Modelling the In Vitro Growth of Phytopathogenic Filamentous Fungi and Oomycetes: The Gompertz Parameters as Robust Indicators of Propolis Antifungal Action
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Propolis Samples Origin and Extraction Procedure
2.3. Chemical Characterization of A16.EE
2.3.1. Total Phenolic Compounds Content
2.3.2. Total Flavonoids Content
2.3.3. Total Ortho-Diphenols Content
2.4. In vitro Activity of A16.EE against Phytopathogenic Fungi and Oomycetes
2.4.1. Fungi and Oomycete Strains, Media and Growth Conditions
2.4.2. Evaluation of A16.EE Antifungal and Anti-Oomycetal Activities
2.4.3. Gompertz Model Applied to Filamentous Growth
2.5. Statistical Analysis
3. Results
3.1. Extraction Yield and Chemical Characterization of A16.EE
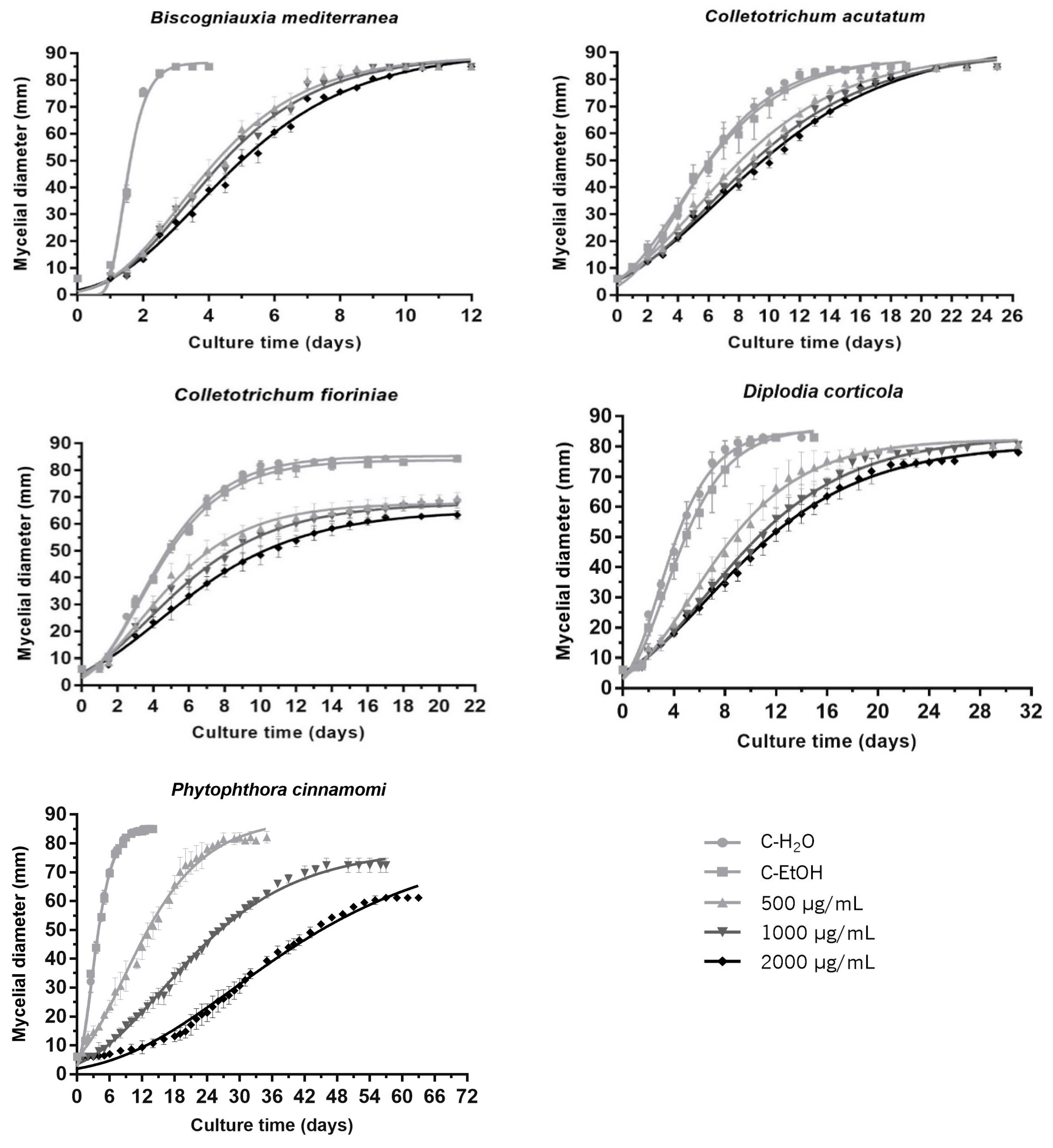
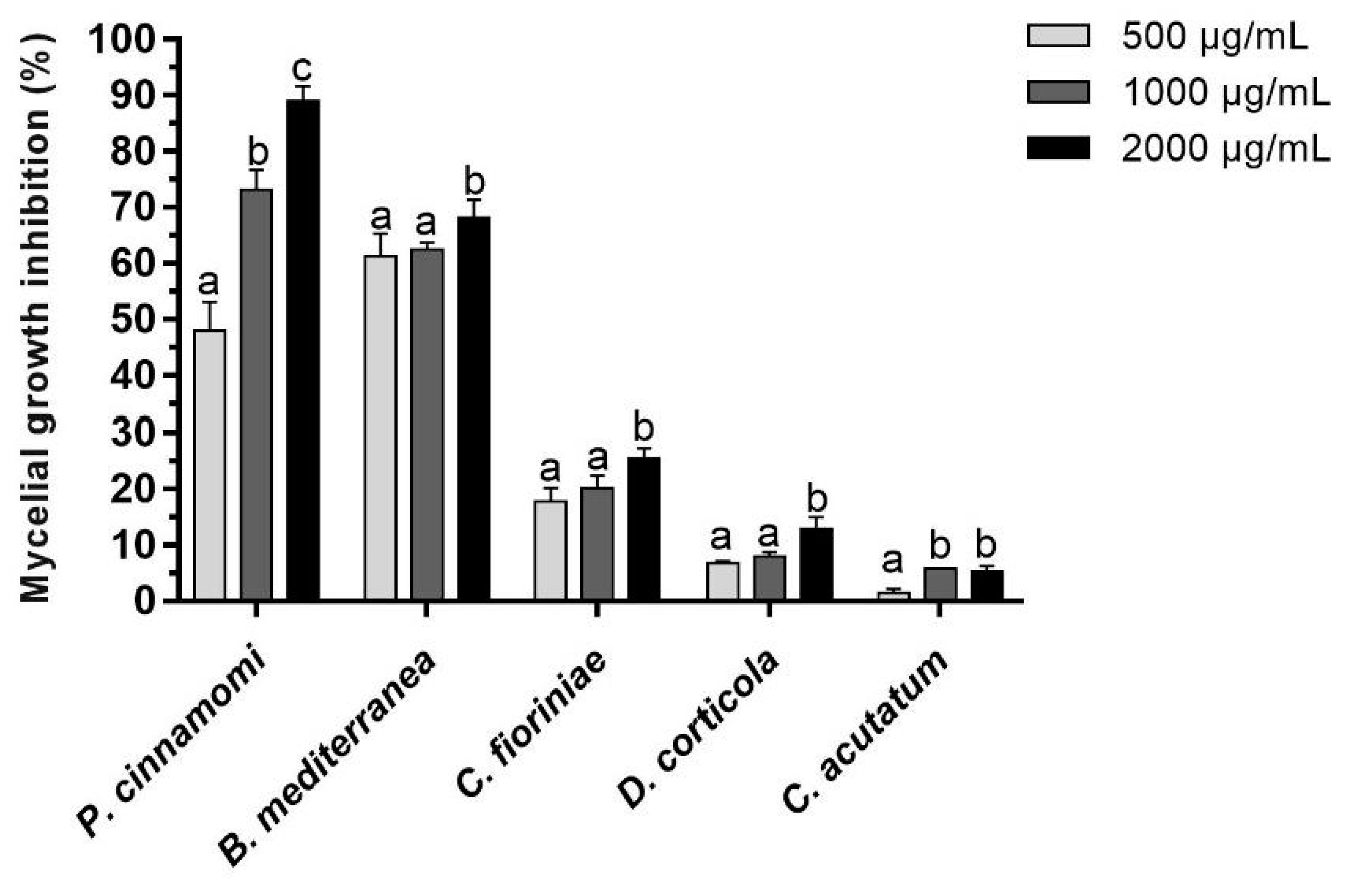
3.2. Quantitative Characterization of the Antimicrobial Activity of Propolis Extract against Phytopathogenic Filamentous Species
4. Discussion
4.1. A16 Shows Chemical Characteristics from Propolis Typical from the South and North of Portugal, Suggesting a Gradient Is More Likely than a Dichotomy
4.2. In Vitro Activity of Propolis against Phytopathogenic Filamentous Species
4.3. Gompertz-Derived Parameters Are Robust Descriptors and Exhibit High Discriminating Power
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Increasing the Resilience of Agricultural Livelihoods. 2016. Available online: http://www.fao.org/3/a-i5615e.pdf (accessed on 6 April 2022).
