Dendritic Cells: Multifunctional Roles in Host Defenses to Cryptococcus Infections
Abstract
1. Introduction
Dendritic Cell Subsets
2. Functional Capacities of DCs
2.1. Recognition
2.2. Initial Degradation and Processing of Cryptococcus by DCs
2.3. Antigen Presenting in Lymphoid Tissues to Prime T Cells: Roles of cDCs
2.4. Restimulation of T Cells
2.5. Terminal Effector Cells
2.6. Role of DCs in Immune Memory
3. DC Modulation by Cryptococcal Factors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Spec, A.; Powderly, W.G. Cryptococcal Meningitis in AIDS. Handb. Clin. Neurol. 2018, 152, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. Dendritic Cells and the Control of Immunity: Enhancing the Efficiency of Antigen Presentation. Mt. Sinai J. Med. N. Y. 2001, 68, 160–166. [Google Scholar]
- Banchereau, J.; Steinman, R.M. Dendritic Cells and the Control of Immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Ramirez-Ortiz, Z.G.; Means, T.K. The Role of Dendritic Cells in the Innate Recognition of Pathogenic Fungi (A. Fumigatus, C. Neoformans and C. Albicans). Virulence 2012, 3, 635–646. [Google Scholar] [CrossRef]
- Jakubzick, C.; Bogunovic, M.; Bonito, A.J.; Kuan, E.L.; Merad, M.; Randolph, G.J. Lymph-Migrating, Tissue-Derived Dendritic Cells Are Minor Constituents within Steady-State Lymph Nodes. J. Exp. Med. 2008, 205, 2839–2850. [Google Scholar] [CrossRef]
- Bozza, S.; Gaziano, R.; Spreca, A.; Bacci, A.; Montagnoli, C.; di Francesco, P.; Romani, L. Dendritic Cells Transport Conidia and Hyphae of Aspergillus Fumigatus from the Airways to the Draining Lymph Nodes and Initiate Disparate Th Responses to the Fungus1. J. Immunol. 2002, 168, 1362–1371. [Google Scholar] [CrossRef]
- Legge, K.L.; Braciale, T.J. Accelerated Migration of Respiratory Dendritic Cells to the Regional Lymph Nodes Is Limited to the Early Phase of Pulmonary Infection. Immunity 2003, 18, 265–277. [Google Scholar] [CrossRef]
- Allam, J.-P.; Stojanovski, G.; Friedrichs, N.; Peng, W.; Bieber, T.; Wenzel, J.; Novak, N. Distribution of Langerhans Cells and Mast Cells within the Human Oral Mucosa: New Application Sites of Allergens in Sublingual Immunotherapy? Allergy 2008, 63, 720–727. [Google Scholar] [CrossRef]
- Massberg, S.; Schaerli, P.; Knezevic-Maramica, I.; Köllnberger, M.; Tubo, N.; Moseman, E.A.; Huff, I.V.; Junt, T.; Wagers, A.J.; Mazo, I.B.; et al. Immunosurveillance by Hematopoietic Progenitor Cells Trafficking through Blood, Lymph, and Peripheral Tissues. Cell 2007, 131, 994–1008. [Google Scholar] [CrossRef]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Wang, Y.; Greter, M.; See, P.; Teo, P.; Malleret, B.; Leboeuf, M.; Low, D.; Oller, G.; Almeida, F.; et al. Adult Langerhans Cells Derive Predominantly from Embryonic Fetal Liver Monocytes with a Minor Contribution of Yolk Sac-Derived Macrophages. J. Exp. Med. 2012, 209, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.N.; Beakley, S.G.; Posey, S.; Conn, B.; Maritz, E.; Seshu, J.; Wozniak, K.L. Antifungal Activity of Dendritic Cell Lysosomal Proteins against Cryptococcus Neoformans. Sci. Rep. 2021, 11, 13619. [Google Scholar] [CrossRef] [PubMed]
- Durai, V.; Murphy, K.M. Functions of Murine Dendritic Cells. Immunity 2016, 45, 719–736. [Google Scholar] [CrossRef]
- Misharin, A.V.; Morales-Nebreda, L.; Mutlu, G.M.; Budinger, G.R.S.; Perlman, H. Flow Cytometric Analysis of Macrophages and Dendritic Cell Subsets in the Mouse Lung. Am. J. Respir. Cell Mol. Biol. 2013, 49, 503–510. [Google Scholar] [CrossRef]
- Shortman, K.; Heath, W.R. The CD8+ Dendritic Cell Subset. Immunol. Rev. 2010, 234, 18–31. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Wu, K.; Azhati, B.; Rexiati, M. Culture and Identification of Mouse Bone Marrow-Derived Dendritic Cells and Their Capability to Induce T Lymphocyte Proliferation. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 244–250. [Google Scholar] [CrossRef]
- Hawkins, A.N.; Determann, B.F.; Nelson, B.N.; Wozniak, K.L. Transcriptional Changes in Pulmonary Phagocyte Subsets Dictate the Outcome Following Interaction with The Fungal Pathogen Cryptococcus Neoformans. Front. Immunol. 2021, 12, 722500. [Google Scholar] [CrossRef]
- Masten, B.J.; Olson, G.K.; Kusewitt, D.F.; Lipscomb, M.F. Flt3 Ligand Preferentially Increases the Number of Functionally Active Myeloid Dendritic Cells in the Lungs of Mice. J. Immunol. 2004, 172, 4077–4083. [Google Scholar] [CrossRef]
- Al Dubayee, M.S.; Alayed, H.; Almansour, R.; Alqaoud, N.; Alnamlah, R.; Obeid, D.; Alshahrani, A.; Zahra, M.M.; Nasr, A.; Al-Bawab, A.; et al. Differential Expression of Human Peripheral Mononuclear Cells Phenotype Markers in Type 2 Diabetic Patients and Type 2 Diabetic Patients on Metformin. Front. Endocrinol. 2018, 9, 537. [Google Scholar] [CrossRef]
- Patel, V.I.; Booth, J.L.; Duggan, E.S.; Cate, S.; White, V.L.; Hutchings, D.; Kovats, S.; Burian, D.M.; Dozmorov, M.; Metcalf, J.P. Transcriptional Classification and Functional Characterization of Human Airway Macrophage and Dendritic Cell Subsets. J. Immunol. 2017, 198, 1183–1201. [Google Scholar] [CrossRef] [PubMed]
- Demedts, I.K.; Brusselle, G.G.; Vermaelen, K.Y.; Pauwels, R.A. Identification and Characterization of Human Pulmonary Dendritic Cells. Am. J. Respir. Cell Mol. Biol. 2005, 32, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.I.; Metcalf, J.P. Airway Macrophage and Dendritic Cell Subsets in the Resting Human Lung. Crit. Rev. Immunol. 2018, 38, 303–331. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.N.; Daugherty, C.S.; Sharp, R.R.; Booth, J.L.; Patel, V.I.; Metcalf, J.P.; Jones, K.L.; Wozniak, K.L. Protective Interaction of Human Phagocytic APC Subsets with Cryptococcus Neoformans Induces Genes Associated with Metabolism and Antigen Presentation. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front. Immunol. 2018, 9, 3176. [Google Scholar] [CrossRef]
- Inflammatory Dendritic Cell Markers: R&D Systems. Available online: https://www.rndsystems.com/resources/cell-markers/immune-cells/dendritic-cells/inflammatory-dendritic-cell-markers (accessed on 15 August 2023).
