MoMaf1 Mediates Vegetative Growth, Conidiogenesis, and Pathogenicity in the Rice Blast Fungus Magnaporthe oryzae
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. Quantification of Gene Expression during Different Phases
2.3. Nucleic Acid Manipulation and qRT-PCR
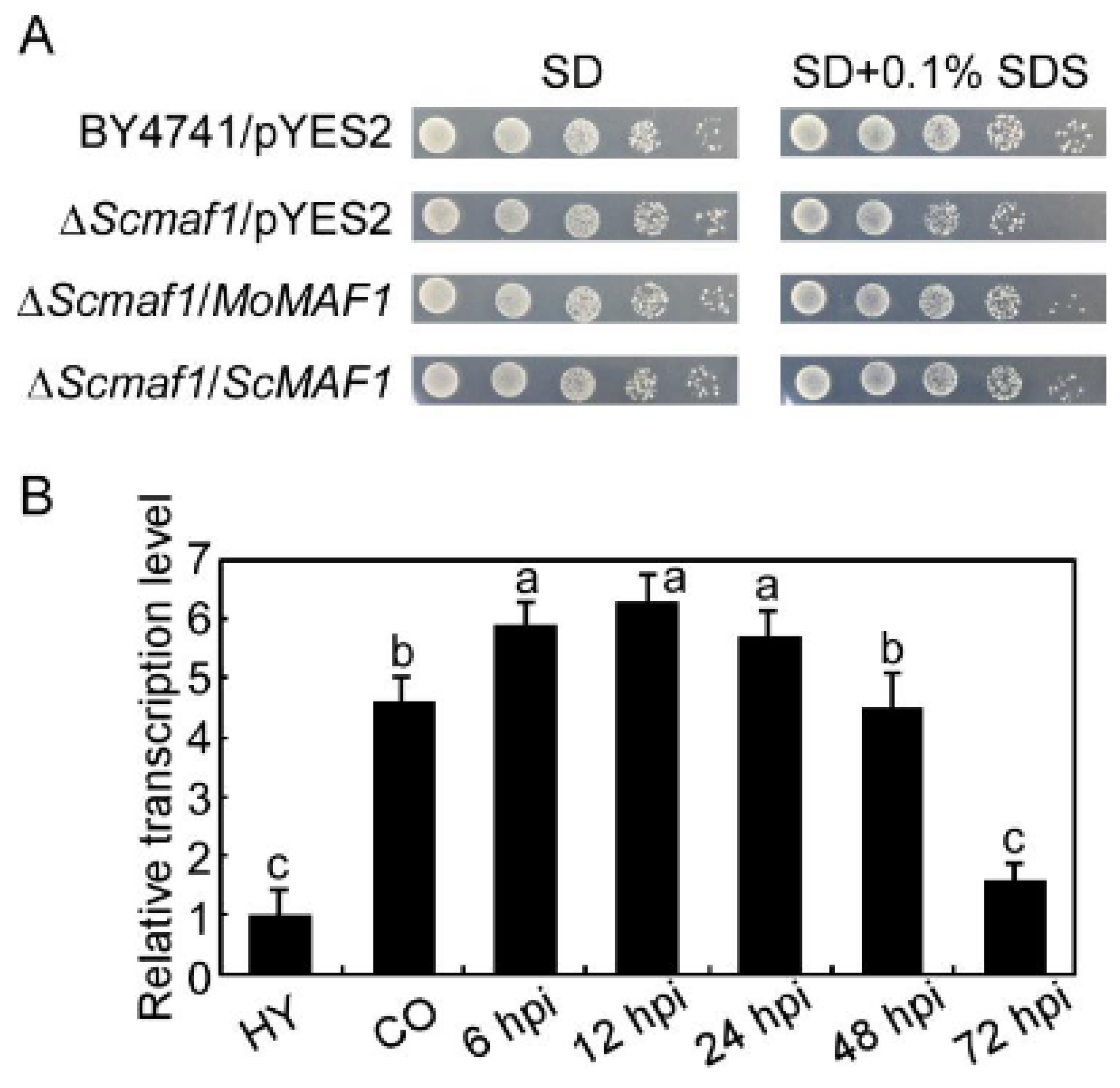
2.4. The Complementation of S. cerevisiae Δmaf1 Mutant
2.5. MoMAF1 Gene Deletion and Complementation
2.6. Conidial Germination and Appressorium Formation
2.7. Host Penetration and Pathogenicity Assay
2.8. Western Blot Analysis of Protein Phosphorylation
2.9. Chitin (N-acetylglucosamine, GlcNAc) Content Assay
2.10. The Observation of Subcellular Localization
2.11. Statistical Analysis
3. Results
3.1. Identification and Expression of MoMAF1
3.2. MoMAF1-Regulated RNA Synthesis
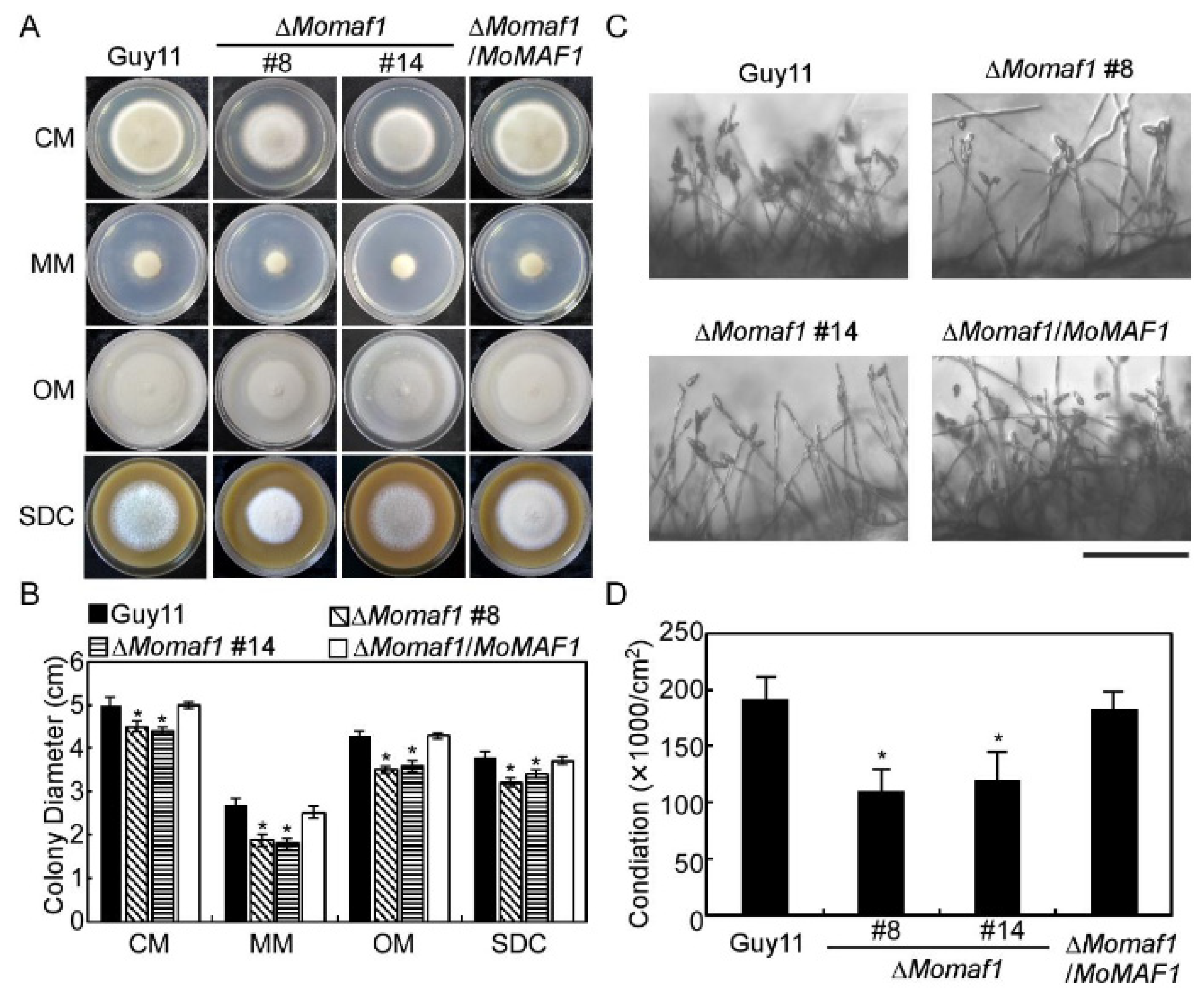
3.3. MoMaf1 Was Involved in Vegetative Growth and Conidiation
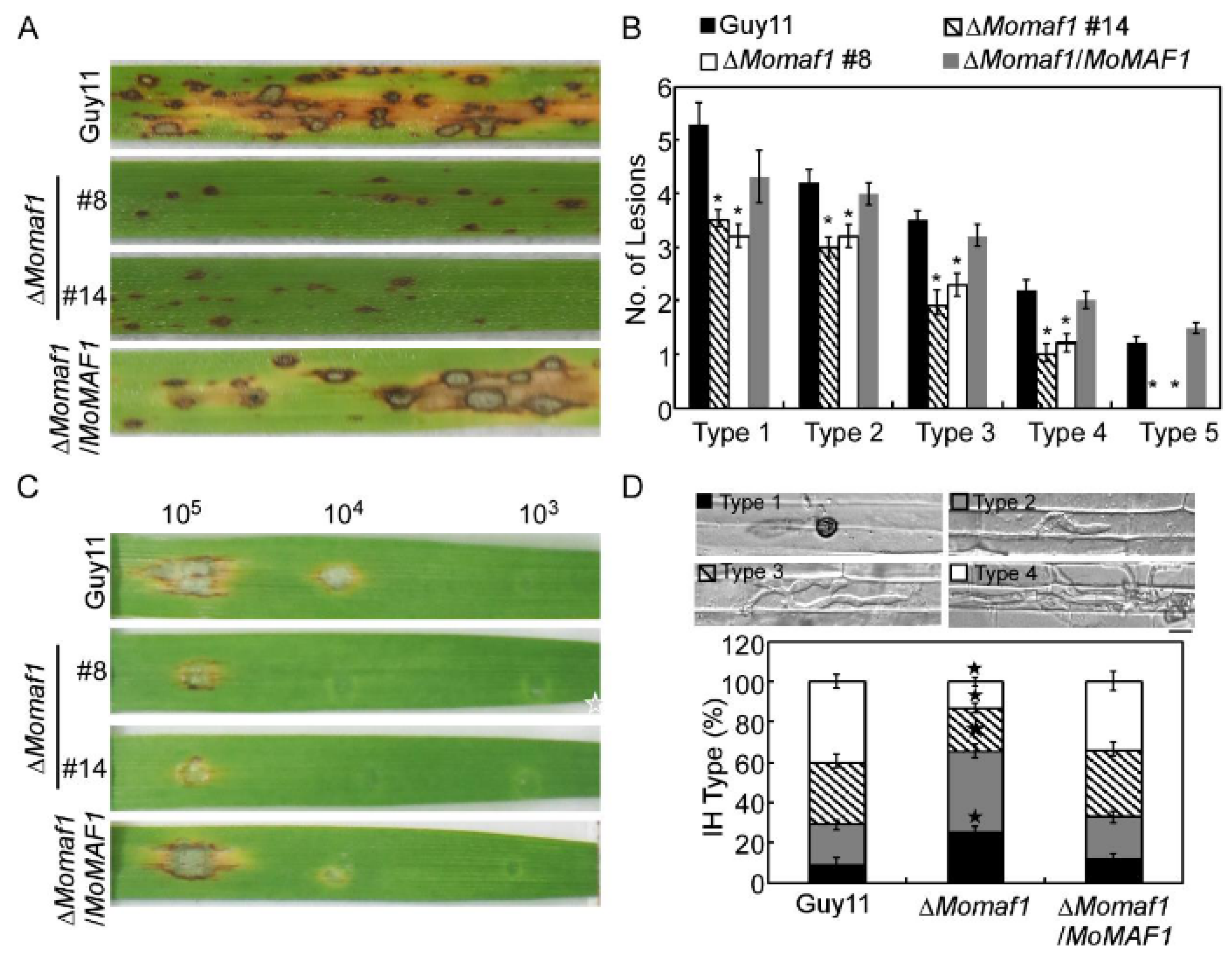
3.4. MoMaf1 Was Required for Penetration and Infectious Growth
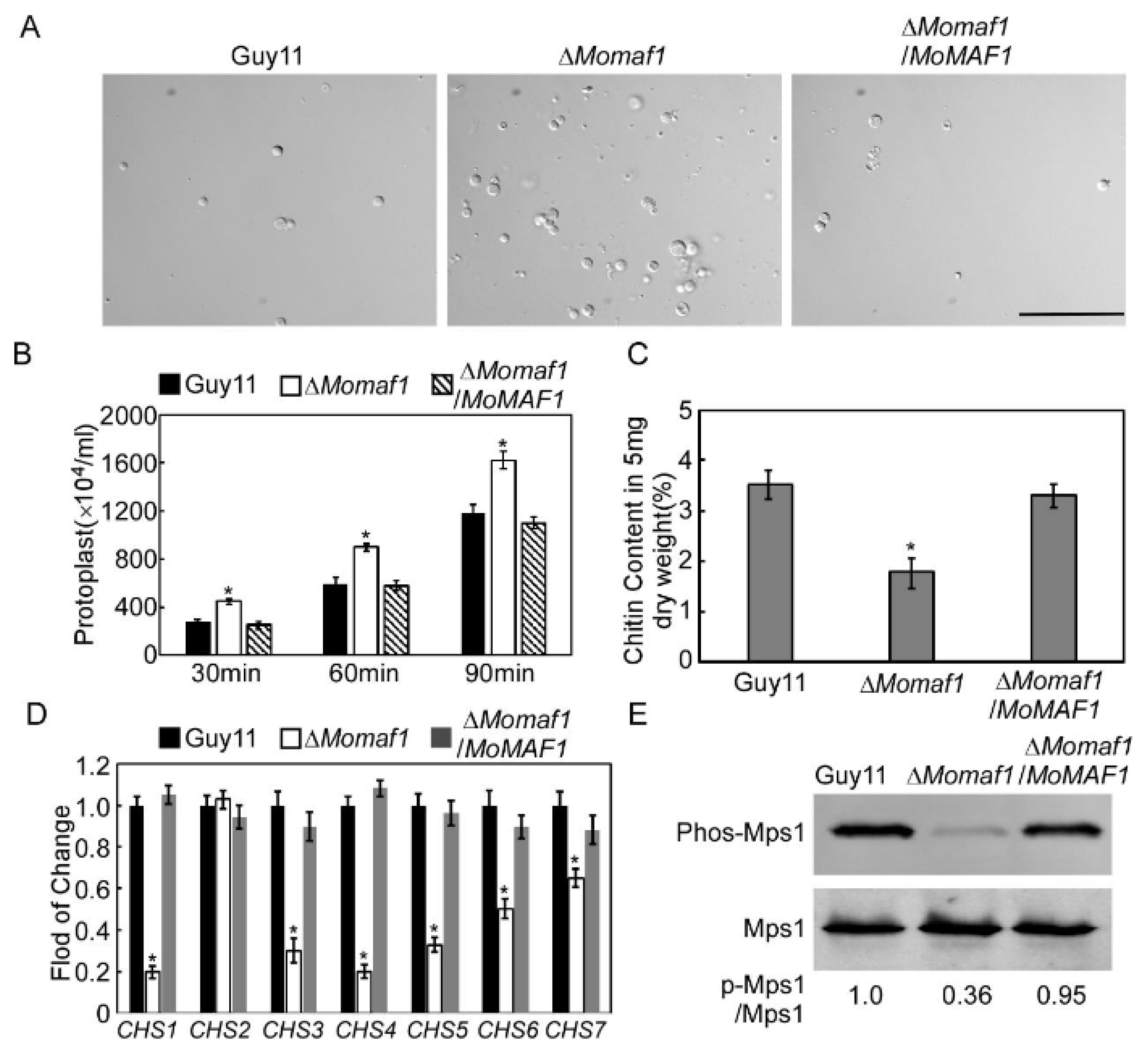
3.5. MoMaf1 Regulated the Generation of Appressorium Turgor Pressure
3.6. MoMaf1 Was Involved in Cell Wall Integrity (CWI)
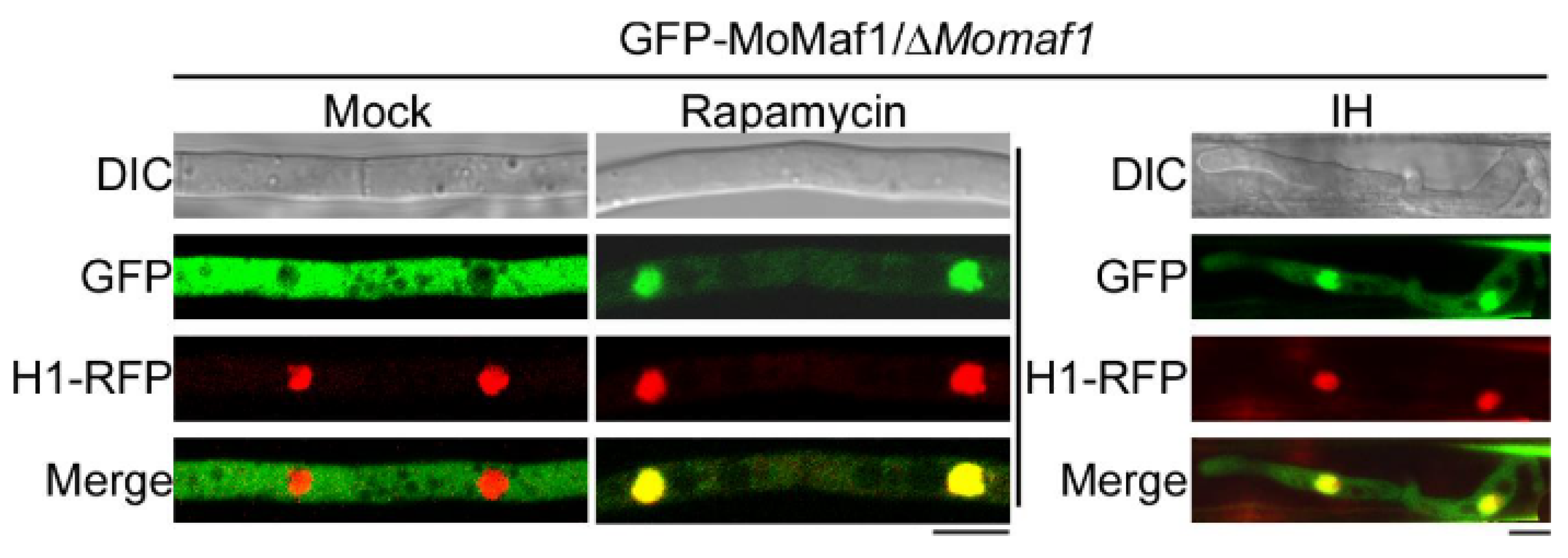
3.7. The Subcellular Localization of MoMaf1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dieci, G.; Fiorino, G.; Castelnuovo, M.; Teichmann, M.; Pagano, A. The expanding RNA polymerase III transcriptome. Trends Genet. 2007, 23, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Ringel, R.; Kusser, A.G.; Berninghausen, O.; Kassavetis, G.A.; Cramer, P. Molecular Basis of RNA Polymerase III Transcription Repression by Maf1. Cell 2010, 143, 59–70. [Google Scholar] [CrossRef]
