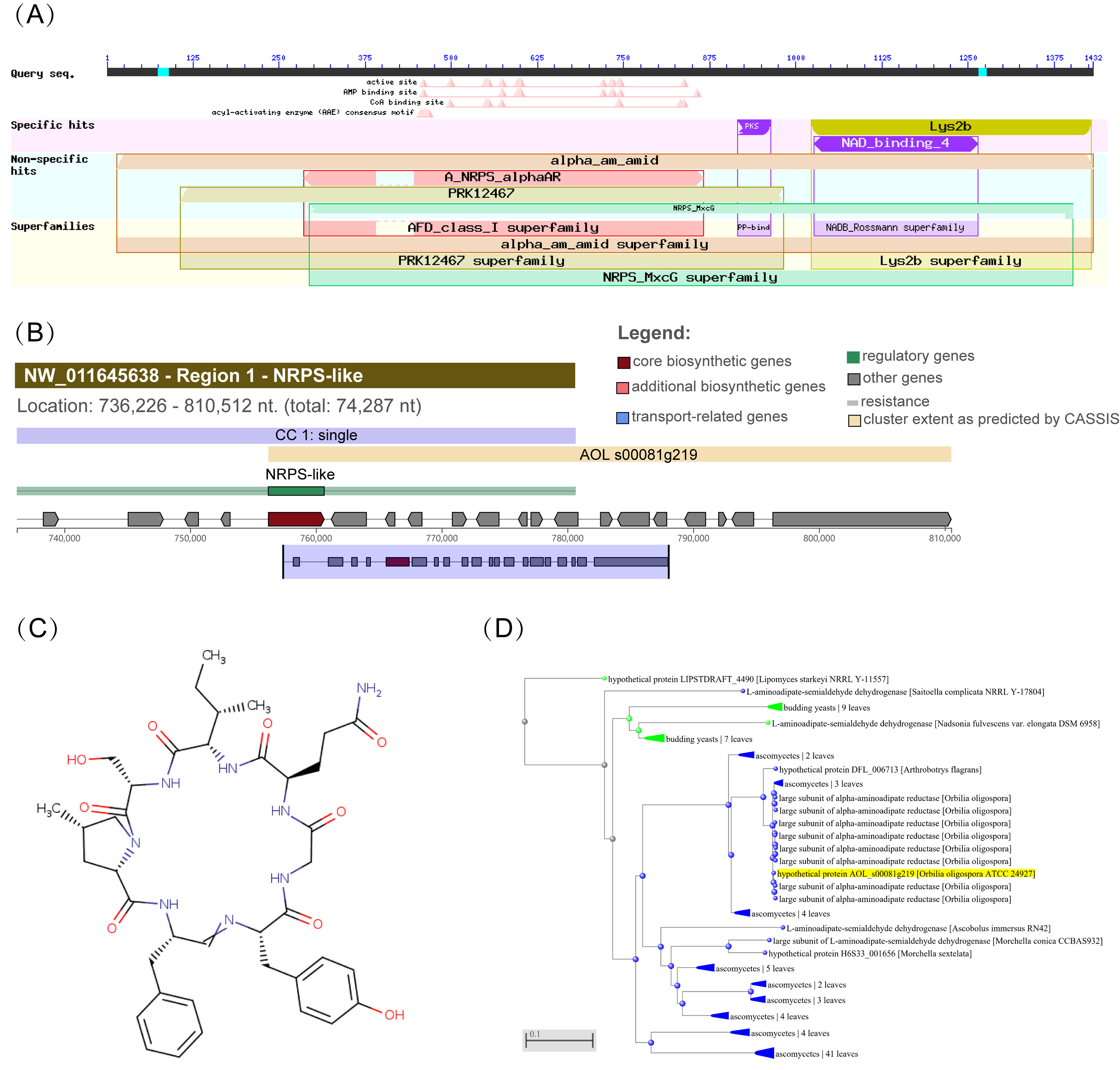
2.2. Sequence Information Analysis
2.3. Construction of the Aoaar Disruption Vector
2.4. Transformation of Protoplasts
2.5. Comparison of Mycelial Growth and Morphology
2.6. Comparison of Conidial Production, Morphology, and Germination
2.7. Trap Formation and Nematicide Activity
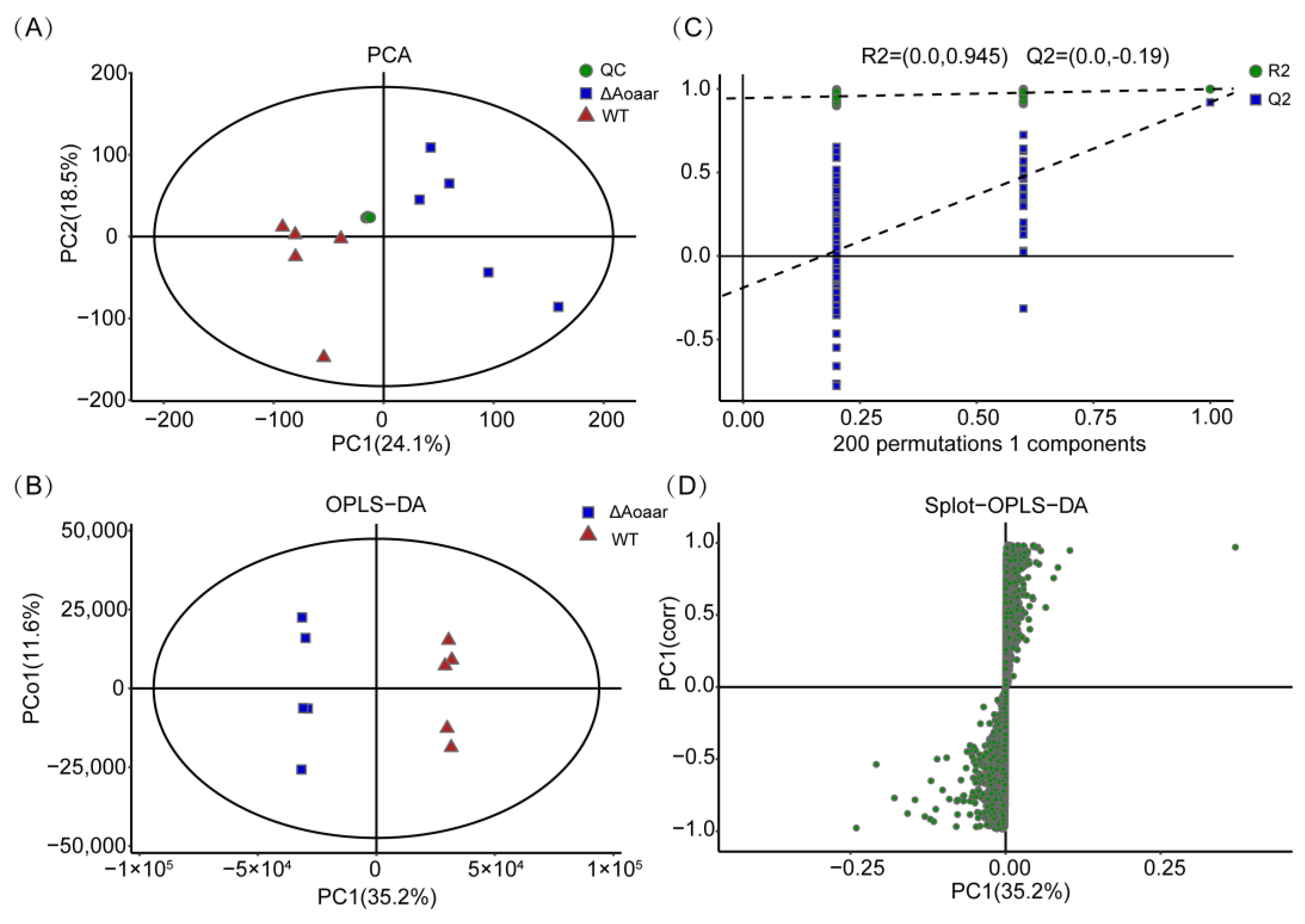
2.8. Metabolomics Analysis
2.9. Statistical Analysis
3. Results
3.1. Sequence Analyses of Aoaar
3.2. The Disruption of Aoaar Affects the Growth and Nematocidal Activity of A. oligospora
3.3. Disruption of Aoaar Reprogrammed Amino Acid-Related Primary and Secondary Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, G.; Khan, A.; Khan, A.A.; Ali, A.; Mohhamad, H.I. Biological control: A novel strategy for the control of the plant parasitic nematodes. Antonie Van Leeuwenhoek 2021, 114, 885–912. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.; Vicente, C.S.L.; Menéndez, E.; Faria, J.M.S.; Rusinque, L.; Camacho, M.J.; Inácio, M.L. The Fight against Plant-Parasitic Nematodes: Current Status of Bacterial and Fungal Biocontrol Agents. Pathogens 2022, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Nordbring-Hertz, B.; Jansson, H.; Tunlid, A. Nematophagous Fungi; eLS: Paris, France, 2006. [Google Scholar]
- Zhang, Y.; Li, G.H.; Zhang, K.Q. A review on the research of nematophagous fungal species. Mycosystema 2011, 30, 836–845. [Google Scholar]
- Hajji-Hedfi, L.; Hlaoua, W.; Al-Judaibi, A.A.; Rhouma, A.; Horrigue-Raouani, N.; Abdel-Azeem, A.M. Comparative Effectiveness of Filamentous Fungi in Biocontrol of Meloidogyne javanica and Activated Defense Mechanisms on Tomato. J. Fungi 2023, 9, 37. [Google Scholar] [CrossRef]
- Jiang, X.; Xiang, M.; Liu, X. Nematode-Trapping Fungi. Microbiol. Spectr. 2017, 5, 1–12. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Ji, X.; Feng, Y.; Li, X.; Zou, C.; Xu, J.; Ren, Y.; Mi, Q.; Wu, J.; et al. Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. PLoS Pathog. 2011, 7, e1002179. [Google Scholar] [CrossRef]