- Osterholzer, J.J.; Curtis, J.L.; Polak, T.; Ames, T.; Chen, G.H.; McDonald, R.; Huffnagle, G.B.; Toews, G.B. CCR2 Mediates Conventional Dendritic Cell Recruitment and the Formation of Bronchovascular Mononuclear Cell Infiltrates in the Lungs of Mice Infected with Cryptococcus Neoformans. J. Immunol. 2008, 181, 610–620. [Google Scholar] [CrossRef]
- Angkasekwinai, P.; Sringkarin, N.; Supasorn, O.; Fungkrajai, M.; Wang, Y.H.; Chayakulkeeree, M.; Ngamskulrungroj, P.; Angkasekwinai, N.; Pattanapanyasat, K. Cryptococcus Gattii Infection Dampens Th1 and Th17 Responses by Attenuating Dendritic Cell Function and Pulmonary Chemokine Expression in the Immunocompetent Hosts. Infect. Immun. 2014, 82, 3880–3890. [Google Scholar] [CrossRef]
- Wozniak, K.L.; Vyas, J.M.; Levitz, S.M. In Vivo Role of Dendritic Cells in a Murine Model of Pulmonary Cryptococcosis. Infect. Immun. 2006, 74, 3817–3824. [Google Scholar] [CrossRef]
- Wozniak, K.L.; Ravi, S.; Macias, S.; Young, M.L.; Olszewski, M.A.; Steele, C.; Wormley, F.L. Insights into the Mechanisms of Protective Immunity against Cryptococcus Neoformans Infection Using a Mouse Model of Pulmonary Cryptococcosis. PLoS ONE 2009, 4, e6854. [Google Scholar] [CrossRef]
- Hole, C.R.; Wager, C.M.L.; Castro-Lopez, N.; Campuzano, A.; Cai, H.; Wozniak, K.L.; Wang, Y.; Wormley, F.L. Induction of Memory-like Dendritic Cell Responses In Vivo. Nat. Commun. 2019, 10, 2955. [Google Scholar] [CrossRef]
- Eastman, A.J.; Xu, J.; Bermik, J.; Potchen, N.; den Dekker, A.; Neal, L.M.; Zhao, G.; Malachowski, A.; Schaller, M.; Kunkel, S.; et al. Epigenetic Stabilization of DC and DC Precursor Classical Activation by TNFα Contributes to Protective T Cell Polarization. Sci. Adv. 2019, 5, eaaw9051. [Google Scholar] [CrossRef]
- Hole, C.R.; Leopold Wager, C.M.; Mendiola, A.S.; Wozniak, K.L.; Campuzano, A.; Lin, X.; Wormley, F.L. Antifungal Activity of Plasmacytoid Dendritic Cells against Cryptococcus Neoformans In Vitro Requires Expression of Dectin-3 (CLEC4D) and Reactive Oxygen Species. Infect. Immun. 2016, 84, 2493–2504. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like Receptors and Innate Immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Holmskov, U.L. Collectins and Collectin Receptors in Innate Immunity. APMIS Suppl. 2000, 100, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.D.; Ramos, R.A.; Tobias, P.S.; Ulevitch, R.J.; Mathison, J.C. CD14, a Receptor for Complexes of Lipopolysaccharide (LPS) and LPS Binding Protein. Science 1990, 249, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Nejad, P.; Häcker, H.; Rutz, M.; Bauer, S.; Vabulas, R.M.; Wagner, H. Bacterial CpG-DNA and Lipopolysaccharides Activate Toll-like Receptors at Distinct Cellular Compartments. Eur. J. Immunol. 2002, 32, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Barton, G.M.; Kagan, J.C.; Medzhitov, R. Intracellular Localization of Toll-like Receptor 9 Prevents Recognition of Self DNA but Facilitates Access to Viral DNA. Nat. Immunol. 2006, 7, 49–56. [Google Scholar] [CrossRef]
- Bieback, K.; Lien, E.; Klagge, I.M.; Avota, E.; Schneider-Schaulies, J.; Duprex, W.P.; Wagner, H.; Kirschning, C.J.; Ter Meulen, V.; Schneider-Schaulies, S. Hemagglutinin Protein of Wild-Type Measles Virus Activates Toll-like Receptor 2 Signaling. J. Virol. 2002, 76, 8729–8736. [Google Scholar] [CrossRef]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; Reis e Sousa, C. Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA. Science 2004, 303, 1529–1531. [Google Scholar] [CrossRef]
- Netea, M.G.; Van der Graaf, C.; Van der Meer, J.W.M.; Kullberg, B.J. Recognition of Fungal Pathogens by Toll-like Receptors. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2004, 23, 672–676. [Google Scholar] [CrossRef]