- Berns, A. A tRNA with oncogenic capacity. Cell 2008, 133, 29–30. [Google Scholar] [CrossRef] [PubMed]
- Seton-Rogers, S. tRNA supply and demand. Nat. Rev. Cancer 2022, 22, 129. [Google Scholar] [CrossRef]
- Boguta, M.; Graczyk, D. RNA polymerase III under control: Repression and de-repression. Trends Biochem. Sci. 2011, 36, 451–456. [Google Scholar] [CrossRef]
- Karkusiewicz, I.; Turowski, T.W.; Graczyk, D.; Towpik, J.; Dhungel, N.; Hopper, A.K.; Boguta, M. Maf1 Protein, Repressor of RNA Polymerase III, Indirectly Affects tRNA Processing. J. Biol. Chem. 2011, 286, 39478–39488. [Google Scholar] [CrossRef] [PubMed]
- Oler, A.J.; Cairns, B.R. PP4 dephosphorylates Maf1 to couple multiple stress conditions to RNA polymerase III repression. EMBO J. 2012, 31, 1440–1452. [Google Scholar] [CrossRef]
- Moir, R.D.; Lee, J.; Haeusler, R.A.; Desai, N.; Engelke, D.R.; Willis, I.M. Protein kinase A regulates RNA polymerase III transcription through the nuclear localization of Maf1. Proc. Natl. Acad. Sci. USA 2006, 103, 15044–15049. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Moir, R.D.; Willis, I.M. Regulation of RNA polymerase III transcription involves SCH9-dependent and SCH9-independent branches of the target of rapamycin (TOR) pathway. J. Biol. Chem. 2009, 284, 12604–12608. [Google Scholar] [CrossRef]
