Multicenter Collaborative Study of the Interaction of Antifungal Combinations against Candida Spp. by Loewe Additivity and Bliss Independence-Based Response Surface Analysis
Abstract
1. Introduction
2. Materials and Methods
- (i)
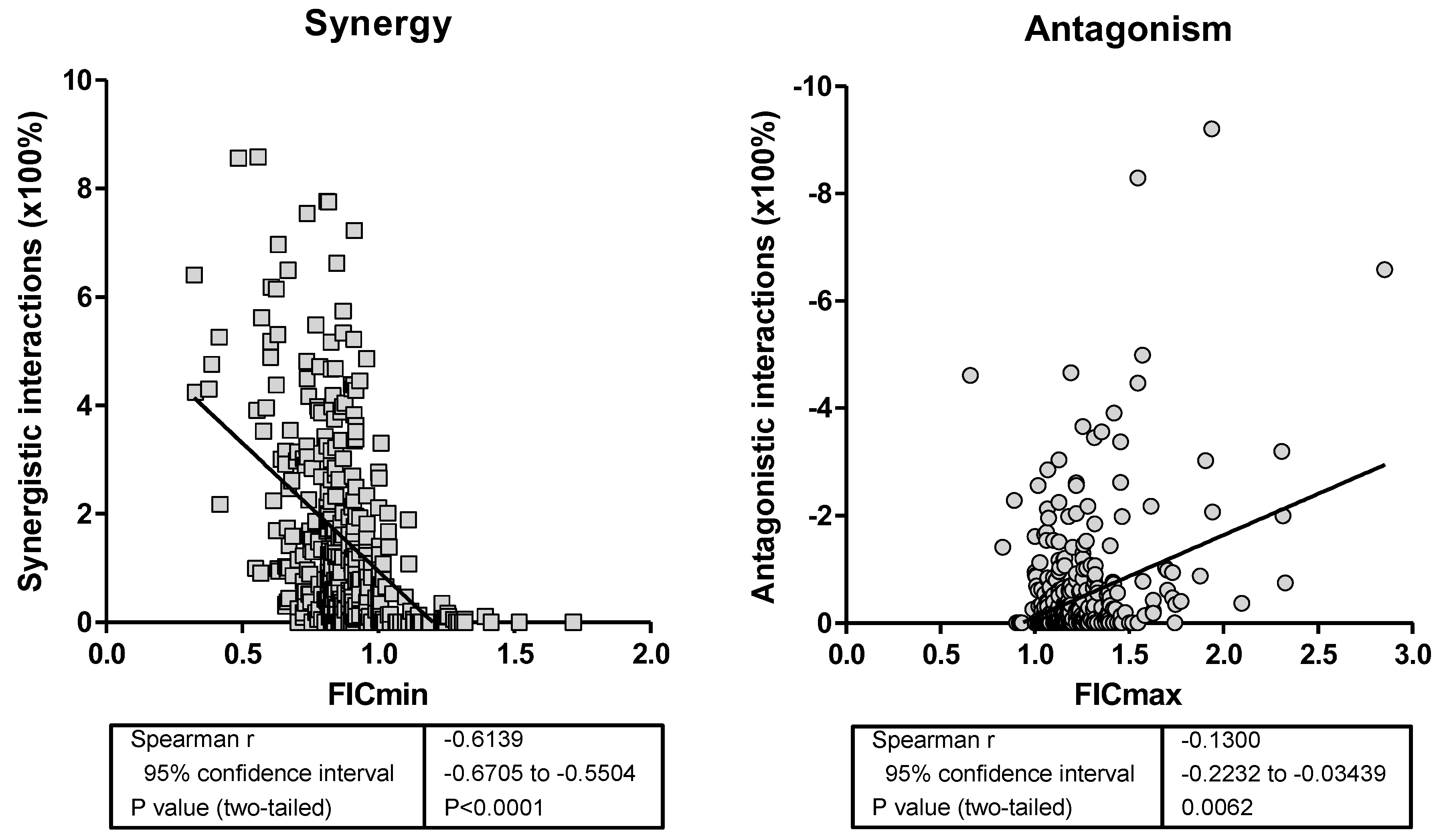
- Fractional inhibitory concentration (FIC) index analysis. The FIC index model is expressed as ∑FIC = FICA + FICB = CAcomb/MICAalone + CBcomb/MICBalone, where MICAalone and MICBalone are the MICs of the drugs A and B when acting alone and CAcomb and CBcomb are concentrations of the drugs A and B at the iso-effective combinations, respectively. In order to capture both synergistic and antagonistic interactions among all ∑FICs calculated for each checkerboard data set, the ∑FICmin and the ∑FICmax were determined as the lowest and highest ∑FIC, respectively. The MIC endpoints were defined as the lowest drug concentration showing <5% of growth compared to that of the growth control. Off-scale MICs were converted to the next-highest or -lowest doubling concentration. Finally, the median and the range of FIC indices among the replicates and centers were determined. In order to analyze statistically, the FICs were transformed to log2 values, and the 95% confidence interval of the FIC of all replicates and centers was calculated for each drug combination and strain based on the t distribution. When the 95% CI of ∑FICmin was smaller than 0.5, significant synergy was claimed; when the 95% CI of ∑FICmax was higher than 4, significant antagonism was claimed; in all other cases, additivity was concluded. The same analysis was performed with the cutoffs 1 for ∑FICmin and 2 for ∑FICmax.
- (ii)