- Shoham, S.; Huang, C.; Chen, J.M.; Golenbock, D.T.; Levitz, S.M. Toll-like Receptor 4 Mediates Intracellular Signaling without TNF-Alpha Release in Response to Cryptococcus Neoformans Polysaccharide Capsule. J. Immunol. 2001, 166, 4620–4626. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Miyagi, K.; Koguchi, Y.; Kinjo, Y.; Uezu, K.; Kinjo, T.; Akamine, M.; Fujita, J.; Kawamura, I.; Mitsuyama, M.; et al. Limited Contribution of Toll-like Receptor 2 and 4 to the Host Response to a Fungal Infectious Pathogen, Cryptococcus Neoformans. FEMS Immunol. Med. Microbiol. 2006, 47, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Yauch, L.E.; Mansour, M.K.; Shoham, S.; Rottman, J.B.; Levitz, S.M. Involvement of CD14, Toll-Like Receptors 2 and 4, and MyD88 in the Host Response to the Fungal Pathogen Cryptococcus Neoformans In Vivo. Infect. Immun. 2004, 72, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Onyishi, C.U.; Desanti, G.E.; Wilkinson, A.L.; Lara-Reyna, S.; Frickel, E.-M.; Fejer, G.; Christophe, O.D.; Bryant, C.E.; Mukhopadhyay, S.; Gordon, S.; et al. Toll-like Receptor 4 and Macrophage Scavenger Receptor 1 Crosstalk Regulates Phagocytosis of a Fungal Pathogen. Nat. Commun. 2023, 14, 4895. [Google Scholar] [CrossRef]
- Nakamura, K.; Miyazato, A.; Xiao, G.; Hatta, M.; Inden, K.; Aoyagi, T.; Shiratori, K.; Takeda, K.; Akira, S.; Saijo, S.; et al. Deoxynucleic Acids from Cryptococcus Neoformans Activate Myeloid Dendritic Cells via a TLR9-Dependent Pathway. J. Immunol. 2008, 180, 4067–4074. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Ishii, K.; Nakamura, Y.; Miyazato, A.; Maki, A.; Abe, Y.; Miyasaka, T.; Yamamoto, H.; Akahori, Y.; Fue, M.; et al. Toll-like Receptor 9-Dependent Activation of Bone Marrow-Derived Dendritic Cells by URA5 DNA from Cryptococcus Neoformans. Infect. Immun. 2012, 80, 778–786. [Google Scholar] [CrossRef]
- Qiu, Y.; Zeltzer, S.; Zhang, Y.; Wang, F.; Chen, G.H.; Dayrit, J.; Murdock, B.J.; Bhan, U.; Toews, G.B.; Osterholzer, J.J.; et al. Early Induction of CCL7 Downstream of TLR9 Signaling Promotes the Development of Robust Immunity to Cryptococcal Infection. J. Immunol. 2012, 188, 3940–3948. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Bhan, U.; Huffnagle, G.B.; Toews, G.B.; Standiford, T.J.; Olszewski, M.A. TLR9 Signaling Is Required for Generation of the Adaptive Immune Protection in Cryptococcus Neoformans-Infected Lungs. Am. J. Pathol. 2010, 177, 754–765. [Google Scholar] [CrossRef]
- da Silva-Junior, E.B.; Firmino-Cruz, L.; Guimarães-de-Oliveira, J.C.; De-Medeiros, J.V.R.; de Oliveira Nascimento, D.; Freire-de-Lima, M.; de Brito-Gitirana, L.; Morrot, A.; Previato, J.O.; Mendonça-Previato, L.; et al. The Role of Toll-like Receptor 9 in a Murine Model of Cryptococcus Gattii Infection. Sci. Rep. 2021, 11, 1407. [Google Scholar] [CrossRef]
- Saijo, S.; Iwakura, Y. Dectin-1 and Dectin-2 in Innate Immunity against Fungi. Int. Immunol. 2011, 23, 467–472. [Google Scholar] [CrossRef]
- Nakamura, K.; Kinjo, T.; Saijo, S.; Miyazato, A.; Adachi, Y.; Ohno, N.; Fujita, J.; Kaku, M.; Iwakura, Y.; Kawakami, K. Dectin-1 Is Not Required for the Host Defense to Cryptococcus Neoformans. Microbiol. Immunol. 2007, 51, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-Q.; Zhu, L.-L.; Chang, Q.; Jiang, C.; You, Y.; Luo, T.; Jia, X.-M.; Lin, X. C-Type Lectin Receptor Dectin-3 Mediates Trehalose 6,6’-Dimycolate (TDM)-Induced Mincle Expression through CARD9/Bcl10/MALT1-Dependent Nuclear Factor (NF)-ΚB Activation. J. Biol. Chem. 2014, 289, 30052–30062. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, A.; Castro-Lopez, N.; Wozniak, K.L.; Leopold Wager, C.M.; Wormley, F.L. Dectin-3 Is Not Required for Protection against Cryptococcus Neoformans Infection. PLoS ONE 2017, 12, e0169347. [Google Scholar] [CrossRef] [PubMed]
- McGreal, E.P.; Rosas, M.; Brown, G.D.; Zamze, S.; Wong, S.Y.C.; Gordon, S.; Martinez-Pomares, L.; Taylor, P.R. The Carbohydrate-Recognition Domain of Dectin-2 Is a C-Type Lectin with Specificity for High Mannose. Glycobiology 2006, 16, 422–430. [Google Scholar] [CrossRef]
- Tanno, D.; Yokoyama, R.; Kawamura, K.; Kitai, Y.; Yuan, X.; Ishii, K.; De Jesus, M.; Yamamoto, H.; Sato, K.; Miyasaka, T.; et al. Dectin-2-Mediated Signaling Triggered by the Cell Wall Polysaccharides of Cryptococcus Neoformans. Microbiol. Immunol. 2019, 63, 500–512. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sato, K.; Yamamoto, H.; Matsumura, K.; Matsumoto, I.; Nomura, T.; Miyasaka, T.; Ishii, K.; Kanno, E.; Tachi, M.; et al. Dectin-2 Deficiency Promotes Th2 Response and Mucin Production in the Lungs after Pulmonary Infection with Cryptococcus Neoformans. Infect. Immun. 2015, 83, 671–681. [Google Scholar] [CrossRef]
