Transcriptional Reprogramming of Candida tropicalis in Response to Isoespintanol Treatment
Abstract
1. Introduction
2. Materials and Methods
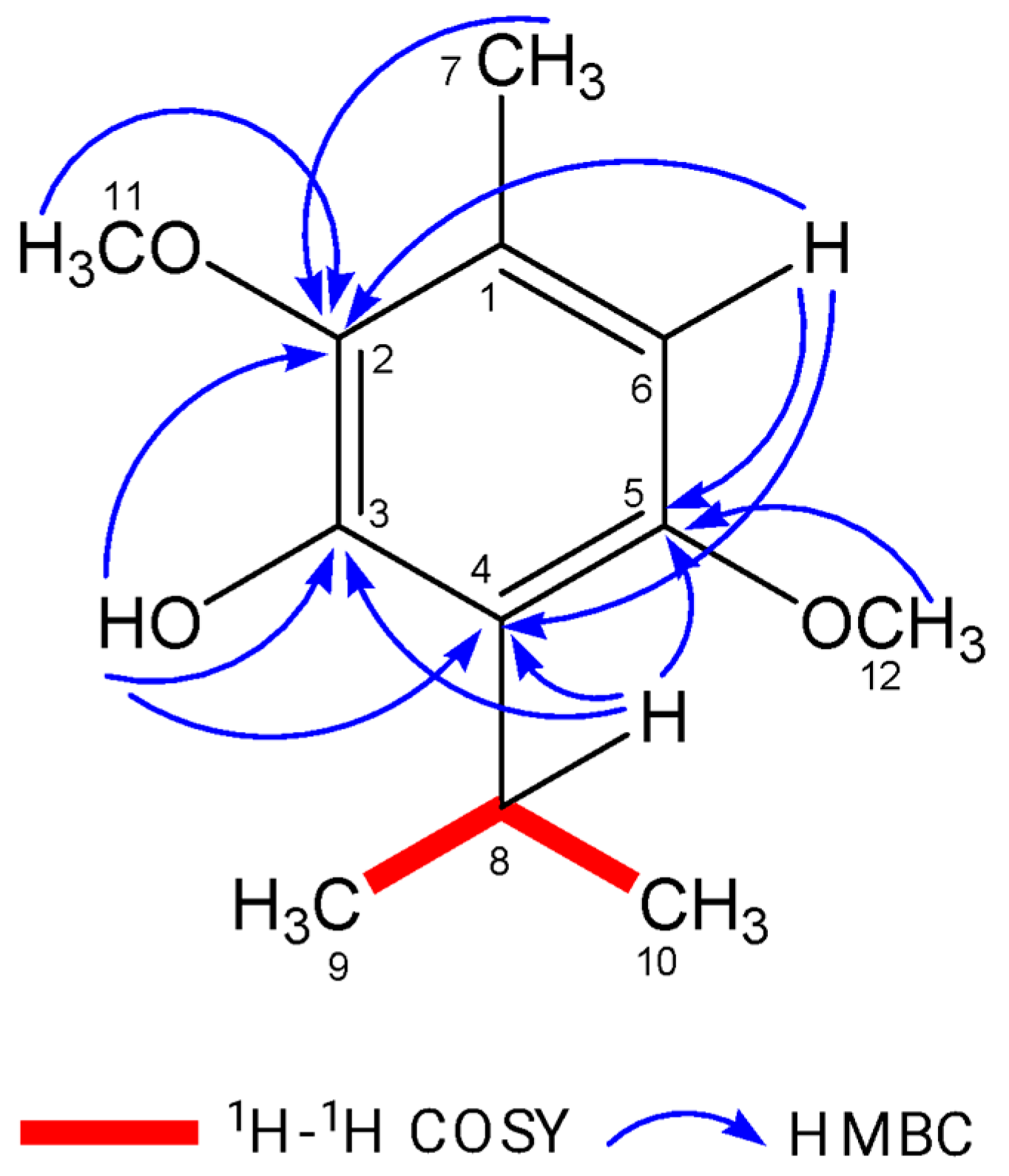
2.1. Isolation and Identification of Isoespintanol
2.2. Yeast Strain and Culture Conditions
2.3. RNA-Sequencing and Read Count Data Acquisition
2.4. Bioinformatics Analyses
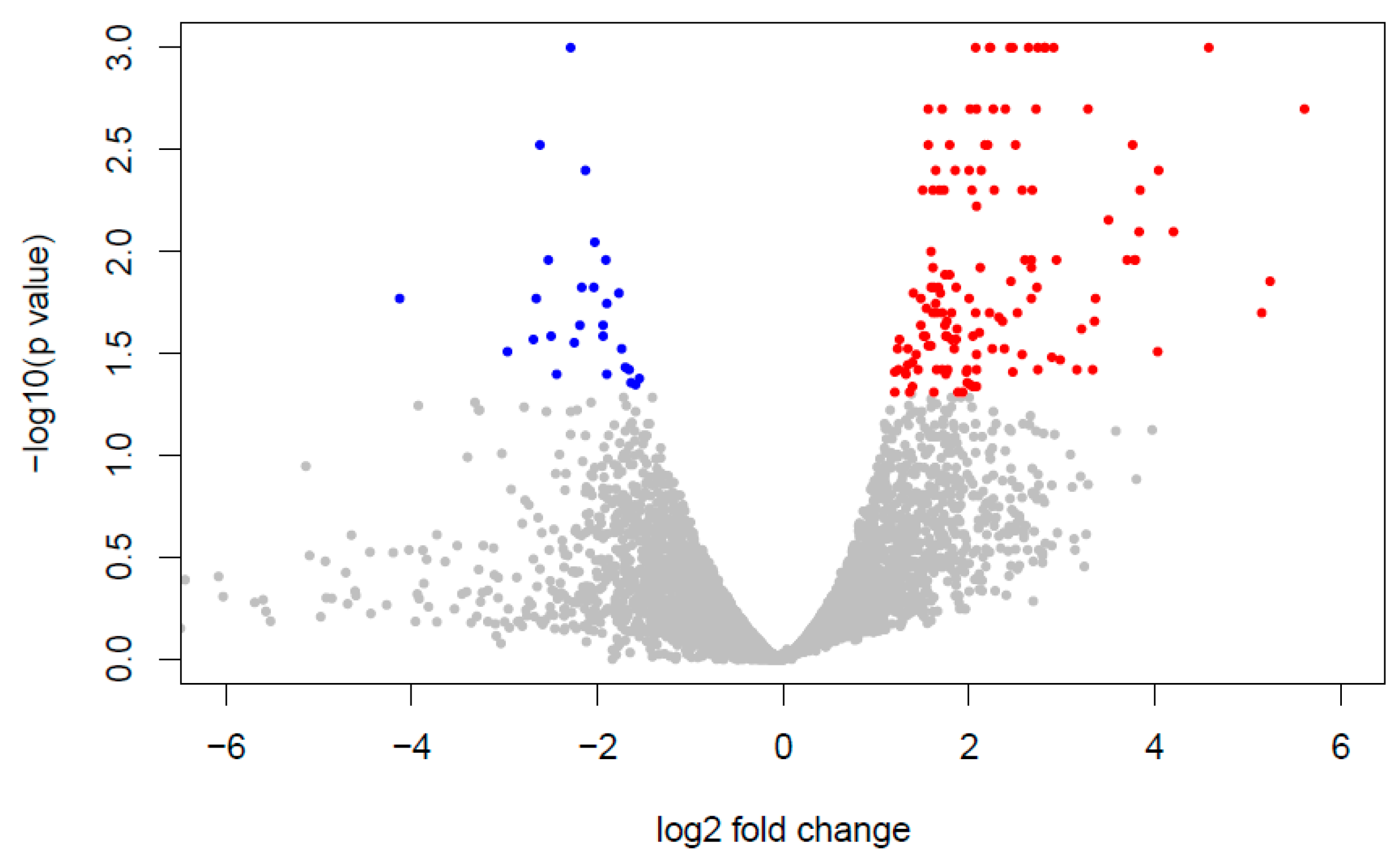
2.4.1. Differential Gene Expression Analysis
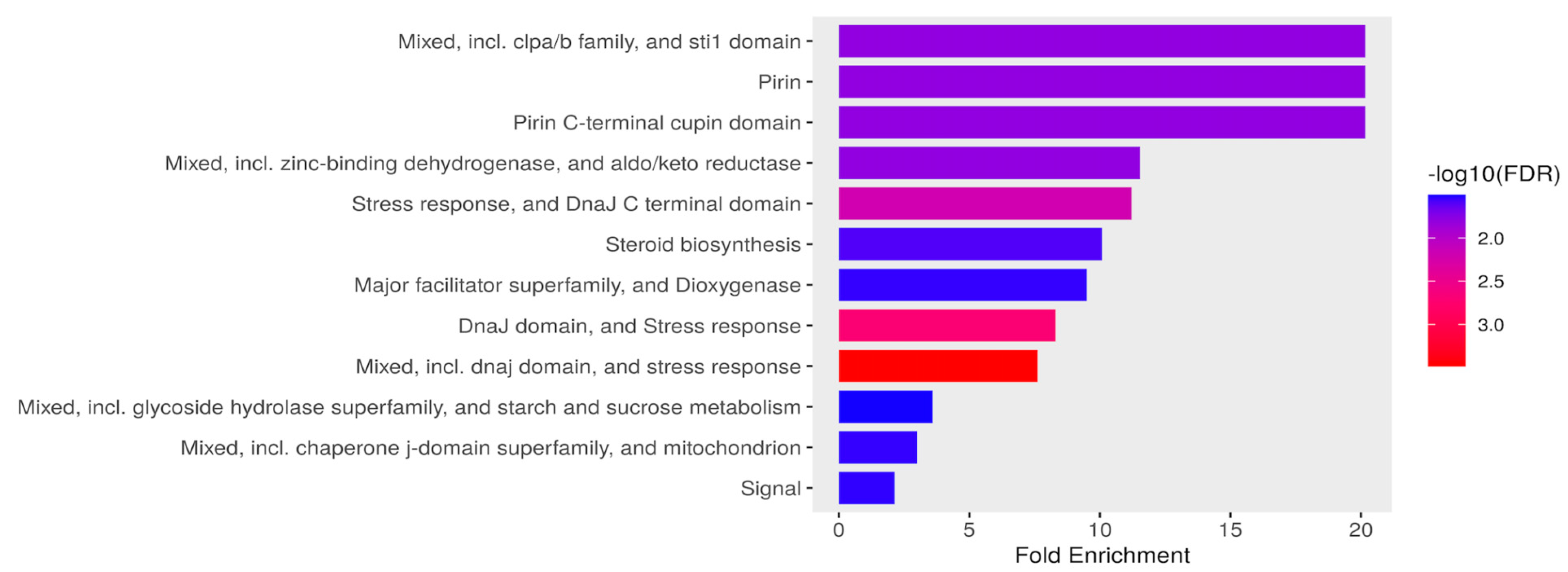
2.4.2. Functional Enrichment Analysis for DEGs
2.5. Computational Prediction of DEGs and Primer Design
2.6. Gene Expression Assessment by Quantitative RT-PCR
2.7. Determination of Total Ergosterol Content
3. Results
3.1. Isolation and Purification of Isoespintanol Molecules
3.2. RNA Preparation, Library Construction, and Sequence Analysis
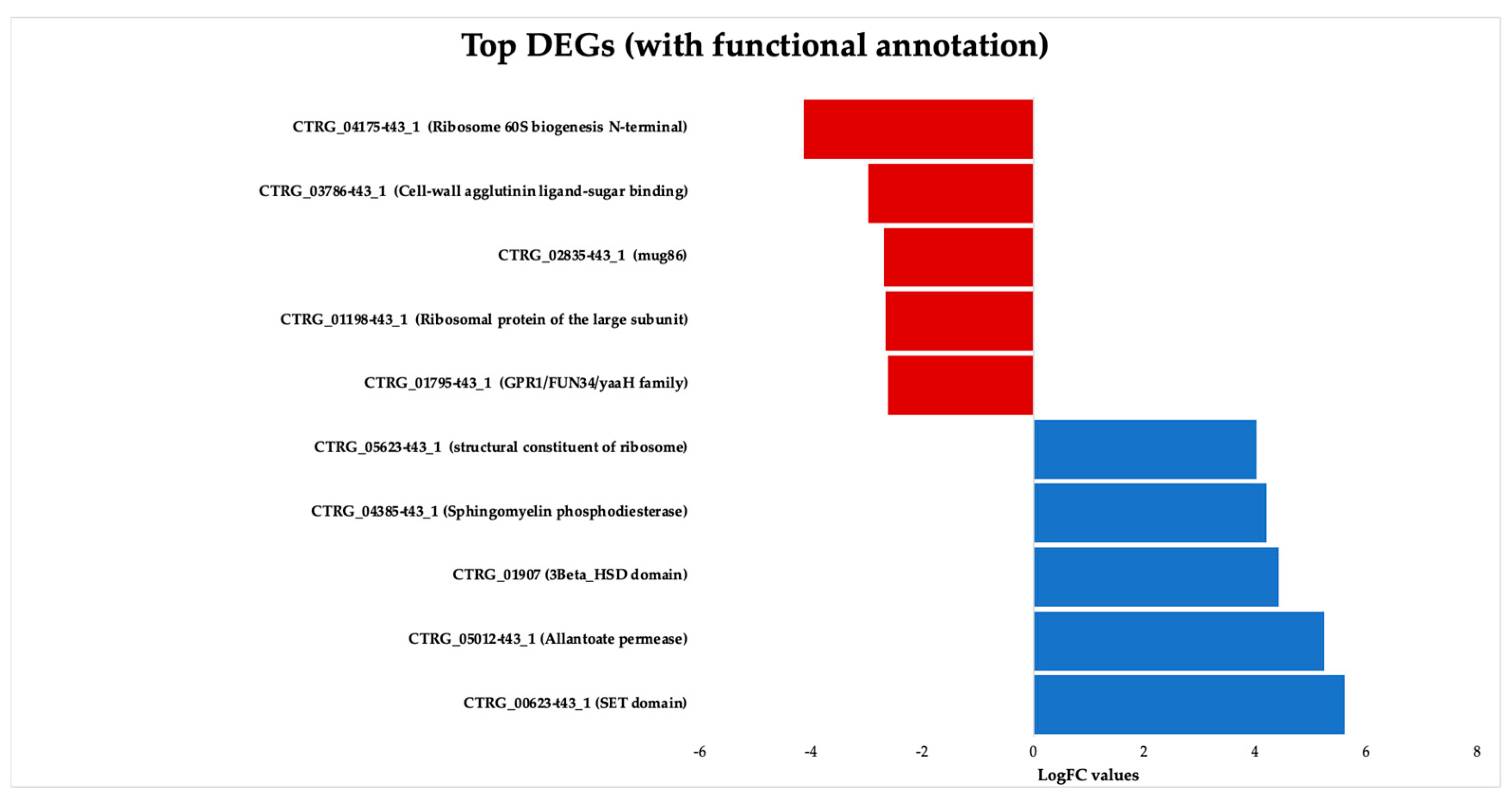
3.3. Analysis of the Transcriptional Profile of Differentially Expressed Genes
3.3.1. Stress Response, Metabolism, and Mitochondrial Functional Processes Are Enriched in Upregulated Genes in C. tropicalis Treated with ISO
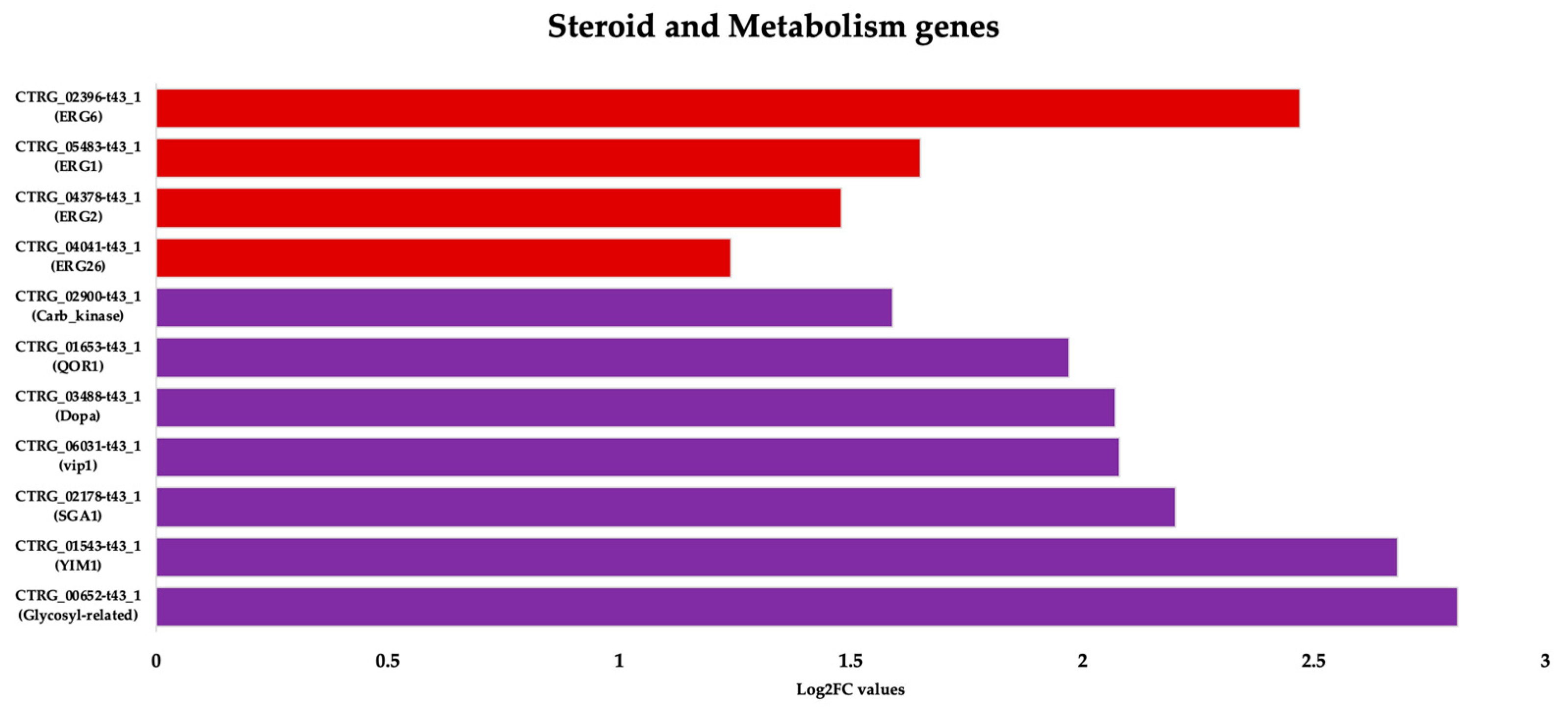
3.3.2. Upregulation of Specific Steroid and Cell Metabolism Genes Is an Effect of C. tropicalis Treated with ISO
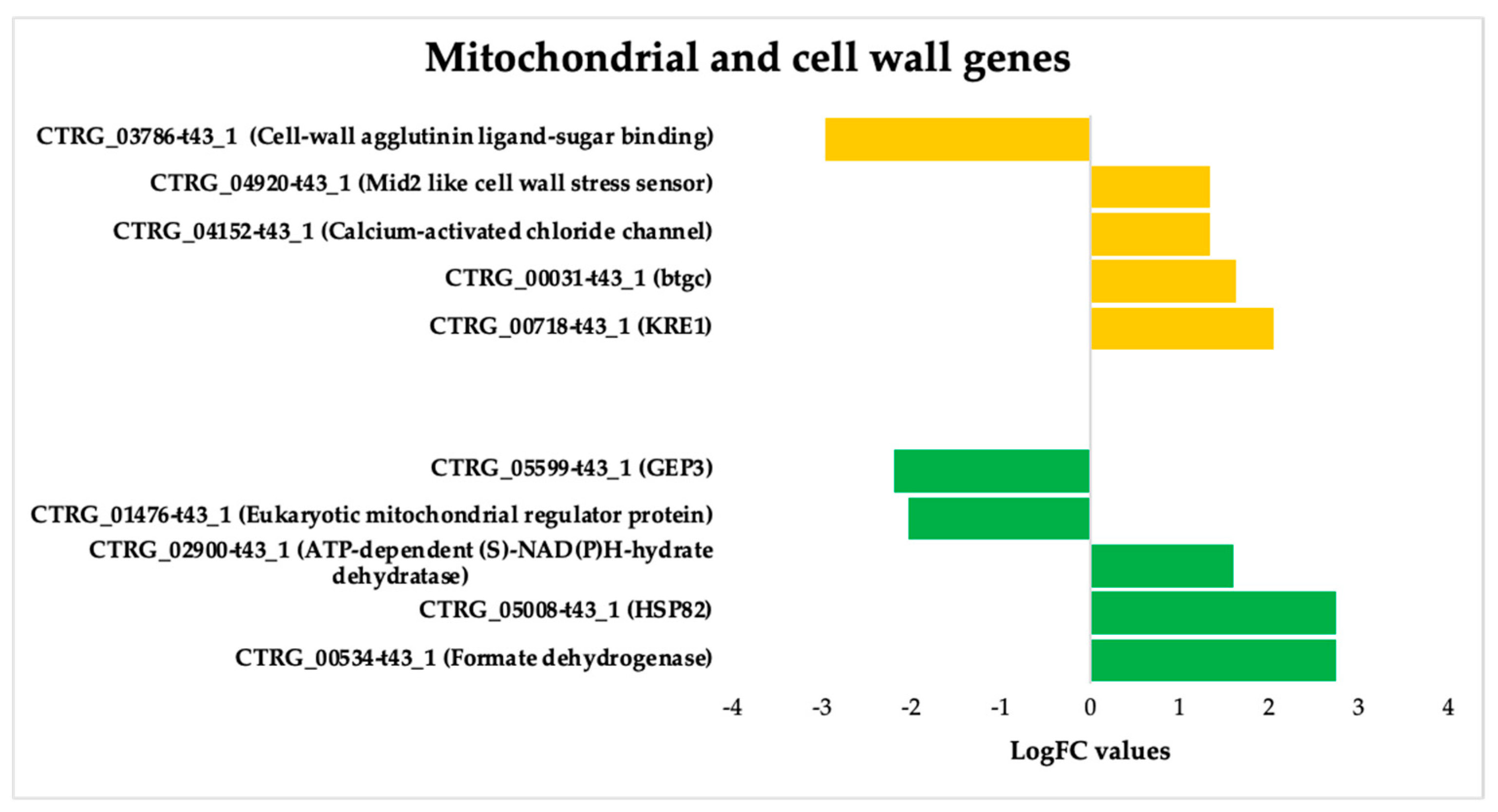
3.3.3. ISO-Induced Dysregulation of Mitochondrial and Cell Wall Genes in C. tropicalis
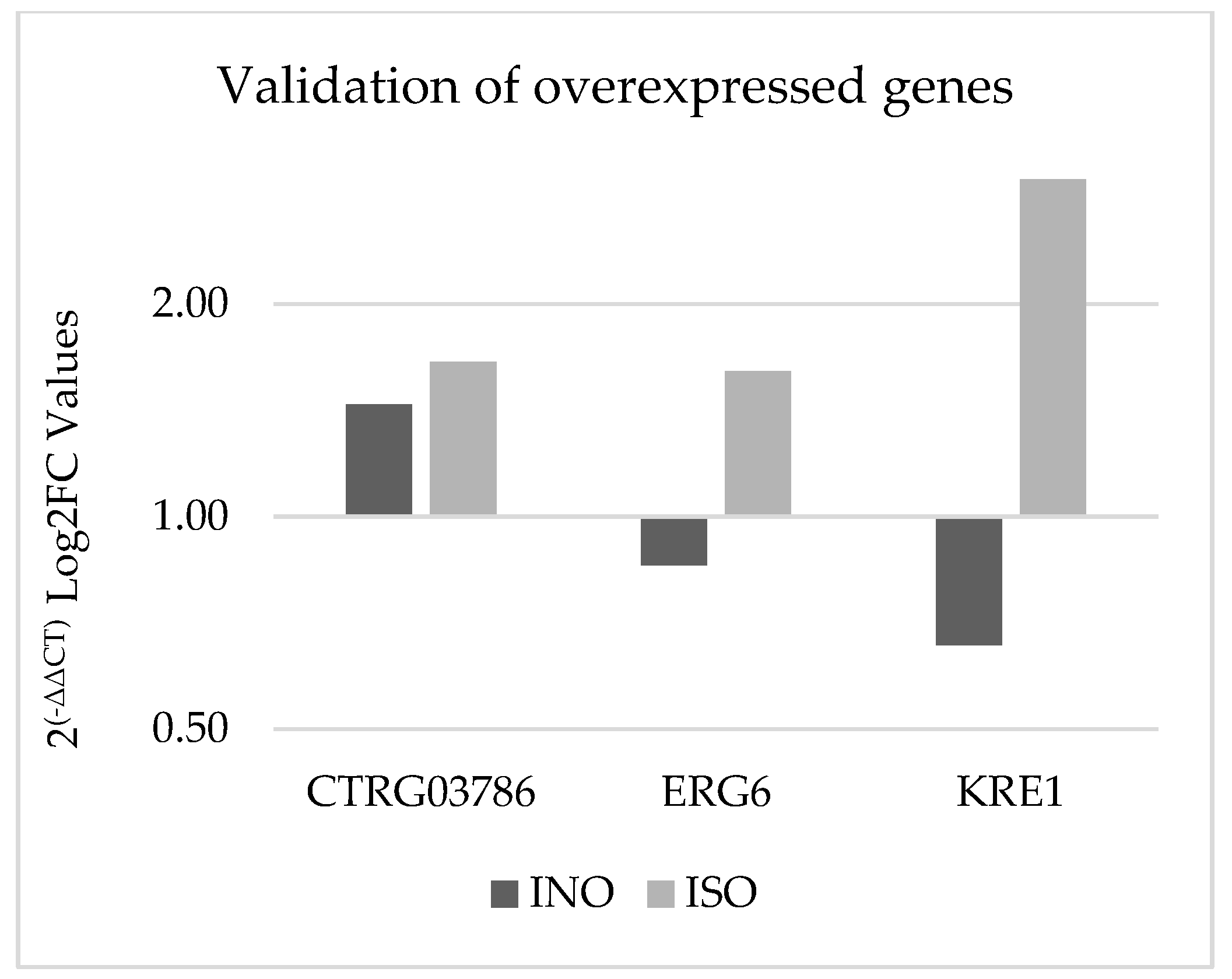
3.3.4. Gene Expression Validation Using qPCR
3.3.5. Methylation-Related Genes Are Downregulated in C. tropicalis after ISO Treatment
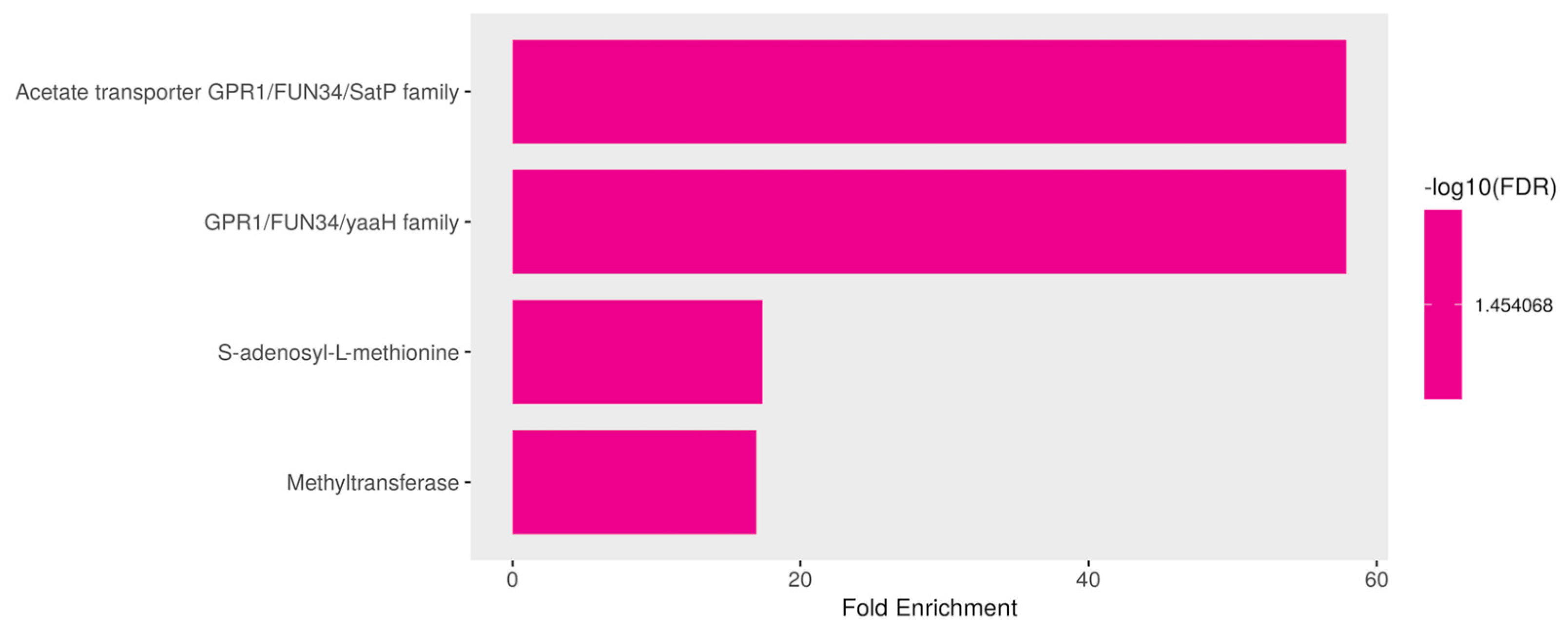
3.3.6. Downregulated Genes Are Enriched in Cell Membrane Roles and S-Adenosyl-L-Methionine (SAM) Pathways in Response to ISO Treatment
3.3.7. Overexpression of Zinc-Regulated Transcription Factors (TFs) in Candida tropicalis Induced by ISO Treatment
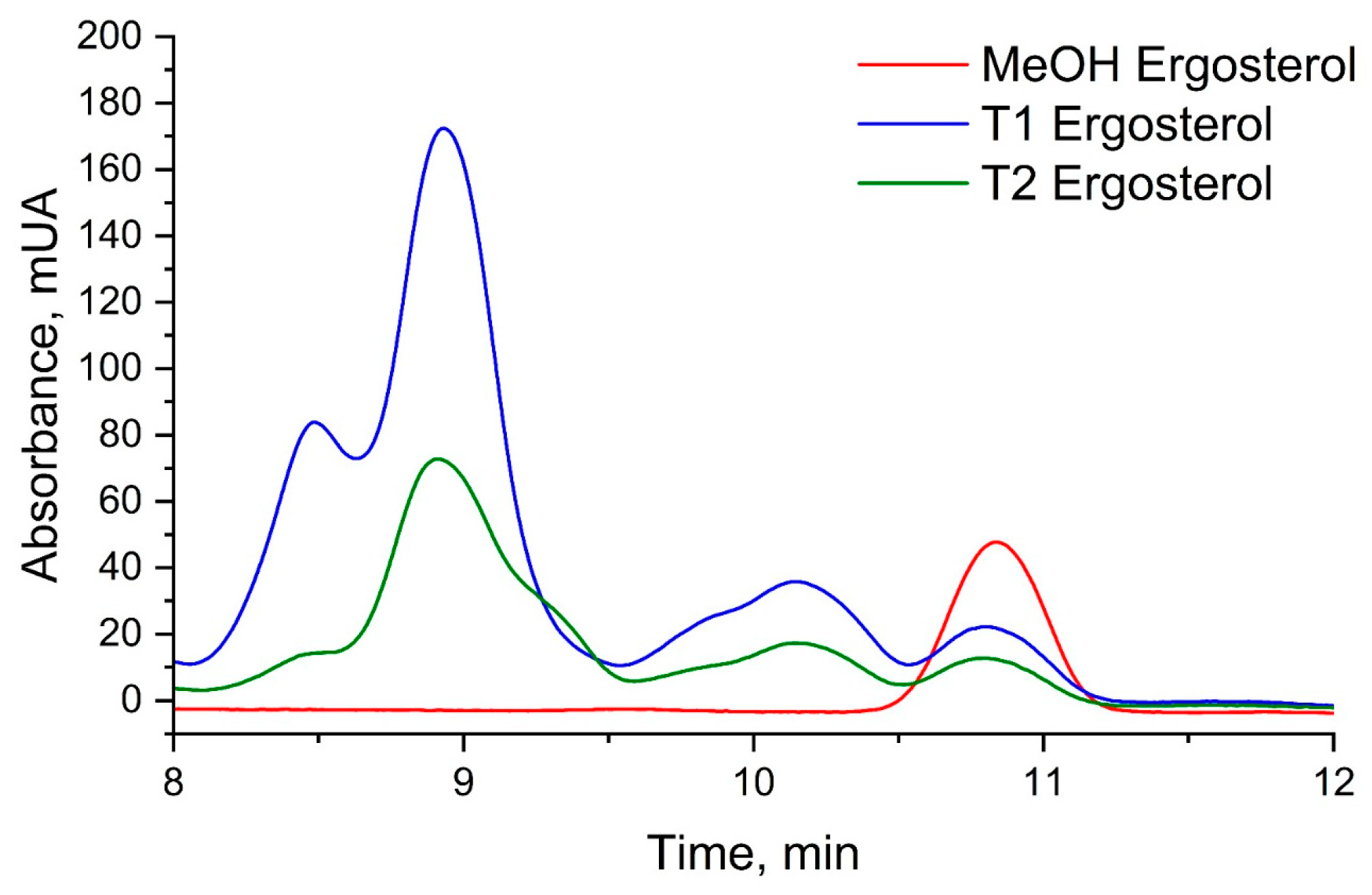
3.4. Disruption of Fungal Membrane Integrity: Ergosterol Content Analysis Reveals ISO’s Antifungal Mechanism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schils, R.; Rampat, R.; Rakic, J.-M.; Crahay, F.-X. Candida Chorioretinitis in Renal Transplant Recipient with Candidemia Related to Contaminated Organ Preservation Fluid: A Role for Dilated Fundus Examination in Its Management. IDCases 2023, 32, e01793. [Google Scholar] [CrossRef]
- Ohashi, Y.; Matono, T.; Suzuki, S.; Yoshino, S.; Alshahni, M.M.; Komori, A.; Makimura, K. The First Case of Clade I Candida auris Candidemia in a Patient with COVID-19 in Japan. J. Infect. Chemother. 2023, 29, 713–717. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Kolonitsiou, F.; Kefala, S.; Spiliopoulou, A.; Aretha, D.; Bartzavali, C.; Siapika, A.; Marangos, M.; Fligou, F. Increased Incidence of Candidemia in Critically Ill Patients during the Coronavirus Disease 2019 (COVID-19) Pandemic. Braz. J. Infect. Dis. 2022, 26, 102353. [Google Scholar] [CrossRef]
- Kimura, S.; Kameda, K.; Harada, K.; Saburi, M.; Okinaka, K.; Shinohara, A.; Uchida, N.; Nishijima, A.; Ozawa, Y.; Tanaka, M.; et al. Risk and Predictive Factors for Candidemia After Allogeneic Hematopoietic Cell Transplantation: JSTCT Transplant Complications Working Group. Transplant. Cell. Ther. 2022, 28, 209.e1–209.e9. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, M.; Chen, J.; Zhang, L.; Lu, M.; Liu, Z. De-Escalation from Echinocandins to Azole Treatment in Critically Ill Patients with Candidemia. Int. J. Infect. Dis. 2022, 121, 69–74. [Google Scholar] [CrossRef]