- Vidal-Diez de Ulzurrun, G.; Hsueh, Y.P. Predator-prey interactions of nematode-trapping fungi and nematodes: Both sides of the coin. Appl. Microbiol. Biotechnol. 2018, 102, 3939–3949. [Google Scholar] [CrossRef]
- Wang, B.L.; Chen, Y.H.; He, J.N.; Xue, H.X.; Yan, N.; Zeng, Z.J.; Bennett, J.W.; Zhang, K.Q.; Niu, X.M. Integrated Metabolomics and Morphogenesis Reveal Volatile Signaling of the Nematode-Trapping Fungus Arthrobotrys oligospora. Appl. Environ. Microbiol. 2018, 84, e02749-17. [Google Scholar] [CrossRef]
- Li, J.; Zou, C.; Xu, J.; Ji, X.; Niu, X.; Yang, J.; Huang, X.; Zhang, K.Q. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Ann. Rev. Phytopathol. 2015, 53, 67–95. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, Y.; Xiang, M.; Kang, S.; Wang, S.; Liu, X. DdaCrz1, a C2H2-Type Transcription Factor, Regulates Growth, Conidiation, and Stress Resistance in the Nematode-Trapping Fungus Drechslerella dactyloides. J. Fungi 2022, 8, 750. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Ma, Y.; Zhang, K.; Yang, J. The Arf-GAP Proteins AoGcs1 and AoGts1 Regulate Mycelial Development, Endocytosis, and Pathogenicity in Arthrobotrys oligospora. J. Fungi 2022, 8, 463. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Chen, S.-A.; Hsueh, Y.-P. The High Osmolarity Glycerol (HOG) Pathway Functions in Osmosensing, Trap Morphogenesis and Conidiation of the Nematode-Trapping Fungus Arthrobotrys oligospora. J. Fungi 2020, 6, 191. [Google Scholar] [CrossRef]
- Xu, H.; Andi, B.; Qian, J.; West, A.H.; Cook, P.F. The alpha-aminoadipate pathway for lysine biosynthesis in fungi. Cell Biochem. Biophys. 2006, 46, 43–64. [Google Scholar] [CrossRef]
- An, K.D.; Nishida, H.; Miura, Y.; Yokota, A. Aminoadipate reductase gene: A new fungal-specific gene for comparative evolutionary analyses. BMC Evol. Biol. 2002, 2, 6. [Google Scholar] [CrossRef]
- Schöbel, F.; Jacobsen, I.D.; Brock, M. Evaluation of lysine biosynthesis as an antifungal drug target: Biochemical characterization of Aspergillus fumigatus homocitrate synthase and virulence studies. Eukaryot. Cell 2010, 9, 878–893. [Google Scholar] [CrossRef]