- Bliss independence response surface (BIRS) analysis. The BI theory is described by the equation Ii = IA + IB − IA × IB, where Ii is the predicted percentage of inhibition of the theoretical non-interactive combination of drugs A and B, and IA, IB are the experimental percentages of inhibition of each drug acting alone, respectively. Since I = 1 − E, where E is the percentage of growth, by substituting into the former equation, the following equation is derived: Ei = EA × EB, where Ei is the predicted percentage of growth of the theoretical noninteractive combination of drugs A and B, respectively, and EA and EB are the experimental percentages of growth of each drug acting alone, respectively. The interaction is described by the difference (ΔE) between the predicted and measured percentages of growth with drugs at various concentrations. Because of the nature of combination testing using microtiter plates with two-fold dilution of either drug, this results in a ΔE for each drug combination. For each combination of the two drugs in each replicate–center experiment, the observed percent growth obtained from the experimental data was subtracted from the predicted percentage, calculated as described above. When the average difference was positive and its 95% CI among the replicates and centers did not include 0, SS synergy was claimed; when the difference was negative and its 95% CI did not include 0, SS antagonism was claimed. In any other case, BI was concluded. In order to summarize the interaction, the sum percentage of all SS synergistic (∑SYN) and antagonistic (∑ANT) interactions was calculated. Interactions with <50% of SS interactions were considered weak, those with 50% to 100% of SS interactions were considered moderate, and those with >100% of SS interactions were considered strong, as was found previously [11]. In addition, the numbers of SS synergistic and antagonistic combinations among the 49 (7 × 7) combinations tested were calculated for each strain.