- Saiprom, N.; Wongsuk, T.; Oonanant, W.; Sukphopetch, P.; Chantratita, N.; Boonsilp, S. Characterization of Virulence Factors in Candida Species Causing Candidemia in a Tertiary Care Hospital in Bangkok, Thailand. J. Fungi 2023, 9, 353. [Google Scholar] [CrossRef]
- Vargas-Espíndola, L.A.; Cuervo-Maldonado, S.I.; Enciso-Olivera, J.; Gómez-Rincón, J.; Jiménez-Cetina, L.; Sánchez-Pedraza, R.; García-Guzmán, K.; López-Mora, M.J.; Álvarez-Moreno, C.; Cortés, J.A.; et al. Fungemia in Hospitalized Adult Patients with Hematological Malignancies: Epidemiology and Risk Factors. J. Fungi 2023, 9, 400. [Google Scholar] [CrossRef]
- Alvarez-Moreno, C.A.; Morales-López, S.; Rodriguez, G.J.; Rodriguez, J.Y.; Robert, E.; Picot, C.; Ceballos-Garzon, A.; Parra-Giraldo, C.M.; Le Pape, P. The Mortality Attributable to Candidemia in C. auris Is Higher than That in Other Candida Species: Myth or Reality? J. Fungi 2023, 9, 430. [Google Scholar] [CrossRef]
- Pappas, P.; Lionakis, M.; Cavling, M.; Ostrosky-Zeichner, L.; Kullberg, B. Invasive Candidiasis. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef]
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2021, 35, 389–413. [Google Scholar] [CrossRef]
- Gómez-Gaviria, M.; Ramírez-Sotelo, U.; Mora-Montes, H.M. Non-Albicans Candida Species: Immune Response, Evasion Mechanisms, and New Plant-Derived Alternative Therapies. J. Fungi 2023, 9, 11. [Google Scholar] [CrossRef]
- Ceballos-Garzon, A.; Peñuela, A.; Valderrama-Beltrán, S.; Vargas-Casanova, Y.; Ariza, B.; Parra-Giraldo, C.M. Emergence and Circulation of Azole-Resistant C. albicans, C. auris and C. parapsilosis Bloodstream Isolates Carrying Y132F, K143R or T220L Erg11p Substitutions in Colombia. Front. Cell. Infect. Microbiol. 2023, 13, 1136217. [Google Scholar] [CrossRef]
- Osset-Trénor, P.; Pascual-Ahuir, A.; Proft, M. Fungal Drug Response and Antimicrobial Resistance. J. Fungi 2023, 9, 565. [Google Scholar] [CrossRef]
- El-kholy, M.A.; Helaly, G.F.; El Ghazzawi, E.F.; El-sawaf, G.; Shawky, S.M. Virulence Factors and Antifungal Susceptibility Profile of C. tropicalis Isolated from Various Clinical Specimens in Alexandria, Egypt. J. Fungi 2021, 7, 351. [Google Scholar] [CrossRef]
- De Oliveira, J.S.; Pereira, V.S.; Castelo-Branco, D.d.S.C.M.; Cordeiro, R.d.A.; Sidrim, J.J.C.; Brilhante, R.S.N.; Rocha, M.F.G. The Yeast, the Antifungal, and the Wardrobe: A Journey into Antifungal Resistance Mechanisms of Candida tropicalis. Can. J. Microbiol. 2020, 66, 377–388. [Google Scholar] [CrossRef]
- Munhoz-Alves, N.; Nishiyama Mimura, L.A.; Viero, R.M.; Bagagli, E.; Schatzmann, J.; Sartori, A.; Fraga-Silva, T.F.d.C. Candida tropicalis Systemic Infection Redirects Leukocyte Infiltration to the Kidneys Attenuating Encephalomyelitis. J. Fungi 2021, 7, 757. [Google Scholar] [CrossRef]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, Epidemiology, Pathogenicity and Antifungal Resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef]
- Wang, D.; An, N.; Yang, Y.; Yang, X.; Fan, Y.; Feng, J. Candida tropicalis Distribution and Drug Resistance Is Correlated with ERG11 and UPC2 Expression. Antimicrob. Resist. Infect. Control 2021, 10, 54. [Google Scholar] [CrossRef]