- United Nations. World Population Prospects—Key Findings and Advance Tables. The 2017 Revision. Department of Economic and Social Affairs. 2017. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/WPP2017_KeyFindings.pdf (accessed on 20 January 2022).
- Santos, J.L. Intensificação sustentável: Um novo modelo tecnológico na agricultura. In Cultivar N.º 3—Cadernos de Análise e Prospetiva. Gabinete de Planeamento, Políticas e Administração Geral; Dimas, B., Diniz, E., Grancinho, M., Morais, A.F., Moura, A.R., Miguel, A.C., Lopes, C., Lobo, H., Gaspar, M., Loureiro, M., et al., Eds.; Gabinete de Planeamento, Políticas e Administração Geral: Lisbon, Portugal, 2016; pp. 13–21. [Google Scholar]
- Organisation for Economic Co-operation and Development, Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2012–2021. 2012. Available online: https://www.oecd-ilibrary.org/agriculture-and-food/oecd-fao-agricultural-outlook-2012_agr_outlook-2012-en (accessed on 20 November 2022).
- Fang, Y.; Ramasamy, R.P. Current and Prospective Methods for Plant Disease Detection. Biosensors 2015, 5, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.; Robson, G.D.; Trinci, A.P.J. 21st Century Guidebook to Fungi, 2nd ed.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium Pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant Pathogenic Fungi. Microbiol. Spectr. 2017, 5, FUNK-0023-2016. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Brar, A.; Yadav, M.; Chawade, A.; Vivekanand, V.; Pareek, N. Chitinases–Potential Candidates for Enhanced Plant Resistance towards Fungal Pathogens. Agriculture 2018, 8, 88. [Google Scholar] [CrossRef]
- Khonglah, D.; Kayang, H. Antagonism of indigenous fungal isolates against Botrytis cineria the causal of gray mold disease of tomato (Solanum lycopersicum L.). Int. J. Curr. Res. Life Sci. 2018, 7, 806–812. [Google Scholar]
- Zadeh, S.M. Pesticides. In More People, More Food, Worse Water? A Global Review of Water Pollution from Agriculture; Mateo-Sagasta, J., Zadeh, S.M., Turral, H., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy; International Water Management Institute on behalf of the Water Land and Ecosystems research program of the CGIAR: Colombo, Sri Lanka, 2018; pp. 77–91. [Google Scholar]
- Plessis, A.D. Freshwater Challenges of South Africa and its Upper Vaal River: Current State and Outlook, 1st ed.; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Gaberell, L.; Hoinkes, C. Highly Hazardous Profits: How Syngenta Makes Billions by Selling Toxic Pesticides; Public Eye Lausanne: Lausanne, Switzerland, 2019. [Google Scholar]
- Darçin, E.S.; Darçin, M. Health effects of agricultural pesticides. Biomed. Res. 2017, S13–S17. [Google Scholar]
- Al-Ahmadi, M.S. Pesticides, Anthropogenic Activities, and the Health of Our Environment Safety. In Pesticides, Anthropogenic Activities and the Health of Our Environment; Larramendy, M.L., Ed.; IntechOpen: London, UK, 2019; pp. 73–88. [Google Scholar] [CrossRef][Green Version]
- Czaja, K.; Góralczyk, K.; Struciński, P.; Hernik, A.; Korcz, W.; Minorczyk, M.; Łyczewska, M.; Ludwicki, J.K. Biopesticides—Towards increased consumer safety in the European Union. Pest Manag. Sci. 2015, 71, 3–6. [Google Scholar] [CrossRef]
- European Commission. Green Deal: Pioneering Proposals to Restore Europe’s Nature by 2050 and Halve Pesticide Use by 2030. 2022. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_22_3746 (accessed on 29 June 2022).
