The Effect of Trichoderma harzianum Hypovirus 1 (ThHV1) and Its Defective RNA ThHV1-S on the Antifungal Activity and Metabolome of Trichoderma koningiopsis T-51
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
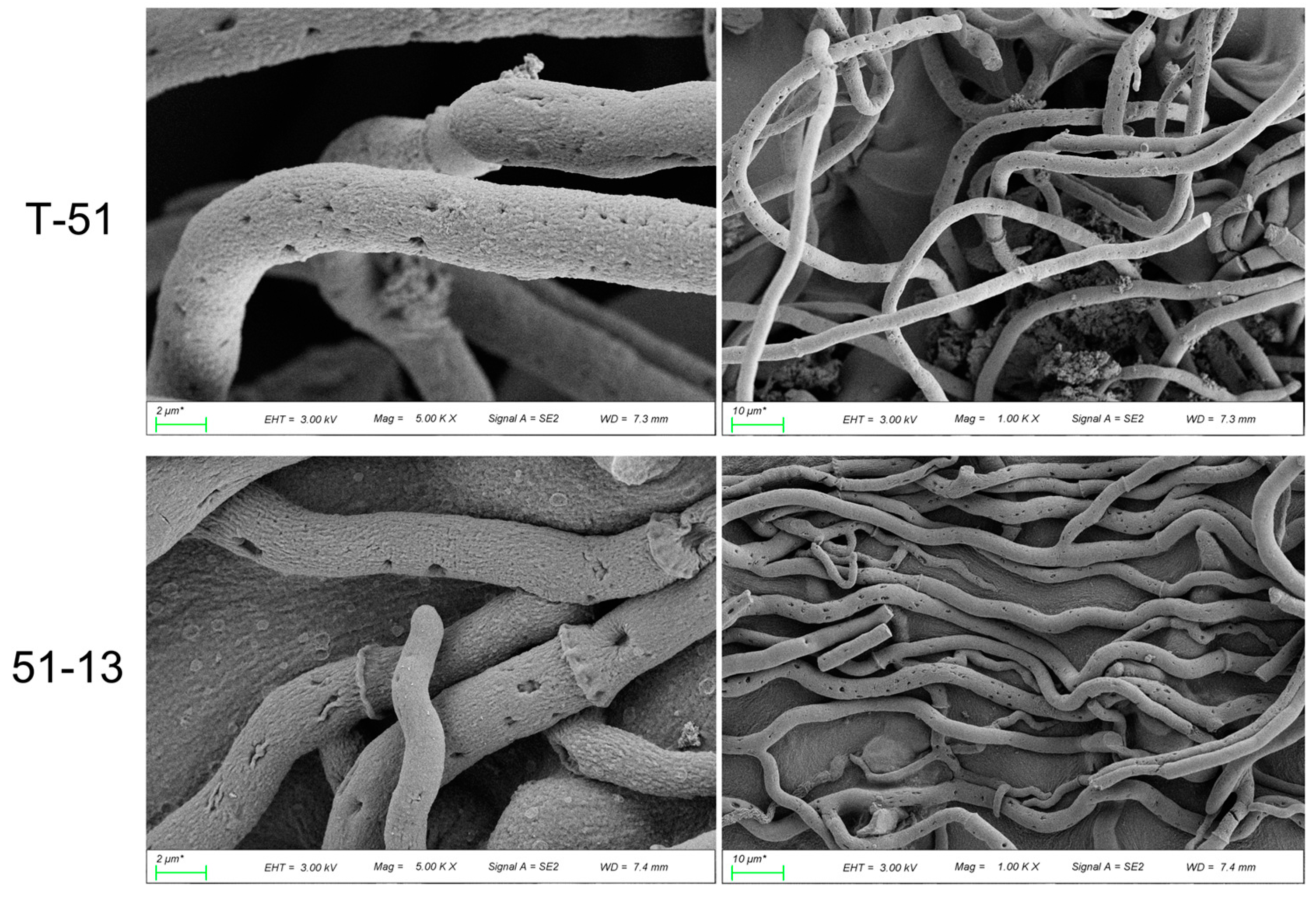
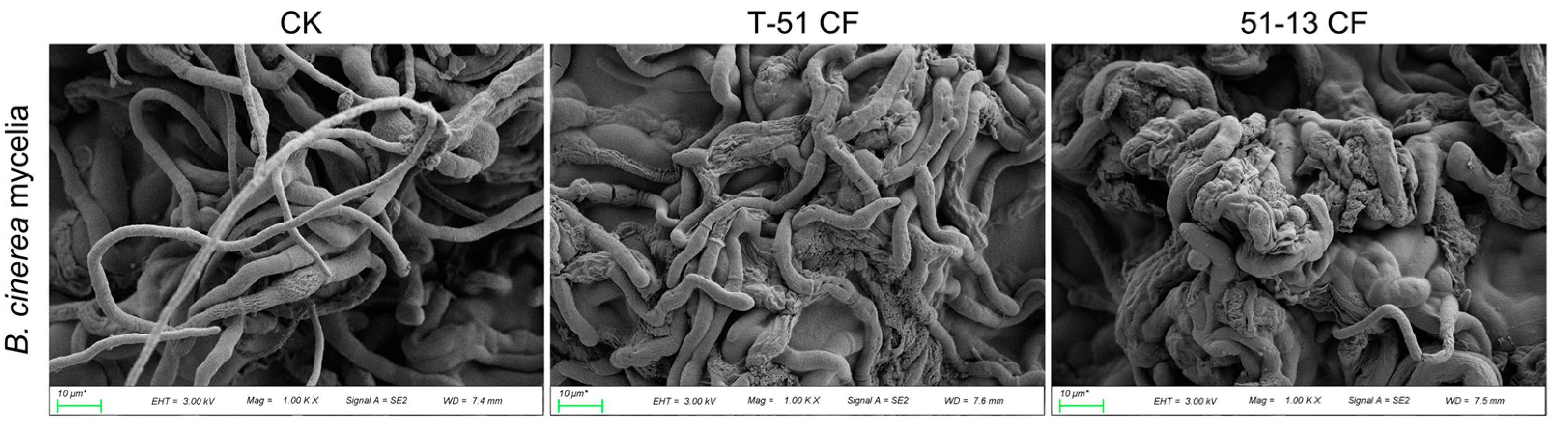
2.2. Scanning Electron Microscopic Observation of Fungal Morphology
2.3. Genomic DNA Sequencing
2.4. Antifungal Activity of CF of Trichoderma Isolates
2.5. Antifungal Activity of the VOCs Produced by T-51 and 51-13
2.6. Transcription Profiling of T-51 and 51-13
2.7. Analysis of Secondary Metabolites in T-51 and 51-13
2.8. Screening of Candidates Producing Antifungal Metabolites
2.9. Real-Time Quantitative PCR (qRT-PCR)
3. Results
3.1. Morphology and Genome of T-51 and 51-13
3.2. Antifungal Activity of the CF of T-51 and 51-13
3.3. The Antifungal Activity of the VOCs of T-51 and 51-13
3.4. Comparison of Transcriptome of T-51 and 51-13
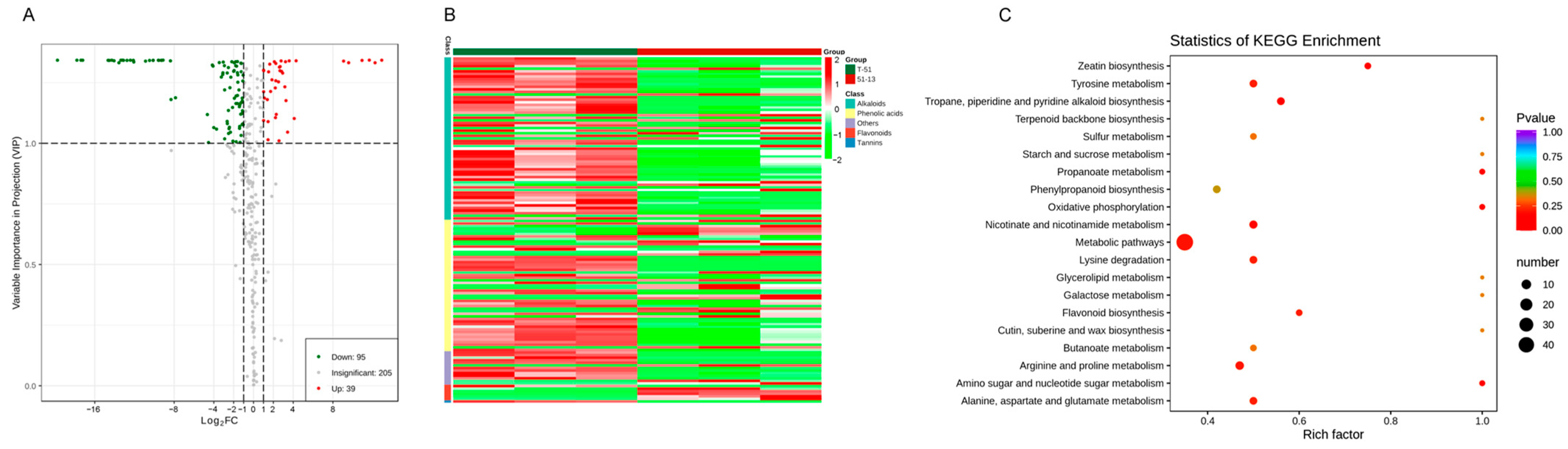
3.5. Metabolic Differences between T-51 and 51-13
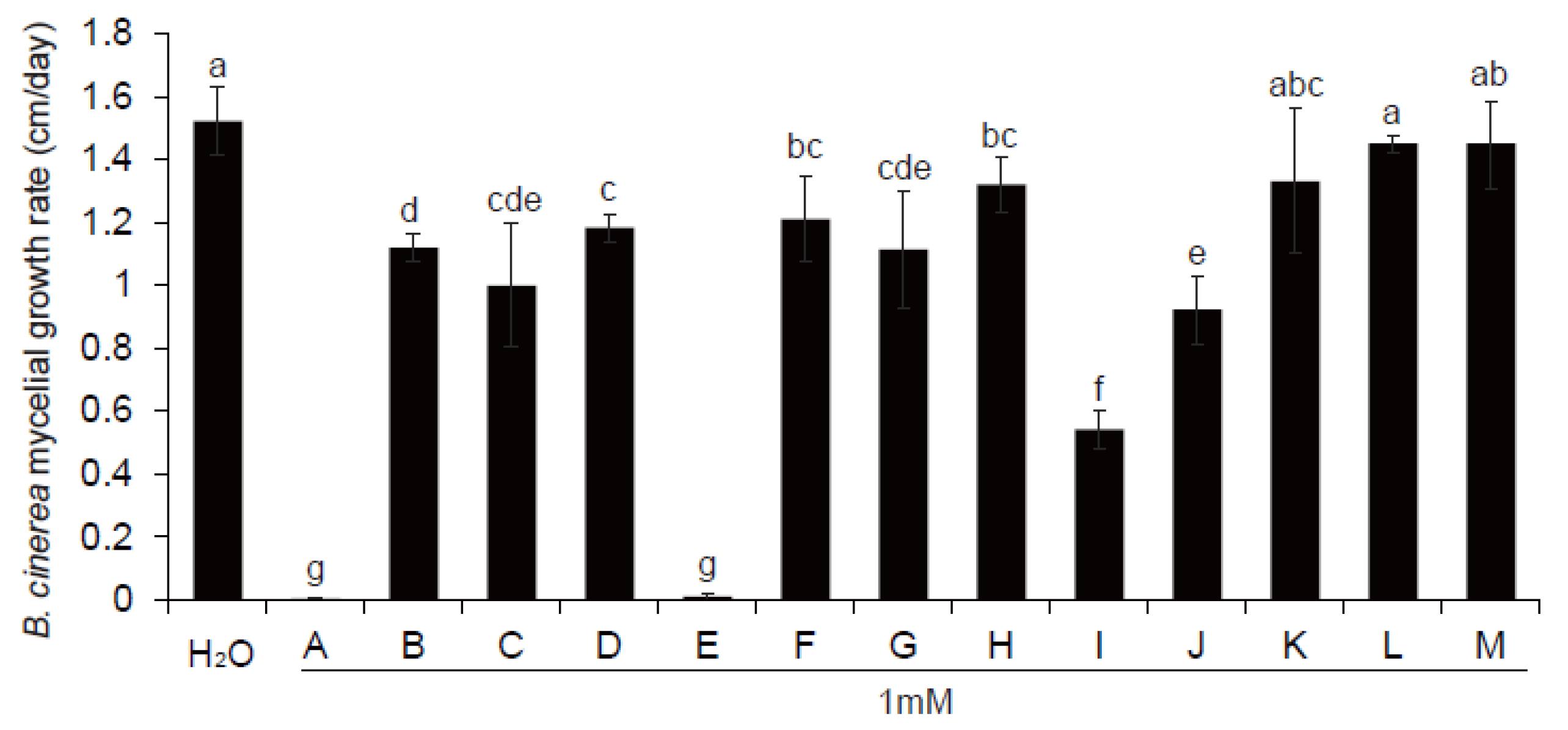
3.6. Antifungal Metabolite Candidates Affected by ThHV1 and ThHV1-S
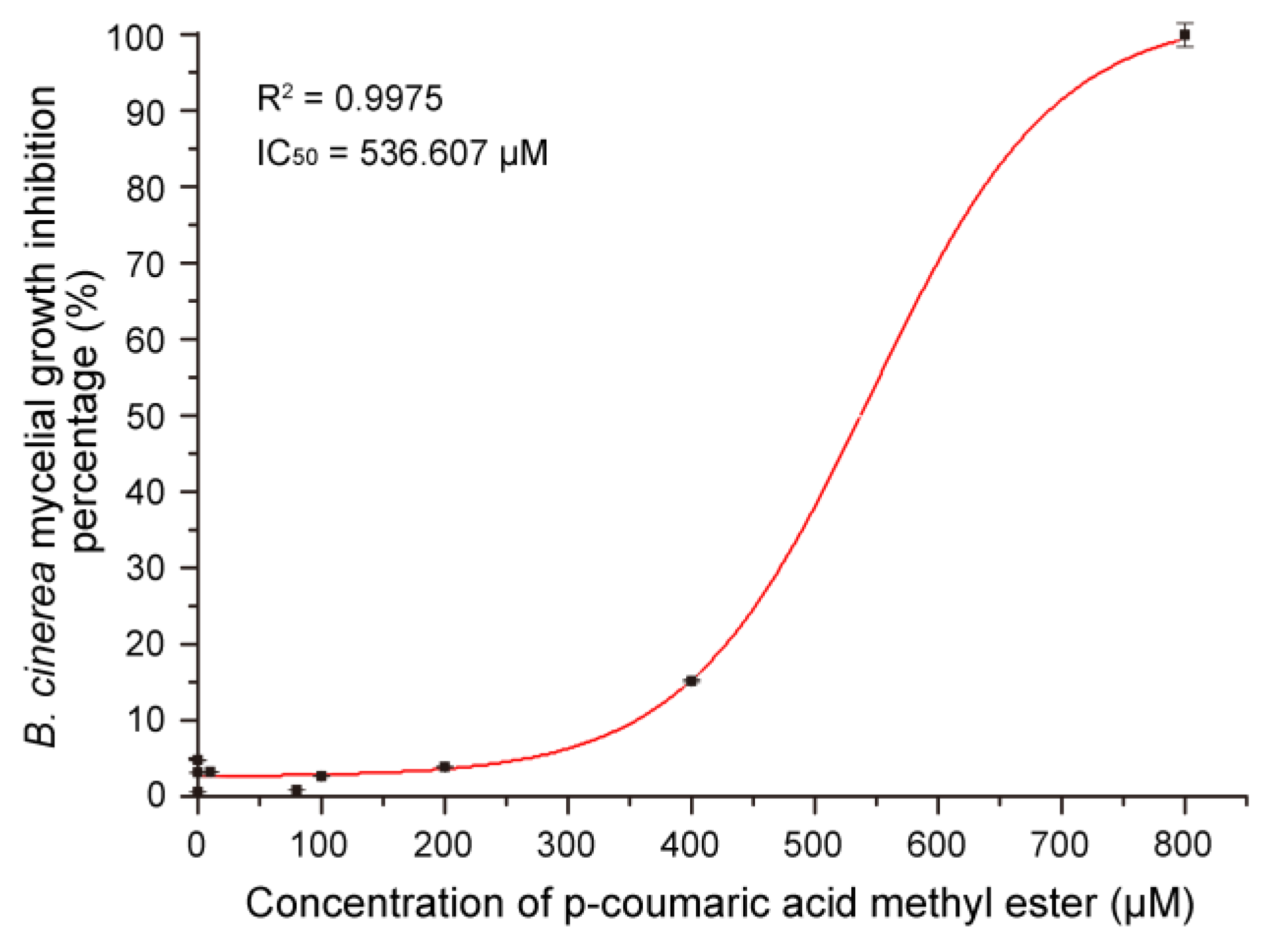
3.7. Antifungal Activity of MeCA In Vitro
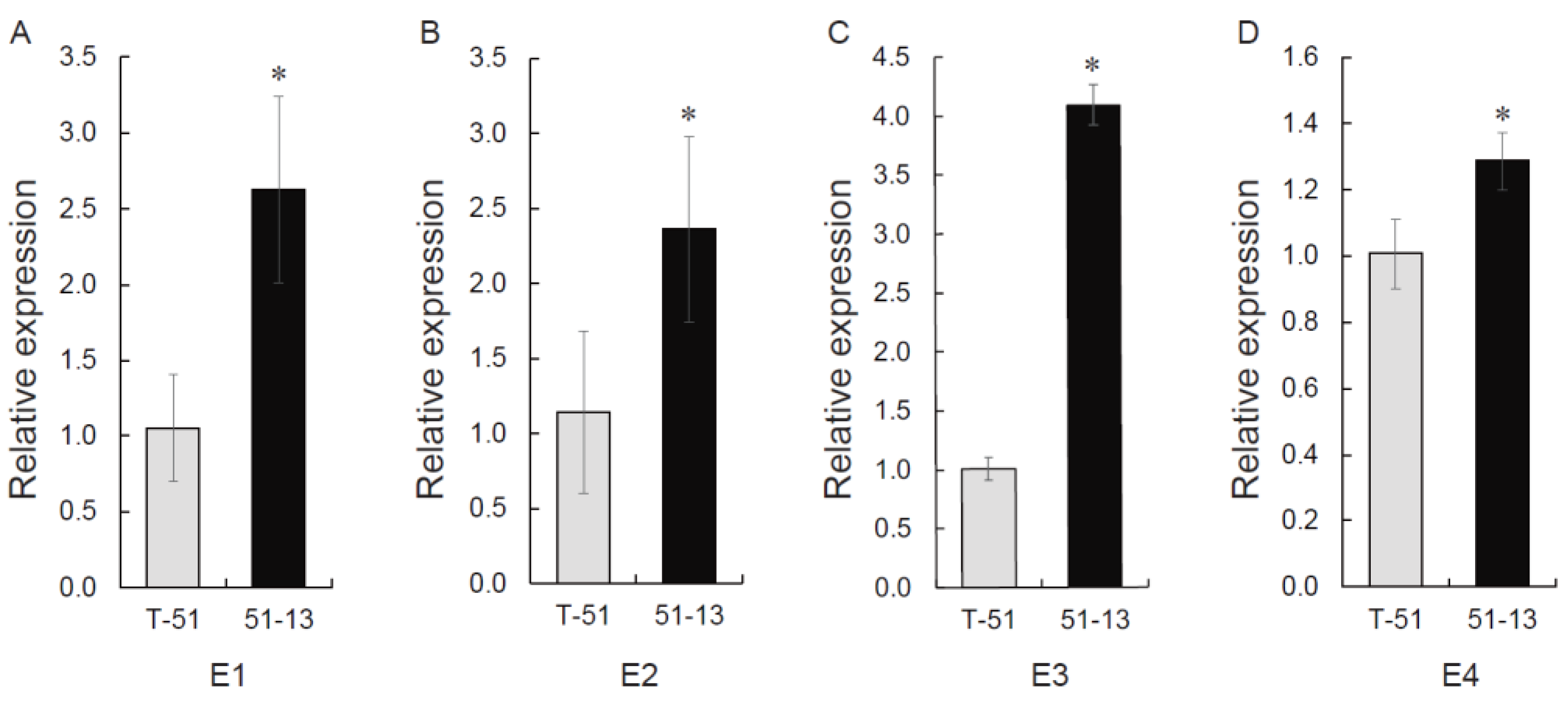
3.8. MeCA Related Gene Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghabrial, S.A.; Suzuki, N. Viruses of plant pathogenic fungi. Annu. Rev. Phytopathol. 2009, 47, 353–384. [Google Scholar] [CrossRef]