- Fan, X.; Xiao, M.; Liao, K.; Kudinha, T.; Wang, H.; Zhang, L.; Hou, X.; Kong, F.; Xu, Y.C. Notable Increasing Trend in Azole Non-Susceptible Candida tropicalis Causing Invasive Candidiasis in China (August 2009 to July 2014): Molecular Epidemiology and Clinical Azole Consumption. Front. Microbiol. 2017, 8, 464. [Google Scholar] [CrossRef]
- Spruijtenburg, B.; Baqueiro, C.C.S.Z.; Colombo, A.L.; Meijer, E.F.J.; de Almeida, J.; Berrio, I.; Fernández, N.B.; Chaves, G.M.; Meis, J.; de Groot, T. Short Tandem Repeat Genotyping and Antifungal Susceptibility Testing of Latin American Candida tropicalis Isolates. J. Fungi 2023, 9, 207. [Google Scholar] [CrossRef]
- Zuza-Alves, D.L.; Sila-Rocha, W.P.; Chaves, G. An Update on Candida tropicalis Based on Basic and Clinical Approaches. Front. Microbiol. 2017, 8, 1927. [Google Scholar] [CrossRef] [PubMed]
- Argüelles, A.; Sánchez-Fresneda, R.; Guirao-Abad, J.P.; Lozano, J.A.; Solano, F.; Argüelles, J.C. Insight into the Antifungal Effects of Propolis and Carnosic Acid—Extension to the Pathogenic Yeast Candida glabrata: New Propolis Fractionation and Potential Synergistic Applications. J. Fungi 2023, 9, 442. [Google Scholar] [CrossRef] [PubMed]
- Iraji, A.; Yazdanpanah, S.; Alizadeh, F.; Mirzamohammadi, S.; Ghasemi, Y.; Pakshir, K.; Yang, Y.; Zomorodian, K. Screening the Antifungal Activities of Monoterpenes and Their Isomers against Candida Species. J. Appl. Microbiol. 2020, 129, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Leite-Andrade, M.C.; de Araújo Neto, L.N.; Buonafina-Paz, M.D.S.; de Assis Graciano dos Santos, F.; da Silva Alves, A.I.; de Castro, M.C.A.B.; Mori, E.; de Lacerda, B.C.G.V.; Araújo, I.M.; Coutinho, H.D.M.; et al. Antifungal Effect and Inhibition of the Virulence Mechanism of D-Limonene against Candida parapsilosis. Molecules 2022, 27, 8884. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, Z.; Xiang, W.; Huang, M.; Tang, J.; Lu, Y.; Zhao, Q.; Zhang, Q.; Rao, Y.; Liu, L. Antifungal Activity and Mechanism of D -Limonene against Foodborne Opportunistic Pathogen Candida tropicalis. LWT-Food Sci. Technol. 2022, 159, 113144. [Google Scholar] [CrossRef]
- Contreras Martínez, O.I.; Angulo Ortíz, A.A.; Santafé Patiño, G. Mechanism of Antifungal Action of Monoterpene Isoespintanol against Clinical Isolates of Candida tropicalis. Molecules 2022, 27, 5808. [Google Scholar] [CrossRef] [PubMed]
- Contreras Martínez, O.I.; Angulo Ortíz, A.A.; Santafé Patiño, G. Antifungal Potential of Isoespintanol Extracted from Oxandra xylopioides Diels (Annonaceae) against Intrahospital Isolations of Candida spp. Heliyon 2022, 8, e11110. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, R.D.; Páez, M.S.; Angulo, A.A. Obtención de Isoespintanol Por Hidrodestilación y Cristalización a Partir Del Extracto Bencínico de Oxandra xylopioides. Inf. Tecnol. 2015, 26, 13–18. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Silva, M.; Cardozo, D.; Fernando, P.; Freitas, F.; Fuzo, C.; Santos, C.; Rodrigues, M.; Santana, J. Modulation of ERG Genes Expression in Clinical Isolates of Candida tropicalis Susceptible and Resistant to Fluconazole and Itraconazole. Mycopathologia 2020, 6, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, T.; Guo, S.; Zhang, Y.; Sheng, R.; Sun, R.; Chen, L.; Lv, R.; Qi, Y. In Vitro Antifungal Activity and Mechanism of Ag3PW12O40 Composites against Candida Species. Molecules 2020, 25, 6012. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.C.; Zhang, X.; Trievel, R.C.; Cheng, X. The SET-Domain Protein Superfamily: Protein Lysine Methyltransferases. Genome Biol. 2005, 6, 227. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Genbauffe, F.; Cooper, T. Structure and Transcription of the Allantoate Permease Gene (DAL5) from Saccharomyces cerevisiae. J. Bacteriol. 1988, 170, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.; Ricketts, M.; Gingras, S.; Soucy, P.; Feltus, A.; Melner, M. Molecular Biology of the 3β-Hydroxysteroid Dehydrogenase/Δ5-Δ4 Isomerase Gene Family. Endocr. Rev. 2005, 26, 525–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Salavaggione, E.; Pelleymounter, L.; Eckloff, B.; Wieben, E.; Weinshilboum, R. Human 3β-Hydroxysteroid Dehydrogenase Types 1 and 2: Gene Sequence Variation and Functional Genomics. J. Steroid Biochem. Mol. Biol. 2007, 107, 88–99. [Google Scholar] [CrossRef][Green Version]
- Han, L.; Ding, G.; Liu, Y.; Huang, J.; Wu, J. Characterization of Sphingomyelin Phosphodiesterase Expression in Bumblebee (Bombus lantschouensis). J. Insect Sci. 2018, 18, 20. [Google Scholar] [CrossRef]
- Ueda, S.; Manabe, Y.; Kubo, N.; Morino, N.; Yuasa, H.; Shiotsu, M.; Tsuji, T.; Sugawara, T.; Kambe, T. Early Secretory Pathway-Resident Zn Transporter Proteins Contribute to Cellular Sphingolipid Metabolism through Activation of Sphingomyelin Phosphodiesterase 1. Am. J. Physiol. Cell Physiol. 2022, 322, C948–C959. [Google Scholar] [CrossRef]
- Sherman, M.Y.S.; Goldberg, A.L. Involvement of Molecular Chaperones in Intracellular Protein Breakdown. Stress-Inducible Cell. Responses 1996, 77, 57–78. [Google Scholar]
- Qiu, X.B.; Shao, Y.M.; Miao, S.; Wang, L. The Diversity of the DnaJ/Hsp40 Family, the Crucial Partners for Hsp70 Chaperones. Cell. Mol. Life Sci. 2006, 63, 2560–2570. [Google Scholar] [CrossRef] [PubMed]
- Fry, M.Y.; Saladi, S.M.; Clemons, W.M. The STI1-Domain Is a Flexible Alpha-Helical Fold with a Hydrophobic Groove. Protein Sci. 2021, 30, 882–898. [Google Scholar] [CrossRef] [PubMed]
- Barski, O.A.; Tipparaju, S.M.; Bhatnagar, A. The Aldo-Keto Reductase Superfamily and Its Role in Drug Metabolism and Detoxification. Drug Metab. Rev. 2008, 40, 553–624. [Google Scholar] [CrossRef] [PubMed]
- Fetzner, S. Ring-Cleaving Dioxygenases with a Cupin Fold. Appl. Environ. Microbiol. 2012, 78, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yu, Q.; Ma, C.; Mao, X.; Liu, Y.; Peng, X.; Li, M. Role of the Inositol Polyphosphate Kinase Vip1 in Autophagy and Pathogenesis in Candida albicans. Future Microbiol. 2020, 15, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.; Sdicu, A.M.; Laroche, M.; Bussey, H. Isolation from Candida albicans of a Functional Homolog of the Saccharomyces cerevisiae KRE1 Gene, Which Is Involved in Cell Wall β-Glucan Synthesis. J. Bacteriol. 1991, 173, 6859–6864. [Google Scholar] [CrossRef]
- Philip, B.; Levin, D.E. Wsc1 and Mid2 Are Cell Surface Sensors for Cell Wall Integrity Signaling That Act through Rom2, a Guanine Nucleotide Exchange Factor for Rho1. Mol. Cell. Biol. 2001, 21, 271–280. [Google Scholar] [CrossRef]
- Hartzell, C.; Putzier, I.; Arreola, J. Calcium-Activated Chloride Channels. Annu. Rev. Physiol. 2005, 67, 719–758. [Google Scholar] [CrossRef]
- Borkovich, K.A.; Farrelly, F.W.; Finkelstein, D.B.; Taulien, J.; Lindquist, S. Hsp82 Is an Essential Protein That Is Required in Higher Concentrations for Growth of Cells at Higher Temperatures. Mol. Cell. Biol. 1989, 9, 3919–3930. [Google Scholar] [CrossRef]
