De Novo Long-Read Whole-Genome Assemblies and the Comparative Pan-Genome Analysis of Ascochyta Blight Pathogens Affecting Field Pea
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Culture
2.2. Nucleic Acid Extraction
2.3. Oxford Nanopore Technologies (ONT) Sequencing
2.4. Transcriptome Sequencing and Alignment
2.5. Repeat Expression Analysis
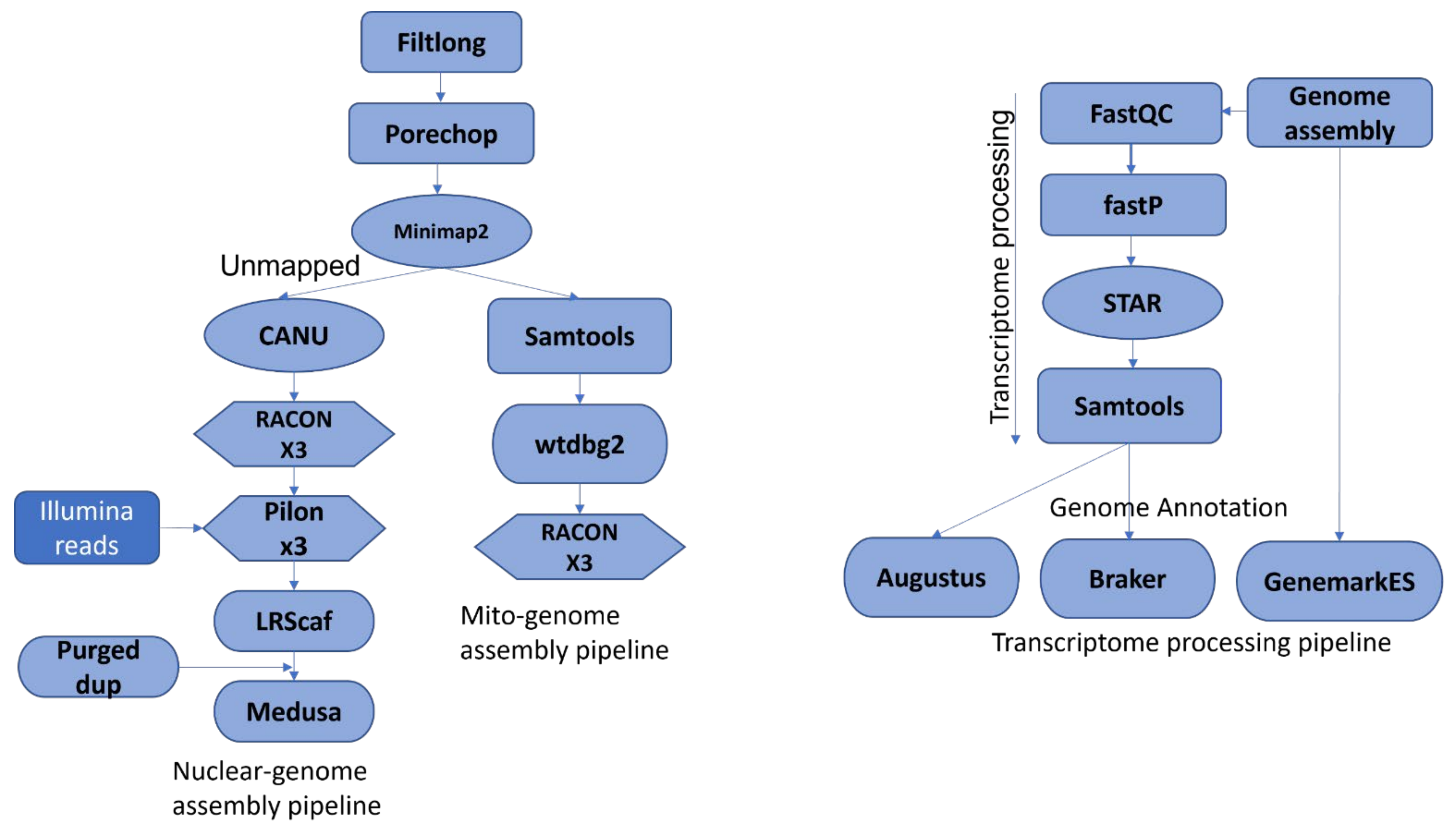
2.6. Nuclear and Mitochondrial Genome Assembly, and Post Assembly Analysis
2.7. Genome Annotation
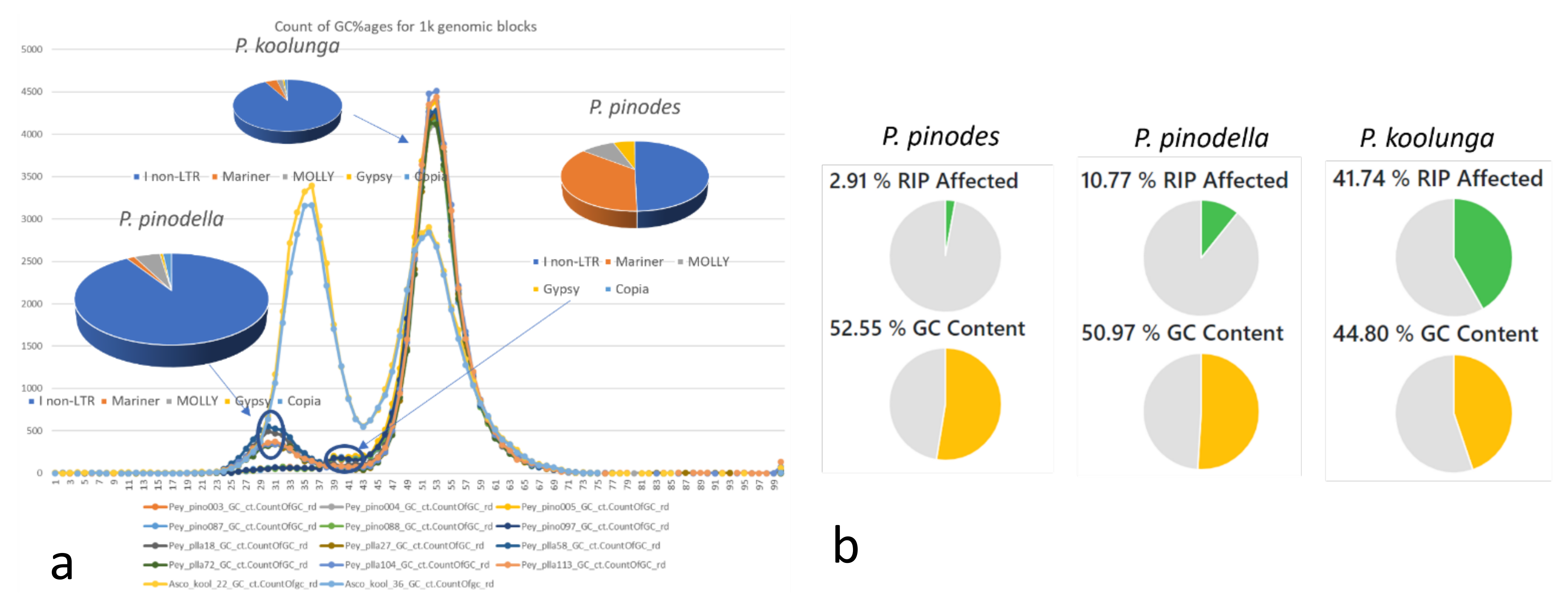
2.8. AB GC Content Variation
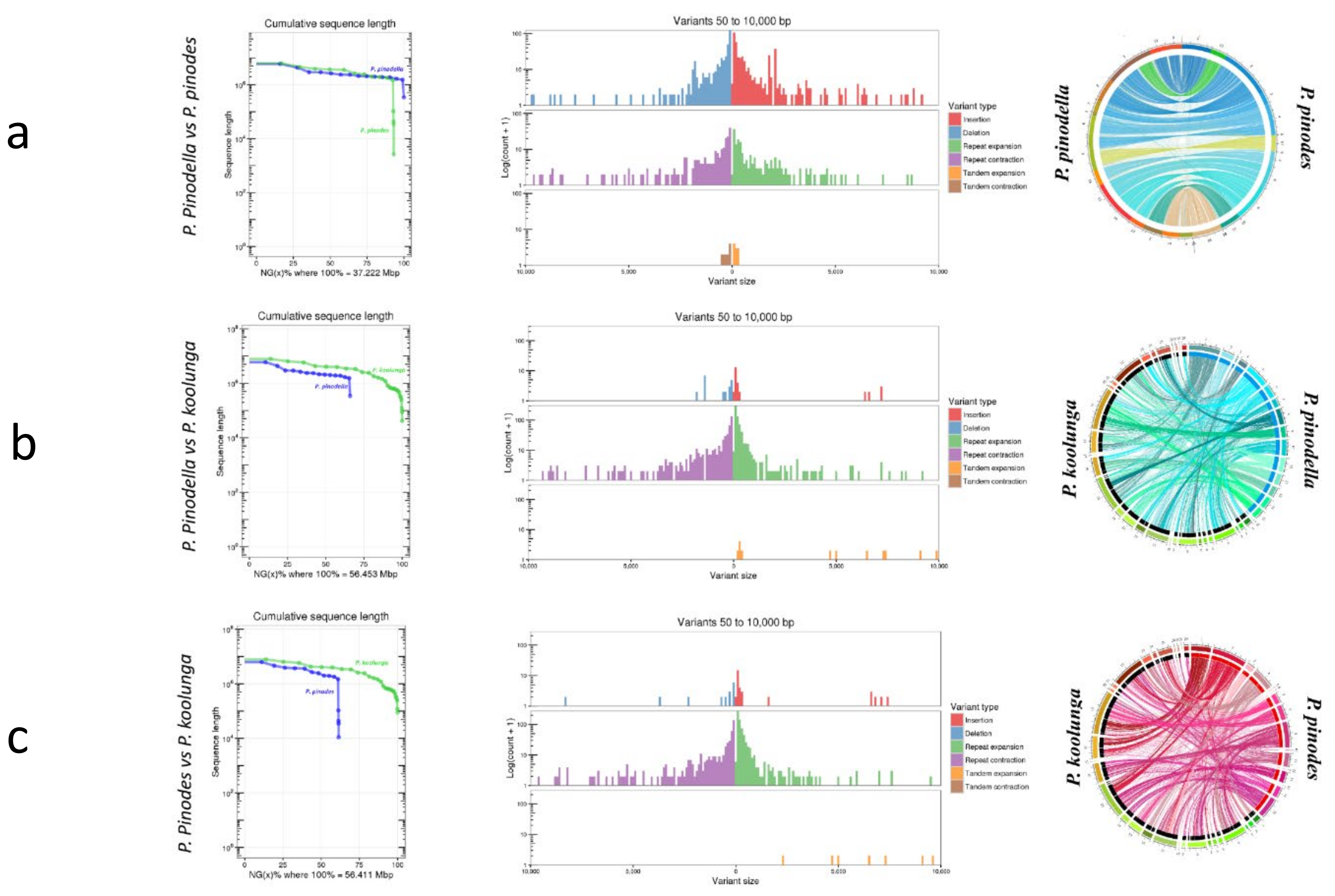
2.9. AB Nuclear Genome Structural Variation and Synteny Analysis
2.10. BLASTn Similarity and Blast2go Analysis
2.11. Mating Type Determination
2.12. Ortholog Analysis
2.13. Phylogenetic Analysis
2.14. Carbohydrate-Active Enzyme (CAZymes) Analysis
2.15. Secondary Metabolite Gene Cluster Analysis
3. Results
3.1. Genome Sequencing
3.2. Genome Assembly Statistics
3.3. Genome Annotation
3.4. Blastn and Blast2go Analysis
3.5. AB Nuclear Genome Structural Variation (SVs) and Synteny Analysis
3.6. Repeat Analysis of Genome Assemblies
3.7. Repeat Analysis using Transcriptome Data
3.8. Mating Type Determination
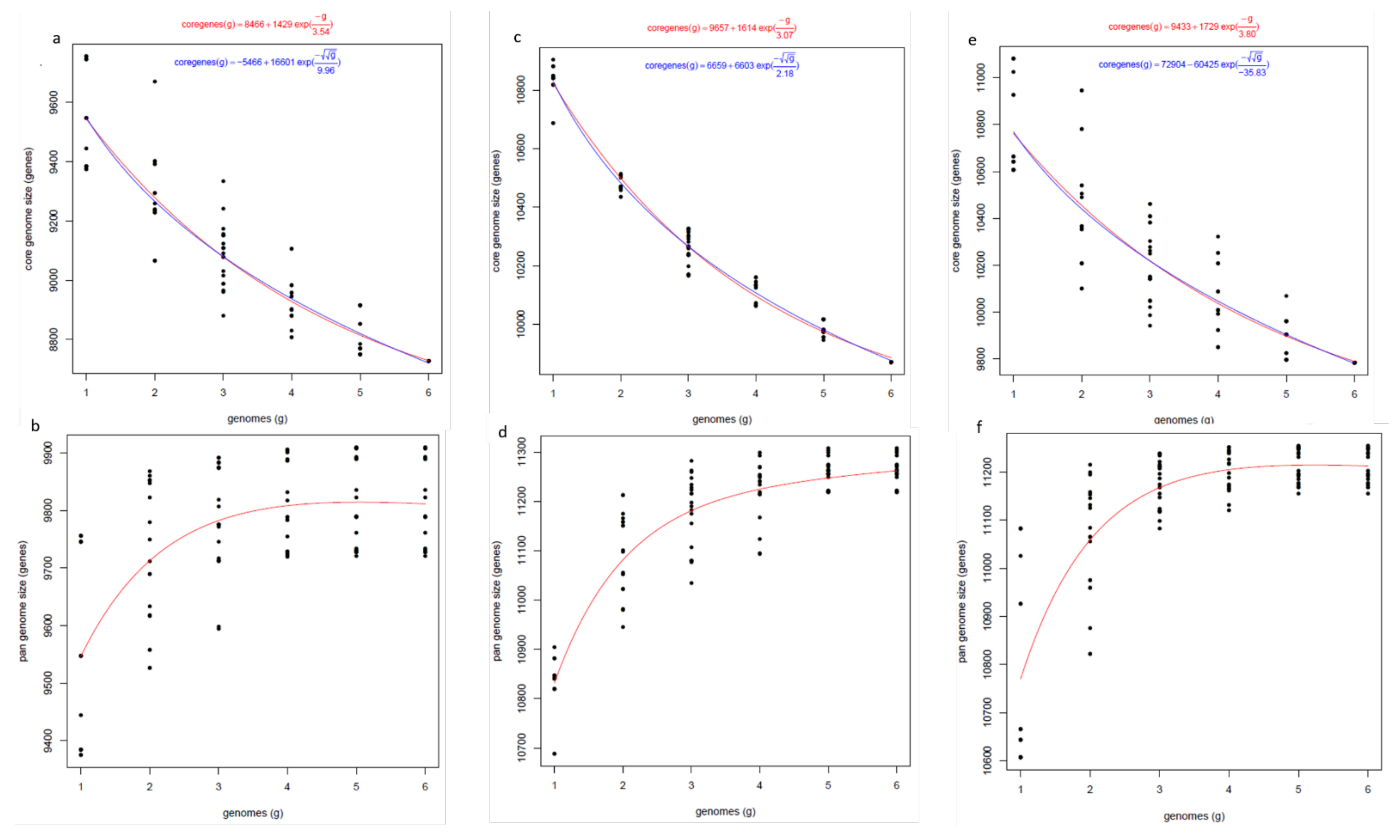
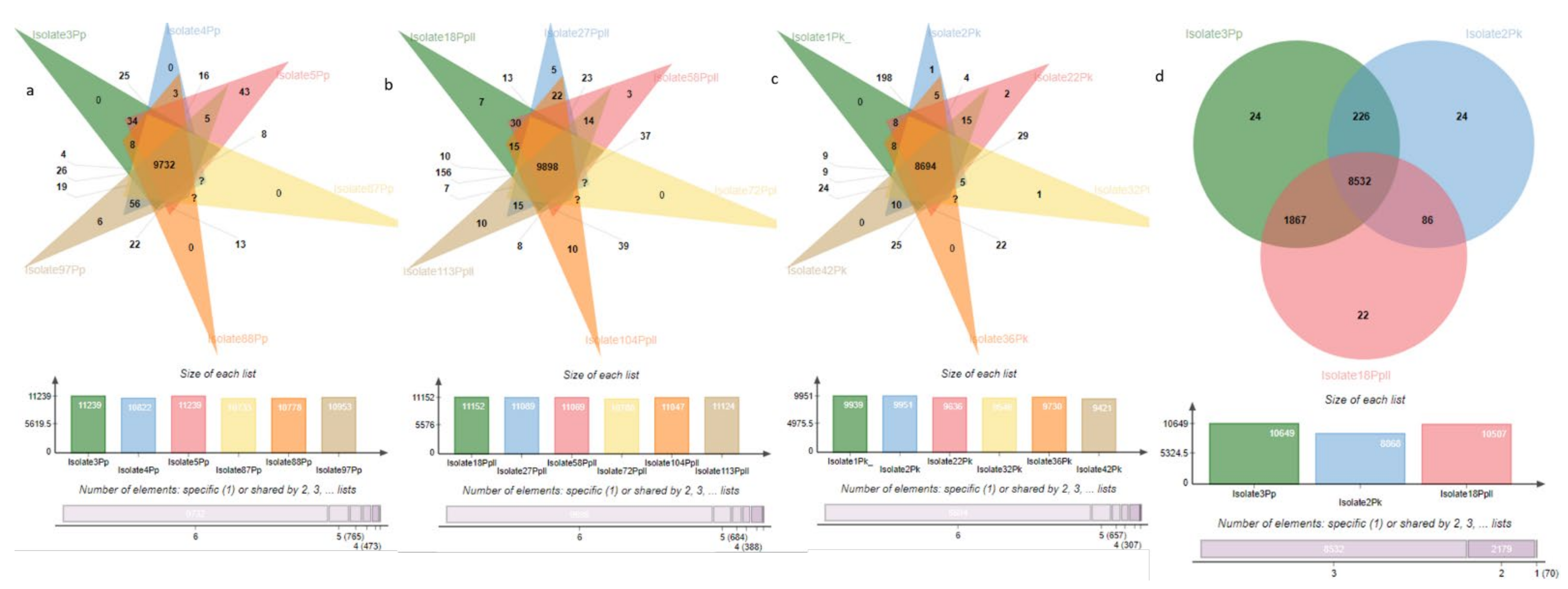
3.9. Orthogroup Analysis
3.10. Phylogeny
3.11. Gene Duplication Events
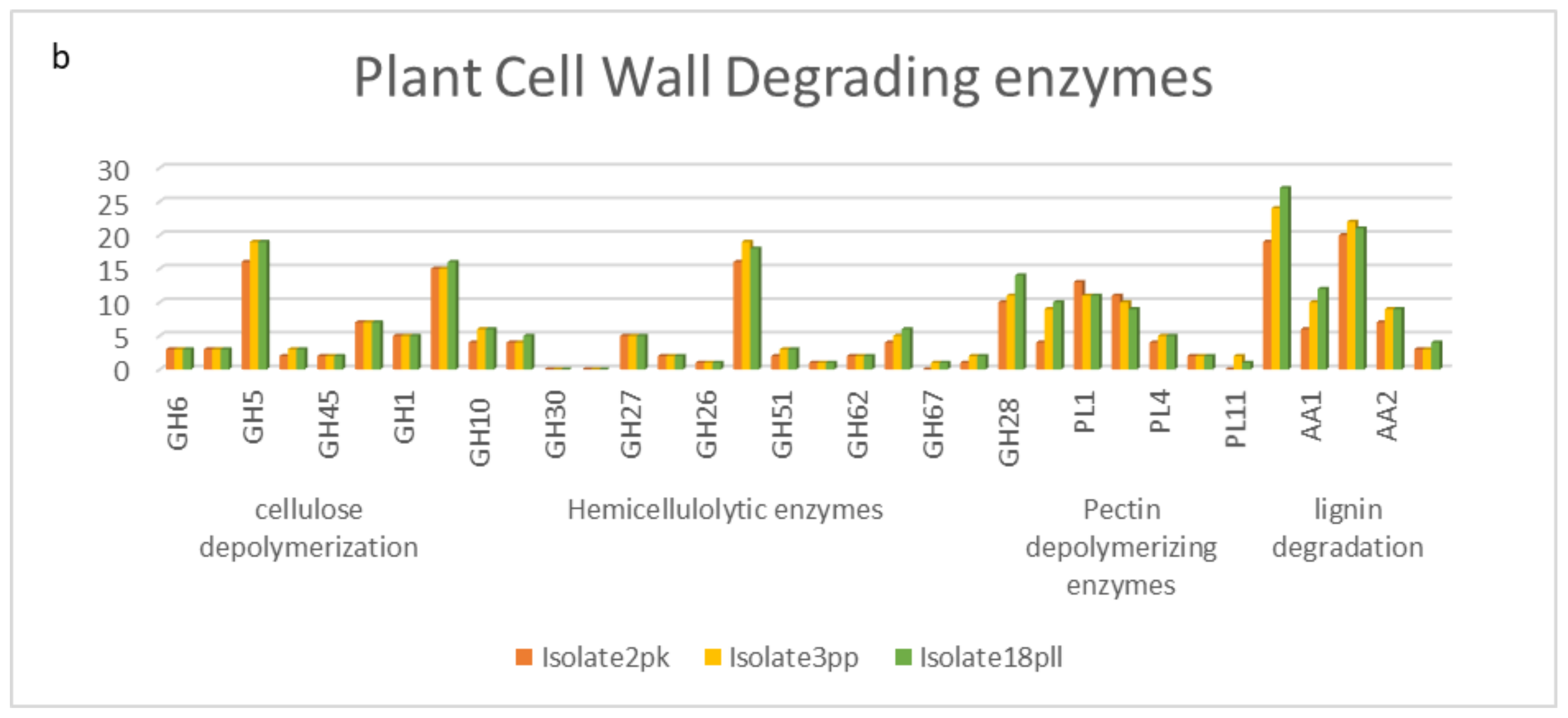
3.12. CAZyme Analysis
3.13. Secondary Metabolite Gene Cluster Analysis
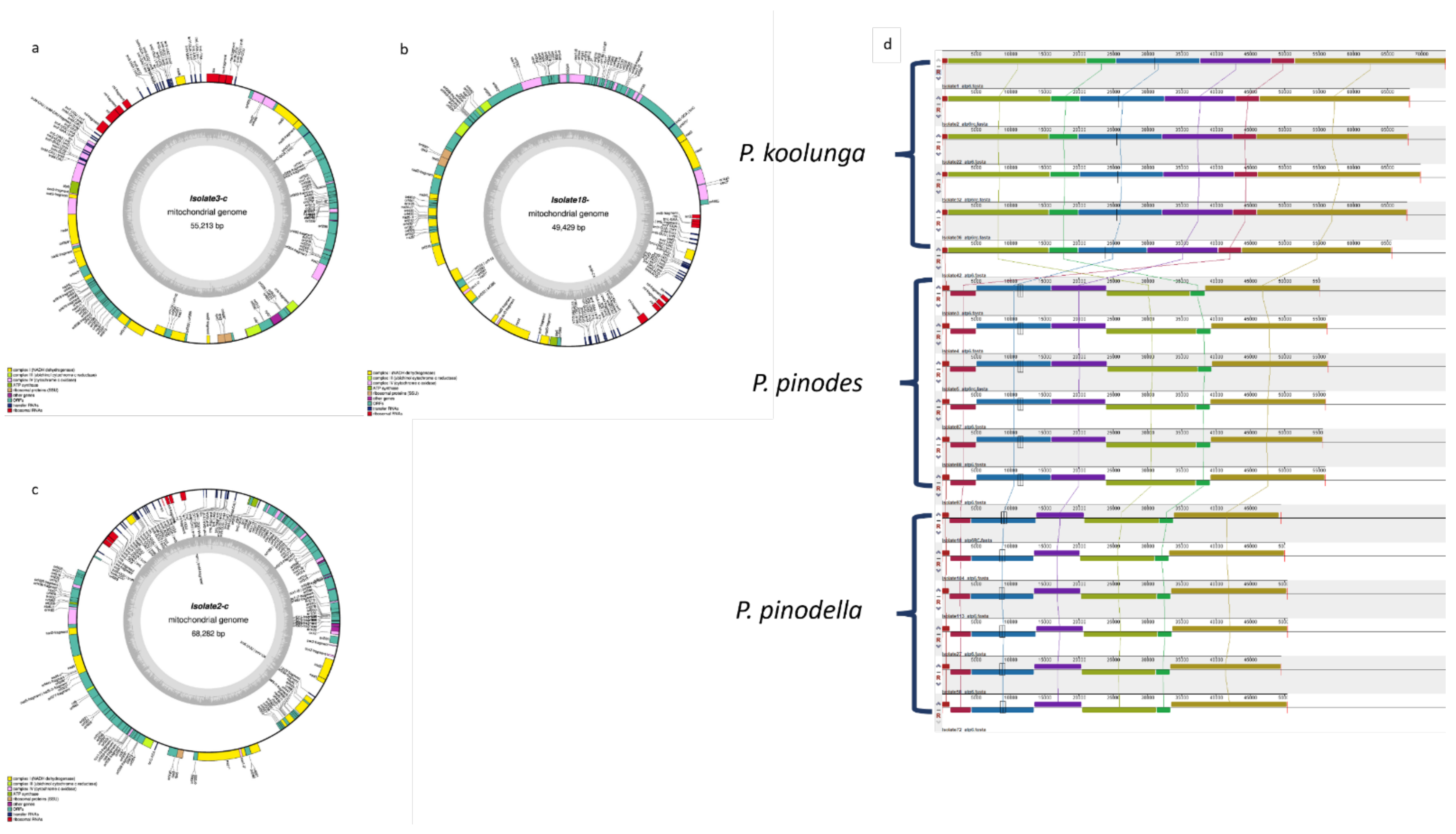
3.14. AB Species Mitochondrial Genomes
4. Diagnostics
5. Discussion
5.1. Near-Chromosome-Level Genome Assembly
5.2. AB Nuclear Genome Structural Variation
5.3. Orthologous Gene Cluster and Pangenome Analysis
5.4. AB Mating Type Determination
5.5. Phylogeny
5.6. CAZyme Analysis
5.7. Secondary Metabolite Analysis
5.8. Mitochondrial Genome Assembly
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johansen, C.; Baldev, B.; Brouwer, J.B.; Erskine, W.; Jermyn, W.A.; Li-Juan, L.; Malik, B.A.; Ahad Miah, A.; Silim, S.N. Biotic and abiotic stresses constraining productivity of cool season food legumes in Asia, Africa and Oceania. In Expanding the Production and Use of Cool Season Food Legumes: A Global Perspective of Peristent Constraints and of Opportunities and Strategies for Further Increasing the Productivity and Use of Pea, Lentil, Faba Bean, Chickpea and Grasspea in Different Farming Systems; Muehlbauer, F.J., Kaiser, W.J., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 175–194. [Google Scholar] [CrossRef]
- Timmerman-Vaughan, G.M.; Frew, T.J.; Russell, A.C.; Khan, T.; Butler, R.; Gilpin, M.; Murray, S.; Falloon, K. QTL Mapping of Partial Resistance to Field Epidemics of Ascochyta Blight of Pea. The research was funded by the New Zealand Foundation for Research, Science and Technology and by the Australian Grains R&D Corporation. Crop Sci. 2002, 42, 2100–2111. [Google Scholar] [CrossRef]