- Baazeem, A.; Alorabi, M.; Manikandan, P.; Alotaibi, S.S.; Almanea, A.; Abdel-Hadi, A.; Vijayaraghavan, P.; Raj, S.R.F.; Kim, Y.O.; Kim, H.-J. Paecilomyces formosus MD12, a Biocontrol Agent to Treat Meloidogyne incognita on Brinjal in Green House. J. Fungi 2021, 7, 632. [Google Scholar] [CrossRef]
- Affenzeller, K.; Jaklitsch, W.M.; Hönlinger, C.; Kubicek, C.P. Lysine biosynthesis in Penicillium chrysogenum is regulated by feedback inhibition of alpha-aminoadipate reductase. FEMS Microbiol. Lett. 1989, 49, 293–297. [Google Scholar] [CrossRef]
- Niu, X.M.; Zhang, K.Q. Arthrobotrys oligospora: A model organism for understanding the interaction between fungi and nematodes. Mycology 2011, 2, 59–78. [Google Scholar] [CrossRef]
- Lin, H.C.; Hsueh, Y.P. Laboratory Maintenance and Culturing of the Nematode-Trapping Fungus Arthrobotrys oligospora. Curr. Protoc. 2021, 1, e41. [Google Scholar] [CrossRef]
- Peng, H.; Dong, X.; Lu, H.; Kong, X.; Zha, X.; Wang, Y. A putative F-box-domain-encoding gene AOL_s00076g207 regulates the development and pathogenicity of Arthrobotrys oligospora. J. Basic Microbiol. 2022, 62, 74–81. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Dong, X.; Zhang, G.; Lu, H.; Tang, K.; Zhang, L.; Kong, X.; Sheng, K.; Wang, J.; Zha, X.; et al. The fucose-specific lectin gene AOL_s00054g276 affects trap formation and nematocidal activity of the nematophagous fungus Arthrobotrys oligospora. FEMS Microbiol. Lett. 2022, 369, fnac013. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chen, H.; Tang, X.; Yang, Q.; Zhang, H.; Chen, Y.Q.; Chen, W. Evaluation of metabolome sample preparation and extraction methodologies for oleaginous filamentous fungi Mortierella alpina. Metabolomics 2019, 15, 50. [Google Scholar] [CrossRef]
