Replacing Mancozeb with Alternative Fungicides for the Control of Late Blight in Potato
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Chambers Experiments
2.2. Tunnel Experiment 1
2.2.1. Plants
2.2.2. Fungicides
2.2.3. Pathogen
2.3. Tunnel Experiment 2
2.4. Disease Assessment and Fungicide Efficiency
2.5. Potato Yield
2.6. Data Analysis
3. Results
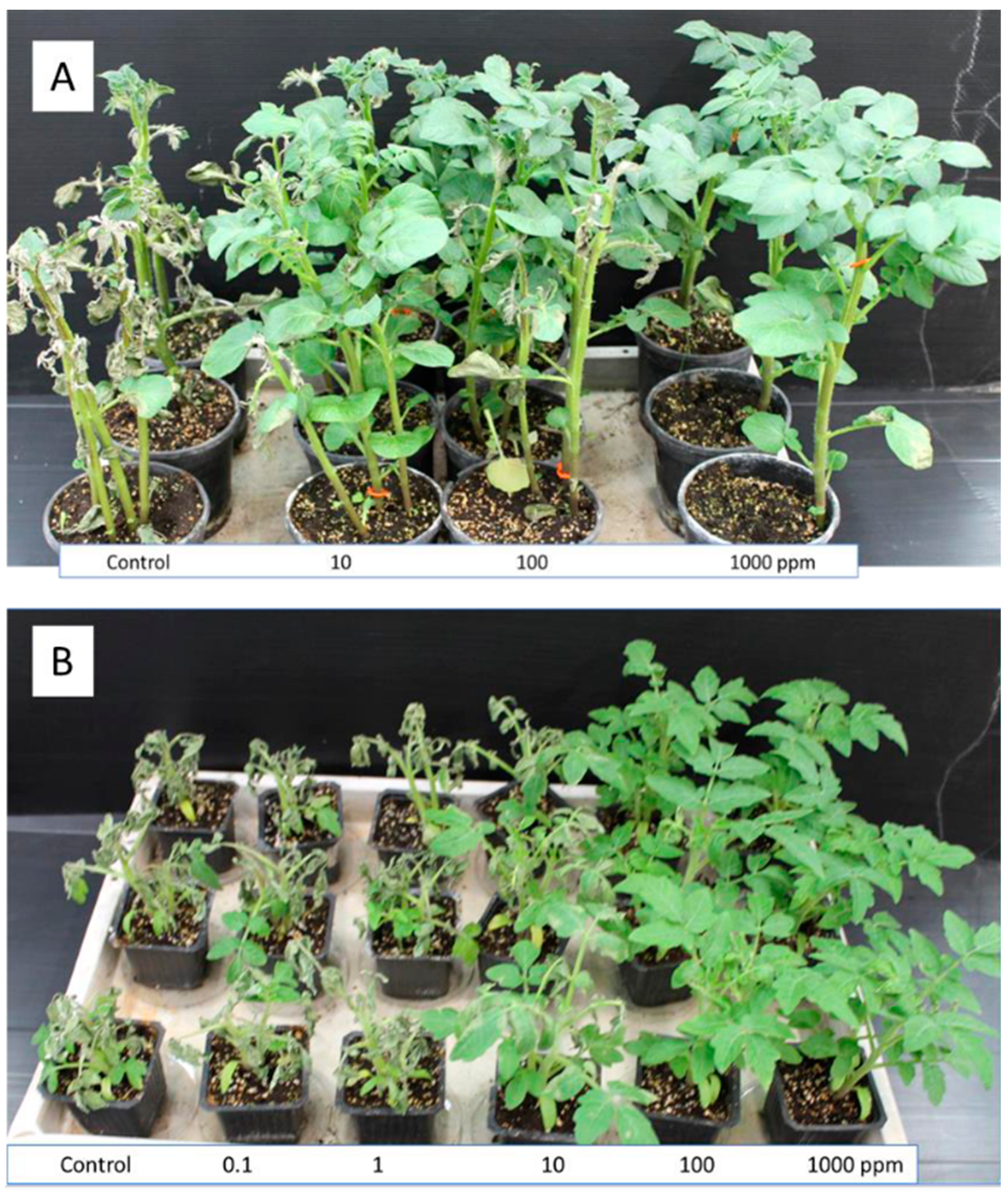
3.1. Growth Chamber Experiments
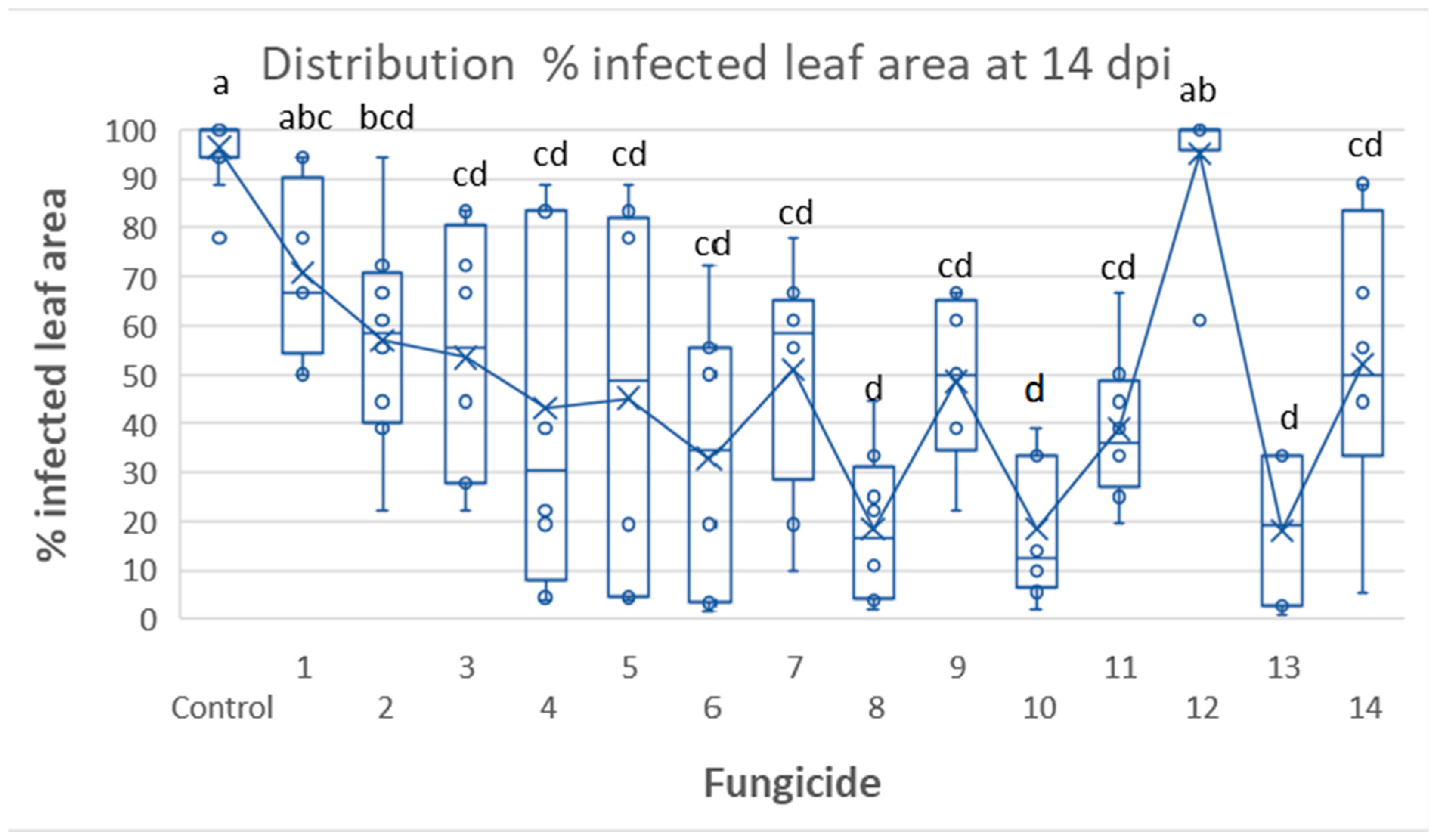
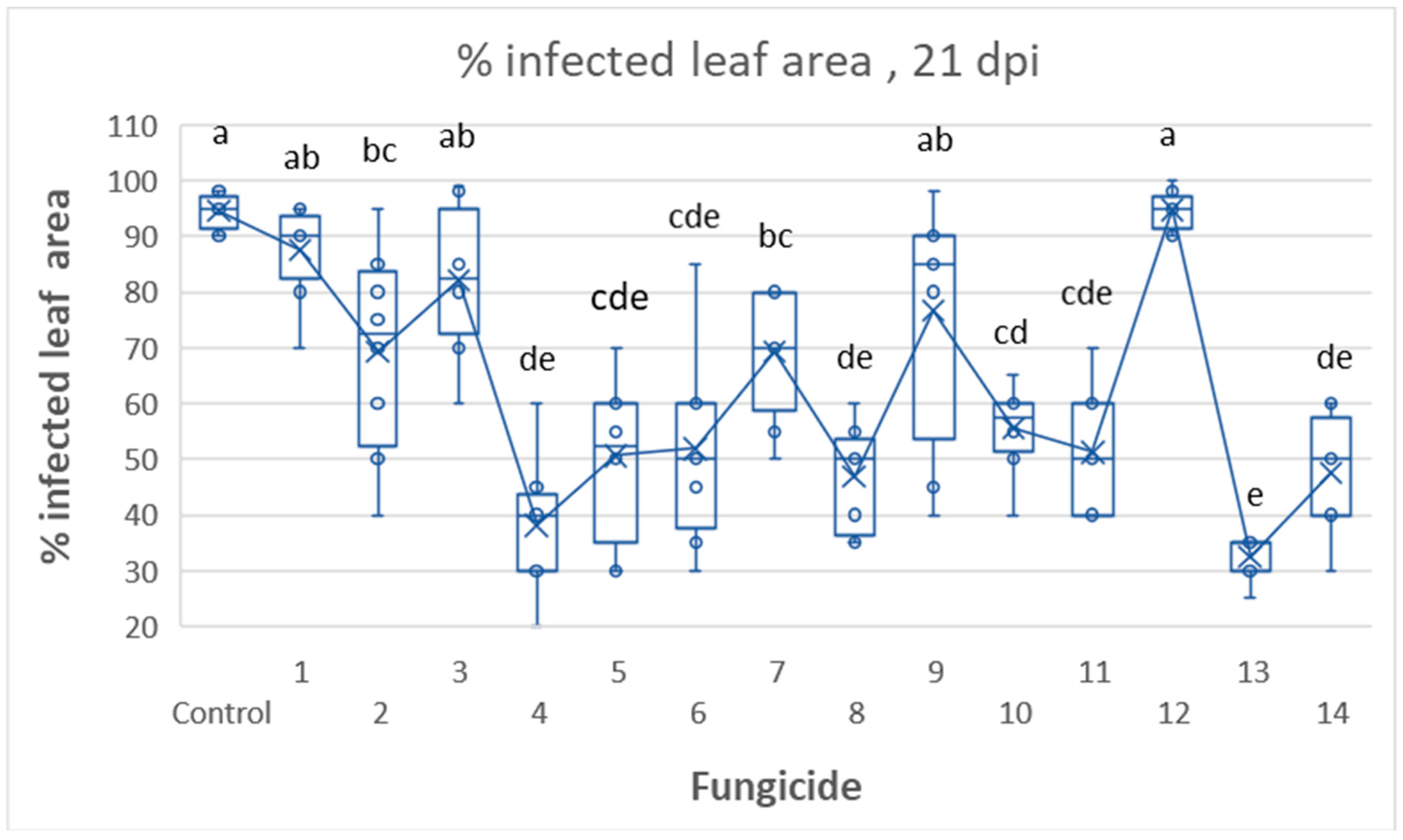
3.2. Tunnel Experiment 1