- Rubiales, D.; Fondevilla, S. Future Prospects for Ascochyta Blight Resistance Breeding in Cool Season Food Legumes. Front. Plant Sci. 2012, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Tivoli, B.; Baranger, A.; Avila, C.M.; Banniza, S.; Barbetti, M.; Chen, W.; Davidson, J.; Lindeck, K.; Kharrat, M.; Rubiales, D.; et al. Screening techniques and sources of resistance to foliar diseases caused by major necrotrophic fungi in grain legumes. Euphytica 2006, 147, 223–253. [Google Scholar] [CrossRef]
- Tivoli, B.; Baranger, A.; Muehlbauer, F.J.; Cooke, B.M. Ascochyta Blights of Grain Legumes; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Aveskamp, M.M.; De Gruyter, J.; Woudenberg, J.H.C.; Verkley, G.J.M.; Crous, P.W. Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Stud. Mycol. 2010, 65, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.A.; Wilmshurst, C.J.; Scott, E.S.; Salam, M.U. Relationship between ascochyta blight on field pea (Pisum sativum) and spore release patterns of Didymella pinodesand other causal agents of ascochyta blight. Plant Pathol. 2013, 62, 1258–1270. [Google Scholar] [CrossRef]
- Jones, L.K. Studies of the Nature and Control of Blight, Leaf and Pod Spot, and Foot-Rot of Peas Caused by Species of ASCO-Chyta; Number 547; Agricultural Experiment Station: New York, NY, USA, 1927; p. 46. [Google Scholar]
- Tran, H.S.; You, M.P.; Lanoiselet, V.; Khan, T.N.; Barbetti, M.J. First Report of Phoma glomerata Associated with the Ascochyta Blight Complex on Field Pea (Pisum sativum) in Australia. Plant Dis. 2014, 98, 427. [Google Scholar] [CrossRef]
- Tran, H.S.; Li, Y.P.; You, M.P.; Khan, T.N.; Pritchard, I.; Barbetti, M.J. Temporal and Spatial Changes in the Pea Black Spot Disease Complex in Western Australia. Plant Dis. 2014, 98, 790–796. [Google Scholar] [CrossRef]
- Liu, N.; Xu, S.; Yao, X.; Zhang, G.; Mao, W.; Hu, Q.; Feng, Z.; Gong, Y. Studies on the Control of Ascochyta Blight in Field Peas (Pisum sativum L.) Caused by Ascochyta pinodes in Zhejiang Province, China. Front. Microbiol. 2016, 7, 481. [Google Scholar] [CrossRef][Green Version]
- Gorfu, D. Yield losses of fieldpea due to ascochyta blight in central Ethiopia. Pest Manag. J. Ethiop. 2000, 4, 89–95. [Google Scholar]
- Warkentin, T.D.; Rashid, K.Y.; Xue, A.G. Fungicidal control of ascochyta blight of field pea. Can. J. Plant Sci. 1996, 76, 67–71. [Google Scholar] [CrossRef]
- Xue, A.; Warkentin, T.; Kenaschuk, E. Effects of timings of inoculation with Mycosphaerella pinodes on yield and seed infection of field pea. Can. J. Plant Sci. 1997, 77, 685–689. [Google Scholar] [CrossRef]
- Tivoli, B.; Beasse, C.; Lemarchand, E.; Masson, E. Effect of ascochyta blight (Mycosphaerella pinodes) on yield components of single pea (Pisum sativum) plants under field conditions. Ann. Appl. Biol. 1996, 129, 207–216. [Google Scholar] [CrossRef]
- Bretag, T.W.; Keane, P.J.; Price, T.V. The epidemiology and control of ascochyta blight in field peas: A review. Aust. J. Agric. Res. 2006, 57, 883–902. [Google Scholar] [CrossRef]
- Davidson, J.A.; Hartley, D.; Priest, M.; Herdina, M.K.; McKay, A.; Scott, E.S. A new species of Phoma causes ascochyta blight symptoms on field peas (Pisum sativum) in South Australia. Mycologia 2009, 101, 120–128. [Google Scholar] [CrossRef]