- Kotta-Loizou, I. Mycoviruses and their role in fungal pathogenesis. Curr. Opin. Microbiol. 2021, 63, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Dawe, A.L.; Nuss, D.L. Hypoviruses and chestnut blight: Exploiting viruses to understand and modulate fungal pathogenesis. Genetics 2001, 35, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, J.; Cheng, J.; Li, B.; Chen, T.; Fu, Y.; Li, G.; Wang, M.; Jin, H.; Wan, H.; et al. Fungal DNA virus infects a mycophagous insect and utilizes it as a transmission vector. Proc. Natl. Acad. Sci. USA 2016, 113, 12803–12808. [Google Scholar] [CrossRef]
- Hao, F.; Ding, T.; Wu, M.; Zhang, J.; Yang, L.; Chen, W.; Li, G. Two Novel hypovirulence-associated mycoviruses in the phytopathogenic fungus Botrytis cinerea: Molecular characterization and suppression of infection cushion formation. Viruses 2018, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzzaman, M.; Wang, Z.; Wu, M.; Yang, L.; Han, Y.; Li, G.; Zhang, J. Promotion of tomato growth by the volatiles produced by the hypovirulent strain QT5-19 of the plant gray mold fungus Botrytis cinerea. Microbiol. Res. 2021, 247, 126731. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzzaman, M.; Lyu, A.; Zhang, J.; Wu, M.; Yang, L.; Chen, W.; Li, G. Competitive saprophytic ability of the hypovirulent isolate QT5-19 of Botrytis cinerea and its importance in biocontrol of necrotrophic fungal pathogens. Biological. Control 2020, 142, 104182. [Google Scholar] [CrossRef]