- Graczyk, D.; Dębski, J.; Muszyńska, G.; Bretner, M.; Lefebvre, O.; Boguta, M. Casein kinase II-mediated phosphorylation of general repressor Maf1 triggers RNA polymerase III activation. Proc. Natl. Acad. Sci. USA 2011, 108, 4926–4931. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, X.; Zhang, Z. The Magnaporthe grisea species complex and plant pathogenesis. Mol. Plant Pathol. 2016, 17, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Feng, W.; Chen, C.; Xu, J.; Li, Y.; Yang, L.; Wang, J.; Liu, X.; Wang, W.; Gao, C.; et al. Shedding light on autophagy coordinating with cell wall integrity signaling to govern pathogenicity of Magnaporthe oryzae. Autophagy 2020, 16, 900–916. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Liu, X.; Jia, J.; Cai, Y.; Chen, C.; Zhang, H.; Zheng, X.; Wang, P.; Zhang, Z. MoPpe1 partners with MoSap1 to mediate TOR and cell wall integrity signalling in growth and pathogenicity of the rice blast fungus Magnaporthe oryzae. Environ. Microbiol. 2018, 20, 3964–3979. [Google Scholar] [CrossRef]
- Qian, B.; Liu, X.; Ye, Z.; Zhou, Q.; Liu, P.; Yin, Z.; Wang, W.; Zheng, X.; Zhang, H.; Zhang, Z. Phosphatase-associated protein MoTip41 interacts with the phosphatase MoPpe1 to mediate crosstalk between TOR and cell wall integrity signalling during infection by the rice blast fungus Magnaporthe oryzae. Environ. Microbiol. 2021, 23, 791–809. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Su, X.; Ye, Z.; Liu, X.; Liu, M.; Shen, D.; Chen, H.; Zhang, H.; Wang, P.; Zhang, Z. MoErv29 promotes apoplastic effector secretion contributing to virulence of the rice blast fungus Magnaporthe oryzae. New Phytol. 2021, 233, 1289–1302. [Google Scholar] [CrossRef]