- Walsh, N.M.; Wuthrich, M.; Wang, H.; Klein, B.; Hull, C.M. Characterization of C-Type Lectins Reveals an Unexpectedly Limited Interaction between Cryptococcus Neoformans Spores and Dectin-1. PLoS ONE 2017, 12, e0173866. [Google Scholar] [CrossRef]
- Kitai, Y.; Sato, K.; Tanno, D.; Yuan, X.; Umeki, A.; Kasamatsu, J.; Kanno, E.; Tanno, H.; Hara, H.; Yamasaki, S.; et al. Role of Dectin-2 in the Phagocytosis of Cryptococcus Neoformans by Dendritic Cells. Infect. Immun. 2021, 89, e0033021. [Google Scholar] [CrossRef]
- Ueno, K.; Otani, Y.; Yanagihara, N.; Nakamura, T.; Shimizu, K.; Yamagoe, S.; Miyazaki, Y. Cryptococcus Gattii Alters Immunostimulatory Potential in Response to the Environment. PLoS ONE 2019, 14, e0220989. [Google Scholar] [CrossRef]
- Ambati, S.; Ellis, E.C.; Lin, J.; Lin, X.; Lewis, Z.A.; Meagher, R.B. Dectin-2-Targeted Antifungal Liposomes Exhibit Enhanced Efficacy. mSphere 2019, 4, e00715-19. [Google Scholar] [CrossRef]
- Mansour, M.K.; Yauch, L.E.; Rottman, J.B.; Levitz, S.M. Protective Efficacy of Antigenic Fractions in Mouse Models of Cryptococcosis. Infect. Immun. 2004, 72, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Syme, R.M.; Spurrell, J.C.; Amankwah, E.K.; Green, F.H.; Mody, C.H. Primary Dendritic Cells Phagocytose Cryptococcus Neoformans via Mannose Receptors and Fcgamma Receptor II for Presentation to T Lymphocytes. Infect. Immun. 2002, 70, 5972–5981. [Google Scholar] [CrossRef] [PubMed]
- Vartivarian, S.E.; Reyes, G.H.; Jacobson, E.S.; James, P.G.; Cherniak, R.; Mumaw, V.R.; Tingler, M.J. Localization of Mannoprotein in Cryptococcus Neoformans. J. Bacteriol. 1989, 171, 6850–6852. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.W.; Mosley, R.L.; Cherniak, R.; Reyes, G.H.; Kozel, T.R.; Reiss, E. Serological, Electrophoretic, and Biological Properties of Cryptococcus Neoformans Antigens. Infect. Immun. 1988, 56, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Pietrella, D.; Cherniak, R.; Strappini, C.; Perito, S.; Mosci, P.; Bistoni, F.; Vecchiarelli, A. Role of Mannoprotein in Induction and Regulation of Immunity to Cryptococcus Neoformans. Infect. Immun. 2001, 69, 2808–2814. [Google Scholar] [CrossRef]
- Chaka, W.; Verheul, A.F.; Vaishnav, V.V.; Cherniak, R.; Scharringa, J.; Verhoef, J.; Snippe, H.; Hoepelman, A.I. Induction of TNF-Alpha in Human Peripheral Blood Mononuclear Cells by the Mannoprotein of Cryptococcus Neoformans Involves Human Mannose Binding Protein. J. Immunol. 1997, 159, 2979–2985. [Google Scholar] [CrossRef]
- Dan, J.M.; Wang, J.P.; Lee, C.K.; Levitz, S.M. Cooperative Stimulation of Dendritic Cells by Cryptococcus Neoformans Mannoproteins and CpG Oligodeoxynucleotides. PLoS ONE 2008, 3, e2046. [Google Scholar] [CrossRef]
- Mansour, M.K.; Latz, E.; Levitz, S.M. Cryptococcus Neoformans Glycoantigens Are Captured by Multiple Lectin Receptors and Presented by Dendritic Cells. J. Immunol. 2006, 176, 3053–3061. [Google Scholar] [CrossRef]
- Mansour, M.K.; Schlesinger, L.S.; Levitz, S.M. Optimal T Cell Responses to Cryptococcus Neoformans Mannoprotein Are Dependent on Recognition of Conjugated Carbohydrates by Mannose Receptors. J. Immunol. 2002, 168, 2872–2879. [Google Scholar] [CrossRef]
- Dan, J.M.; Kelly, R.M.; Lee, C.K.; Levitz, S.M. Role of the Mannose Receptor in a Murine Model of Cryptococcus Neoformans Infection. Infect. Immun. 2008, 76, 2362–2367. [Google Scholar] [CrossRef]
- Cross, C.E.; Collins, H.L.; Bancroft, G.J. CR3-Dependent Phagocytosis by Murine Macrophages: Different Cytokines Regulate Ingestion of a Defined CR3 Ligand and Complement-Opsonized Cryptococcus Neoformans. Immunology 1997, 91, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Flaczyk, A.; Neal, L.M.; Fa, Z.; Eastman, A.J.; Malachowski, A.N.; Cheng, D.; Moore, B.B.; Curtis, J.L.; Osterholzer, J.J.; et al. Scavenger Receptor MARCO Orchestrates Early Defenses and Contributes to Fungal Containment during Cryptococcal Infection. J. Immunol. 2017, 198, 3548–3557. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.L.; Holly, A. Candida Albicans Is Phagocytosed, Killed, and Processed for Antigen Presentation by Human Dendritic Cells. Infect. Immun. 2001, 69, 6813–6822. [Google Scholar] [CrossRef]