- Beasse, C.; Ney, B.; Tivoli, B. Effects of pod infection by Mycosphaerella pinodes on yield components of pea (Pisum sativum). Ann. Appl. Biol. 1999, 135, 359–367. [Google Scholar] [CrossRef]
- Beasse, C.; Ney, B.; Tivoli, B. A simple model of pea (Pisum sativum) growth affected by Mycosphaerella pinodes. Plant Pathol. 2000, 49, 187–200. [Google Scholar] [CrossRef]
- Davidson, J.A.; Ramsey, M.D. Pea yield decline syndrome in South Australia: The role of diseases and the impact of agronomic practices. Aust. J. Agric. Res. 2000, 51, 347–354. [Google Scholar] [CrossRef]
- Garry, G.; Jeuffroy, M.H.; Ney, B.; Tivoli, B. Effects of Ascochyta blight (Mycosphaerella pinodes) on the photosynthesizing leaf area and the photosynthetic efficiency of the green leaf area of dried-pea (Pisum sativum). Plant Pathol. 1998, 47, 473–479. [Google Scholar] [CrossRef]
- May, C.L.; Schoeny, A.; Tivoli, B.; Ney, B. Improvement and validation of a pea crop growth model to simulate the growth of cultivars infected with Ascochyta blight (Mycosphaerella pinodes). Eur. J. Plant Pathol. 2005, 112, 1–12. [Google Scholar] [CrossRef]
- Warkentin, T.; Xue, A.; McAndrew, D. Effect of mancozeb on the control of Mycosphaerella blight of field pea. Can. J. Plant Sci. 2000, 80, 403–406. [Google Scholar] [CrossRef]
- McMurray, L.; Davidson, J.; Lines, M.; Leonforte, A.; Salam, M. Combining management and breeding advances to improve field pea (Pisum sativum L.) grain yields under changing climatic conditions in south-eastern Australia. Euphytica 2011, 180, 69–88. [Google Scholar] [CrossRef]
- Davidson, J.A.; Kimber, R.B.E. Integrated disease management of ascochyta blight in pulse crops. Eur. J. Plant Pathol. 2007, 119, 99–110. [Google Scholar] [CrossRef]
- Rodda, M.S.; Davidson, J.; Javid, M.; Sudheesh, S.; Blake, S.; Forster, J.W.; Kaur, S. Molecular Breeding for Ascochyta Blight Resistance in Lentil: Current Progress and Future Directions. Front. Plant Sci. 2017, 8, 1136. [Google Scholar] [CrossRef]
- Sharma, M.; Ghosh, R. An update on genetic resistance of chickpea to ascochyta blight. Agronomy 2016, 6, 18. [Google Scholar] [CrossRef]
- Islam, W.; Qasim, M.; Noman, A.; Idrees, A.; Wang, L. Genetic resistance in chickpea against Ascochyta blight: Historical efforts and recent accomplishments. J. Anim. Plant Sci. 2017, 27, 1941–1957. [Google Scholar]
- Faris-Mokaiesh, S.; Boccara, M.; Denis, J.B.; Derrien, A.; Spire, D. Differentiation of the “Ascochyta complex” fungi of pea by biochemical and molecular markers. Curr. Genet. 1996, 29, 182–190. [Google Scholar] [CrossRef]
- Madhosingh, C.; Wallen, V.R. Serological differentiation of the Ascochyta species on peas. Can. J. Microbiol. 1968, 14, 449–451. [Google Scholar] [CrossRef]
- Padder, B.A.; Kapoor, V.; Kaushal, R.P.; Sharma, P.N. Identification and Genetic Diversity Analysis of Ascochyta Species Associated with Blight Complex of Pea in a Northwestern Hill State of India. Agric. Res. 2012, 1, 325–337. [Google Scholar] [CrossRef]
- Tadja, A.; Benkada, M.Y.; Rickauer, M.; Bendahmane, B.S.; Benkhelifa, M. Characterization of Ascochyta as Pathological Species of Pea (Pisum sativum L.) at the North-West of Algeria. J. Agron. 2009, 8, 100–106. [Google Scholar] [CrossRef]