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. New emerging fields of application of propolis. Maced. J. Chem. Chem. Eng. 2016, 35, 1–11. [Google Scholar] [CrossRef]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development. Evid. Based Complementary Altern. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Šturm, L.; Ulrih, N.P. Advances in the Propolis Chemical Composition between 2013 and 2018: A Review. eFood 2019, 1, 24–37. [Google Scholar] [CrossRef]
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.; Antunes, P.; Paulo, L.; Ferreira, A.M.; Cunha, A.; Almeida-Aguiar, C.; Oliveira, R. Antioxidant and dual dose-dependent antigenotoxic and genotoxic properties of an ethanol extract of propolis. RSC Adv. 2016, 6, 49806–49816. [Google Scholar] [CrossRef]
- Graikou, K.; Popova, M.; Gortzi, O.; Bankova, V.; Chinou, I. Characterization and biological evaluation of selected Mediterranean propolis samples. Is it a new type? LWT—Food Sci. Technol. 2016, 65, 261–267. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2018, 26, 1695–1703. [Google Scholar] [CrossRef]
- Curifuta, M.; Vidal, J.; Sánchez-Venegas, J.; Contreras, A.; Salazar, L.A.; Alvear, M. The in vitro antifungal evaluation of a commercial extract of Chilean propolis against six fungi of agricultural importance. Cien. Investig. Agrar. 2012, 39, 347–359. [Google Scholar] [CrossRef]
- Cibanal, I.; Fernández, L.; Krepper, G.; Pellegrini, C.; Gallez, L. Avances en el desarrollo de un biofungicida: Caracterización físico-química y actividad antifúngica de propóleos. Agrocienc. Urug. 2019, 23, 99–108. [Google Scholar] [CrossRef]
- Bankova, V.S.; Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Fokt, H.; Pereira, A.; Ferreira, A.M.; Cunha, A.; Aguiar, C. How do bees prevent hive infections? The antimicrobial properties of propolis. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 1, 481–493. [Google Scholar]
- Silva-Carvalho, R.; Miranda-Gonçalves, V.; Ferreira, A.M.; Cardoso, S.M.; Sobral, A.J.F.N.; Almeida-Aguiar, C.; Baltazar, F. Antitumoural and antiangiogenic activity of Portuguese propolis in in vitro and in vivo models. J. Funct. Foods 2014, 11, 160–171. [Google Scholar] [CrossRef]
- Freitas, A.S.; Cunha, A.; Cardoso, S.M.; Oliveira, R.; Almeida-Aguiar, C. Constancy of the bioactivities of propolis samples collected on the same apiary over four years. Food Res. Int. 2019, 119, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.S.; Cunha, A.; Parpot, P.; Cardoso, S.M.; Oliveira, R.; Almeida-Aguiar, C. Propolis efficacy: The Quest for Eco-Friendly Solvents. Molecules 2022, 27, 7531. [Google Scholar] [CrossRef] [PubMed]
- Falcão, S.I.; Vale, N.; Gomes, P.; Domingues, M.R.M.; Freire, C.; Cardoso, S.M.; Vilas-Boas, M. Phenolic Profiling of Portuguese Propolis by LC–MS Spectrometry: Uncommon Propolis Rich in Flavonoid Glycosides. Phytochem. Anal. 2012, 24, 309–318. [Google Scholar] [CrossRef]
- Falcão, S.I.; Freire, C.; Vilas-Boas, M. A Proposal for Physicochemical Standards and Antioxidant Activity of Portuguese Propolis. J. Am. Oil Chem. Soc. 2013, 90, 1729–1741. [Google Scholar] [CrossRef]
- Falcão, S.I.; Tomás, A.; Vale, N.; Gomes, P.; Freire, C.; Vilas-Boas, M. Phenolic quantification and botanical origin of Portuguese propolis. Ind. Crops Prod. 2013, 49, 805–812. [Google Scholar] [CrossRef]
- Casaca, J.D. Manual de Produção de Pólen e Própolis em Portugal, 1st ed.; Federação Nacional dos Apicultores de Portugal: Lisbon, Portugal, 2010. [Google Scholar]
- Vilas-Boas, M. Própolis: Um Negócio Por Explorar. Available online: http://esa.ipb.pt/blogs/noticiasesa/2015/propolis-um-negocio-por-explorar/ (accessed on 5 January 2022).
- Gouvinhas, I.; Martins-Lopes, P.; Carvalho, T.; Barros, A.; Gomes, S. Impact of Colletotrichum acutatum Pathogen on Olive Phenylpropanoid Metabolism. Agriculture 2019, 9, 173. [Google Scholar] [CrossRef]