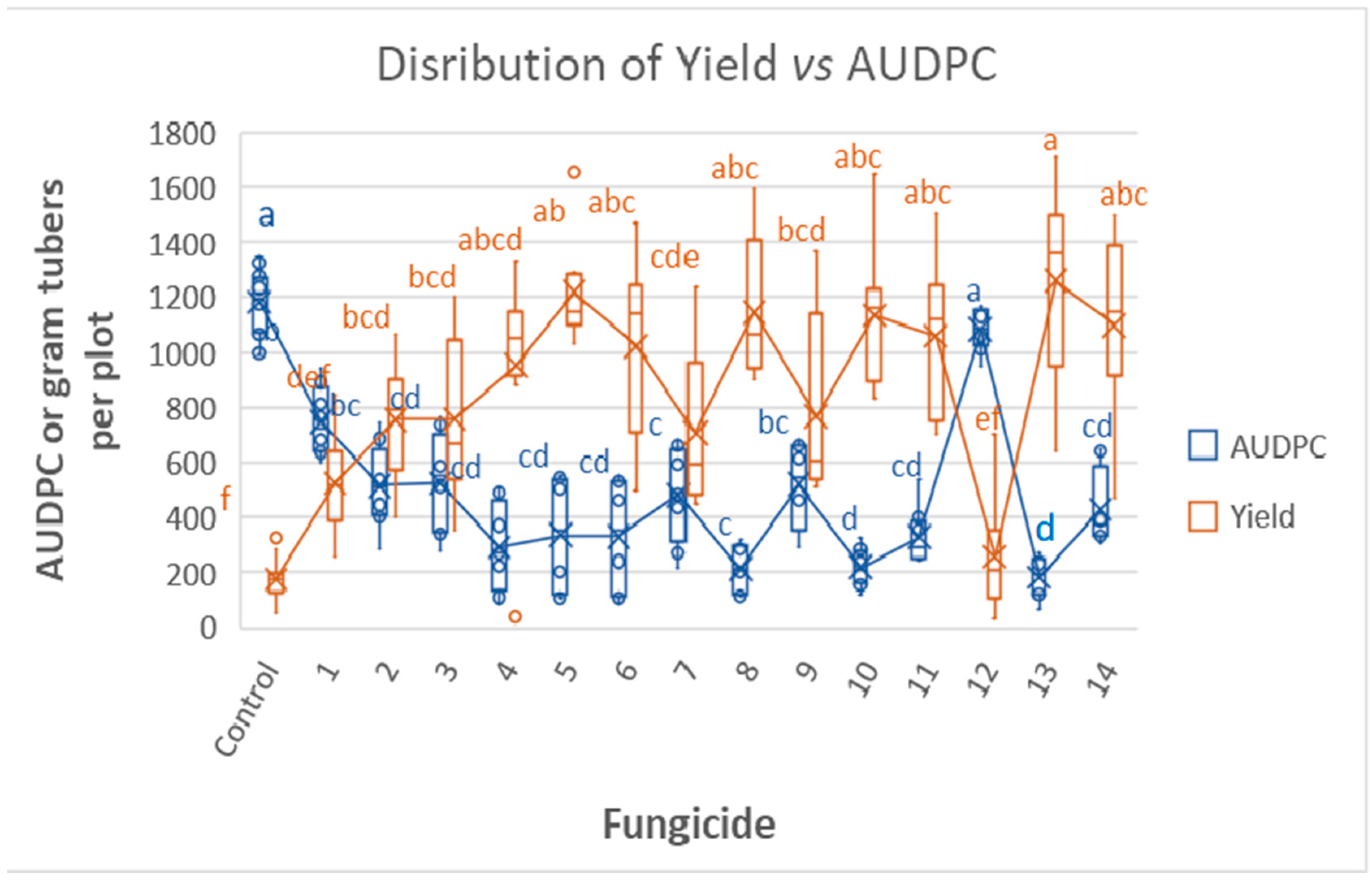
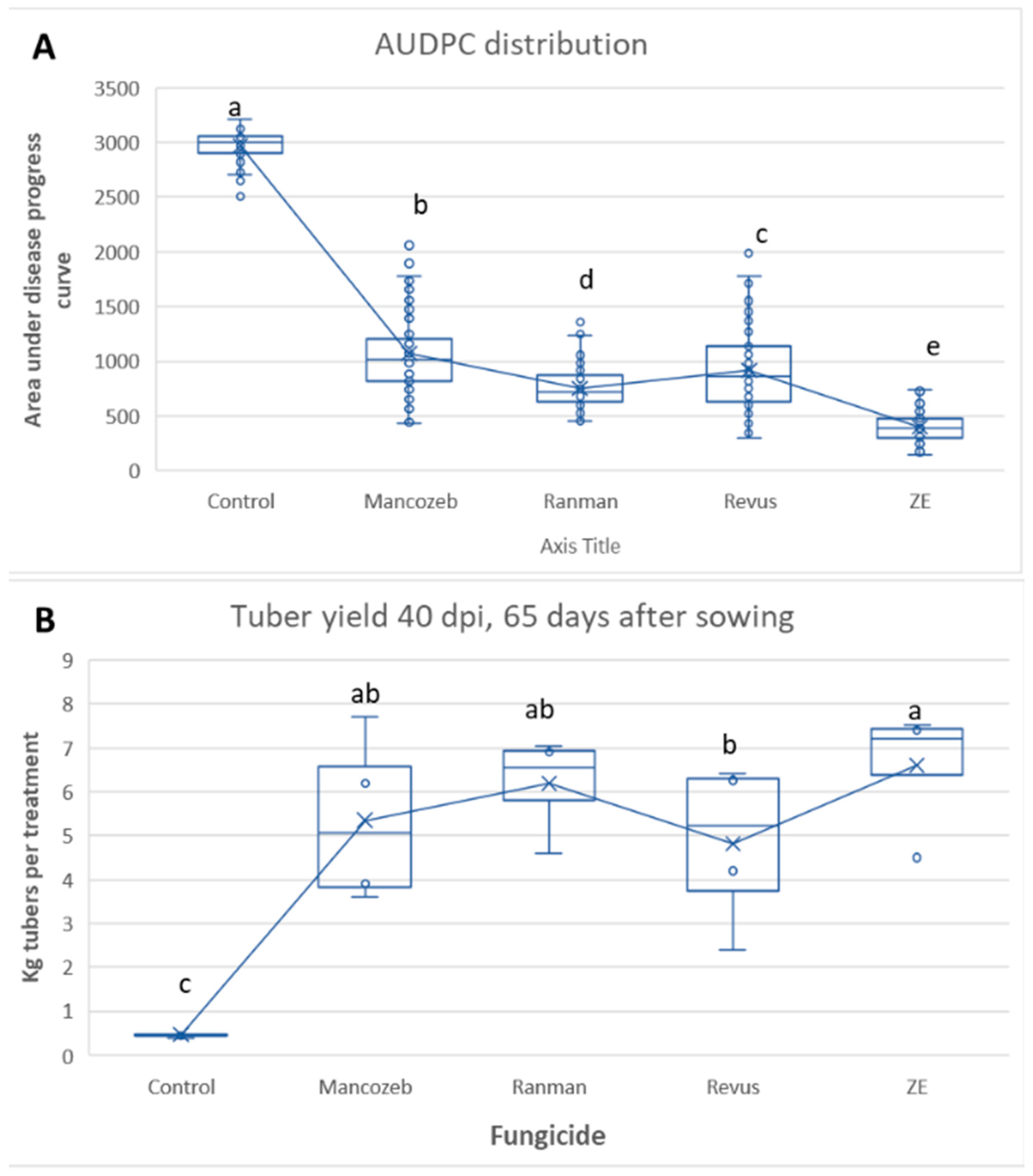
3.3. Tunnel Experiment 2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fry, W. Phytophthora infestans: The plant (and R gene) destroyer. Mol. Plant Pathol. 2008, 9, 385–402. [Google Scholar] [CrossRef]
- Fungicide Resistance Action Committee. FRAC Code List: Fungal Control agents Sorted by Cross Resistance Pattern and Mode of Action. 2020. Available online: https://cpb-us-w2.wpmucdn.com/u.osu.edu/dist/b/28945/files/2020/02/frac-code-list-2020-final.pdf (accessed on 20 October 2023).