- Tayeh, N.; Aubert, G.; Pilet-Nayel, M.-L.; Lejeune-Hénaut, I.; Warkentin, T.D.; Burstin, J. Genomic Tools in Pea Breeding Programs: Status and Perspectives. Front. Plant Sci. 2015, 6, 1037. [Google Scholar] [CrossRef]
- Le May, C.; Chilvers, M.I.; Saucereau, A.L.; Guibert, M.; Peever, T.L. Spatiotemporal distribution of Ascochyta pinodes and Ascochyta pinodella during the winter growing season in France. Plant Pathol. 2018, 67, 1031–1045. [Google Scholar] [CrossRef]
- Spanu, P.D.; Abbott, J.C.; Amselem, J.; Burgis, T.A.; Soanes, D.M.; Stüber, K.; van Themaat, E.V.L.; Brown, J.K.; Butcher, S.A.; Gurr, S.J. Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism. Science 2010, 330, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, S.; Kamoun, S. Genome evolution in filamentous plant pathogens: Why bigger can be better. Nat. Rev. Microbiol. 2012, 10, 417–430. [Google Scholar] [CrossRef]
- Chénais, B.; Caruso, A.; Hiard, S.; Casse, N. The impact of transposable elements on eukaryotic genomes: From genome size increase to genetic adaptation to stressful environments. Gene 2012, 509, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Abby, S.; Daubin, V. Comparative genomics and the evolution of prokaryotes. Trends Microbiol. 2007, 15, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, L.D.; Moreno-Hagelsieb, G.; Eguiarte, L.E.; Souza, V.; Herrera-Estrella, L.; Olmedo, G. Understanding the evolutionary relationships and major traits of Bacillus through comparative genomics. BMC Genom. 2010, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.C.; de Castro Soares, S.; Trost, E.; Blom, J.; Ramos, R.T.J.; Silva, A.; Barh, D.; Azevedo, V. Genome informatics and vaccine targets in Corynebacterium urealyticum using two whole genomes, comparative genomics, and reverse vaccinology. BMC Genom. 2015, 16, S7. [Google Scholar] [CrossRef][Green Version]
- Kato, R.B.; Jaiswal, A.K.; Tiwari, S.; Barh, D.; Azevedo, V.; Góes-Neto, A. Pan-genomics of fungi and its applications. In Pan-genomics: Applications, Challenges, and Future Prospects; Elsevier: Amsterdam, The Netherlands, 2020; pp. 251–260. [Google Scholar]
- Snipen, L.; Almøy, T.; Ussery, D.W. Microbial comparative pan-genomics using binomial mixture models. BMC Genom. 2009, 10, 385. [Google Scholar] [CrossRef][Green Version]
- Lapierre, P.; Gogarten, J.P. Estimating the size of the bacterial pan-genome. Trends Genet. 2009, 25, 107–110. [Google Scholar] [CrossRef]
- Muzzi, A.; Masignani, V.; Rappuoli, R. The pan-genome: Towards a knowledge-based discovery of novel targets for vaccines and antibacterials. Drug Discov. Today 2007, 12, 429–439. [Google Scholar] [CrossRef]
- Tettelin, H.; Masignani, V.; Cieslewicz, M.J.; Donati, C.; Medini, D.; Ward, N.L.; Angiuoli, S.V.; Crabtree, J.; Jones, A.L.; Durkin, A.S. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: Implications for the microbial “pan-genome”. Proc. Natl. Acad. Sci. USA 2005, 102, 13950–13955. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.G.P.; Fitzpatrick, D.A. Pan-genome analyses of model fungal species. Microb. Genom. 2019, 5, e000243. [Google Scholar] [CrossRef] [PubMed]