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Vazquez, J.A. Combination antifungal therapy against Candida species: The new frontier—Are we there yet? Med. Mycol. 2003, 41, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, C.A. Clinical efficacy of new antifungal agents. Curr. Opin. Microbiol. 2006, 9, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; te Dorsthorst, D.T.A.; Verweij, P.E. The concentration-dependent nature of in vitro amphotericin B-itraconazole interaction against Aspergillus fumigatus: Isobolographic and response surface analysis of complex pharmacodynamic interactions. Int. J. Antimicrob. Agents 2006, 28, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The search for synergy: A critical review from a response surface perspective. Pharmacol. Rev. 1995, 47, 331–385. [Google Scholar] [PubMed]
- Meletiadis, J.; Mouton, J.W.; Meis, J.F.G.M.; Verweij, P.E. Methodological issues related to antifungal drug interaction modelling for filamentous fungi. Rev. Med. Microbiol. 2003, 13, 101–117. [Google Scholar] [CrossRef]
- Johnson, M.D.; MacDougall, C.; Ostrosky-Zeichner, L.; Perfect, J.R.; Rex, J.H. Combination antifungal therapy. Antimicrob. Agents Chemother. 2004, 48, 693–715. [Google Scholar] [CrossRef]
- Meletiadis, J.; Pournaras, S.; Roilides, E.; Walsh, T.J. Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2010, 54, 602–609. [Google Scholar] [CrossRef]
- Meletiadis, J.; Petraitis, V.; Petraitiene, R.; Lin, P.; Stergiopoulou, T.; Kelaher, A.; Sein, T.; Schaufele, R.; Bacher, J.; Walsh, T.J. Triazole-polyene antagonism in experimental invasive pulmonary aspergillosis: In vitro and in vivo correlation. J. Infect. Dis. 2006, 194, 1008–1018. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Hope, W.; Meletiadis, J.; Mickiene, D.; Hughes, J.; Cotton, M.; Stergiopoulou, T.; Kasai, M.; Francesconi, A.; et al. Combination therapy in treatment of experimental pulmonary aspergillosis: In vitro and in vivo correlations of the concentration- and dose- dependent interactions between anidulafungin and voriconazole by Bliss independence drug interaction analysis. Antimicrob. Agents Chemother. 2009, 53, 2382–2391. [Google Scholar] [CrossRef]
- Meletiadis, J.; Stergiopoulou, T.; O’Shaughnessy, E.M.; Peter, J.; Walsh, T.J. Concentration-dependent synergy and antagonism within a triple antifungal drug combination against Aspergillus species: Analysis by a new response surface model. Antimicrob. Agents Chemother. 2007, 51, 2053–2064. [Google Scholar] [CrossRef]