- Dong, G.; Ryde, U. Reaction Mechanism of Formate Dehydrogenase Studied by Computational Methods. J. Biol. Inorg. Chem. 2018, 23, 1243–1254. [Google Scholar] [CrossRef]
- Ljungdahl, L.G. Formate Dehydrogenases: Role of Molybdenum, Tungsten and Selenium; Pergamon Press Ltd.: Oxford, UK, 1980; pp. 463–486. [Google Scholar] [CrossRef]
- Hirata, A.; Okada, K.; Yoshii, K.; Shiraishi, H.; Saijo, S.; Yonezawa, K.; Shimizu, N.; Hori, H. Structure of TRNA Methyltransferase Complex of Trm7 and Trm734 Reveals a Novel Binding Interface for TRNA Recognition. Nucleic Acids Res. 2019, 47, 10942–10955. [Google Scholar] [CrossRef] [PubMed]
- Pintard, L.; Lecointe, F.; Bujnicki, J.M.; Bonnerot, C.; Grosjean, H.; Lapeyre, B. Trm7p Catalyses the Formation of Two 2¢-O-Methylriboses in Yeast TRNA Anticodon Loop Lionel. EMBO J. Vol. 2002, 21, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, M.E.; Johansson, J.O.M.; Von Pawel-Rammingen, U.; Byström, A.S. Identification of the TRM2 Gene Encoding the TRNA(M5U54) Methyltransferase of Saccharomyces cerevisiae. RNA 2000, 6, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, F.; Gafken, P.R.; Gottschling, D.E. Dot1p Modulates Silencing in Yeast by Methylation of the Nucleosome Core. Cell 2002, 109, 745–756. [Google Scholar] [CrossRef]
- San-segundo, P.A.; Roeder, G.S. Role for the Silencing Protein Dot1 in Meiotic Checkpoint Control. Mol. Biol. Cell 2000, 11, 3601–3615. [Google Scholar] [CrossRef]
- Wysocki, R.; Javaheri, A.; Sha, F.; Co, J. Role of Dot1-Dependent Histone H3 Methylation in G 1 and S Phase DNA Damage Checkpoint Functions of Rad9. Mol. Cell. Biol. 2005, 25, 8430–8443. [Google Scholar] [CrossRef]
- Frederiks, F.; Heynen, G.J.J.E.; Van Deventer, S.J.; Janssen, H.; Van Leeuwen, F. Two Dot1 Isoforms in Saccharomyces cerevisiae as a Result of Leaky Scanning by the Ribosome. Nucleic Acids Res. 2009, 37, 7047–7058. [Google Scholar] [CrossRef]
- Robellet, X.; Flipphi, M.; Pégot, S.; MacCabe, A.; Vélot, C. AcpA, a Member of the GPR1/FUN34/YaaH Membrane Protein Family, Is Essential for Acetate Permease Activity in the Hyphal Fungus Aspergillus nidulans. Biochem. J. 2008, 493, 485–493. [Google Scholar] [CrossRef]
- Miwa, T.; Takagi, Y.; Shinozaki, M.; Yun, C.W.; Schell, W.A.; Perfect, J.R.; Kumagai, H.; Tamaki, H. Gpr1, a Putative G-Protein-Coupled Receptor, Regulates Morphogenesis and Hypha Formation in the Pathogenic Fungus Candida albicans. Eukaryot. Cell 2004, 3, 919–931. [Google Scholar] [CrossRef]
- Maidan, M.M.; De Rop, L.; Serneels, J.; Exler, S.; Rupp, S.; Tournu, H.; Thevelein, J.; Van Dijck, P. The G Protein-Coupled Receptor Gpr1 and the Gα Protein Gpa2 Act through the CAMP-Protein Kinase A Pathway to Induce Morphogenesis in Candida albicans. Mol. Biol. Cell 2005, 16, 1971–1986. [Google Scholar] [CrossRef]
- Fontecave, M.; Atta, M.; Mulliez, E. S-Adenosylmethionine: Nothing Goes to Waste. Trends Biochem. Sci. 2004, 29, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Z.; Cai, H.; Zhou, C. Progress in the Microbial Production of S-Adenosyl-l-Methionine. World J. Microbiol. Biotechnol. 2016, 32, 153. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Coleman, J.E. GAL4 Transcription Factor Is Not a “Zinc Finger” but Forms a Zn(II)2Cys6 Binuclear Cluster. Proc. Natl. Acad. Sci. USA 1990, 87, 2077–2081. [Google Scholar] [CrossRef] [PubMed]