- Brynildsrud, O.; Bohlin, J.; Scheffer, L.; Eldholm, V. Rapid scoring of genes in microbial pan-genome-wide association studies with Scoary. Genome Biol. 2016, 17, 238. [Google Scholar] [CrossRef] [PubMed]
- Plissonneau, C.; Hartmann, F.E.; Croll, D. Pangenome analyses of the wheat pathogen Zymoseptoria tritici reveal the structural basis of a highly plastic eukaryotic genome. BMC Biol. 2018, 16, 5. [Google Scholar] [CrossRef]
- Hartmann, F.E.; McDonald, B.A.; Croll, D. Genome-wide evidence for divergent selection between populations of a major agricultural pathogen. Mol. Ecol. 2018, 27, 2725–2741. [Google Scholar] [CrossRef]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef]
- Lu, H.; Giordano, F.; Ning, Z. Oxford Nanopore MinION sequencing and genome assembly. Genom. Proteom. Bioinform. 2016, 14, 265–279. [Google Scholar] [CrossRef]
- Jain, M.; Fiddes, I.T.; Miga, K.H.; Olsen, H.E.; Paten, B.; Akeson, M. Improved data analysis for the MinION nanopore sequencer. Nat. Methods 2015, 12, 351–356. [Google Scholar] [CrossRef]
- Jain, M.; Olsen, H.E.; Paten, B.; Akeson, M. The Oxford Nanopore MinION: Delivery of nanopore sequencing to the genomics community. Genome Biol. 2016, 17, 239. [Google Scholar] [CrossRef]
- Schlaeppi, K.; Bender, S.F.; Mascher, F.; Russo, G.; Patrignani, A.; Camenzind, T.; Hempel, S.; Rillig, M.C.; van der Heijden, M.G. High-resolution community profiling of arbuscular mycorrhizal fungi. New Phytol. 2016, 212, 780–791. [Google Scholar] [CrossRef]
- Walder, F.; Schlaeppi, K.; Wittwer, R.; Held, A.Y.; Vogelgsang, S.; van der Heijden, M.G. Community profiling of Fusarium in combination with other plant-associated fungi in different crop species using SMRT sequencing. Front. Plant Sci. 2017, 8, 2019. [Google Scholar] [CrossRef]
- Heeger, F.; Bourne, E.C.; Baschien, C.; Yurkov, A.; Bunk, B.; Spröer, C.; Overmann, J.; Mazzoni, C.J.; Monaghan, M.T. Long-read DNA metabarcoding of ribosomal RNA in the analysis of fungi from aquatic environments. Mol. Ecol. Resour. 2018, 18, 1500–1514. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Albertsen, M.; Anslan, S.; Callahan, B. Perspectives and benefits of high-throughput long-read sequencing in microbial ecology. Appl. Environ. Microbiol. 2021, 87, e0062621. [Google Scholar] [CrossRef]
- Rhoads, A.; Au, K.F. PacBio sequencing and its applications. Genom. Proteom. Bioinform. 2015, 13, 278–289. [Google Scholar] [CrossRef]
- Ashton, P.M.; Nair, S.; Dallman, T.; Rubino, S.; Rabsch, W.; Mwaigwisya, S.; Wain, J.; O’grady, J. MinION nanopore sequencing identifies the position and structure of a bacterial antibiotic resistance island. Nat. Biotechnol. 2015, 33, 296–300. [Google Scholar] [CrossRef]
- Norris, A.L.; Workman, R.E.; Fan, Y.; Eshleman, J.R.; Timp, W. Nanopore sequencing detects structural variants in cancer. Cancer Biol. Ther. 2016, 17, 246–253. [Google Scholar] [CrossRef]
- Bolisetty, M.T.; Rajadinakaran, G.; Graveley, B.R. Determining exon connectivity in complex mRNAs by nanopore sequencing. Genome Biol. 2015, 16, 204. [Google Scholar] [CrossRef]