- Loureiro, A.; Talhinhas, P.; Oliveira, H. Olive anthracnose is caused by different species of fungi, with distinct geographic distribution, virulence and preference for the cultivar [Special issue]. Rev. Ciências Agrárias 2018, 41, 102–109. [Google Scholar]
- Talhinhas, P.; Loureiro, A.; Oliveira, H. Olive anthracnose: A yield- and oil quality-degrading disease caused by several species of Colletotrichum that differ in virulence, host preference and geographical distribution. Mol. Plant Pathol. 2018, 19, 1797–1807. [Google Scholar] [CrossRef]
- Henriques, J.; Nóbrega, F.; Sousa, E.; Lima, A. Analysis of the genetic diversity and phylogenetic relationships of Biscogniauxia mediterranea isolates associated with cork oak. Phytoparasitica 2016, 44, 19–34. [Google Scholar] [CrossRef]
- Moricca, S.; Linaldeddu, B.T.; Ginetti, B.; Scanu, B.; Franceschini, A.; Ragazzi, A. Endemic and Emerging Pathogens Threatening Cork Oak Trees: Management Options for Conserving a Unique Forest Ecosystem. Plant Dis. 2016, 100, 2184–2193. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Machado, H.; Correia, I.; Gomes, F.; Gomes-Laranjo, J.; Costa, R. Phenotyping Castanea hybrids for Phytophthora cinnamomi resistance. Plant Pathol. 2015, 64, 901–910. [Google Scholar] [CrossRef]
- Rivas, E.M.; Gil de Prado, E.; Wrent, P.; Silóniz, M.I.; Barreiro, P.; Correa, E.C.; Conejero, F.; Murciano, A.; Peinado, J.M. A simple mathematical model that describes the growth of the area and the number of total and viable cells in yeast colonies. Lett. Appl. Microbiol. 2014, 59, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Tjørve, K.M.C.; Tjørve, E. The use of Gompertz models in growth analyses, and new Gompertz-model approach: An addition to the Unified-Richards family. PLoS ONE 2017, 12, e0178691. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.W.; Sampers, I.; Meeren, P.V. Influence of virgin coconut oil on the inhibitory effect of emulsion-based edible coatings containing cinnamaldehyde against the growth of Colletotrichum gloeosporioides (Glomerella cingulata). Food Control 2021, 121, 107622. [Google Scholar] [CrossRef]
- Camenzind, T.; Weimershaus, P.; Lehmann, A.; Aguilar-Trigueros, C.; Rillig, M.C. Soil fungi invest into asexual sporulation under resource scarcity, but trait spaces of individual isolates are unique. Environ. Microbiol. 2022, 24, 2962–2978. [Google Scholar] [CrossRef]
- Savary, O.; Coton, M.; Jany, J.L.; Coroller, L.; Coton, E. Effect of abiotic factors and culture media on the growth of cheese-associated Nectriaceae species. Int. J. Food Microbiol. 2022, 364, 109509. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Durán, R.M.; Padilla, R.M.; Martín, A.M.; Ursinos, F.R.; Mendoza, A. Biodegradación de los compuestos fenólicos presentes en el alpechín. Grasas Aceites 1991, 42, 71–276. [Google Scholar]
- Gallez, L.; Kiehr, M.; Fernández, L.; Delhey, R.; Stikar, D. Antifungal activity in vitro of propolis solutions from Argentina against two plant pathogenic fungi: Didymella bryoniae and Rhizotocnia solani. J. Apic. Res. 2014, 53, 438–440. [Google Scholar] [CrossRef]
- Caetano, A.R.; Oliveira, R.D.; Celeiro, S.P.; Freitas, A.S.; Cardoso, S.M.; Gonçalves, M.S.T.; Baltazar, F.; Almeida-Aguiar, C. Phenolic Compounds Contribution to Portuguese Propolis Anti-Melanoma Activity. Molecules 2023, 28, 3107. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid. Based Complement. Alternat. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Ye, S.R.; Ting, C.; Yu, Y.H. Antibacterial activity of propolins from Taiwanese green propolis. J. Food Drug Anal. 2018, 26, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Rodrigues, S.; Feás, X.; Estevinho, L.M. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem. Toxicol. 2012, 50, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Gajger, I.T.; Pavlović, I.; Bojić, M.; Kosalec, I.; Srečec, S.; Vlainić, T.; Vlainić, J. The Components Responsible for the Antimicrobial Activity of Propolis from Continental and Mediterranean Regions in Croatia. Czech J. Food Sci. 2017, 35, 376–385. [Google Scholar] [CrossRef]