- Bevan, E.A.; Herring, A.J.; Mitchell, D.J. Preliminary characterization of two species of dsRNA in yeast and their relationship to the “killer” character. Nature 1973, 245, 81–86. [Google Scholar] [CrossRef]
- Icho, T.; Wickner, R.B. The double-stranded RNA genome of yeast virus L-A encodes its own putative RNA polymerase by fusing two open reading frames. J. Biol. Chem. 1989, 264, 6716–6723. [Google Scholar] [CrossRef]
- Rodríguez-Cousiño, N.; Maqueda, M.; Ambrona, J.; Zamora, E.; Esteban, R.; Ramírez, M. A new wine Saccharomyces cerevisiae killer toxin (Klus), encoded by a double-stranded rna virus, with broad antifungal activity is evolutionarily related to a chromosomal host gene. Appl. Environ. Microbiol. 2011, 77, 1822–1832. [Google Scholar] [CrossRef]
- Rodríguez-Cousiño, N.; Gómez, P.; Esteban, R. Variation and distribution of L-A helper totiviruses in Saccharomyces sensustricto yeasts producing different killer toxins. Toxins 2017, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Ichinose, S.; Takeshita, K.; Urayama, S.-i.; Fukuhara, T.; Komatsu, K.; Arie, T.; Ishihara, A.; Egusa, M.; Kodama, M.; et al. Molecular characterization of a novel mycovirus in Alternaria alternata manifesting two-sided effects: Down-regulation of host growth and up-regulation of host plant pathogenicity. Virology 2018, 519, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Nerva, L.; Chitarra, W.; Siciliano, I.; Gaiotti, F.; Ciuffo, M.; Forgia, M.; Varese, G.C.; Turina, M. Mycoviruses mediate mycotoxin regulation in Aspergillus ochraceus. Environ. Microbiol. 