- Lalo, D.; Steffan, J.S.; Dodd, J.A.; Nomura, M. RRN11 Encodes the Third Subunit of the Complex Containing Rrn6p and Rrn7p That Is Essential for the Initiation of RDNA Transcription by Yeast RNA Polymerase I. J. Biol. Chem. 1996, 271, 21062–21067. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Varshavsky, A. RPN4 Is a Ligand, Substrate, and Transcriptional Regulator of the 26S Proteasome: A Negative Feedback Circuit. Proc. Natl. Acad. Sci. USA 2001, 98, 3056–3061. [Google Scholar] [CrossRef] [PubMed]
- Mannhaupt, G.; Schnall, R.; Karpov, V.; Vetter, I.; Feldmann, H. Rpn4p Acts as a Transcription Factor by Binding to PACE, a Nonamer Box Found Upstream of 26S Proteasomal and Other Genes in Yeast. FEBS Lett. 1999, 450, 27–34. [Google Scholar] [CrossRef]
- Böhm, S.; Frishman, D.; Werner Mewes, H. Variations of the C2H2 Zinc Finger Motif in the Yeast Genome and Classification of Yeast Zinc Finger Proteins. Nucleic Acids Res. 1997, 25, 2464–2469. [Google Scholar] [CrossRef]
- Hlynialuk, C.; Schierholtz, R.; Vernooy, A.; van der Merwe, G. Nsf1/Ypl230w Participates in Transcriptional Activation during Non-Fermentative Growth and in Response to Salt Stress in Saccharomyces cerevisiae. Microbiology 2008, 154, 2482–2491. [Google Scholar] [CrossRef]
- Gangwani, L.; Mikrut, M.; Galcheva-Gargova, Z.; Davis, R.J. Interaction of ZPR1 with Translation Elongation Factor-1α in Proliferating Cells. J. Cell Biol. 1998, 143, 1471–1484. [Google Scholar] [CrossRef]
- Ribar, B.; Prakash, L.; Prakash, S. ELA1 and CUL3 Are Required Along with ELC1 for RNA Polymerase II Polyubiquitylation and Degradation in DNA-Damaged Yeast Cells. Mol. Cell. Biol. 2007, 27, 3211–3216. [Google Scholar] [CrossRef]
- Marie, C.; Leyde, S.; White, T.C. Cytoplasmic Localization of Sterol Transcription Factors Upc2p and Ecm22p in S. cerevisiae. Fungal Genet. Biol. 2008, 45, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Vik, A.; Rine, J. Upc2p and Ecm22p, Dual Regulators of Sterol Biosynthesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 2001, 21, 6395–6405. [Google Scholar] [CrossRef] [PubMed]
- Truernit, E.; Schmid, J.; Epple, P.; Illig, J.; Sauer, N. The Sink-Specific and Stress-Regulated Arabidopsis SPP4 Gene: Enhanced Expression of a Gene Encoding a Monosaccharide Transporter by Wounding, Elicitors, and Pathogen Challenge. Plant Cell 1996, 8, 2169–2182. [Google Scholar] [PubMed]
- Chen, P.; Chuang, Y.; Wu, U.; Sun, H.; Wang, J.; Sheng, W.; Chen, Y.; Chang, S. Mechanisms of Azole Resistance and Trailing in Candida tropicalis Bloodstream Isolates. J. Fungi 2021, 7, 612. [Google Scholar] [CrossRef] [PubMed]
- Maikan, H.K.; Jabbar, S.; Al-Haishawi, H. Isolation and Identification of Candida tropicalis as a Cause of Cutaneous Candidiasis in Kalar District, Iraq. Arch. Razi Inst. 2022, 77, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Beattie, S.R.; Krysan, D.J. Antifungal Drug Screening: Thinking Outside the Box to Identify Novel Antifungal Scaffolds. Curr. Opin. Microbiol. 2020, 57, 1–6. [Google Scholar] [CrossRef]
- Houšť, J.; Spížek, J.; Havlíček, V. Antifungal Drugs. Metabolites 2020, 10, 106. [Google Scholar] [CrossRef]
- Scorneaux, B.; Angulo, D.; Borroto-Esoda, K.; Ghannoum, M.; Peel, M.; Wring, S. SCY-078 Is Fungicidal against Candida Species in Time-Kill Studies. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef]
- Avato, P. Editorial to the Special Issue—“Natural Products and Drug Discovery”. Molecules 2020, 25, 1128. [Google Scholar] [CrossRef]