- Moreira, L.; Dias, L.G.; Pereira, J.A.; Estevinho, L. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem. Toxicol. 2008, 46, 3482–3485. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Gutiérrez, A.L. Antioxidant Activity and Total Phenolics of Propolis from the Basque Country (Northeastern Spain). J. Am. Oil Chem. Soc. 2011, 88, 1387–1395. [Google Scholar] [CrossRef]
- Maia, S.I.C. Avaliação da Atividade Antioxidante de Extratos de Própolis e Determinação de Componentes com Relevância Farmacológica. Master’s Thesis, Master in Laboratory Medicine, Lisbon, Portugal, 2014. [Google Scholar]
- Pereira, L.; Cunha, A.; Almeida-Aguiar, C. Portuguese propolis from Caramulo as a biocontrol agent of the apple blue mold. Food Control 2022, 139, 109071. [Google Scholar] [CrossRef]
- Gonçalves, A.; Silva, E.; Brito, C.; Martins, S.; Pinto, L.; Dinis, L.T.; Luzio, A.; Martins-Gomes, C.; Fernandes-Silva, A.; Ribeiro, C.; et al. Olive tree physiology and chemical composition of fruits are modulated by different deficit irrigation strategies. J. Sci. Food Agric. 2019, 100, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Carbas, B.; Machado, N.; Oppolzer, D.; Ferreira, L.; Queiroz, M.; Brites, C.; Rosa, E.A.S.; Barros, A.I. Nutrients, Antinutrients, Phenolic Composition, and Antioxidant Activity of Common Bean Cultivars and their Potential for Food Applications. Antioxidants 2020, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- Soufi, O.; Romero, C.; Louaileche, H. Ortho-diphenol profile and antioxidant activity of Algerian black olive cultivars: Effect of dry salting process. Food Chem. 2014, 157, 504–510. [Google Scholar] [CrossRef]
- Araújo, S.; Matos, C.; Correia, E.; Antunes, M.C. Evaluation of phytochemicals content, antioxidant activity and mineral composition of selected edible flowers. Qual. Assur. Saf. Crops Foods 2019, 11, 471–478. [Google Scholar] [CrossRef]
- Yusuf, Y.; Durdane, Y.; Servet, A. Antifungal Activity of Turkish Propolis Against Phytophthora Species. Plant Pathol. J. 2005, 4, 58–60. [Google Scholar]
- Yang, S.; Peng, L.; Cheng, Y.; Chen, F.; Pan, S. Control of Citrus Green and Blue Molds by Chinese Propolis. Food Sci. Biotechnol. 2010, 19, 1303–1308. [Google Scholar] [CrossRef]
- Sánchez, C.; Duarte, P.; Vasilenko, P.; Santos, M.; Loebler, M.; Cruz, A.S.; Gonçalves, M. Potential application of Portuguese propolis to control blue mould disease in ‘Rocha’ pear. Acta Hortic. 2016, 1144, 359–364. [Google Scholar] [CrossRef]
- Loebler, M.; Sánchez, C.; Santos, M.; Vasilenko, P.; Duarte, P.; Cruz, A.; Gonçalves, M. Aplicação de extratos de própolis para conservação pós-colheita de morangos. Vida Rural 2018, 1837, 38–40. [Google Scholar]
- Loebler, M.; Sánchez, C.; Maurício, E.M.; Diogo, E.; Santos, M.; Vasilenko, P.; Cruz, A.S.; Mendes, B.; Gonçalves, M.; Duarte, M.P. Potential Application of Propolis Extracts to Control the Growth of Stemphylium vesicarium in “Rocha” Pear. Appl. Sci. 2020, 10, 1990. [Google Scholar] [CrossRef]
- García, L.R.P.; Galán, J.P.M.; Pajón, C.M.G.; González, J.H.G.; Restrepo, D.L.D. Caracterización Fisicoquímica y Actividad Antimicrobiana del Propóleos en el Municipio de La Unión (Antioquia, Colombia). Rev. Fac. Nac. Agron. Medellín 2010, 63, 5373–5383. [Google Scholar]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential Use of Phenolic Acids as Anti-Candida Agents: A Review. Front. Microbiol. 2015, 6, 1420. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Z.; Peng, L.T.; Su, X.J.; Chen, F.; Cheng, Y.J.; Fan, G.; Pan, S.Y. Bioassay-guided isolation and identification of antifungal components from propolis against Penicillium italicum. Food Chem. 2011, 127, 210–215. [Google Scholar] [CrossRef]
