Preservation Methods in Isolates of Sporothrix Characterized by Polyphasic Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Preservation Methods
2.3. Recoverage
2.4. Phenotype Stability
2.5. Genotypic Stability
2.6. Proteomic Stability
2.7. Statistical Analysis
3. Results
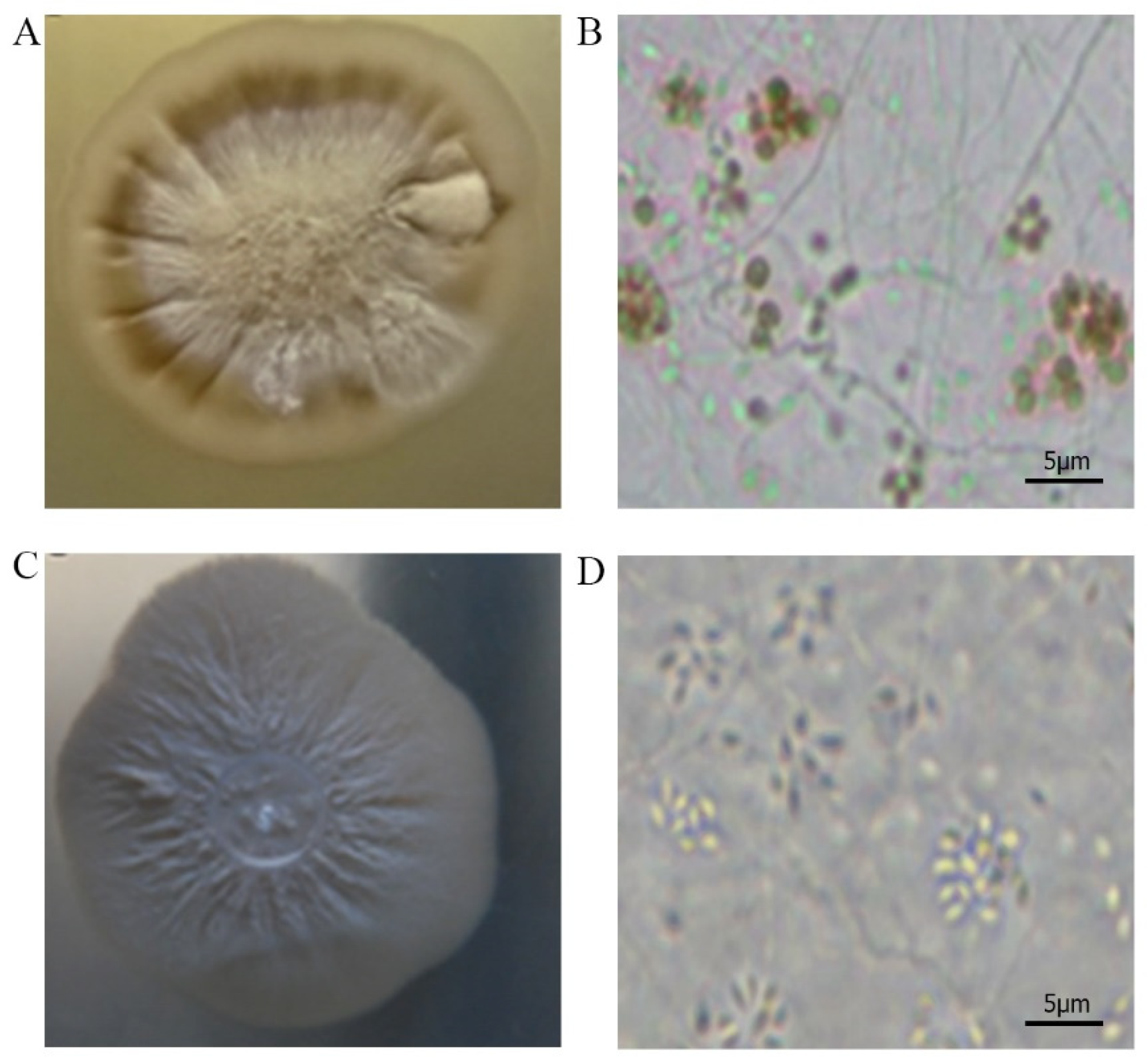
3.1. Phenotypic Characterization before Preservation
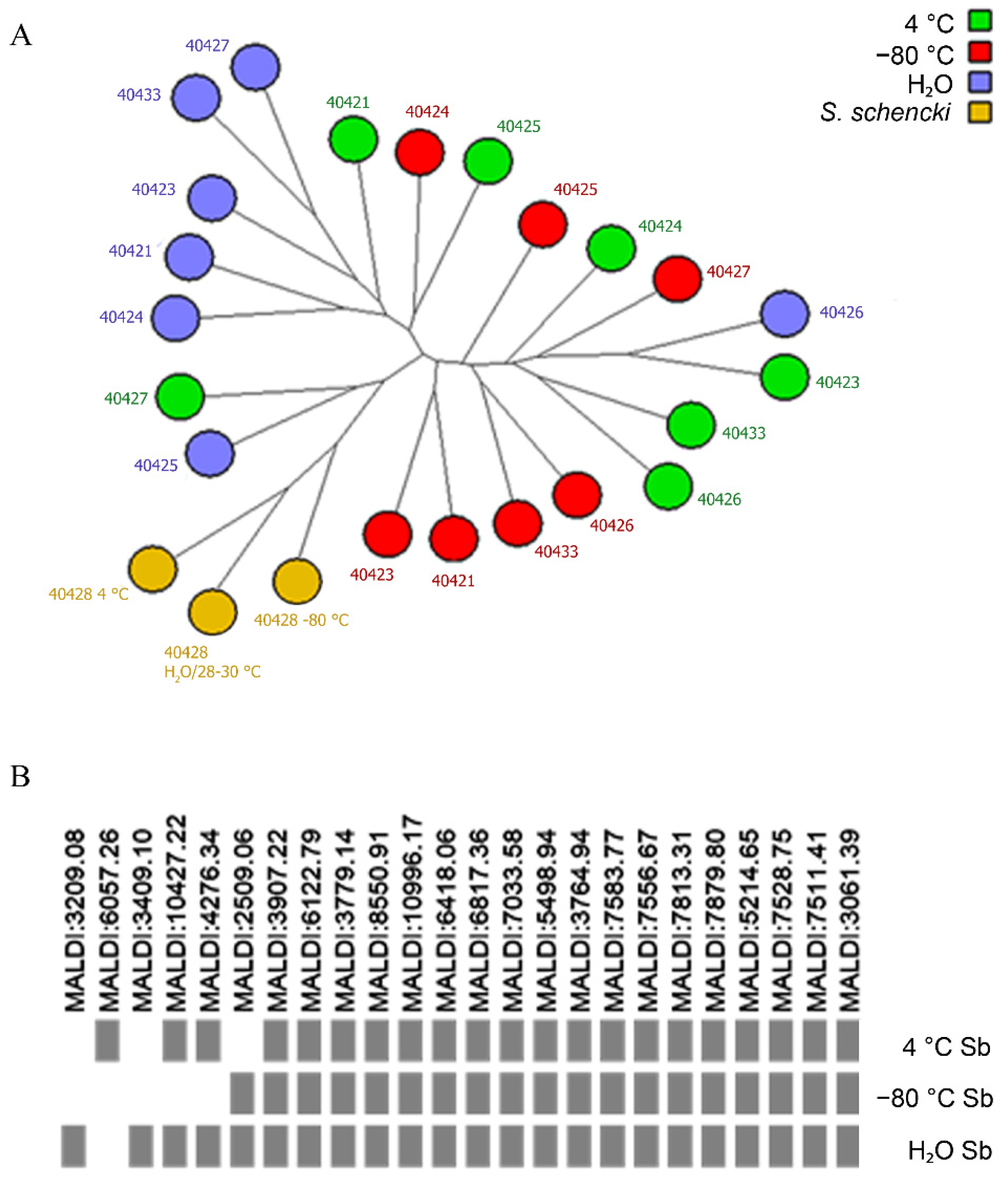
3.2. Genotypic Stability
3.3. Proteomic Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marimon, R.; Cano, J.; Gené, J.; Sutton, D.A.; Kawasaki, M.; Guarro, J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J. Clin. Microbiol. 2007, 45, 3198–3206. [Google Scholar] [CrossRef] [PubMed]
- Marimon, R.; Gené, J.; Cano, J.; Guarro, J. Sporothrix luriei: A rare fungus from clinical origin. Med. Mycol. 2008, 46, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Romeo, O.; Scordino, F.; Criseo, G. New insight into molecular phylogeny and epidemiology of Sporothrix schenckii species complex based on calmodulin-encoding gene analysis of Italian isolates. Mycopathologia 2011, 172, 179–186. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Cruz-Choappa, R.; Fernandes, G.F.; de Hoog, G.S.; de Camargo, Z.P. Sporothrix chilensis sp. nov. (Ascomycota: Ophiostomatales), a soil-borne agent of human sporotrichosis with mild-pathogenic potential to mammals. Fungal Biol. 2016, 120, 246–264. [Google Scholar] [CrossRef] [PubMed]
- Makri, N.; Paterson, G.K.; Greggem, F.; Urquhart, C.; Nuttall, T. First case report of cutaneous sporotrichosis (Sporothrix species) in a cat in the UK. JFMS Open Rep. 2020, 6, 2055116920906001. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Bonifaz, A.; Gutierrez-Galhardo, M.C.; Mochizuki, T.; Li, S. Global epidemiology of sporotrichosis. Med. Mycol. 2015, 53, 3–14. [Google Scholar] [CrossRef]
- Gremião, I.D.F.; Oliveira, M.M.E.; Monteiro de Miranda, L.H.; Saraiva Freitas, D.F.; Pereira, S.A. Geographic Expansion of Sporotrichosis, Brazil. Emerg. Infect. Dis. 2020, 26, 621–624. [Google Scholar] [CrossRef]