- Kelly, R.M.; Chen, J.; Yauch, L.E.; Levitz, S.M. Opsonic Requirements for Dendritic Cell-Mediated Responses to Cryptococcus Neoformans. Infect. Immun. 2005, 73, 592–598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wozniak, K.L.; Levitz, S.M. Cryptococcus Neoformans Enters the Endolysosomal Pathway of Dendritic Cells and Is Killed by Lysosomal Components. Infect. Immun. 2008, 76, 4764–4771. [Google Scholar] [CrossRef] [PubMed]
- Artavanis-Tsakonas, K.; Love, J.C.; Ploegh, H.L.; Vyas, J.M. Recruitment of CD63 to Cryptococcus Neoformans Phagosomes Requires Acidification. Proc. Natl. Acad. Sci. USA 2006, 103, 15945–15950. [Google Scholar] [CrossRef] [PubMed]
- Hole, C.R.; Bui, H.; Wormley, F.L.; Wozniak, K.L. Mechanisms of Dendritic Cell Lysosomal Killing of Cryptococcus. Sci. Rep. 2012, 2, 739. [Google Scholar] [CrossRef] [PubMed]
- Levitz, S.M.; Nong, S.H.; Seetoo, K.F.; Harrison, T.S.; Speizer, R.A.; Simons, E.R. Cryptococcus Neoformans Resides in an Acidic Phagolysosome of Human Macrophages. Infect. Immun. 1999, 67, 885–890. [Google Scholar] [CrossRef]
- De Leon-Rodriguez, C.M.; Rossi, D.C.P.; Fu, M.S.; Dragotakes, Q.; Coelho, C.; Ros, I.G.; Caballero, B.; Nolan, S.J.; Casadevall, A. The Outcome of the Cryptococcus Neoformans-Macrophage Interaction Depends on Phagolysosomal Membrane Integrity. J. Immunol. 2018, 201, 583–603. [Google Scholar] [CrossRef]
- Smith, L.M.; Dixon, E.F.; May, R.C. The Fungal Pathogen Cryptococcus Neoformans Manipulates Macrophage Phagosome Maturation. Cell Microbiol. 2015, 17, 702–713. [Google Scholar] [CrossRef]
- Orsi, C.F.; Colombari, B.; Ardizzoni, A.; Peppoloni, S.; Neglia, R.; Posteraro, B.; Morace, G.; Fadda, G.; Blasi, E. The ABC Transporter-Encoding Gene AFR1 Affects the Resistance of Cryptococcus Neoformans to Microglia-Mediated Antifungal Activity by Delaying Phagosomal Maturation. FEMS Yeast Res. 2009, 9, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Burgos, E.J.; Stuckey, P.V.; Santiago-Tirado, F.H. Real-Time Visualization of Phagosomal PH Manipulation by Cryptococcus Neoformans in an Immune Signal-Dependent Way. Front. Cell Infect. Microbiol. 2022, 12, 967486. [Google Scholar] [CrossRef] [PubMed]
- De Leon-Rodriguez, C.M.; Fu, M.S.; Çorbali, M.O.; Cordero, R.J.B.; Casadevall, A. The Capsule of Cryptococcus Neoformans Modulates Phagosomal PH through Its Acid-Base Properties. mSphere 2018, 3, e00437-18. [Google Scholar] [CrossRef]
- Xu, J.; Eastman, A.J.; Flaczyk, A.; Neal, L.M.; Zhao, G.; Carolan, J.; Malachowski, A.N.; Stolberg, V.R.; Yosri, M.; Chensue, S.W.; et al. Disruption of Early Tumor Necrosis Factor Alpha Signaling Prevents Classical Activation of Dendritic Cells in Lung-Associated Lymph Nodes and Development of Protective Immunity against Cryptococcal Infection. mBio 2016, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; Traynor, T.R.; McDonald, R.A.; Olszewski, M.A.; Lindell, D.M.; Herring, A.C.; Toews, G.B. Leukocyte Recruitment during Pulmonary Cryptococcus Neoformans Infection. Immunopharmacology 2000, 48, 231–236. [Google Scholar] [CrossRef]
- Lindell, D.M.; Moore, T.A.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Distinct Compartmentalization of CD4+ T-Cell Effector Function versus Proliferative Capacity during Pulmonary Cryptococcosis. Am. J. Pathol. 2006, 168, 847–855. [Google Scholar] [CrossRef]
- Xu, J.; Flaczyk, A.; Neal, L.M.; Fa, Z.; Cheng, D.; Ivey, M.; Moore, B.B.; Curtis, J.L.; Osterholzer, J.J.; Olszewski, M.A. Exploitation of Scavenger Receptor, Macrophage Receptor with Collagenous Structure, by Cryptococcus Neoformans Promotes Alternative Activation of Pulmonary Lymph Node CD11b+ Conventional Dendritic Cells and Non-Protective Th2 Bias. Front. Immunol. 2017, 8, 1231. [Google Scholar] [CrossRef]
- Bauman, S.K.; Huffnagle, G.B.; Murphy, J.W. Effects of Tumor Necrosis Factor Alpha on Dendritic Cell Accumulation in Lymph Nodes Draining the Immunization Site and the Impact on the Anticryptococcal Cell-Mediated Immune Response. Infect. Immun. 2003, 71, 68–74. [Google Scholar] [CrossRef]