- Feng, W.; Yin, Z.; Wu, H.; Liu, P.; Liu, X.; Liu, M.; Yu, R.; Gao, C.; Zhang, H.; Zheng, X.; et al. Balancing of the mitotic exit network and cell wall integrity signaling governs the development and pathogenicity in Magnaporthe oryzae. PLoS Pathog. 2021, 17, e1009080. [Google Scholar] [CrossRef]
- Chen, D.; Liang, J.; Jiang, C.; Wu, D.; Huang, B.; Teng, X.; Tang, Y. Mitochondrion Participated in Effect Mechanism of Manganese Poisoning on Heat Shock Protein and Ultrastructure of Testes in Chickens. Biol. Trace Elem. Res. 2022, 1–10. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Y.; Teng, X.; Luan, P.; Yin, X. Immunosuppression participated in complement activation-mediated inflammatory injury caused by 4-octylphenol via TLR7/IkappaBalpha/NF-kappaB pathway in common carp (Cyprinus carpio) gills. Aquat. Toxicol. 2022, 249, 106211. [Google Scholar] [CrossRef]
- Guo, M.; Chen, Y.; Du, Y.; Dong, Y.; Guo, W.; Zhai, S.; Zhang, H.; Dong, S.; Zhang, Z.; Wang, Y.; et al. The bZIP Transcription Factor MoAP1 Mediates the Oxidative Stress Response and Is Critical for Pathogenicity of the Rice Blast Fungus Magnaporthe oryzae. PLoS Pathog. 2011, 7, e1001302. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, Q.; Yu, M.; Liu, Y.; Teng, X.; Gu, X. 4-tert-butylphenol triggers common carp hepatocytes ferroptosis via oxidative stress, iron overload, SLC7A11/GSH/GPX4 axis, and ATF4/HSPA5/GPX4 axis. Ecotoxicol. Environ. Saf. 2022, 242, 113944. [Google Scholar] [CrossRef]