- Available online: www.proficientmarketinsights.com (accessed on 20 October 2023).
- Minnesota Department of Agriculture. Pesticide Sales Database. 2017. Available online: http://www2.mda.state.mn.us/webapp/lis/chemsold_default.jsp (accessed on 7 May 2019).
- Gullino, M.L.; Tinivella, F.; Garibaldi, A.; Kemmitt, G.M.; Bacci, L.; Sheppard, B. Mancozeb: Past, present, and future. Plant Dis. 2010, 94, 1076–1087. [Google Scholar]
- Housenger, J.; Shamim, M.; Snyderman, S. Registration Review—Problem Formulation for the Ecological Risk Assessment and Drinking Water Exposure Assessment to be Conducted for MZ; USEPA: Triangle Park, NC, USA, 2015.
- Dall’agnol, J.C.; Ferri Pezzini, M.; Suarez Uribe, N.; Joveleviths, D. Systemic effects of the pesticide mancozeb—A literature review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4113–4120. [Google Scholar] [PubMed]
- Bianchi, S.; Nottola, S.A.; Torge, D.; Palmerini, M.G.; Necozione, S.; Macchiarelli, G. Association between Female Reproductive Health and Mancozeb: Systematic Review of Experimental Models. Int. J. Environ. Res. Public Health 2020, 17, 2580. [Google Scholar] [CrossRef] [PubMed]
- Fatma, F.; Verma, S.; Kamal, A.; Srivastava, A. Monitoring of morphotoxic, cytotoxic and genotoxic potential of mancozeb using Allium assay. Chemosphere 2018, 195, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Quds, R.; Iqbal, Z.; Arif, A.; Mahmood, R. Mancozeb-induced cytotoxicity in human erythrocytes: Enhanced gen-eration of reactive species, hemoglobin oxidation, diminished antioxidant power, membrane damage and morphological changes. Pestic. Biochem. Physiol. 2023, 193, 105453. [Google Scholar]
- Abdourahime, H.; Anastassiadou, M.; Arena, M.; Auteri, D.; Barmaz, S.; Brancato, A.; Bura, L.; Carrasco Cabrera, L.; Chaideftou, E.; Chiusolo, A.; et al. Conclusion on the peer review of the pesticide risk assessment of the active substance mancozeb. EFSA J. 2020, 18, 5755. [Google Scholar] [CrossRef]
- Anonymous. Mancozeb Non-Renewal and MRL Review. USDA GAIN Report Number: E42020-0099. Official Journal of the European Union L 160/89. 2020. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Mancozeb%20Non-Renewal%20and%20MRL%20Review%20%20%20%20%20_Brussels%20USEU_European%20Union_12-14-2020 (accessed on 20 October 2023).
- Anonymous. Commission Implementing Regulation (EU) 2021/745. 2021. Available online: https://eur-lex.europa.eu/eli/reg_impl/2021/745/oj (accessed on 20 October 2023).
- Muskett, A.E.; Colhoun, J. Prevention of Seedling Blight in the Flax Crop. Nature 1940, 146, 32. [Google Scholar] [CrossRef]
- Harrington, G.E. Thiram sulfide for turf diseases. Science 1941, 93, 311. [Google Scholar] [CrossRef]
- Anderson, P.J. A successful spray for blue mold of tobacco. Plant Dis. Rep. 1942, 26, 201–202. [Google Scholar]
- Kincaid, R.R. Disease in cigar-wrapper tobacco plant beds in Florida in 1942. Plant Dis. Rep. 1942, 26, 223. [Google Scholar]
- Heuberger, J.W.; Wolfenbarger, D.O. Zinc dimethyl dithiocar-bamate and the control of early blight and anthracnose on tomatoes and of leaf hoppers and early blight on potatoes. Phytopathology 1944, 34, 1003. [Google Scholar]
- Wilson, J.D. The zinc salt of dimethyl dithiocarbamic acid (metasan and zincate) as a fungicide on vegetables. Phytopathology 1944, 34, 1014. [Google Scholar]
- Brandes, G.A. The history and development of the Ethylene Bisdi-thiocarbamate fungicides. Am. J. Potato Res. 1953, 30, 137–140. [Google Scholar] [CrossRef]
- Flenner, A.L. Manganous Ethylene–Bis-Dithiocarbamate and Fungicidal Compositions Containing Same. U.S. Patent 2,504,404, 18 April 1950. [Google Scholar]
- Tomlin, C.D.S. Propineb p. 884–885 and Metiram p. 714–715 Profiles. In The Pesticide Manual, 14th ed.; British Crop Protection Council: Farnham, UK, 2006. [Google Scholar]