- Boechat, J.S.; Oliveira, M.M.; Almeida-Paes, R.; Gremião, I.D.; Machado, A.C.; Oliveira, R.D.; Figueiredo, A.B.; Rabello, V.B.; Silva, K.B.; Zancopé-Oliveira, R.M.; et al. Feline sporotrichosis: Associations between clinical-epidemiological profiles and phenotypic-genotypic characteristics of the etiological agents in the Rio de Janeiro epizootic area. Mem. Inst. Oswaldo Cruz. 2018, 113, 185–196. [Google Scholar] [CrossRef]
- OECD—Organisation for Economic Co-Operation and Development. OECD Best Practice Guidelines for Biological Resource Centres; OECD: Paris, France, 2007. [Google Scholar]
- WFCC. World Federation for Culture Collections Guidelines: For the Establishment and Operation of Collections of Cultures of Microorganisms; WFCC: Paris, France, 1999. [Google Scholar]
- Homolka, L. Preservation of live cultures of basidiomycetes—recent methods. Fungal Biol. 2014, 118, 107–125. [Google Scholar] [CrossRef]
- Castellani, A. Viability of some pathogenic fungi in distilled water. J. Trop. Med. Hyg. 1993, 24, 270–276. [Google Scholar]
- Borba, C.M.; da Silva, A.M.; de Oliveira, P.C. Long-time survival and morphological stability of preserved Sporothrix schenckii strains. Mycoses 1992, 35, 185–188. [Google Scholar] [CrossRef]
- Capriles, C.C.; Mata Essayag, S.; Lander, A.; Camacho, R. Experimental pathogenicity of Sporothrix schenckii preserved in water (Castellani). Mycopathologia 1993, 122, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Qiangqiang, Z.; Jiajun, W.; Li, L. Storage of fungi using sterile distilled water or lyophilization: Comparison after 12 years. Mycoses 1998, 41, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.; Alvarado, P.; Díaz de Torres, E.; Lucena, L.; de Albornoz, M.C. Comportamiento fisiológico y de sensibilidad in vitro de aislamientos de Sporothrix schenckii mantenidos 18 años por dos métodos de preservación. Rev. Iberoam. Micol. 2005, 22, 151–156. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Szekely, A.; Campbell, C.K.; Johnson, E.M. Evaluation of the viability of pathogenic filamentous fungi after prolonged storage in sterile water and review of recent published studies on storage methods. Mycopathologia 2006, 161, 361–368. [Google Scholar] [CrossRef]
- Guimarães, L.C.; Fernandes, A.P.; Chalfoun, S.M.; Batista, L.R. Methods to preserve potentially toxigenic fungi. Braz. J. Microbiol. 2014, 19, 43–47. [Google Scholar] [CrossRef][Green Version]