- Atanasov, A.; Zotchev, S.; Dirsch, V.; Taskforce, T.I.N.P.S.; Supuran, C. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Aylate, A.; Agize, M.; Ekero, D.; Kiros, A.; Ayledo, G.; Gendiche, K. In-Vitro and In-Vivo Antibacterial Activities of Croton macrostachyus Methanol Extract against E. coli and S. aureus. Adv. Anim. Vet. Sci. 2017, 5, 107–114. [Google Scholar] [CrossRef]
- Contreras Martínez, O.I.; Angulo Ortíz, A.; Santafé Patiño, G.; Peñata-Taborda, A.; Berrio Soto, R. Isoespintanol Antifungal Activity Involves Mitochondrial Dysfunction, Inhibition of Biofilm Formation, and Damage to Cell Wall Integrity in Candida tropicalis. Int. J. Mol. Sci. 2023, 24, 10187. [Google Scholar] [CrossRef] [PubMed]
- Jordá, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef]
- Sun, L.; Liao, K. The Effect of Honokiol on Ergosterol Biosynthesis and Vacuole Function in Candida albicans. J. Microbiol. Biotechnol. 2020, 30, 1835–1842. [Google Scholar] [CrossRef]
- Liu, J.F.; Xia, J.J.; Nie, K.L.; Wang, F.; Deng, L. Outline of the Biosynthesis and Regulation of Ergosterol in Yeast. World J. Microbiol. Biotechnol. 2019, 35, 98. [Google Scholar] [CrossRef]
- Cheng, R.; Xu, Q.; Hu, F.; Li, H.; Yang, B.; Duan, Z.; Zhang, K.; Wu, J.; Li, W.; Luo, Z. Antifungal Activity of MAF-1A Peptide against Candida albicans. Int. Microbiol. 2021, 24, 233–242. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Esquivel, B.D.; White, T.C. Overexpression or Deletion of Ergosterol Biosynthesis Genes Alters Doubling Time, Response to Stress Agents, and Drug Susceptibility in Saccharomyces cerevisiae. MBio 2018, 9, 10–1128. [Google Scholar] [CrossRef]
- Quiles-Melero, I.; García-Rodríguez, J. Antifúngicos de Uso Sistémico. Rev. Iberoam. Micol. 2021, 38, 42–46. [Google Scholar] [CrossRef]
- De Backer, M.D.; Ilyina, T.; Ma, X.J.; Vandoninck, S.; Luyten, W.H.M.L.; Bossche, H. Vanden. Genomic Profiling of the Response of Candida albicans to Itraconazole Treatment Using a DNA Microarray. Antimicrob. Agents Chemother. 2001, 45, 1660–1670. [Google Scholar] [CrossRef]
- Florio, A.R.; Ferrari, S.; De Carolis, E.; Torelli, R.; Fadda, G.; Sanguinetti, M.; Sanglard, D.; Posteraro, B. Genome-Wide Expression Profiling of the Response to Short-Term Exposure to Fluconazole in Cryptococcus neoformans Serotype A. BMC Microbiol. 2011, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Kodedová, M.; Sychrová, H. Changes in the Sterol Composition of the Plasma Membrane Affect Membrane Potential, Salt Tolerance and the Activity of Multidrug Resistance Pumps in Saccharomyces cerevisiae. PLoS ONE 2015, 10, e0139306. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Pergakes, K.L.; Kennedy, M.A.; Lees, N.D.; Barbuch, R.; Koegel, C.; Bard, M. Sequencing, Disruption, and Characterization of the Candida Albicans Sterol Methyltransferase (ERG6) Gene: Drug Susceptibility Studies in Erg6 Mutants. Antimicrob. Agents Chemother. 1998, 42, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Parks, L.W.; Smith, S.J.; Crowley, J.H. Biochemical and Physiological Effects of Sterol Alterations in Yeast-A Review. Lipids 1995, 30, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Ahmadipour, S.; Field, R.A.; Miller, G.J. Prospects for Anti-Candida Therapy through Targeting the Cell Wall: A Mini-Review. Cell Surf. 2021, 7, 100063. [Google Scholar] [CrossRef] [PubMed]