- McCallan, S.E.A. History of Fungicides. In Fungicides, An Advanced Treatise; Academic Press: New York, NY, USA, 1967; Volume 1, pp. 1–37. [Google Scholar]
- Bradshaw, N.J.; Vaughan, T.B. The effect of phenylamide fungicides on the control of potato late-blight (Phytophthora infestans) in England and Wales from 1978 to 1992. Plant Pathol. 1996, 45, 249–269. [Google Scholar] [CrossRef]
- Cohen, Y.; Samoucha, Y. Selection for metalaxyl resistance in potato crops infected with Phytophtora infestans: Effects of fungicides and initial frequency of resistant sporangia. Plant Pathol. 1989, 38, 382–390. [Google Scholar] [CrossRef]
- Evenhuis, A.; Spits, H.; Schepers, H.T.A.M. Efficacy of fungicidal protection of newly developing potato leaves against Phytophthora infestans. Crop. Prot. 2006, 25, 562–568. [Google Scholar] [CrossRef]
- Mitani, S.; Kamachi, K.; Sugimoto, K. Control of Potato Late Blight by Cyazofamid. J. Pestic. Sci. 2005, 30, 116–119. [Google Scholar] [CrossRef]
- Becktell, M.C.; Daughtrey, M.L.; Fry, W.E. Epidemiology and Management of Petunia and Tomato Late Blight in the Greenhouse. Plant Dis. 2005, 89, 1000–1008. [Google Scholar] [CrossRef][Green Version]
- Tumwine, J.; Frinking, H.D.; Jeger, M.J. Integrating cultural control methods for tomato late blight (Phytophthora infestans) in Uganda. Ann. Appl. Biol. 2002, 141, 225–236. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z.; Ullah, Z.; Ullah, R.; Ahmad, H. Late blight of potato (Phytophthora infestans) I: Fungi-cides application and associated challenges. Turk. J. Agric.-Food Sci. Technol. 2017, 5, 261–266. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Ukladov, E.O.; Golubeva, T.S. Phytophthora infestans: An Overview of Methods and Attempts to Combat Late Blight. J. Fungi 2021, 7, 1071. [Google Scholar] [CrossRef]
- Horsfield, A.; Wicks, T.; Davies, K.; Wilson, D.; Paton, S. Effect of fungicide use strategies on the control of early blight (Alternaria solani) and potato yield. Australas. Plant Pathol. 2010, 39, 368–375. [Google Scholar] [CrossRef]
- Samen, F.A.-E.; Goussous, S.; Jendi, A.; Makhadmeh, I. Evaluation of tomato early blight management using reduced application rates and frequencies of fungicide applications. Int. J. Pest Manag. 2015, 61, 320–328. [Google Scholar] [CrossRef]
- Kashyap, A.S. Studies on Leaf Spot of Tomato Caused by Septoria Lycopersici Speg; Diss UAS: Dharwad, India, 2013. [Google Scholar]
- Anand, Y.R.; Begum, S.; Dangmei, R.; Nath, P.S. Evaluation of fungicide azoxystrobin 23% SC (Coromandel) against leaf spot diseases of tomato. J. Agric. Technol. 2014, 1, 77–79. [Google Scholar]
- Veloukas, T.; Bardas, G.; Karaoglanidis, G.; Tzavella-Klonari, K. Management of tomato leaf mould caused by Cladosporium fulvum with trifloxystrobin. Crop. Prot. 2007, 26, 845–851. [Google Scholar] [CrossRef]
- Chapin, L.J.G.; Wang, Y.; Lutton, E.; Gardener, B.B.M. Distribution and Fungicide Sensitivity of Fungal Pathogens Causing Anthracnose-like Lesions on Tomatoes Grown in Ohio. Plant Dis. 2006, 90, 397–403. [Google Scholar] [CrossRef]
- Ingram, J.; Cummings, T.F.; Johnson, D.A. Response of colletotrichum coccodes to selected fungicides using a plant inoculation assay and efficacy of azoxystrobin applied by chemigation. Am. J. Potato Res. 2011, 88, 309–317. [Google Scholar] [CrossRef]
- McLeod, A.; Masimba, T.; Jensen, T.; Serfontein, K.; Coertze, S. Evaluating spray programs for managing copper resistant Pseudomonas syringae pv. tomato populations on tomato in the Limpopo region of South Africa. Crop. Prot. 2017, 102, 32–42. [Google Scholar] [CrossRef]