2019, 21, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Li, J.; Chen, K.; Yang, H.; Huang, Y.; Zhang, G. Research progress in antifungal metabolites of Trichoderma spp. Pesticides 2010, 10, 713–716. (In Chinese) [Google Scholar]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in Trichoderma—Chemistry meets genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- You, J.; Zhang, J.; Wu, M.; Yang, L.; Chen, W.; Li, G. Multiple criteria-based screening of Trichoderma isolates for biological control of Botrytis cinerea on tomato. Biological. Control 2016, 101, 31–38. [Google Scholar] [CrossRef]
- You, J.; Li, G.; Li, C.; Zhu, L.; Yang, H.; Song, R.; Gu, W. Biological control and plant growth promotion by volatile organic compounds of Trichoderma koningiopsis T-51. J. Fungi 2022, 8, 131. [Google Scholar] [CrossRef]
- You, J.; Zhou, K.; Liu, X.; Wu, M.; Yang, L.; Zhang, J.; Chen, W.; Li, G. Defective RNA of a Novel mycovirus with high transmissibility detrimental to biocontrol properties of Trichoderma spp. Microorganisms 2019, 7, 507. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, L.; Zhang, J.; Wu, M.; Chen, W.; Jiang, D.; Li, G. Production of anti-fungal volatiles by non-pathogenic Fusarium oxysporum and its efficacy in suppression of Verticillium wilt of cotton. Plant Soil 2015, 392, 101–114. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, Y.; He, G.; Cao, Y.; Bi, M.; Song, M.; Yang, P.; Xu, L.; Ming, J. Comprehensive analysis of secondary metabolites in the extracts from different lily bulbs and their antioxidant ability. Antioxidants 2021, 10, 1634. [Google Scholar] [CrossRef] [PubMed]
- Koltin, Y.; Kandel, J. Killer phenomenon in Ustilago maydis: The organization of the viral genome. Genetics 1978, 88, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Lebuhn, M.; Heulin, T.; Hartmann, A. Production of auxin and other indolic and phenolic compounds by Paenibacillus polymyxa strains isolated from different proximity to plant roots. FEMS Microbiol. Ecol. 1997, 22, 325–334. [Google Scholar] [CrossRef]
- Meng, D.; Sommella, E.; Salviati, E.; Campiglia, P.; Ganguli, K.; Djebali, K.; Zhu, W.; Walker, W.A. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr. Res. 2020, 88, 209–217. [Google Scholar] [CrossRef]
- Sprunck, S.; Jacobsen, H.; Reinard, T. Indole-3-lactic acid is a weak auxin analogue but not an anti-auxin. J. Plant Growth Regul. 1995, 14, 191. [Google Scholar] [CrossRef]
- Daayf, F.; Bel-Rhlid, R.; Bélanger, R.R. Methyl ester of p-coumaric acid: A phytoalexin-like compound from long english cucumber leaves. J. Chem. Ecol. 1997, 23, 1517–1526. [Google Scholar] [CrossRef]