- Meletiadis, J.; Mouton, J.W.; Meis, J.F.G.M.; Verweij, P.E. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother. 2003, 47, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Verweij, P.E.; te Dorsthorst, D.T.; Meis, J.F.G.M.; Mouton, J.W. Assessing in vitro combinations of antifungal drugs against yeasts and filamentous fungi: Comparison of different drug interaction models. Med. Mycol. 2005, 43, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.; Ramani, R.; Andes, D.; Diekema, D.J.; Pfaller, M.A.; Ghannoum, M.A.; Knapp, C.; Lockhart, S.R.; Ostrosky-Zeichner, L.; Walsh, T.J.; et al. Multilaboratory testing of two-drug combinations of antifungals against Candida albicans, Candida glabrata, and Candida parapsilosis. Antimicrob. Agents Chemother. 2011, 55, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.; Ramani, R.; Ghannoum, M.A.; Killian, S.B.; Holliday, N.; Knapp, C.; Ostrosky-Zeichner, L.; Messer, S.A.; Pfaller, M.A.; Iqbal, N.J.; et al. Multilaboratory testing of antifungal combinations against a quality control isolate of Candida krusei. Antimicrob. Agents Chemother. 2008, 52, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- CLSI Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 2nd ed.; Approved Standard M38-A2; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2008; Volume 28.
- American Society for Microbiology. Instructions to authors. Antimicrob. Agents Chemother. 2004, 48, i–xxi. [Google Scholar]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Serena, C.; Marine, M.; Quindos, G.; Carrillo, A.J.; Cano, J.F.; Pastor, F.J.; Guarro, J. In vitro interactions of micafungin with amphotericin B against clinical isolates of Candida spp. Antimicrob. Agents Chemother. 2008, 52, 1529–1532. [Google Scholar] [CrossRef]
- Barchiesi, F.; Spreghini, E.; Tomassetti, S.; Giannini, D.; Scalise, G. Caspofungin in combination with amphotericin B against Candida parapsilosis. Antimicrob. Agents Chemother. 2007, 51, 941–945. [Google Scholar] [CrossRef]
- Barchiesi, F.; Spreghini, E.; Fothergill, A.W.; Arzeni, D.; Greganti, G.; Giannini, D.; Rinaldi, M.G.; Scalise, G. Caspofungin in combination with amphotericin B against Candida glabrata. Antimicrob. Agents Chemother. 2005, 49, 2546–2549. [Google Scholar] [CrossRef]
- Olson, J.A.; Adler-Moore, J.P.; Smith, P.J.; Proffitt, R.T. Treatment of Candida glabrata infection in immunosuppressed mice by using a combination of liposomal amphotericin B with caspofungin or micafungin. Antimicrob. Agents Chemother. 2005, 49, 4895–4902. [Google Scholar] [CrossRef]