- Ana, K.L.; Amaury, D.S.; Ivanete, D.L.; Alita, M.D.; Luciana, F.B.; Liliane, C.D.; Joo, B.D.; Rica, S.D. Availability and morphological characteristics of endophytic fungi held in different methods of preservation. Scient Res. Essays 2016, 11, 76–79. [Google Scholar] [CrossRef]
- Brilhante, R.S.; Silva, N.F.; de Lima, R.A.; Caetano, É.P.; de Alencar, L.P.; de Souza Collares Maia Castelo-Branco, D.; Moreira, J.L.; Bandeira, S.P.; de Camargo, Z.P.; Rodrigues, A.M.; et al. Easy storage strategies for Sporothrix spp. strains. Biopreserv. Biobank 2015, 13, 131–134. [Google Scholar] [CrossRef]
- Johnson, G.C.; Martin, A.K. Survival of wood-inhabiting fungi stored for 10 years in water and under oil. Can. J. Microbiol. 1992, 8, 861–864. [Google Scholar] [CrossRef]
- Pasarell, L.; McGinnis, M.R. Viability of fungal cultures maintained at −70 °C. J. Clin. Microbiol. 1992, 30, 1000–1004. [Google Scholar] [CrossRef]
- Lima, R.F.; Borba, C.M. Viability, morphological characteristics and dimorphic ability of fungi preserved by different methods. Rev. Iberoam. Micol. 2001, 18, 191–196. [Google Scholar]
- Oliveira, M.M.; Almeida-Paes, R.; Muniz, M.M.; Gutierrez-Galhardo, M.C.; Zancope-Oliveira, R.M. Phenotypic and molecular identification of Sporothrix isolates from an epidemic area of sporotrichosis in Brazil. Mycopathologia 2011, 172, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.M.E.; Almeida-Paes, R.; Medeiros, M.M.; Lima Barros, M.B.; Galhardo, M.C.; Zancope-Oliveira, R.M. Sporotrichosis caused by Sporothrix globosa in Rio De Janeiro, Brazil: Case report. Mycopathologia 2010, 169, 359–363. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.M.; Veríssimo, C.; Sabino, R.; Aranha, J.; Zancopé-Oliveira, R.M.; Sampaio, P.; Pais, C. First autochthone case of sporotrichosis by Sporothrix globosa in Portugal. Diagn. Microbiol. Infect. Dis. 2014, 78, 388–390. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Nirenberg, H.I.; Aoki, T.; Cigelnik, E. A multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additional phylogenetically distinct species. Mycoscience 2000, 41, 61–78. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molec. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar]