- Shaik, A.B.; Ahil, S.B.; Govardhanam, R.; Senthi, M.; Khan, R.; Sojitra, R.; Kumar, S.; Srinivas, A. Antifungal effect and protective role of ursolic acid and three phenolic derivatives in the management of sorghum grain mold under field conditions. Chem. Biodivers. 2016, 13, 1158–1164. [Google Scholar] [CrossRef]
- Yuan, S.; Li, W.; Li, Q.; Wang, L.; Cao, J.; Jiang, W. Defense Responses, Induced by p-coumaric acid and methyl p-coumarate, of jujube (Ziziphus jujuba Mill.) fruit against black spot rot caused by Alternaria alternata. J. Agric. Food Chem. 2019, 67, 2801–2810. [Google Scholar] [CrossRef]
- Daayf, F.; Ongena, M.; Boulanger, R.; El Hadrami, I.; Bélanger, R.R. Induction of Phenolic Compounds in two cultivars of cucumber by treatment of healthy and powdery mildew-infected plants with extracts of Reynoutria sachalinensis. J. Chem. Ecol. 2000, 26, 1579–1593. [Google Scholar] [CrossRef]



| Formula | Compounds | CAS | Class I | Log2 FC | Bioassay No. |
|---|---|---|---|---|---|
| C4H6N2O2 | 5,6-Dihydrouracil | 504-07-4 | Alkaloids | 12.92 | F |
| C10H13N5O4 | 2′-Deoxyguanosine | 961-07-9 | Alkaloids | 12.25 | M |
| C16H12O7 | 3-O-Methylquercetin | 1486-70-0 | Flavonoids | 10.94 | - |
| C13H12O8 | Cis-Coutaric acid | 67920-37-0 | Phenolic acids | 9.59 | - |
| C2H7NO3S | 2-Aminoethanesulfonic acid | 107-35-7 | Phenolic acids | 9.08 | K |
| C12H14O5 | 3,4,5-Trimethoxycinnamic acid | 90-50-6 | Phenolic acids | 4.29 | I |