- Miao, Z.; Teng, X.; Xu, S. Melatonin alleviates lead-induced intestinal epithelial cell pyroptosis in the common carps (Cyprinus carpio) via miR-17-5p/TXNIP axis. Fish Shellfish Immunol. 2022, 131, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, C.; Yang, J.; Feng, W.; Liu, X.; Zuo, R.; Zhang, Z. Histone acetyltransferase MoHat1 acetylates autophagy-related proteins MoAtg3 and MoAtg9 to orchestrate functional appressorium formation and pathogenicity in Magnaporthe oryzae. Autophagy 2019, 15, 1234–1257. [Google Scholar] [CrossRef]
- Pluta, K.; Lefebvre, O.; Martin, N.C.; Smagowicz, W.J.; Stanford, D.R.; Ellis, S.R.; Hopper, A.K.; Sentenac, A.; Boguta, M. Maf1p, a Negative Effector of RNA Polymerase III in Saccharomyces cerevisiae. Mol. Cell. Biol. 2001, 21, 5031–5040. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Li, X.; Le, X.; Kong, X.; Zhang, H.; Zheng, X.; Wang, P.; Zhang, Z. MoDnm1 Dynamin Mediating Peroxisomal and Mitochondrial Fission in Complex with MoFis1 and MoMdv1 Is Important for Development of Functional Appressorium in Magnaporthe oryzae. PLoS Pathog. 2016, 12, e1005823. [Google Scholar] [CrossRef] [PubMed]
- Veneault-Fourrey, C.; Barooah, M.; Egan, M.; Wakley, G.; Talbot, N.J. Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science 2006, 312, 580–583. [Google Scholar] [CrossRef]
- deJong, J.C.; MCCormack, B.J.; Smirnoff, N.; Talbot, N.J. Glycerol generates turgor in rice blast. Nature 1997, 389, 244–245. [Google Scholar] [CrossRef]
- Howard, R.J.; Valent, B. Breaking and entering: Host penetration by the fungal rice blast pathogen Magnaporthe grisea. Annu. Rev. Microbiol. 1996, 50, 491–512. [Google Scholar] [CrossRef]