- Peng, L.; Yang, S.; Cheng, Y.J.; Chen, F.; Pan, S.; Fan, G. Antifungal Activity and Action Mode of Pinocembrin from Propolis against Penicillium italicum. Food Sci. Biotechnol. 2012, 21, 1533–1539. [Google Scholar] [CrossRef]
- Chudapongse, N.; Klahan, K.; Kamkhunthod, M.; Ratchawong, C.; Nantapong, N. Antifungal activity against Candida albicans and effect on mitochondrial NADH oxidation of galangin. Planta Med. 2010, 76, P415. [Google Scholar] [CrossRef]
- De Castro, P.A.; Bom, V.L.P.; Brown, N.A.; De Almeida, R.S.C.; Ramalho, L.N.Z.; Savoldi, M.; Goldman, M.H.S.; Berretta, A.A.; Goldman, G.H. Identification of the cell targets important for propolis-induced cell death in Candida albicans. Fungal Genet. Biol. 2013, 60, 74–86. [Google Scholar] [CrossRef]
- Boisard, S.; Le Ray, A.M.; Landreau, A.; Kempf, M.; Cassisa, V.; Flurin, C.; Richomme, P. Antifungal and antibacterial me-tabolites from a French poplar type propolis. Evid. Based Complementary Altern. Med. 2015, 2015, 319240. [Google Scholar] [CrossRef]
- Agüero, M.B.; Gonzalez, M.; Lima, B.; Svetaz, L.; Sánchez, M.; Zacchino, S.; Feresin, G.E.; Shmeda-Hirschmann, G.; Palermo, J.; Wunderlin, D.; et al. Argentinean propolis from Zuccagnia punctata Cav.(Caesalpinieae) exudates: Phytochemical characterization and antifungal activity. J. Agric. Food Chem. 2010, 58, 194–201. [Google Scholar] [CrossRef]
- López, J.M.; Jensen, H.J. Generic model of morphological changes in growing colonies of fungi. Phys. Rev. E 2002, 65, 021903. [Google Scholar] [CrossRef]
- Holmström-Ruddick, B.; Mortensen, K. In vitro formation and survival of appressoria of a mycoherbicide agent, Colletotrichum gloeosporioides f. sp. malvae and a benomyl-resistant strain, HP4.5RR. Mycol. Res. 1995, 99, 1103–1107. [Google Scholar]
- Suthar, M.; Dufossé, L.; Singh, S.K. The Enigmatic World of Fungal Melanin: A Comprehensive Review. J. Fungi 2023, 9, 891. [Google Scholar] [CrossRef]
- Yu, S.M.; Ramkumar, G.; Lee, Y.H. Light quality influences the virulence and physiological responses of Colletotrichum acutatum causing anthracnose in pepper plants. J. Appl. Microbiol. 2013, 115, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, D.; Tian, C. Analysis of melanin biosynthesis in the plant pathogenic fungus Colletotrichum gloeosporioides. Fungal Biol. 2021, 125, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xu, W.; Zong, Y.; Wang, X.; Li, Y.; Bi, Y.; Prusky, D.B. Melanin synthesis gene Aapks contributes to appressorium formation, stress response, cell well integrity and virulence in Alternaria alternata. Postharvest Biol. Technol. 2023, 198, 112247. [Google Scholar] [CrossRef]
- Gamir, J.; Darwiche, R.; Van’t Hof, P.; Choudhary, V.; Stumpe, M.; Schneiter, R.; Mauch, F. The sterol-binding activity of PATHOGENESIS-RELATED PROTEIN 1 reveals the mode of action of an antimicrobial protein. Plant J. 2017, 89, 502–509. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Govers, F. The mysterious route of sterols in oomycetes. PLOS Pathog. 2021, 17, e1009591. [Google Scholar] [CrossRef]
- Wang, Y.; Tyler, B.M.; Wang, Y. Defense and Counterdefense During Plant-Pathogenic Oomycete Infection. Annu. Rev. Microbiol. 2019, 73, 667–696. [Google Scholar] [CrossRef]
- Miljanović, A.; Bhat, R.A.H.; Tandel, R.S.; Pavić, D.; Grbin, D.; Dent, M.; Marijanović, Z.; Jerković, I.; Pedisic, S.; Bielen, A. Bioactive compounds in fluid propolis preparations inhibit different life stages of pathogenic oomycetes Aphanomyces astaci and Saprolegnia parasitica. Aquaculture 2022, 552, 737982. [Google Scholar] [CrossRef]
- Robinson, S.M.; Bostock, R.M. β-glucans and eicosapolyenoic acids as MAMPs in plant–oomycete interactions: Past and present. Front. Plant Sci. 2015, 5, 797. [Google Scholar] [CrossRef][Green Version]
- Castillo-Reyes, F.; Hernández-Castillo, F.D.; Clemente-Constantino, J.A.; Gallegos-Morales, G.; Rodríguez-Herrera, R.; Aguilar, C.N. In vitro antifungal activity of polyphenols-rich plant extracts against Phytophthora cinnamomi Rands. Afr. J. Agric. Res. 2015, 10, 4554–4560. [Google Scholar]