- Bauman, S.K.; Nichols, K.L.; Murphy, J.W. Dendritic Cells in the Induction of Protective and Nonprotective Anticryptococcal Cell-Mediated Immune Responses. J. Immunol. 2000, 165, 158–167. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Rivera, A.; Xue, C. Development of a Heat-Killed Fbp1 Mutant Strain as a Therapeutic Agent To Treat Invasive Cryptococcus Infection. Microbiol. Spectr. 2023, 11, e0495522. [Google Scholar] [CrossRef]
- Movahed, E.; Cheok, Y.Y.; Tan, G.M.Y.; Lee, C.Y.Q.; Cheong, H.C.; Velayuthan, R.D.; Tay, S.T.; Chong, P.P.; Wong, W.F.; Looi, C.Y. Lung-Infiltrating T Helper 17 Cells as the Major Source of Interleukin-17A Production during Pulmonary Cryptococcus Neoformans Infection. BMC Immunol. 2018, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, D.L.; Specht, C.A.; Lee, C.K.; Smith, K.D.; Mukaremera, L.; Lee, S.T.; Lee, C.G.; Elias, J.A.; Nielsen, J.N.; Boulware, D.R.; et al. Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection. PLoS Pathog. 2015, 11, e1004701. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic Cells, Monocytes and Macrophages: A Unified Nomenclature Based on Ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef]
- Plantinga, M.; Guilliams, M.; Vanheerswynghels, M.; Deswarte, K.; Branco-Madeira, F.; Toussaint, W.; Vanhoutte, L.; Neyt, K.; Killeen, N.; Malissen, B.; et al. Conventional and Monocyte-Derived CD11b(+) Dendritic Cells Initiate and Maintain T Helper 2 Cell-Mediated Immunity to House Dust Mite Allergen. Immunity 2013, 38, 322–335. [Google Scholar] [CrossRef]
- Herring, A.C.; Falkowski, N.R.; Chen, G.H.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Transient Neutralization of Tumor Necrosis Factor Alpha Can Produce a Chronic Fungal Infection in an Immunocompetent Host: Potential Role of Immature Dendritic Cells. Infect. Immun. 2005, 73, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Fa, Z.; Xu, J.; Yi, J.; Sang, J.; Pan, W.; Xie, Q.; Yang, R.; Fang, W.; Liao, W.; Olszewski, M.A. TNF-α-Producing Cryptococcus Neoformans Exerts Protective Effects on Host Defenses in Murine Pulmonary Cryptococcosis. Front. Immunol. 2019, 10, 1725. [Google Scholar] [CrossRef]
- Coillard, A.; Segura, E. Antigen Presentation by Mouse Monocyte-Derived Cells: Re-Evaluating the Concept of Monocyte-Derived Dendritic Cells. Mol. Immunol. 2021, 135, 165–169. [Google Scholar] [CrossRef]
- Osterholzer, J.J.; Chen, G.H.; Olszewski, M.A.; Curtis, J.L.; Huffnagle, G.B.; Toews, G.B. Accumulation of CD11b+ Lung Dendritic Cells in Response to Fungal Infection Results from the CCR2-Mediated Recruitment and Differentiation of Ly-6Chigh Monocytes. J. Immunol. 2009, 183, 8044–8053. [Google Scholar] [CrossRef]
- Huston, S.M.; Li, S.S.; Stack, D.; Timm-McCann, M.; Jones, G.J.; Islam, A.; Berenger, B.M.; Xiang, R.F.; Colarusso, P.; Mody, C.H. Cryptococcus Gattii is Killed by Dendritic Cells, but Evades Adaptive Immunity by Failing to Induce Dendritic Cell Maturation. J. Immunol. 2013, 191, 249–261. [Google Scholar] [CrossRef]
- Kidd, S.E.; Hagen, F.; Tscharke, R.L.; Huynh, M.; Bartlett, K.H.; Fyfe, M.; Macdougall, L.; Boekhout, T.; Kwon-Chung, K.J.; Meyer, W. A Rare Genotype of Cryptococcus Gattii Caused the Cryptococcosis Outbreak on Vancouver Island (British Columbia, Canada). Proc. Natl. Acad. Sci. USA 2004, 101, 17258–17263. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.J.; Xavier, R.J. Trained Immunity: A Program of Innate Immune Memory in Health and Disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Leopold Wager, C.M.; Hole, C.R.; Campuzano, A.; Castro-Lopez, N.; Cai, H.; Caballero Van Dyke, M.C.; Wozniak, K.L.; Wang, Y.; Wormley, F.L. IFN-γ Immune Priming of Macrophages in Vivo Induces Prolonged STAT1 Binding and Protection against Cryptococcus Neoformans. PLoS Pathog. 2018, 14, e1007358. [Google Scholar] [CrossRef]
- Nami, S.; Mohammadi, R.; Vakili, M.; Khezripour, K.; Mirzaei, H.; Morovati, H. Fungal Vaccines, Mechanism of Actions and Immunology: A Comprehensive Review. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 109, 333–344. [Google Scholar] [CrossRef]
- Caballero Van Dyke, M.C.; Wormley, F.L. A Call to Arms: Quest for a Cryptococcal Vaccine. Trends Microbiol. 2018, 26, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Urai, M.; Takatsuka, S.; Abe, M.; Miyazaki, Y.; Kinjo, Y. Immunization with Antigen-Pulsed Dendritic Cells Against Highly Virulent Cryptococcus Gattii Infection: Analysis of Cytokine-Producing T Cells. Methods Mol. Biol. Clifton NJ 2017, 1625, 327–339. [Google Scholar] [CrossRef]