- Tang, W.; Ru, Y.; Hong, L.; Zhu, Q.; Zuo, R.; Guo, X.; Wang, J.; Zhang, H.; Zheng, X.; Wang, P.; et al. System-wide characterization of bZIP transcription factor proteins involved in infection-related morphogenesis of Magnaporthe oryzae. Environ. Microbiol. 2014, 17, 1377–1396. [Google Scholar] [CrossRef]
- Yin, Z.; Tang, W.; Wang, J.; Liu, X.; Yang, L.; Gao, C.; Zhang, J.; Zhang, H.; Zheng, X.; Wang, P.; et al. Phosphodiesterase MoPdeH targets MoMck1 of the conserved mitogen-activated protein (MAP) kinase signalling pathway to regulate cell wall integrity in rice blast fungus Magnaporthe oryzae. Mol. Plant Pathol. 2016, 17, 654–668. [Google Scholar] [CrossRef]
- Lesniewska, E.; Boguta, M. Novel layers of RNA polymerase III control affecting tRNA gene transcription in eukaryotes. Open Biol. 2017, 7, 170001. [Google Scholar] [CrossRef]
- Oficjalska-Pham, D.; Harismendy, O.; Smagowicz, W.J.; de Peredo, A.G.; Boguta, M.; Sentenac, A.; Lefebvre, O. General Repression of RNA Polymerase III Transcription Is Triggered by Protein Phosphatase Type 2A-Mediated Dephosphorylation of Maf1. Mol. Cell 2006, 22, 623–632. [Google Scholar] [CrossRef]
- Johnson, S.S.; Zhang, C.; Fromm, J.; Willis, I.M.; Johnson, D.L. Mammalian Maf1 is a negative regulator of transcription by all three nuclear RNA polymerases. Mol. Cell 2007, 26, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Reina, J.H.; Azzouz, T.N.; Hernandez, N. Maf1, a New Player in the Regulation of Human RNA Polymerase III Transcription. PLoS ONE 2006, 1, e134. [Google Scholar] [CrossRef] [PubMed]