| Filamentous Fungi/Oomycete | Reference | Environmental Isolates | Origin |
|---|---|---|---|
| Biscogniauxia mediterranea | Br41 | Quercus suber L. | Teresa Lino Neto (University of Minho) |
| Colletotrichum acutatum | PT227 | Olea europaea L | Rui Oliveira (University of Minho) |
| Colletotrichum fioriniae | unknown | Olea europaea L. | Paula Baptista (Polytechnic Institute of Bragança) |
| Diplodia corticola | CAA008 | Quercus suber L. | Rui Oliveira (University of Minho) |
| Phytophthora cinnamomi | PH107 | Castanea sativa Mill. | Rui Oliveira (University of Minho) |
| Extract | Yield (%) | TPC (mg GAE/g Extract) | TFC (mg QE/g Extract) | TOC (mg GAE/g Extract) |
|---|---|---|---|---|
| A16.EE | 83.4 | 89.58 ± 3.49 | 30.47 ± 1.01 | 352.25 ± 3.43 |
| B. mediterranea | µ (mm/day) | ti (days) | Ymax (mm) |
|---|---|---|---|
| C-H2O | 80.74 (±1.53) a | 1.37 (±0.03) a | 86.47 (±0.15) a |
| C-EtOH | 79.90 (±1.67) a | 1.36 (±0.03) a | 86.51 (±0.08) a |
| 500 µg/mL | 15.01 (±0.92) b | 3.19 (±0.16) b | 85.42 (±1.91) b |
| 1000 µg/mL | 14.51 (±0.35) b | 3.31 (±0.03) bc | 85.34 (±1.96) b |
| 2000 µg/mL | 12.90 (±0.46) b | 3.61 (±0.20) c | 85.21 (±0.94) b |
| C. acutatum | µ (mm/day) | ti (days) | Ymax (mm) |
| C-H2O | 9.84 (±0.19) a | 4.07 (±0.07) a | 86.10 (±0.17) a |
| C-EtOH | 9.05 (±1.45) a | 3.86 (±0.45) a | 86.48 (±1.23) a |
| 500 µg/mL | 6.38 (±0.10) b | 5.35 (±0.55) b | 85.70 (±1.04) b |
| 1000 µg/mL | 6.01 (±0.13) b | 6.02 (±0.28) bc | 85.51 (±1.05) b |
| 2000 µg/mL | 5.50 (±0.24) b | 6.44 (±0.32) c | 85.33 (±0.66) b |
| C. fioriniae | µ (mm/day) | ti (days) | Ymax (mm) |
| C-H2O | 11.78 (±0.40) a | 3.11 (±0.09) a | 85.13 (±0.10) a |
| C-EtOH | 11.22 (±1.10) a | 3.14 (±0.09) a | 84.10 (±0.66) a |
| 500 µg/mL | 7.54 (±0.89) b | 3.43 (±0.24) a | 68.77 (±2.28) b |
| 1000 µg/mL | 6.30 (±0.65) bc | 3.75 (±0.33) ab | 68.14 (±1.93) b |
| 2000 µg/mL | 5.24 (±0.61) c | 4.35 (±0.45) b | 65.09 (±0.71) c |
| D. corticola | µ (mm/day) | ti (days) | Ymax (mm) |
| C-H2O | 13.69 (±1.23) a | 2.78 (±0.12) a | 85.33 (±0.17) a |
| C-EtOH | 11.77 (±1.46) a | 3.23 (±0.30) a | 86.32 (±0.30) a |
| 500 µg/mL | 6.45 (±0.94) b | 5.53 (±0.67) b | 81.10 (±1.67) b |
| 1000 µg/mL | 5.06 (±0.51) b | 6.66 (±0.38) bc | 80.94 (±1.26) b |
| 2000 µg/mL | 4.54 (±0.42) b | 6.83 (±0.57) c | 78.81 (±0.83) c |
| P. cinnamomi | µ (mm/day) | ti (days) | Ymax (mm) |
| C-H2O | 14.47 (±0.32) a | 2.62 (±0.12) a | 86.68 (±0.31) a |
| C-EtOH | 14.15 (±0.64) a | 2.58 (±0.06) a | 86.03 (±0.57) a |
| 500 µg/mL | 3.90 (±0.24) b | 8.54 (±1.14) ab | 90.23 (±1.41) a |
| 1000 µg/mL | 2.22 (±0.13) c | 14.86 (±1.11) b | 78.91 (±2.72) b |
| 2000 µg/mL | 1.55 (±0.12) c | 22.67 (±5.12) c | 68.68 (±2.34) c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passão, C.; Almeida-Aguiar, C.; Cunha, A. Modelling the In Vitro Growth of Phytopathogenic Filamentous Fungi and Oomycetes: The Gompertz Parameters as Robust Indicators of Propolis Antifungal Action. J. Fungi 2023, 9, 1161. https://doi.org/10.3390/jof9121161
Passão C, Almeida-Aguiar C, Cunha A. Modelling the In Vitro Growth of Phytopathogenic Filamentous Fungi and Oomycetes: The Gompertz Parameters as Robust Indicators of Propolis Antifungal Action. Journal of Fungi. 2023; 9(12):1161. https://doi.org/10.3390/jof9121161
Chicago/Turabian StylePassão, Catarina, Cristina Almeida-Aguiar, and Ana Cunha. 2023. "Modelling the In Vitro Growth of Phytopathogenic Filamentous Fungi and Oomycetes: The Gompertz Parameters as Robust Indicators of Propolis Antifungal Action" Journal of Fungi 9, no. 12: 1161. https://doi.org/10.3390/jof9121161
APA StylePassão, C., Almeida-Aguiar, C., & Cunha, A. (2023). Modelling the In Vitro Growth of Phytopathogenic Filamentous Fungi and Oomycetes: The Gompertz Parameters as Robust Indicators of Propolis Antifungal Action. Journal of Fungi, 9(12), 1161. https://doi.org/10.3390/jof9121161