- Ueno, K.; Urai, M.; Sadamoto, S.; Shinozaki, M.; Takatsuka, S.; Abe, M.; Otani, Y.; Yanagihara, N.; Shimizu, K.; Iwakura, Y.; et al. A Dendritic Cell-Based Systemic Vaccine Induces Long-Lived Lung-Resident Memory Th17 Cells and Ameliorates Pulmonary Mycosis. Mucosal. Immunol. 2019, 12, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Coelho, C.; Cordero, R.J.B.; Dragotakes, Q.; Jung, E.; Vij, R.; Wear, M.P. The Capsule of Cryptococcus Neoformans. Virulence 2019, 10, 822–831. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, T.R.; Alspaugh, J.A. The Cryptococcus Neoformans Capsule: A Sword and a Shield. Clin. Microbiol. Rev. 2012, 25, 387–408. [Google Scholar] [CrossRef]
- Wang, Z.A.; Li, L.X.; Doering, T.L. Unraveling Synthesis of the Cryptococcal Cell Wall and Capsule. Glycobiology 2018, 28, 719–730. [Google Scholar] [CrossRef]
- Yauch, L.E.; Lam, J.S.; Levitz, S.M. Direct Inhibition of T-Cell Responses by the Cryptococcus Capsular Polysaccharide Glucuronoxylomannan. PLoS Pathog. 2006, 2, e120. [Google Scholar] [CrossRef]
- Huston, S.M.; Ngamskulrungroj, P.; Xiang, R.F.; Ogbomo, H.; Stack, D.; Li, S.S.; Timm-McCann, M.; Kyei, S.K.; Oykhman, P.; Kwon-Chung, K.J.; et al. Cryptococcus Gattii Capsule Blocks Surface Recognition Required for Dendritic Cell Maturation Independent of Internalization and Antigen Processing. J. Immunol. 2016, 196, 1259–1271. [Google Scholar] [CrossRef]
- Urai, M.; Kaneko, Y.; Ueno, K.; Okubo, Y.; Aizawa, T.; Fukazawa, H.; Sugita, T.; Ohno, H.; Shibuya, K.; Kinjo, Y.; et al. Evasion of Innate Immune Responses by the Highly Virulent Cryptococcus Gattii by Altering Capsule Glucuronoxylomannan Structure. Front. Cell Infect. Microbiol. 2015, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Teitz-Tennenbaum, S.; Viglianti, S.P.; Roussey, J.A.; Levitz, S.M.; Olszewski, M.A.; Osterholzer, J.J. Autocrine IL-10 Signaling Promotes Dendritic Cell Type-2 Activation and Persistence of Murine Cryptococcal Lung Infection. J. Immunol. 2018, 201, 2004–2015. [Google Scholar] [CrossRef] [PubMed]
- Vecchiarelli, A.; Pietrella, D.; Lupo, P.; Bistoni, F.; McFadden, D.C.; Casadevall, A. The Polysaccharide Capsule of Cryptococcus Neoformans Interferes with Human Dendritic Cell Maturation and Activation. J. Leukoc. Biol. 2003, 74, 370–378. [Google Scholar] [CrossRef]
- Vecchiarelli, A.; Retini, C.; Pietrella, D.; Monari, C.; Tascini, C.; Beccari, T.; Kozel, T.R. Downregulation by Cryptococcal Polysaccharide of Tumor Necrosis Factor Alpha and Interleukin-1 Beta Secretion from Human Monocytes. Infect. Immun. 1995, 63, 2919–2923. [Google Scholar] [CrossRef] [PubMed]
- Lupo, P.; Chang, Y.C.; Kelsall, B.L.; Farber, J.M.; Pietrella, D.; Vecchiarelli, A.; Leon, F.; Kwon-Chung, K.J. The Presence of Capsule in Cryptococcus Neoformans Influences the Gene Expression Profile in Dendritic Cells during Interaction with the Fungus. Infect. Immun. 2008, 76, 1581–1589. [Google Scholar] [CrossRef]
- Kawakami, K.; Qifeng, X.; Tohyama, M.; Qureshi, M.H.; Saito, A. Contribution of Tumour Necrosis Factor-Alpha (TNF-Alpha) in Host Defence Mechanism against Cryptococcus Neoformans. Clin. Exp. Immunol. 1996, 106, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Grijpstra, J.; Tefsen, B.; van Die, I.; de Cock, H. The Cryptococcus Neoformans Cap10 and Cap59 Mutant Strains, Affected in Glucuronoxylomannan Synthesis, Differentially Activate Human Dendritic Cells. FEMS Immunol. Med. Microbiol. 2009, 57, 142–150. [Google Scholar] [CrossRef]
- Osterholzer, J.J.; Surana, R.; Milam, J.E.; Montano, G.T.; Chen, G.H.; Sonstein, J.; Curtis, J.L.; Huffnagle, G.B.; Toews, G.B.; Olszewski, M.A. Cryptococcal Urease Promotes the Accumulation of Immature Dendritic Cells and a Non-Protective T2 Immune Response within the Lung. Am. J. Pathol. 2009, 174, 932–943. [Google Scholar] [CrossRef]
- Li, L.X.; Hole, C.R.; Rangel-Moreno, J.; Khader, S.A.; Doering, T.L. Cryptococcus Neoformans Evades Pulmonary Immunity by Modulating Xylose Precursor Transport. Infect. Immun. 2020, 88, 10–1128. [Google Scholar] [CrossRef]