- Asghar, F.; Yan, H.; Jiang, L. The putative transcription factor CaMaf1 controls the sensitivity to lithium and rapamycin and represses RNA polymerase III transcription in Candida albicans. FEMS Yeast Res. 2018, 18, foy068. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Zhang, C.; Yu, F.; Yin, Y.; Shim, W.B.; Ma, Z. Protein kinase FgSch9 serves as a mediator of the target of rapamycin and high osmolarity glycerol pathways and regulates multiple stress responses and secondary metabolism in Fusarium graminearum. Environ. Microbiol. 2015, 17, 2661–2676. [Google Scholar] [CrossRef]
- Yang, J.; Liu, M.; Liu, X.; Yin, Z.; Sun, Y.; Zhang, H.; Zhang, Z. Heat-Shock Proteins MoSsb1, MoSsz1, and MoZuo1 Attenuate MoMkk1-Mediated Cell-Wall Integrity Signaling and Are Important for Growth and Pathogenicity of Magnaporthe oryzae. Mol. Plant-Microbe Interact. 2018, 31, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, J.; Zhou, W.; Chen, X.L.; Huang, J.G.; Cheng, Z.H.; Peng, Y.L. A spindle pole antigen gene MoSPA2 is important for polar cell growth of vegetative hyphae and conidia, but is dispensable for pathogenicity in Magnaporthe oryzae. Curr. Genet. 2014, 60, 255–263. [Google Scholar] [CrossRef]
- Michels, A.A. MAF1: A new target of mTORC1. Biochem. Soc. Trans. 2011, 39, 487–491. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Qian, B.; Cai, Y.; Zou, X.; Zhang, H.; Zheng, X.; Wang, P.; Zhang, Z. MoYvh1 subverts rice defense through functions of ribosomal protein MoMrt4 in Magnaporthe oryzae. PLoS Pathog. 2018, 14, e1007016. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, L.; Zhang, T.; Zhou, R.; Ren, Y.; Li, X.; Zhang, H. Transcription factor MoMsn2 targets the putative 3-methylglutaconyl-CoA hydratase-encoding gene MoAUH1 to govern infectious growth via mitochondrial fusion/fission balance in Magnaporthe oryzae. Environ. Microbiol. 2021, 23, 774–790. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Guo, X.; Guo, M.; Qi, Z.; Tang, W.; Dong, Y.; Ye, W.; Zheng, X.; Wang, P.; et al. Pleiotropic Function of the Putative Zinc-Finger Protein MoMsn2 in Magnaporthe oryzae. Mol. Plant-Microbe Interact. 2014, 27, 446–460. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, B.; Guo, L.; Song, C.; Ji, H. MoMaf1 Mediates Vegetative Growth, Conidiogenesis, and Pathogenicity in the Rice Blast Fungus Magnaporthe oryzae. J. Fungi 2023, 9, 106. https://doi.org/10.3390/jof9010106
Qian B, Guo L, Song C, Ji H. MoMaf1 Mediates Vegetative Growth, Conidiogenesis, and Pathogenicity in the Rice Blast Fungus Magnaporthe oryzae. Journal of Fungi. 2023; 9(1):106. https://doi.org/10.3390/jof9010106
Chicago/Turabian StyleQian, Bin, Lingyuan Guo, Chi Song, and Hong Ji. 2023. "MoMaf1 Mediates Vegetative Growth, Conidiogenesis, and Pathogenicity in the Rice Blast Fungus Magnaporthe oryzae" Journal of Fungi 9, no. 1: 106. https://doi.org/10.3390/jof9010106
APA StyleQian, B., Guo, L., Song, C., & Ji, H. (2023). MoMaf1 Mediates Vegetative Growth, Conidiogenesis, and Pathogenicity in the Rice Blast Fungus Magnaporthe oryzae. Journal of Fungi, 9(1), 106. https://doi.org/10.3390/jof9010106