- Marine, M.; Serena, C.; Pastor, F.J.; Guarro, J. Combined antifungal therapy in a murine infection by Candida glabrata. J. Antimicrob. Chemother. 2006, 58, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Heyn, K.; Tredup, A.; Salvenmoser, S.; Müller, F.-M.C.; Muller, F.M. Effect of voriconazole combined with micafungin against Candida, Aspergillus, and Scedosporium spp. and Fusarium solani. Antimicrob. Agents Chemother. 2005, 49, 5157–5159. [Google Scholar] [CrossRef] [PubMed]
- Kiraz, N.; Dag, I.; Yamac, M.; Kiremitci, A.; Kasifoglu, N.; Oz, Y. Synergistic activities of three triazoles with caspofungin against Candida glabrata isolates determined by time-kill, Etest, and disk diffusion methods. Antimicrob. Agents Chemother. 2010, 54, 2244–2247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pfeiffer, C.D.; Garcia-Effron, G.; Zaas, A.K.; Perfect, J.R.; Perlin, D.S.; Alexander, B.D. Breakthrough Invasive Candidiasis in Patients on Micafungin. J. Clin. Microbiol. 2010, 48, 2373–2380. [Google Scholar] [CrossRef]
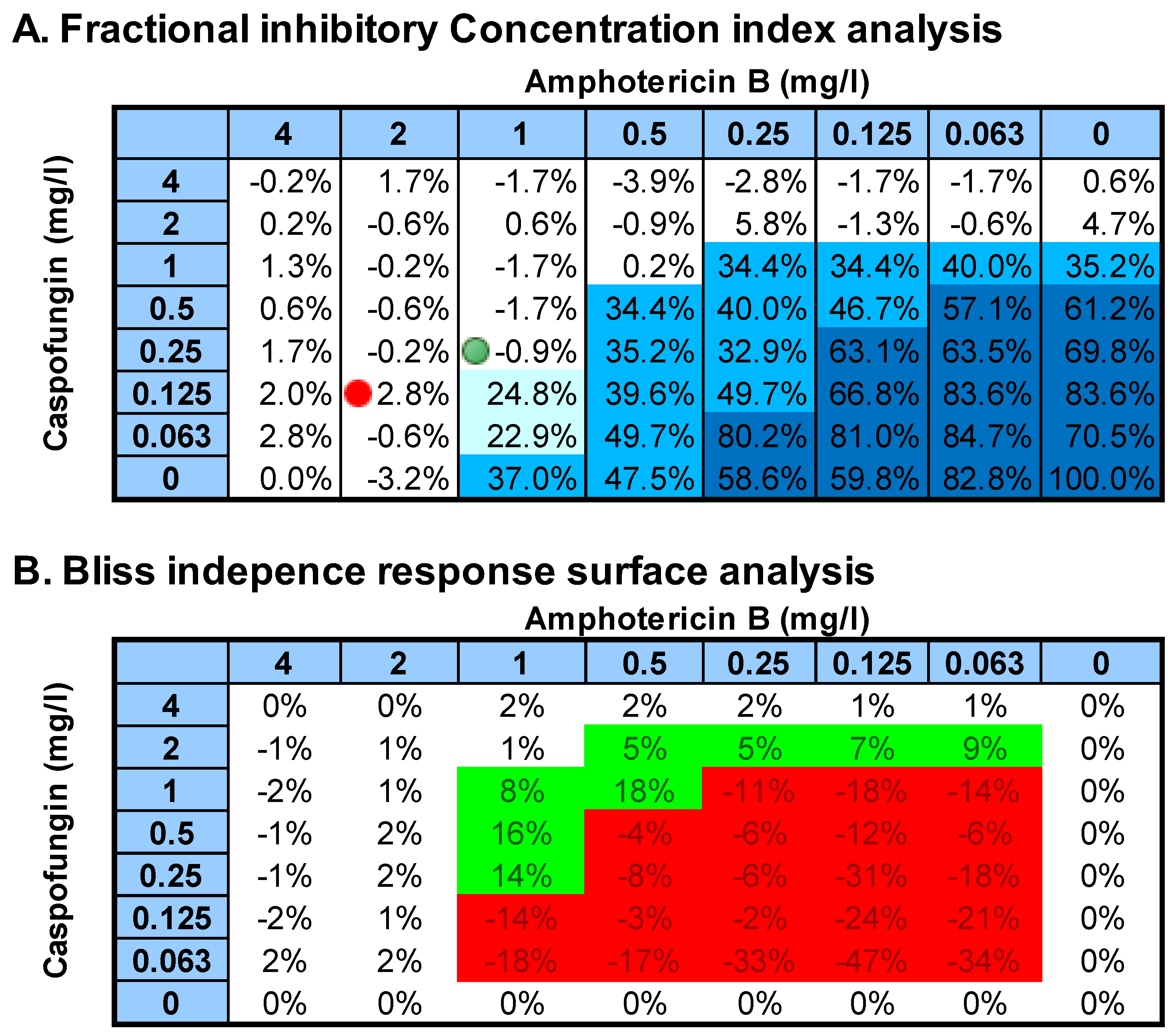
| Antifungal Drug | C. albicans #91 (20533.043) | C. albicans #92 (20464.007) | C. glabrata #93 (20205.075) | C. parapsilosis #94 (20580.070) | C. parapsilosis QC ATCC 22019 |
|---|---|---|---|---|---|
| Amphotericin B | 1 (0.5–2) | 1 (0.5–2) | 1 (0.5–2) | 1 (0.5–4) | 1 (0.5–2) |
| Anidulafungin | 0.06 (0.03–0.06) | 0.25 (0.12–0.5) | 0.06 (0.03–0.06) | 2 (2–4) | 2 (0.5–2) |
| Caspofungin | 0.12 (0.06–1) | 1 (1–2) | 0.06 (0.06–0.12) | 1 (0.5–1) | 1 (0.5–2) |
| Micafungin | 0.06 (0.03–0.12) | 0.12 (0.12–0.5) | 0.06 (0.06–0.5) | 2 (1–2) | 2 (1–4) |
| Posaconazole | 1 (0.5–2) | 0.5 (0.25–1) | 2 | 0.12 (0.06–0.25) | 0.25 (0.12–0.5) |
| Voriconazole | 2 (1–2) | 2 | 2 | 0.015 | 0.03 (0.03–0.12) |
| Combinations a | Drugs b | C. albicans #91 | C. albicans #92 | C. glabrata #93 | C. parapsilosis #94 | C. parapsilosis QC |
|---|---|---|---|---|---|---|
| -------------------------------------------------------LOEWE SYNERGISTIC INTERACTIONS (∑FICmin)------------------------------ | ||||||
| Interclass combinations | ||||||
| ECH+AB | ANI+AB | 0.44 (0.09–0.63) c | 0.5 (0.31–0.75) c | 0.63 (0.5–1.13) | 0.52 (0.04–1) c | 0.75 (0.16–1) c |
| CAS+AB | 0.56 (0.19–1.06) c | 0.63 (0.53–1.06) c | 1 (0.5–1.06) | 0.75 (0.13–1.25) c | 0.66 (0.19–1.13) c | |
| MIF+AB | 0.53 (0.31–0.56) c | 0.5 (0.16–0.75) c | 0.63 (0.63–0.63) | 0.56 (0.16–1.13) c | 0.55 (0.19–1.06) c | |
| AZO+AB | POS+AB | 0.64 (0.14–1.03) c | 0.34 (0.19–1.03) c | 0.52 (0.04–1) c | 0.53 (0.25–1.13) c | 1 (0.31–1.25) |
| VOR+AB | 0.78 (0.13–2.01) | 1.01 (0.51–1.06) | 1.01 (0.31–1.02) | 0.28 (0.19–0.56) c | 0.81 (0.38–1.5) | |