- Liu, T.-B.; Xue, C. Fbp1-Mediated Ubiquitin-Proteasome Pathway Controls Cryptococcus Neoformans Virulence by Regulating Fungal Intracellular Growth in Macrophages. Infect. Immun. 2014, 82, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-B.; Wang, Y.; Stukes, S.; Chen, Q.; Casadevall, A.; Xue, C. The F-Box Protein Fbp1 Regulates Sexual Reproduction and Virulence in Cryptococcus Neoformans. Eukaryot. Cell 2011, 10, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-B.; Xue, C. The Ubiquitin-Proteasome System and F-Box Proteins in Pathogenic Fungi. Mycobiology 2011, 39, 243–248. [Google Scholar] [CrossRef]
- Masso-Silva, J.; Espinosa, V.; Liu, T.B.; Wang, Y.; Xue, C.; Rivera, A. The F-Box Protein Fbp1 Shapes the Immunogenic Potential of Cryptococcus neoformans. mBio 2018, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Abe, Y.; Miyazato, A.; Tanno, D.; Tanaka, M.; Miyasaka, T.; Ishii, K.; Kawakami, K. Cryptococcus Neoformans Suppresses the Activation of Bone Marrow-Derived Dendritic Cells Stimulated with Its Own DNA, but Not with DNA from Other Fungi. FEMS Immunol. Med. Microbiol. 2011, 63, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Jamil, K.; Polyak, M.J.; Feehan, D.D.; Surmanowicz, P.; Stack, D.; Li, S.S.; Ogbomo, H.; Olszewski, M.; Ganguly, A.; Mody, C.H. Phagosomal F-Actin Retention by Cryptococcus Gattii Induces Dendritic Cell Immunoparalysis. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef]

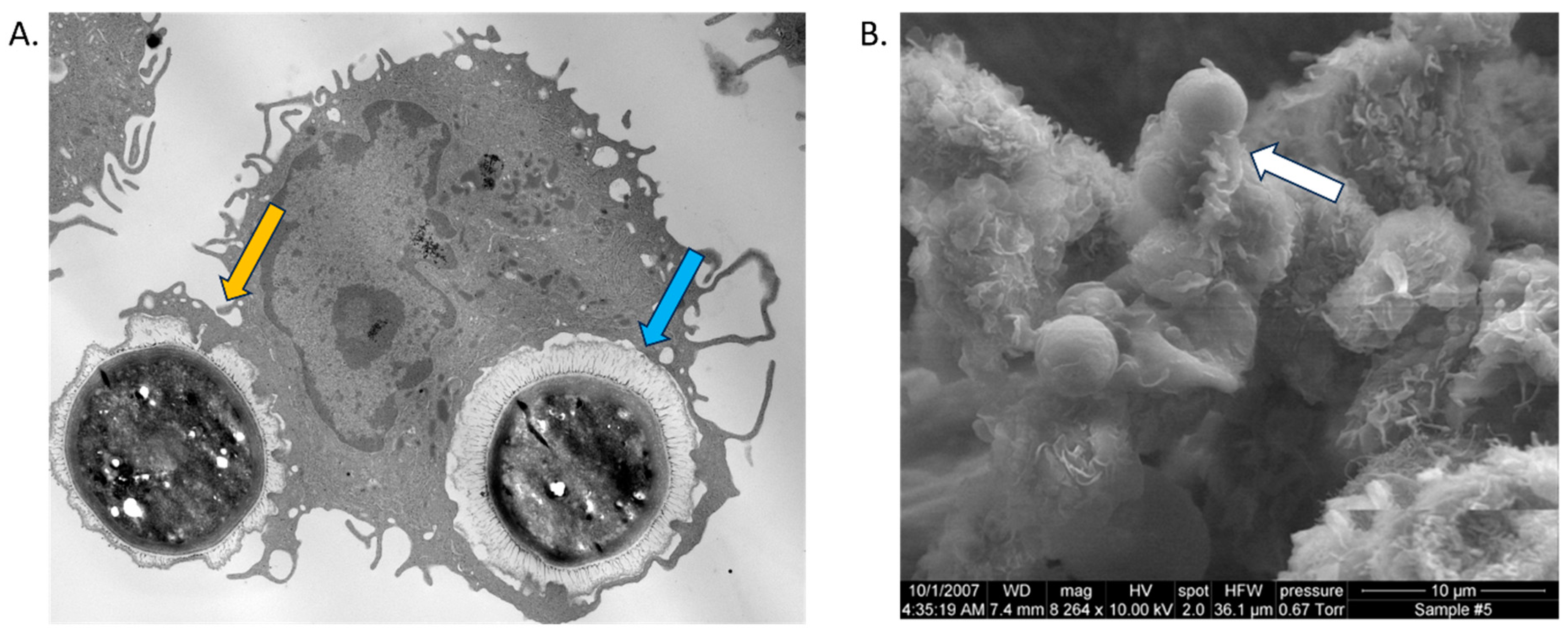

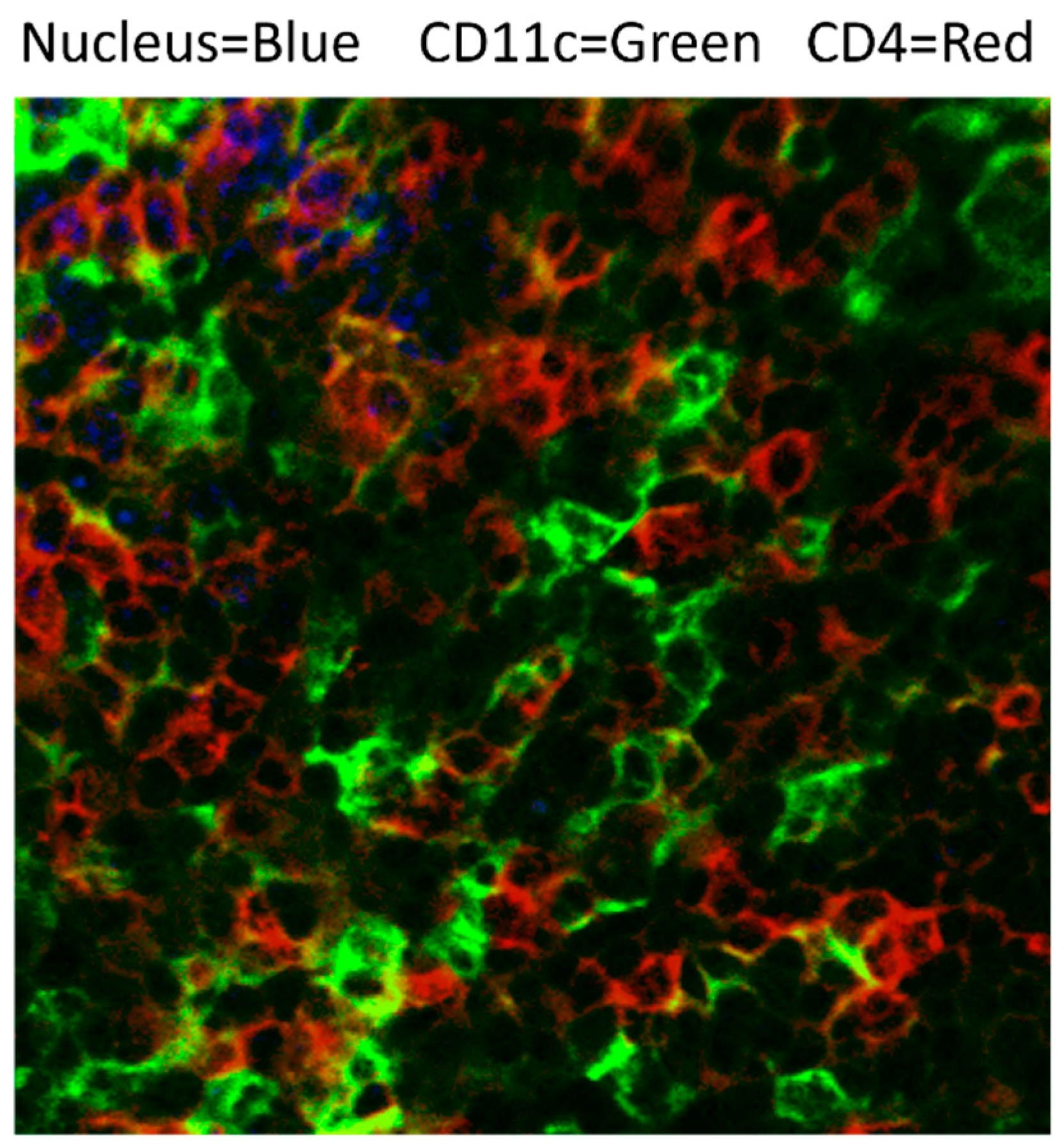
| DC Subset | Description | Location | Surface Markers | References |
|---|---|---|---|---|
| cDC2 | CD11b+ Conventional Pulmonary DCs | Lungs | CD11c+/CD24+/CD11b+/CD172α+ | [11,14,25] |
| cDC1 | CD103+ Conventional Pulmonary DCs | Lungs | CD11c+/CD24+/CD11b−/CD103+ | [11,14,25] |
| pDCs | Plasmacytoid DCs (pDCs) | Lungs and circulation | CD11cint/PDCA1+/B220+ | [15] |
| MoDCs | Inflammatory monocyte-derived DCs (MoDCs) | Lungs, from circulation | CD11b+, CD11c+, F4/80+, Ly-6C+, MHC II+, and CD64+(Fc gamma RI+) | [25,26] |
| inf-cDC2 | Inflammatory cDC | Lungs (only during inflammation) | CD26+, CD172A+, | [27] |
| LCs | Langerhans DCs | Epidermal layer of skin | CD11chi, Langerin+ (CD207+), EpCam+, CLEC9a−, CD209+, DEC205+, F4/80+, CD103−, CD11b+, CD207+ | [11] |
| LDC | Lymphoid DCs | Peripheral lymphoid organs | CD11c+ CD11b− DEC205hiCD8hi | [16] |
| BMDCs | Bone marrow derived dendritic cells | Bone marrow | CD11c+ F4/80− | [17] |
| DC Subset | Description | Location | Surface Markers | References |
|---|---|---|---|---|
| CD1c+ CD14− DC | Conventional pulmonary DC subset | Lungs | BDCA1+(CD1c+)/CD14−/CD11c+ | [21,23,25] |
| CD1c+ CD14+ DC | Conventional pulmonary DC subset | Lungs | BDCA1+(CD1c+)/ CD14+/CD11c+ | [21,23,25] |
| Langerin+ DC | Langerin DCs | Lungs | HLA-DR+/langerin+ | [21] |
| pDC | Plasmacytoid DCs (pDCs) | Lungs | BDCA2+(CD303+)/ CD11c−/CD123+ | [23] |
| DC precursor | Classical monocytes | Circulation | CD14hi CD16− | [20] |
| DC precursor | Non-classical monocytes | Circulation | CD16hi CD14lo | [20] |
| DC precursor | Intermediate monocytes | Circulation | CD14hi CD16hi | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goughenour, K.D.; Nair, A.S.; Xu, J.; Olszewski, M.A.; Wozniak, K.L. Dendritic Cells: Multifunctional Roles in Host Defenses to Cryptococcus Infections. J. Fungi 2023, 9, 1050. https://doi.org/10.3390/jof9111050
Goughenour KD, Nair AS, Xu J, Olszewski MA, Wozniak KL. Dendritic Cells: Multifunctional Roles in Host Defenses to Cryptococcus Infections. Journal of Fungi. 2023; 9(11):1050. https://doi.org/10.3390/jof9111050
Chicago/Turabian StyleGoughenour, Kristie D., Ayesha S. Nair, Jintao Xu, Michal A. Olszewski, and Karen L. Wozniak. 2023. "Dendritic Cells: Multifunctional Roles in Host Defenses to Cryptococcus Infections" Journal of Fungi 9, no. 11: 1050. https://doi.org/10.3390/jof9111050
APA StyleGoughenour, K. D., Nair, A. S., Xu, J., Olszewski, M. A., & Wozniak, K. L. (2023). Dendritic Cells: Multifunctional Roles in Host Defenses to Cryptococcus Infections. Journal of Fungi, 9(11), 1050. https://doi.org/10.3390/jof9111050