- Laver, T.; Harrison, J.; O’neill, P.; Moore, K.; Farbos, A.; Paszkiewicz, K.; Studholme, D.J. Assessing the performance of the oxford nanopore technologies minion. Biomol. Detect. Quantif. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Ip, C.L.; Loose, M.; Tyson, J.R.; de Cesare, M.; Brown, B.L.; Jain, M.; Leggett, R.M.; Eccles, D.A.; Zalunin, V.; Urban, J.M. MinION Analysis and Reference Consortium: Phase 1 data release and analysis. F1000Research 2015, 4, 1075. [Google Scholar] [CrossRef]
- Goodwin, S.; Gurtowski, J.; Ethe-Sayers, S.; Deshpande, P.; Schatz, M.C.; McCombie, W.R. Oxford Nanopore sequencing, hybrid error correction, and de novo assembly of a eukaryotic genome. Genome Res. 2015, 25, 1750–1756. [Google Scholar] [CrossRef]
- Jamy, M.; Foster, R.; Barbera, P.; Czech, L.; Kozlov, A.; Stamatakis, A.; Bending, G.; Hilton, S.; Bass, D.; Burki, F. Long-read metabarcoding of the eukaryotic rDNA operon to phylogenetically and taxonomically resolve environmental diversity. Mol. Ecol. Resour. 2020, 20, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Kõljalg, U.; Abarenkov, K. Identifying the ‘unidentified’ fungi: A global-scale long-read third-generation sequencing approach. Fungal Divers. 2020, 103, 273–293. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2012, 29, 15–21. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Huang, Y.; Niu, B.; Gao, Y.; Fu, L.; Li, W. CD-HIT Suite: A web server for clustering and comparing biological sequences. Bioinformatics 2010, 26, 680–682. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Okorski, A.; Pszczolkowska, A.; Jastrzebski, J.P.; Paukszto, L.; Okorska, S. The complete mitogenome of Mycosphaerella pinodes (Ascomycota, Mycosphaerellaceae). Mitochondrial DNA B Resour. 2016, 1, 48–49. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Subgroup, G.P.D.P. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Li, H. Fast and accurate long-read assembly with wtdbg2. Nat. Methods 2020, 17, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of Long Error-Prone Reads Using Repeat Graphs. bioRxiv 2018. [Google Scholar] [CrossRef] [PubMed]
- Vaser, R.; Sović, I.; Nagarajan, N.; Šikić, M. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 2017, 27, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Wu, S.; Li, A.; Zhao, F.; Feng, H.; Ding, L.; Ruan, J. LRScaf: Improving draft genomes using long noisy reads. BMC Genom. 2019, 20, 955. [Google Scholar] [CrossRef]
- Bosi, E.; Donati, B.; Galardini, M.; Brunetti, S.; Sagot, M.F.; Lió, P.; Crescenzi, P.; Fani, R.; Fondi, M. MeDuSa: A multi-draft based scaffolder. Bioinformatics 2015, 31, 2443–2451. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Guan, D.; McCarthy, S.A.; Wood, J.; Howe, K.; Wang, Y.; Durbin, R. Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics 2020, 36, 2896–2898. [Google Scholar] [CrossRef]
- Nishimura, D. RepeatMasker. Biotech Softw. Internet Rep. 2000, 1, 36–39. [Google Scholar] [CrossRef]