- Lu, H.; Chen, H.; Tang, X.; Yang, Q.; Zhang, H.; Chen, Y.Q.; Chen, W. Ultra Performance Liquid Chromatography-Q Exactive Orbitrap/Mass Spectrometry-Based Lipidomics Reveals the Influence of Nitrogen Sources on Lipid Biosynthesis of Mortierella alpina. J. Agric. Food Chem. 2019, 67, 10984–10993. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, J.; Raftery, D. Five Easy Metrics of Data Quality for LC-MS-Based Global Metabolomics. Anal. Chem. 2020, 92, 12925–12933. [Google Scholar] [CrossRef]
- Luesch, H.; Hoffmann, D.; Hevel, J.M.; Becker, J.E.; Golakoti, T.; Moore, R.E. Biosynthesis of 4-methylproline in cyanobacteria: Cloning of nosE and nosF genes and biochemical characterization of the encoded dehydrogenase and reductase activities. J. Org. Chem. 2003, 68, 83–91. [Google Scholar] [CrossRef]
- Suvarna, K.; Seah, L.; Bhattacherjee, V.; Bhattacharjee, J.K. Molecular analysis of the LYS2 gene of Candida albicans: Homology to peptide antibiotic synthetases and the regulation of the alpha-aminoadipate reductase. Curr. Genet. 1998, 33, 268–275. [Google Scholar] [CrossRef]
- Bhattacherjee, V.; Bhattacharjee, J.K. Nucleotide sequence of the Schizosaccharomyces pombe lys1+ gene and similarities of the lys1+ protein to peptide antibiotic synthetases. Yeast 1998, 14, 479–484. [Google Scholar] [CrossRef]
- Casqueiro, J.; Gutiérrez, S.; Bañuelos, O.; Fierro, F.; Velasco, J.; Martín, J.F. Characterization of the lys2 gene of Penicillium chrysogenum encoding alpha-aminoadipic acid reductase. Mol. Gen. Genet. 1998, 259, 549–556. [Google Scholar] [CrossRef]
- Kalb, D.; Lackner, G.; Hoffmeister, D. Fungal peptide synthetases: An update on functions and specificity signatures—ScienceDirect. Fungal Biol. Rev. 2013, 27, 43–50. [Google Scholar] [CrossRef]
- Zabriskie, T.M.; Jackson, M.D. Lysine biosynthesis and metabolism in fungi. Nat. Prod. Rep. 2000, 17, 85–97. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Xie, M.; Bai, N.; Yang, J.; Jiang, K.; Zhang, K.Q.; Yang, J. Pleiotropic roles of Ras GTPases in the nematode-trapping fungus Arthrobotrys oligospora identified through multi-omics analyses. iScience 2021, 24, 102820. [Google Scholar] [CrossRef]