- Oliveira, M.M.; Santos, C.; Sampaio, P.; Romeo, O.; Almeida-Paes, R.; Pais, C.; Lima, N.; Zancopé-Oliveira, R.M. Development and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res. Microbiol. 2015, 166, 102–110. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Melo Teixeira, M.; de Hoog, G.S.; Schubach, T.M.; Pereira, S.A.; Fernandes, G.F.; Bezerra, L.M.; Felipe, M.S.; de Camargo, Z.P. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl. Trop. Dis. 2013, 20, e2281. [Google Scholar] [CrossRef]
- Ottonelli Stopiglia, C.D.; Magagnin, C.M.; Castrillon, M.R.; Mendes, S.D.; Heidrich, D.; Valente, P.; Scroferneker, M.L. Antifungal susceptibilities and identification of species of the Sporothrix schenckii complex isolated in Brazil. Med. Mycol. 2014, 52, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Barreira, T.; Corrêa-Moreira, D.; Moraes, A.; Borba, C.; Oliveira, M.M.E. Molecular and phenotypic reidentification of Sporothrix schenckii clinical isolates preserved under mineral oil for 34 to 64 years in a culture collection in Brazil. Cur. Res. Microbial. Sci. 2022, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sandven, P. Laboratory identification and sensitivity testing of yeast isolates. Acta Odontol. Scand. 1990, 48, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.; Lortholary, O.; Dromer, F.; Dannaoui, E. Carbon assimilation profiles as a tool for identification of zygomycetes. J. Clin. Microbiol. 2007, 45, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Broughton, R.; Buddie, A.G.; Smith, D.; Ryan, M.J. The effect of cryopreservation on genomic stability in strains of the fungus Trichoderma. Cryo Lett. 2012, 33, 299–306. [Google Scholar]
- Raff, E.C. Genetics of microtubule systems. J. Cell Biol. 1984, 99 Pt 1, 1–10. [Google Scholar] [CrossRef]
- Smith, H.A.; Allaudeen, H.S.; Whitman, M.H.; Koltin, Y.; Gorman, J.A. Isolation and characterization of a beta-tubulin gene from Candida albicans. Gene 1988, 63, 53–63. [Google Scholar] [CrossRef]
- Cleveland, D.W.; Sullivan, K.F. Molecular biology and genetics of tubulin. Annu. Rev. Biochem. 1985, 54, 331–365. [Google Scholar] [CrossRef]
- McKean, P.G.; Vaughan, S.; Gull, K. The extended tubulin superfamily. J. Cell Sci. 2001, 114 Pt 15, 2723–2733. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, K.; Xia, Y. Contributions of β-tubulin to cellular morphology, sporulation and virulence in the insect-fungal pathogen, Metarhizium acridum. Fungal Genet. Biol. 2017, 103, 16–24. [Google Scholar] [CrossRef]