| AZO+ECH | VOR+CAS | 0.59 (0.31–1) c | 0.53 (0.51–1.01) c | 0.51 (0.26–1) c | 0.13 (0.05–0.56) c | 0.55 (0.28–1.06) c |
| POS+CAS | 1 (0.28–2) | 0.28 (0.09–1.5) c | 0.63 (0.31–1.03) | 0.52 (0.09–1.5) c | 1 (0.07–2) | |
| POS+MIF | 0.53 (0.51–1.02) c | 0.51 (0.25–1.03) c | 0.54 (0.51–0.56) c | 0.55 (0.05–0.75) c | 0.59 (0.38–1.06) c | |
| VOR+MIF | 0.52 (0.51–0.75) c | 0.51 (0.26–1.01) c | 0.51 (0.51–0.52) c | 0.06 (0.04–0.53) c | 0.75 (0.25–1.06) c | |
| POS+ANI | 0.51 (0.03–1.06) c | 0.44 (0.09–1.03) c | 0.51 (0.13–2.01) c | 0.41 (0.02–0.56) c | 0.53 (0.28–1) c | |
| VOR+ANI | 0.52 (0.05–1.02) c | 0.51 (0.26–1.01) c | 0.53 (0.14–2.01) c | 0.05 (0.02–0.52) c | 1.02 (0.52–2.06) | |
| Intraclass combinations | ||||||
| AZO+AZO | POS+VOR | 1 (0.08–2.01) | 1 (0.13–1.5) | ND | 0.63 (0.38–3) | 1.25 (0.63–2.06) |
| ECH+ECH | CAS+MIF | 0.56 (0.5–1) c | 0.5 (0.28–0.63) c | 1 | 0.75 (0.25–1) c | 0.59 (0.14–2) c |
| MIF+ANI | 0.75 (0.52–1.5) | 0.5 (0.38–0.56) c | 0.88 (0.75–1) | 0.53 (0.38–1) c | 0.69 (0.14–1.25) c | |
| ANI+CAS | 0.56 (0.16–1) c | 0.38 (0.25–0.63) c | 1 (0.38–1) | 0.63 (0.06–1.03) c | 0.75 (0.5–1.25) c | |
| ------------------------------------------------------LOEWE ANTAGONISTIC INTERACTIONS (∑FICmax)--------------------------- | ||||||
| Interclass combinations | ||||||
| ECH+AB | ANI+AB | 1.25 (1.02–1.5) | 1.25 (1.06–2.06) | 1.5 (1.25–3) | 1.25 (1.06–2.03) | 1.5 (1.02–4.25) |
| CAS+AB | 1.25 (1.01–4.25) | 1.25 (1.13–4.25) | 1.5 (1.5–5) | 2.13 (1.06–4.25) | 1.5 (1.03–5) | |
| MIF+AB | 1.5 (1.25–1.5) | 1.25 (1.03–4.13) | 1.81 (1.5–2.13) | 1.5 (1.13–2.5) | 1.25 (1.03–4.25) | |
| AZO+AB | POS+AB | 1.38 (0.56–2.5) | 1.19 (1.01–4.06) | 1.25 (1.01–2.5) | 1.25 (1.01–4.02) | 2.25 (1.13–4.13) |
| VOR+AB | 1.5 (0.63–2.5) | 2.13 (1.5–4.02) | 1.5 (1.01–2.5) | 1.5 (1.01–2.5) | 2.5 (1.25–4.03) | |
| AZO+ECH | VOR+CAS | 1.5 (1.01–2.5) | 1.5 (1.01–4.06) | 1.5 (1.01–2.1) | 1.01 (1.01–1.5) | 2.13 (1.5–4.5) |
| POS+CAS | 1.5 (1.01–4.13) | 1.03 (1.01–3) | 1.13 (1.01–2.5) | 1.5 (1.01–4.5) | 2 (1.06–4.5) | |
| POS+MIF | 3 (1.5–8.5) d | 1.13 (1.01–2.5) | 1.77 (1.5–2.03) | 1.25 (1.06–2.5) | 1.78 (1.06–2.5) | |
| VOR+MIF | 1.5 (1.01–4.5) | 1.5 (1.01–2.25) | 1.01 (1.01–1.02) | 1.27 (1.01–2.01) | 2 (1.13–4) | |
| POS+ANI | 1.5 (1.01–4.25) | 1.05 (1.01–4.02) | 1.01 (1.01–4.06) | 1.19 (0.53–2.13) | 1.5 (1.5–4.5) | |
| VOR+ANI | 2.25 (1.01–4.5) | 1.02 (1.01–2.5) | 1.13 (1.01–4.02) | 1.03 (0.51–1.5) | 2.19 (1.5–5) | |
| Intraclass combinations | ||||||
| AZO+AZO | POS+VOR | 1.5 (1–2.5) | 1 (0.63–2.25) | ND | 1.88 (1.13–4.13) | 2.75 (1.5–9) d |