- Lenardon, M.D.; Sood, P.; Dorfmueller, H.C.; Brown, A.J.P.; Gow, N.A.R. Scalar Nanostructure of the Candida albicans Cell Wall; a Molecular, Cellular and Ultrastructural Analysis and Interpretation. Cell Surf. 2020, 6, 100047. [Google Scholar] [CrossRef] [PubMed]
- Ibe, C.; Munro, C.A. Fungal Cell Wall Proteins and Signaling Pathways Form a Cytoprotective Network to Combat Stresses. J. Fungi 2021, 7, 739. [Google Scholar] [CrossRef]
- Popolo, L.; Gualtieri, T.; Ragni, E. The Yeast Cell-Wall Salvage Pathway. Med. Mycol. Suppl. 2001, 39, 111–121. [Google Scholar] [CrossRef]
- Boone, C.; Sommer, S.S.; Hensel, A.; Bussey, H. Yeast KRE Genes Provide Evidence for a Pathway of Cell Wall β-Glucan Assembly. J. Cell Biol. 1990, 110, 1833–1843. [Google Scholar] [CrossRef]
- Nagahash, S.; Lussier, M.; Bussey, H. Isolation of Candida glabrata Homologs of the Saccharomyces cerevisiae KRE9 and KNH1 Genes and Their Involvement in Cell Wall β-1,6-Glucan Synthesis. J. Bacteriol. 1998, 180, 5020–5029. [Google Scholar] [CrossRef]
- García, R.; Botet, J.; Rodríguez-Peña, J.M.; Bermejo, C.; Ribas, J.C.; Revuelta, J.L.; Nombela, C.; Arroyo, J. Genomic Profiling of Fungal Cell Wall-Interfering Compounds: Identification of a Common Gene Signature. BMC Genom. 2015, 16, 683. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Zhou, X.; Xiang, W.; Huang, M.; Lin, Z.; Tang, J.; Cai, T.; Zhang, Q. Integrated Transcriptome Reveals That D -Limonene Inhibits Candida tropicalis by Disrupting Metabolism. LWT-Food Sci. Technol. 2023, 176, 114535. [Google Scholar] [CrossRef]
- Sun, F.-J.; Li, M.; Gu, L.; Wang, M.; Yang, M. Recent Progress on Anti-Candida Natural Products. Chin. J. Nat. Med. 2021, 19, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Hymbaugh Bergman, S.J.; Comstock, L.R. N-Mustard Analogs of S-Adenosyl-l-Methionine as Biochemical Probes of Protein Arginine Methylation. Bioorganic Med. Chem. 2015, 23, 5050–5055. [Google Scholar] [CrossRef]
- Sun, B.; Dong, Y.; Lei, K.; Wang, J.; Zhao, L.; Liu, M. Design, Synthesis and Biological Evaluation of Amide-Pyridine Derivatives as Novel Dual-Target (SE, CYP51)Antifungal Inhibitors. Bioorganic Med. Chem. 2019, 27, 2427–2437. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Martínez, O.I.; Angulo-Ortíz, A.; Santafé-Patiño, G.; Aviña-Padilla, K.; Velasco-Pareja, M.C.; Yasnot, M.F. Transcriptional Reprogramming of Candida tropicalis in Response to Isoespintanol Treatment. J. Fungi 2023, 9, 1199. https://doi.org/10.3390/jof9121199
Contreras-Martínez OI, Angulo-Ortíz A, Santafé-Patiño G, Aviña-Padilla K, Velasco-Pareja MC, Yasnot MF. Transcriptional Reprogramming of Candida tropicalis in Response to Isoespintanol Treatment. Journal of Fungi. 2023; 9(12):1199. https://doi.org/10.3390/jof9121199
Chicago/Turabian StyleContreras-Martínez, Orfa Inés, Alberto Angulo-Ortíz, Gilmar Santafé-Patiño, Katia Aviña-Padilla, María Camila Velasco-Pareja, and María Fernanda Yasnot. 2023. "Transcriptional Reprogramming of Candida tropicalis in Response to Isoespintanol Treatment" Journal of Fungi 9, no. 12: 1199. https://doi.org/10.3390/jof9121199
APA StyleContreras-Martínez, O. I., Angulo-Ortíz, A., Santafé-Patiño, G., Aviña-Padilla, K., Velasco-Pareja, M. C., & Yasnot, M. F. (2023). Transcriptional Reprogramming of Candida tropicalis in Response to Isoespintanol Treatment. Journal of Fungi, 9(12), 1199. https://doi.org/10.3390/jof9121199