- Breen, J.; Mur, L.; Sivakumaran, A.; Akinyemi, A.; Wilkinson, M.J.; Rodriguez Lopez, C.M. Botrytis cinerea Loss and Restoration of Virulence during In Vitro Culture Follows Flux in Global DNA Methylation. Int. J. Mol. Sci. 2022, 23, 3034. [Google Scholar] [CrossRef] [PubMed]
- Hatta, R.; Ito, K.; Hosaki, Y.; Tanaka, T.; Tanaka, A.; Yamamoto, M.; Akimitsu, K.; Tsuge, T. A conditionally dispensable chromosome controls host-specific pathogenicity in the fungal plant pathogen Alternaria alternata. Genetics 2002, 161, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Kramer, K.; Valdivia, L.; Ortiz, S.; Castillo, A. A double-stranded RNA mycovirus confers hypovirulence-associated traits to Botrytis cinerea. FEMS Microbiol. Lett. 2003, 228, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Lima-Neto, R.; Santos, C.; Lima, N.; Sampaio, P.; Pais, C.; Neves, R.P. Application of MALDI-TOF MS for requalification of a Candida clinical isolates culture collection. Braz. J. Microbiol. 2014, 29, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Santos, C.; Simões, M.F.; Soares, C.; Santos, C.; Lima, N. Polyphasic, Including MALDI-TOF MS, Evaluation of Freeze-Drying Long-Term Preservation on Aspergillus (Section Nigri) Strains. Microorganisms 2019, 7, 291. [Google Scholar] [CrossRef] [PubMed]


| Diameter of Colonies (mm) | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Preservation Method | Time of Preservation (Months) | Min | Median | Max | |||
| Growth temperature | 30 °C | H2O | 6 | 19.00 | 41.25 | 50.00 | 1.000 |
| 12 | 21.00 | 31.00 | 49.50 | 0.800 | |||
| 18 | 21.00 | 36.25 | 46.25 | 1.000 | |||
| 24 | 18.25 | 39.38 | 54.00 | 1.000 | |||
| 4 °C | 6 | 22.00 | 36.50 | 63.00 | ** | ||
| 12 | 20.00 | 32.50 | 40.50 | 0.310 | |||
| 18 | 17.25 | 33.50 | 45.75 | 0.940 | |||
| 24 | 19.75 | 37.50 | 53.75 | 1.000 | |||
| −80 °C | 6 | 21.25 | 29.50 | 49.00 | 0.140 | ||
| 12 | 25.00 | 34.00 | 42.50 | 1.000 | |||
| 18 | 21.25 | 41.12 | 46.75 | 1.000 | |||
| 24 | 28.25 | 41.62 | 51.25 | 1.000 | |||
| 37 °C | H2O | 6 | 8.00 | 11.00 | 14.50 | 0.740 | |