- Chen, Y.H.; Liu, X.; Dai, R.; Ou, X.; Xu, Z.F.; Zhang, K.Q.; Niu, X.M. Novel Polyketide-Terpenoid Hybrid Metabolites and Increased Fungal Nematocidal Ability by Disruption of Genes 277 and 279 in Nematode-Trapping Fungus Arthrobotrys oligospora. J. Agric. Food Chem. 2020, 68, 7870–7879. [Google Scholar] [CrossRef]
- Teng, L.L.; Song, T.Y.; Chen, Y.H.; Chen, Y.G.; Zhang, K.Q.; Li, S.H.; Niu, X.M. Novel Polyketide-Terpenoid Hybrid Metabolites from a Potent Nematicidal Arthrobotrys oligospora Mutant ΔAOL_s00215g278. J. Agric. Food Chem. 2020, 68, 11449–11458. [Google Scholar] [CrossRef]
- Wei, L.X.; Zhang, H.X.; Tan, J.L.; Chu, Y.S.; Li, N.; Xue, H.X.; Wang, Y.L.; Niu, X.M.; Zhang, Y.; Zhang, K.Q. Arthrobotrisins A-C, oligosporons from the nematode-trapping fungus Arthrobotrys oligospora. J. Nat. Prod. 2011, 74, 1526–1530. [Google Scholar] [CrossRef]
- He, Z.Q.; Wang, L.J.; Wang, Y.J.; Chen, Y.H.; Wen, Y.; Zhang, K.Q.; Niu, X.M. Polyketide Synthase-Terpenoid Synthase Hybrid Pathway Regulation of Trap Formation through Ammonia Metabolism Controls Soil Colonization of Predominant Nematode-Trapping Fungus. J. Agric. Food Chem. 2021, 69, 4464–4479. [Google Scholar] [CrossRef]
- Zhang, H.X.; Tan, J.L.; Wei, L.X.; Wang, Y.L.; Zhang, C.P.; Wu, D.K.; Zhu, C.Y.; Zhang, Y.; Zhang, K.Q.; Niu, X.M. Morphology regulatory metabolites from Arthrobotrys oligospora. J. Nat. Prod. 2012, 75, 1419–1423. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Q.-F.; Li, S.-H.; Yan, J.-X.; Wu, L.; Cheng, Q.-Y.; He, Z.-Q.; Yue, X.-T.; Zhang, K.-Q.; Zhang, L.-L.; et al. The Multifaceted Gene 275 Embedded in the PKS-PTS Gene Cluster Was Involved in the Regulation of Arthrobotrisin Biosynthesis, TCA Cycle, and Septa Formation in Nematode-Trapping Fungus Arthrobotrys oligospora. J. Fungi 2022, 8, 1261. [Google Scholar] [CrossRef]
- Jiang, K.-X.; Liu, Q.-Q.; Bai, N.; Zhu, M.-C.; Zhang, K.-Q.; Yang, J.-K. AoSsk1, a Response Regulator Required for Mycelial Growth and Development, Stress Responses, Trap Formation, and the Secondary Metabolism in Arthrobotrys oligospora. J. Fungi 2022, 8, 260. [Google Scholar] [CrossRef]