- Van Wyk, S.; Harrison, C.H.; Wingfield, B.D.; De Vos, L.; van Der Merwe, N.A.; Steenkamp, E.T. The RIPper, a web-based tool for genome-wide quantification of Repeat-Induced Point (RIP) mutations. PeerJ 2019, 7, e7447. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Kent, W.J. BLAT—The BLAST-like alignment tool. Genome Res. 2002, 12, 656–664. [Google Scholar]
- Haas, B.J.; Delcher, A.L.; Mount, S.M.; Wortman, J.R.; Smith, R.K., Jr.; Hannick, L.I.; Maiti, R.; Ronning, C.M.; Rusch, D.B.; Town, C.D. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic Acids Res. 2003, 31, 5654–5666. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Stanke, M.; Morgenstern, B. AUGUSTUS: A web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 2005, 33, W465–W467. [Google Scholar] [CrossRef]
- Lomsadze, A.; Ter-Hovhannisyan, V.; Chernoff, Y.O.; Borodovsky, M. Gene identification in novel eukaryotic genomes by self-training algorithm. Nucleic Acids Res. 2005, 33, 6494–6506. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P.; Rødland, E.A.; Stærfeldt, H.-H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Kurtz, S.; Phillippy, A.; Delcher, A.L.; Smoot, M.; Shumway, M.; Antonescu, C.; Salzberg, S.L. Versatile and open software for comparing large genomes. Genome Biol. 2004, 5, R12. [Google Scholar] [CrossRef]
- Nattestad, M.; Schatz, M.C. Assemblytics: A web analytics tool for the detection of variants from an assembly. Bioinformatics 2016, 32, 3021–3023. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Wang, Y.; Coleman-Derr, D.; Chen, G.; Gu, Y.Q. OrthoVenn: A web server for genome wide comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2015, 43, W78–W84. [Google Scholar] [CrossRef]
- Li, L.; Stoeckert, C.J.; Roos, D.S. OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res. 2003, 13, 2178–2189. [Google Scholar] [CrossRef]
- Gardner, S.N.; Slezak, T.; Hall, B.G. kSNP3. 0: SNP detection and phylogenetic analysis of genomes without genome alignment or reference genome. Bioinformatics 2015, 31, 2877–2878. [Google Scholar] [CrossRef]
- Emms, D.; Kelly, S. OrthoFinder2: Fast and accurate phylogenomic orthology analysis from gene sequences. BioRxiv 2018. [Google Scholar] [CrossRef]
- Zhang, H.; Yohe, T.; Huang, L.; Entwistle, S.; Wu, P.; Yang, Z.; Busk, P.K.; Xu, Y.; Yin, Y. dbCAN2: A meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2018, 46, W95–W101. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Hidden markov models. Curr. Opin. Struct. Biol. 1996, 6, 361–365. [Google Scholar] [CrossRef]
- Busk, P.K.; Pilgaard, B.; Lezyk, M.J.; Meyer, A.S.; Lange, L. Homology to peptide pattern for annotation of carbohydrate-active enzymes and prediction of function. BMC Bioinform. 2017, 18, 214. [Google Scholar] [CrossRef]
- Medema, M.H.; Blin, K.; Cimermancic, P.; De Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. antiSMASH: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef]
- Seidl, M.F.; Thomma, B.P. Sex or no sex: Evolutionary adaptation occurs regardless. Bioessays 2014, 36, 335–345. [Google Scholar] [CrossRef]