| C5H9NO3S | N-Acetyl-L-Cysteine | 616-91-1 | Alkaloids | 4.10 | C |
| C11H11NO4 | Methyl dioxindole-3-acetate | 57061-18-4 | Others | 3.59 | - |
| C6H5NO2 | 2-Picolinic acid | 98-98-6 | Phenolic acids | 3.43 | L |
| C23H39NO8 | Trichodermoside | 1226878-09-6 | Others | 3.27 | - |
| C10H15NO | Hordenine | 539-15-1 | Alkaloids | 3.22 | D |
| C20H20O5 | 2,4,2′,4′-tetrahydroxy-3′-prenylchalcone | - | Flavonoids | 3.16 | - |
| C7H7NO2 | Salicylamide | 65-45-2 | Alkaloids | 2.94 | J |
| C7H12N2O4 | N-Acetyl-L-Glutamine | 2490-97-3 | Alkaloids | 2.83 | B |
| C16H30O4 | Hexadecanedioic acid | 505-54-4 | Phenolic acids | 2.78 | G |
| C10H10O3 | p-Coumaric acid methyl ester | 3943-97-3 | Phenolic acids | 2.68 | E |
| C11H11NO3 | Indole-3-lactic acid | 1821-52-9 | Phenolic acids | 2.66 | A |
| C17H16O6 | 4′,6-Dihydroxy-5,7-dimethoxyflavanone | 6951-57-1 | Flavonoids | 2.60 | - |
| C9H10O4 | Methyl 2,4-dihydroxyphenylacetate | 67828-42-6 | Phenolic acids | 2.52 | - |
| C12H14N2O | Acetryptine | 3551-18-6 | Alkaloids | 2.29 | - |
| C22H18O9 | Bis(p-Coumaroyl)malic acid | - | Phenolic acids | 2.25 | - |
| C5H10O4 | 2,3-Dihydroxy-3-Methylbutanoic Acid | 1756-18-9 | Phenolic acids | 2.24 | - |
| C21H26O6 | 5-Hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)heptan-3-one | - | Others | 2.21 | - |
| C9H10O4 | (S)-2-Hydroxy-3-(4-Hydroxyphenyl)Propanoic Acid | 23508-35-2 | Phenolic acids | 2.19 | - |
| C15H10O7 | Quercetin | 117-39-5 | Flavonoids | 2.10 | H |