- Kuo, T.H.; Yang, C.T.; Chang, H.Y.; Hsueh, Y.P.; Hsu, C.C. Nematode-Trapping Fungi Produce Diverse Metabolites during Predator-Prey Interaction. Metabolites 2020, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Bordallo, J.J.; Lopez-Llorca, L.V.; Jansson, H.B.; Salinas, J.; Persmark, L.; Asensio, L. Colonization of plant roots by egg-parasitic and nematode-trapping fungi. New Phytol. 2002, 154, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Matheson, V.G.; Munakata-Marr, J.; Hopkins, G.D.; McCarty, P.L.; Tiedje, J.M.; Forney, L.J. A novel means to develop strain-specific DNA probes for detecting bacteria in the environment. Appl. Environ. Microbiol. 1997, 63, 2863–2869. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Deng, Y.; Zhou, J. Development of functional gene microarrays for microbial community analysis. Curr. Opin. Biotechnol. 2012, 23, 49–55. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Wang, S.; Gu, T.; Sun, L.; Wang, Y. Roles of the Fungal-Specific Lysine Biosynthetic Pathway in the Nematode-Trapping Fungus Arthrobotrys oligospora Identified through Metabolomics Analyses. J. Fungi 2023, 9, 206. https://doi.org/10.3390/jof9020206
Lu H, Wang S, Gu T, Sun L, Wang Y. Roles of the Fungal-Specific Lysine Biosynthetic Pathway in the Nematode-Trapping Fungus Arthrobotrys oligospora Identified through Metabolomics Analyses. Journal of Fungi. 2023; 9(2):206. https://doi.org/10.3390/jof9020206
Chicago/Turabian StyleLu, Hengqian, Shuai Wang, Tiantian Gu, Liangyin Sun, and Yongzhong Wang. 2023. "Roles of the Fungal-Specific Lysine Biosynthetic Pathway in the Nematode-Trapping Fungus Arthrobotrys oligospora Identified through Metabolomics Analyses" Journal of Fungi 9, no. 2: 206. https://doi.org/10.3390/jof9020206
APA StyleLu, H., Wang, S., Gu, T., Sun, L., & Wang, Y. (2023). Roles of the Fungal-Specific Lysine Biosynthetic Pathway in the Nematode-Trapping Fungus Arthrobotrys oligospora Identified through Metabolomics Analyses. Journal of Fungi, 9(2), 206. https://doi.org/10.3390/jof9020206