| ECH+ECH | CAS+MIF | 1.5 (1.25–1.5) | 1.13 (1.13–4.25) | 1.5 (1.5–1.5) | 1.5 (1.03–4.03) | 1.5 (0.63–4.5) |
| MIF+ANI | 1.5 (1.25–2.5) | 1.25 (1.13–4.25) | 1.5 (1.5–1.5) | 1.25 (1.01–1.5) | 1.5 (1.25–4.5) | |
| ANI+CAS | 1.5 (1.25–2.5) | 1.13 (1.03–2.25) | 1.5 (1.25–4.5) | 1.19 (1.01–3) | 1.5 (1.13–4.25) | |
| Combination c | Drugs b | C. albicans #91 | C. albicans #92 | C. glabrata #93 | C. parapsilosis #94 | C. parapsilosis QC | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum | (N) | Sum | (N) | Sum | (N) | Sum | (N) | Sum | (N) | ||
| ------------------------------------------------------------------BLISS SYNERGISTIC INTERACTIONS----------------------------------- | |||||||||||
| Interclass combinations | |||||||||||
| ECH+AB | ANI+AB | 138% | (23) | 74% | (30) | 10% | (19) | 364% | (35) | 169% | (29) |
| CAS+AB | 64% | (16) | 34% | (20) | 21% | (26) | 116% | (34) | 60% | (27) | |
| MIF+AB | 74% | (17) | 88% | (31) | 14% | (24) | 221% | (30) | 281% | (32) | |
| AZO+AB | POS+AB | 143% | (20) | 35% | (17) | 131% | (38) | 44% | (24) | 35% | (26) |
| VOR+AB | 93% | (14) | 13% | (16) | 42% | (15) | 70% | (33) | 14% | (11) | |
| AZO+ECH | VOR+CAS | 110% | (13) | 34% | (14) | 44% | (22) | 398% | (47) | 116% | (25) |
| POS+CAS | 23% | (9) | 60% | (18) | 35% | (29) | 321% | (37) | 83% | (35) | |
| POS+MIF | 22% | (6) | 158% | (40) | 56% | (36) | 269% | (30) | 175% | (21) | |
| VOR+MIF | 40% | (11) | 249% | (38) | 57% | (33) | 490% | (37) | 70% | (28) | |
| POS+ANI | 171% | (24) | 136% | (30) | 25% | (12) | 452% | (40) | 135% | (24) | |
| VOR+ANI | 135% | (28) | 234% | (42) | 51% | (23) | 578% | (49) | 10% | (19) | |
| Intraclass combinations | |||||||||||
| AZO+AZO | POS+VOR | 84% | (7) | 117% | (21) | 16% | (4) | 0% | (2) | 4% | (4) |
| ECH+ECH | CAS+MIF | 27% | (8) | 150% | (28) | 21% | (31) | 223% | (36) | 161% | (19) |
| MIF+ANI | 0% | (0) | 138% | (28) | 6% | (23) | 397% | (33) | 260% | (19) | |
| ANI+CAS | 38% | (10) | 112% | (27) | 15% | (25) | 177% | (24) | 70% | (14) | |
| ----------------------------------------------------------------------BLISS ANTAGONISTIC INTERACTIONS--------------------------- | |||||||||||
| ECH+AB | ANI+AB | −2% | (1) | 0% | (0) | 0% | (0) | −2% | (2) | −7% | (1) |
| CAS+AB | −40% | (6) | −33% | (2) | −23% | (3) | 0% | (0) | −30% | (2) | |
| MIF+AB | −2% | (2) | 0% | (0) | 0% | (0) | 0% | (0) | −2% | (1) | |
| AZO+AB | POS+AB | −100% | (9) | −68% | (4) | 0% | (0) | −6% | (1) | −14% | (1) |
| VOR+AB | −71% | (8) | −172% | (12) | 0% | (0) | 0% | (0) | −2% | (2) | |
| AZO+ECH | VOR+CAS | −234% | (17) | −161% | (10) | 0% | (0) | 0% | (0) | 0% | (0) |