| C15H24N2O17P2 | Uridine 5′-diphospho-D-glucose | 133-89-1 | Alkaloids | 1.86 | - |
| C15H10O6 | Isoscutellarein | 41440-05-5 | Flavonoids | 1.46 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, J.; Hu, Z.; Li, C.; Yang, H.; Zhu, L.; Cao, B.; Song, R.; Gu, W. The Effect of Trichoderma harzianum Hypovirus 1 (ThHV1) and Its Defective RNA ThHV1-S on the Antifungal Activity and Metabolome of Trichoderma koningiopsis T-51. J. Fungi 2023, 9, 175. https://doi.org/10.3390/jof9020175
You J, Hu Z, Li C, Yang H, Zhu L, Cao B, Song R, Gu W. The Effect of Trichoderma harzianum Hypovirus 1 (ThHV1) and Its Defective RNA ThHV1-S on the Antifungal Activity and Metabolome of Trichoderma koningiopsis T-51. Journal of Fungi. 2023; 9(2):175. https://doi.org/10.3390/jof9020175
Chicago/Turabian StyleYou, Jiaqi, Zheng Hu, Chaohan Li, Hongjuan Yang, Lihua Zhu, Biting Cao, Ronghao Song, and Weihong Gu. 2023. "The Effect of Trichoderma harzianum Hypovirus 1 (ThHV1) and Its Defective RNA ThHV1-S on the Antifungal Activity and Metabolome of Trichoderma koningiopsis T-51" Journal of Fungi 9, no. 2: 175. https://doi.org/10.3390/jof9020175
APA StyleYou, J., Hu, Z., Li, C., Yang, H., Zhu, L., Cao, B., Song, R., & Gu, W. (2023). The Effect of Trichoderma harzianum Hypovirus 1 (ThHV1) and Its Defective RNA ThHV1-S on the Antifungal Activity and Metabolome of Trichoderma koningiopsis T-51. Journal of Fungi, 9(2), 175. https://doi.org/10.3390/jof9020175