| 12 | 6.00 | 9.25 | 12.00 | 1.000 | |||
| 18 | 7.25 | 10.12 | 12.50 | 1.000 | |||
| 24 | 6.25 | 8.79 | 11.00 | 0.800 | |||
| 4 °C | 6 | 6.50 | 10.50 | 11.50 | ** | ||
| 12 | 6.00 | 7.00 | 11.50 | 1.000 | |||
| 18 | 6.75 | 8.38 | 12.75 | 1.000 | |||
| 24 | 5.25 | 9.25 | 72.75 | 1.000 | |||
| −80 °C | 6 | 4.50 | 9.62 | 15.50 | 1.000 | ||
| 12 | 4.50 | 8.50 | 10.50 | 1.000 | |||
| 18 | 7.25 | 9.62 | 10.50 | 1.000 | |||
| 24 | 8.25 | 11.30 | 12.75 | 1.000 | |||
| H2O | 4 °C | −80 °C | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate | T0 | 6M | 12M | 18M | 24M | 6M | 12M | 18M | 24M | 6M | 12M | 18M | 24M |
| CMRVS 40421 | - | + | + | + | + | - | + | + | + | + | + | + | + |
| CMRVS 40423 | - | + | + | + | + | - | - | + | + | - | + | + | + |
| CMRVS 40425 | - | - | - | + | + | - | - | + | + | - | - | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Rabello, V.B.; Corrêa-Moreira, D.; Santos, C.; Abreu Pinto, T.C.; Procopio-Azevedo, A.C.; Boechat, J.; Coelho, R.A.; Almeida-Paes, R.; Costa, G.; Lima, N.; et al. Preservation Methods in Isolates of Sporothrix Characterized by Polyphasic Approach. J. Fungi 2023, 9, 34. https://doi.org/10.3390/jof9010034
de Souza Rabello VB, Corrêa-Moreira D, Santos C, Abreu Pinto TC, Procopio-Azevedo AC, Boechat J, Coelho RA, Almeida-Paes R, Costa G, Lima N, et al. Preservation Methods in Isolates of Sporothrix Characterized by Polyphasic Approach. Journal of Fungi. 2023; 9(1):34. https://doi.org/10.3390/jof9010034
Chicago/Turabian Stylede Souza Rabello, Vanessa Brito, Danielly Corrêa-Moreira, Cledir Santos, Tatiana Casto Abreu Pinto, Anna Carolina Procopio-Azevedo, Jéssica Boechat, Rowena Alves Coelho, Rodrigo Almeida-Paes, Gisela Costa, Nelson Lima, and et al. 2023. "Preservation Methods in Isolates of Sporothrix Characterized by Polyphasic Approach" Journal of Fungi 9, no. 1: 34. https://doi.org/10.3390/jof9010034
APA Stylede Souza Rabello, V. B., Corrêa-Moreira, D., Santos, C., Abreu Pinto, T. C., Procopio-Azevedo, A. C., Boechat, J., Coelho, R. A., Almeida-Paes, R., Costa, G., Lima, N., Zancopé-Oliveira, R. M., & Marques Evangelista Oliveira, M. (2023). Preservation Methods in Isolates of Sporothrix Characterized by Polyphasic Approach. Journal of Fungi, 9(1), 34. https://doi.org/10.3390/jof9010034