| POS+CAS | −333% | (24) | −99% | (5) | 0% | (0) | 0% | (0) | 0% | (0) | |
| POS+MIF | −66% | (13) | 0% | (0) | −1% | (1) | 0% | (0) | −5% | (3) | |
| VOR+MIF | −51% | (9) | 0% | (0) | 0% | (0) | 0% | (0) | −4% | (3) | |
| POS+ANI | −12% | (4) | 0% | (0) | 0% | (0) | 0% | (0) | 0% | (0) | |
| VOR+ANI | −47% | (7) | 0% | (0) | 0% | (0) | 0% | (0) | −2% | (1) | |
| AZO+AZO | POS+VOR | −15% | (1) | −78% | (5) | −35% | (3) | 0% | (0) | −293% | (11) |
| ECH+ECH | CAS+MIF | −5% | (3) | 0% | (0) | 0% | (0) | 0% | (0) | 0% | (0) |
| MIF+ANI | −9% | (5) | 0% | (0) | −2% | (1) | 0% | (0) | 0% | (0) | |
| ANI+CAS | −4% | (3) | 0% | (0) | −2% | (1) | 0% | (0) | −93% | (5) | |
| Loewe Additivity Interactions | Bliss Independence Interactions | ||
|---|---|---|---|
| SYN (≥10%) | IND (<10%, >−10%) | ANT (≤−10%) | |
| SYN (FIC < 1) | 293 | 31 | 4 |
| ADD (FIC 1–2) | 38 | 320 | 167 |
| ANT (FIC ≥ 2) | 0 | 6 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meletiadis, J.; Andes, D.R.; Lockhart, S.R.; Ghannoum, M.A.; Knapp, C.C.; Ostrosky-Zeichner, L.; Pfaller, M.A.; Chaturvedi, V.; Walsh, T.J. Multicenter Collaborative Study of the Interaction of Antifungal Combinations against Candida Spp. by Loewe Additivity and Bliss Independence-Based Response Surface Analysis. J. Fungi 2022, 8, 967. https://doi.org/10.3390/jof8090967
Meletiadis J, Andes DR, Lockhart SR, Ghannoum MA, Knapp CC, Ostrosky-Zeichner L, Pfaller MA, Chaturvedi V, Walsh TJ. Multicenter Collaborative Study of the Interaction of Antifungal Combinations against Candida Spp. by Loewe Additivity and Bliss Independence-Based Response Surface Analysis. Journal of Fungi. 2022; 8(9):967. https://doi.org/10.3390/jof8090967
Chicago/Turabian StyleMeletiadis, Joseph, David R. Andes, Shawn R. Lockhart, Mahmoud A. Ghannoum, Cindy C. Knapp, Luis Ostrosky-Zeichner, Michael A. Pfaller, Vishnu Chaturvedi, and Thomas J. Walsh. 2022. "Multicenter Collaborative Study of the Interaction of Antifungal Combinations against Candida Spp. by Loewe Additivity and Bliss Independence-Based Response Surface Analysis" Journal of Fungi 8, no. 9: 967. https://doi.org/10.3390/jof8090967
APA StyleMeletiadis, J., Andes, D. R., Lockhart, S. R., Ghannoum, M. A., Knapp, C. C., Ostrosky-Zeichner, L., Pfaller, M. A., Chaturvedi, V., & Walsh, T. J. (2022). Multicenter Collaborative Study of the Interaction of Antifungal Combinations against Candida Spp. by Loewe Additivity and Bliss Independence-Based Response Surface Analysis. Journal of Fungi, 8(9), 967. https://doi.org/10.3390/jof8090967