- Momol, T.; Jones, J.; Olson, S.; Obradovic, A.; Balogh, B.; King, P. Integrated Management of Bacterial Spot on Tomato in Florida PP110/PP110, 9/2002; EDIS: Pyeongtaek, Republic of Korea, 2002. [Google Scholar]
- Fayette, J.; Roberts, P.D.; Pernezny, K.L.; Jones, J.B. The role of cymoxanil and famoxadone in the management of bacterial spot on tomato and pepper and bacterial leaf spot on lettuce. Crop. Prot. 2012, 31, 107–112. [Google Scholar] [CrossRef]
- Strayer-Scherer, A.; Liao, Y.Y.; Abrahamian, P.; Timilsina, S.; Paret, M.; Momol, T.; Vallad, G.E. Integrated Management of Bacterial Spot on Tomato in Florida PP353, 11/2019; EDIS: Pyeongtaek, Republic of Korea, 2019. [Google Scholar]
- Mizubuti, E.S.; Júnior, V.L.; Forbes, G.A. Management of late blight with alternative products. Pest Technol. 2007, 1, 106–116. [Google Scholar]
- Islam, M.R.; Mondal, C.; Hossain, I.; Meah, M.B. Organic management: An alternative to control late blight of potato and tomato caused by Phytophthora infestans. Int. J. Theor. Appl. Sci. 2013, 5, 32–42. [Google Scholar]
- Gopi, R.; Avasthe, R.K.; Kalita, H.; Yadav, A.; DAS, S.K.; Rai, D. Eco-friendly management of tomato late blight using botanicals, bio-control agents, compost tea and copper fungicides. Indian J. Agric. Sci. 2020, 90, 35–39. [Google Scholar] [CrossRef]
- Fry, W.E.; Birch, P.R.J.; Judelson, H.S.; Grünwald, N.J.; Danies, G.; Everts, K.L.; Gevens, A.J.; Gugino, B.K.; Johnson, D.A.; Johnson, S.B.; et al. Five Reasons to Consider Phytophthora infestans a Reemerging Pathogen. Phytopathology 2015, 105, 966–981. [Google Scholar] [CrossRef] [PubMed]
- Haverkort, A.J.; Boonekamp, P.M.; Hutten, R.; Jacobsen, E.; Lotz, L.A.P.; Kessel, G.J.Y.; Visser, R.G.F.; van der Vosson, E.A.G. Societal costs of late blight in potato and prospects for durable resistance through cisgenic modifcations. Potato Res. 2008, 51, 47–57. [Google Scholar] [CrossRef]
- Cohen, Y.; Weitman, M. Mobility of oxathiapiprolin in and between tomato plants. Pest Manag. Sci. 2022, 79, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Rubin, A.E.; Galperin, M. Effective control of two genotypes of Phytophthora infestans in the field by three oxa-thiapiprolin fungicidal mixtures. PLoS ONE 2021, 16, e0258280. [Google Scholar]
- Cohen, Y.; Rubin, A.E.; Galperin, M. Oxathiapiprolin-based fungicides provide enhanced control of tomato late blight induced by mefenoxam-insensitive Phytophthora infestans. PLoS ONE 2018, 13, e0204523. [Google Scholar] [CrossRef]
- Lu, F.; Zhao, J.; Liu, X.; Meng, R.; Wu, J.; Han, X.; Wang, W. Monitoring of resistance of Phytophthora infestans on potato to metalaxyl and the control efficacy of alternative fungicides. Sci. Agric. Sin. 2018, 51, 2700–2710. [Google Scholar]
- Khadka, R.B.; Chaulagain, B.; Subedi, S.; Marasini, M.; Rawal, R.; Pathak, N.; Sharma-Poudyal, D. Evaluation of fungicides to control potato late blight (Phytophthora infestans) in the plains of Nepal. J. Phytopathol. 2020, 168, 245–253. [Google Scholar] [CrossRef]
- Dowley, L.J.; O’Sullivan, E. Activity of fluazinam against late blight of potatoes. Ir. J. Agric. Food Res. 1995, 34, 33–37. [Google Scholar]
- Najdabbasi, N.; Mirmajlessi, S.M.; Dewitte, K.; Mänd, M.; Landschoot, S.; Haesaert, G. Combination of Potassium Phosphite and Reduced Doses of Fungicides Encourages Protection against Phytophthora infestans in Potatoes. Agriculture 2022, 12, 189. [Google Scholar] [CrossRef]
- Babli, D.; Yadav, P.S.; Sheeren Parveen, V.K. Efficacy of different eco-friendly methods against late blight of potato, Phytophthora infestans: A review. Pharma Innov. J. 2022, 11, 1949–1961. [Google Scholar]
- D’Arcangelo, K.N.; Adams, M.L.; Kerns, J.P.; Quesada-Ocampo, L.M. Assessment of fungicide product applications and program approaches for control of downy mildew on pickling cucumber in North Carolina. Crop. Prot. 2020, 140, 105412. [Google Scholar] [CrossRef]
- Miao, J.; Cai, M.; Dong, X.; Liu, L.; Lin, D.; Zhang, C.; Pang, Z.; Liu, X. Resistance Assessment for oxathiapiprolin in Phytophthora capsici and the detection of a point mutation (G769W) in PcORP1 that confers resistance. Front. Microbiol. 2016, 7, 615. [Google Scholar] [CrossRef]
- Mboup, M.K.; Sweigard, J.W.; Carroll, A.; Jaworska, G.; Genet, J. Genetic mechanism, baseline sensitivity and risk of resistance to oxathiapiprolin in oomycetes. Pest Manag. Sci. 2021, 78, 905–913. [Google Scholar] [CrossRef]
- Sakai, J.; Miura, I.; Shibata, M.; Yonekura, N.; Hiyoshi, H.; Takagaki, M.; Nagayama, K. Development of a new fungicide, benthiavalicarb-isopropyl. J. Pestic. Sci. 2010, 35, 488–489. [Google Scholar] [CrossRef]
- Cohen, Y.; Rubin, E.; Hadad, T.; Gotlieb, D.; Sierotzki, H.; Gisi, U. Sensitivity of Phytophthora infestans to mandi-propamid and the effect of enforced selection pressure in the field. Plant Pathol. 2007, 56, 836–842. [Google Scholar] [CrossRef]
- Abuley, I.K.; Lynott, J.S.; Hansen, J.G.; Cooke, D.E.L.; Lees, A.K. The EU43 genotype of Phytophthora infestans displays resistance to mandipropamid. Plant Pathol. 2023, 72, 1305–1313. [Google Scholar] [CrossRef]


| Code | Product | Active Ingredients gr/Kg | Chemical Group * | FRAC Group ** | gram/h | Dose, % |
|---|---|---|---|---|---|---|
| 1 | AS 121 | Valifenalate 60 + CuOH 150 + CuOCl 150 | CAA + M | H5 | 200 | 0.4 |
| 2 | Infinito | Fluopicolide 62.5 + Propamocarb 625 | Benzamide + Carbamate | B5 + F4 | 150 | 0.3 |
| 3 | Cabrio | Dimethomorph 72 + Pyraclostrobin 40 | CAA + QoI | H5 + C3 | 250 | 0.5 |
| 4 | Carial Plus | Cymoxanil 180 + Mandipropamid 250 | CO + CAA | UN + H5 | 60 | 0.12 |
| 5 | CYO 719 | Fluazinam 300 + Cymoxanil 200 | Phenyl-pyridineamine + CO | UN + C5 | 100 | 0.2 |
| 6 | Banjo | Fluazinam 500 | Phenyl-pyridineamine | C5 | 100 | 0.2 |
| 7 | Banjo Forte | Fluazinam 200 + Dimethomorph 200 | Phenyl-pyridineamine + CAA | C5 + H5 | 50 | 0.1 |
| 8 | Revus | Mandipropamid 250 | CAA | H5 | 60 | 0.12 |
| 9 | Electis | Cymoxanil 390 + Zoxamid 330 | CO + Benzamide | UN + B3 | 45 | 0.1 |
| 10 | Ranman | Cyazofamid 500 | QiI | C4 | 50 | 0.1 |
| 11 | Mancozeb | Mancozeb 750 | M | M | 300 | 0.6 |
| 12 | Polyram | Zineb700 | M | M | 300 | 0.6 |
| 13 | Zorvec Endavia | Oxathiapiprolin 30 + Bethiavalicarb 70 | OSBPI + CAA | C9 + H5 | 50 | 0.1 |
| 14 | AGF 273 | Fluopicolide 200 + Cymoxanil 240 | Benzamide + CO | B5 + UN | 60 | 0.12 |
| Fungicide | Kg/h | Sprays | Interval | Dates of Spray | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mancozeb | 3 | 6 | 7 days | 8.12.22 | 15.12.22 | 22.12.22 | 29.12.22 | 5.1.23 | 12.1.23 |
| Ranman | 0.5 | 6 | 7 days | 8.12.22 | 15.12.22 | 22.12.22 | 29.12.22 | 5.1.23 | 12.1.23 |
| Revus | 0.6 | 4 | 9 days | 8.12.22 | 16.12.22 | 25.12.22 | 2.1.23 | ||
| ZE | 0.5 | 2 | 21 days | 8.12.22 | 29.12.22 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Naim, Y.; Cohen, Y. Replacing Mancozeb with Alternative Fungicides for the Control of Late Blight in Potato. J. Fungi 2023, 9, 1046. https://doi.org/10.3390/jof9111046
Ben Naim Y, Cohen Y. Replacing Mancozeb with Alternative Fungicides for the Control of Late Blight in Potato. Journal of Fungi. 2023; 9(11):1046. https://doi.org/10.3390/jof9111046
Chicago/Turabian StyleBen Naim, Yariv, and Yigal Cohen. 2023. "Replacing Mancozeb with Alternative Fungicides for the Control of Late Blight in Potato" Journal of Fungi 9, no. 11: 1046. https://doi.org/10.3390/jof9111046
APA StyleBen Naim, Y., & Cohen, Y. (2023). Replacing Mancozeb with Alternative Fungicides for the Control of Late Blight in Potato. Journal of Fungi, 9(11), 1046. https://doi.org/10.3390/jof9111046