- Walker, J.C.; Hare, W.W. Ascochyta Diseases of Canning Pea. Agricultural Experiment Station University of Wisconsin Research Bulletin. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201300438150 (accessed on 11 January 2021).
- Mohanta, T.K.; Bae, H. The diversity of fungal genome. Biol. Proced. Online 2015, 17, 8. [Google Scholar] [CrossRef]
- Aylward, J.; Steenkamp, E.T.; Dreyer, L.L.; Roets, F.; Wingfield, B.D.; Wingfield, M.J. A plant pathology perspective of fungal genome sequencing. IMA Fungus 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Castanera, R.; Lopez-Varas, L.; Borgognone, A.; LaButti, K.; Lapidus, A.; Schmutz, J.; Grimwood, J.; Perez, G.; Pisabarro, A.G.; Grigoriev, I.V. Transposable elements versus the fungal genome: Impact on whole-genome architecture and transcriptional profiles. PLoS Genet. 2016, 12, e1006108. [Google Scholar] [CrossRef]
- Shah, R.M.; Williams, A.H.; Hane, J.K.; Lawrence, J.A.; Farfan-Caceres, L.M.; Debler, J.W.; Oliver, R.P.; Lee, R.C. Reference Genome Assembly for Australian Ascochyta rabiei Isolate ArME14. G3 Genes Genomes Genet. 2020, 10, 2131–2140. [Google Scholar] [CrossRef]
- Lee, R.C.; Farfan-Caceres, L.; Debler, J.W.; Williams, A.H.; Syme, R.A.; Henares, B.M. Reference genome assembly for Australian Ascochyta lentis isolate Al4. G3 Genes Genomes Genet. 2021, 11, jkab006. [Google Scholar] [CrossRef]
- Grandaubert, J.; Balesdent, M.-H.; Rouxel, T. Evolutionary and adaptive role of transposable elements in fungal genomes. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 70, pp. 79–107. [Google Scholar]
- Ohm, R.A.; Feau, N.; Henrissat, B.; Schoch, C.L.; Horwitz, B.A.; Barry, K.W.; Condon, B.J.; Copeland, A.C.; Dhillon, B.; Glaser, F. Diverse lifestyles and strategies of plant pathogenesis encoded in the genomes of eighteen Dothideomycetes fungi. PLoS Pathog. 2012, 8, e1003037. [Google Scholar] [CrossRef]
- Möller, M.; Stukenbrock, E.H. Evolution and genome architecture in fungal plant pathogens. Nat. Rev. Microbiol. 2017, 15, 756–771. [Google Scholar] [CrossRef]
- Syme, R.A.; Martin, A.; Wyatt, N.A.; Lawrence, J.A.; Muria-Gonzalez, M.J.; Friesen, T.L.; Ellwood, S.R. Transposable element genomic fissuring in Pyrenophora teres is associated with genome expansion and dynamics of host-pathogen genetic interactions. Front. Genet. 2018, 9, 130. [Google Scholar] [CrossRef]
- Zolan, M.E. Chromosome-length polymorphism in fungi. Microbiol. Rev. 1995, 59, 686–698. [Google Scholar] [CrossRef]
- Akamatsu, H.O.; Chilvers, M.I.; Kaiser, W.J.; Peever, T.L. Karyotype polymorphism and chromosomal rearrangement in populations of the phytopathogenic fungus. Ascochyta Rabiei. Fungal Biol. 2012, 116, 1119–1133. [Google Scholar] [CrossRef]
- Sen, D.; Paul, K.; Saha, C.; Mukherjee, G.; Nag, M.; Ghosh, S.; Das, A.; Seal, A.; Tripathy, S. A unique life-strategy of an endophytic yeast Rhodotorula mucilaginosa JGTA-S1—A comparative genomics viewpoint. DNA Res. 2019, 26, 131–146. [Google Scholar] [CrossRef]
- Weiß, C.L.; Pais, M.; Cano, L.M.; Kamoun, S.; Burbano, H.A. nQuire: A statistical framework for ploidy estimation using next generation sequencing. BMC Bioinform. 2018, 19, 122. [Google Scholar] [CrossRef]
- Garber, R.C.; Gillian Turgeon, B.; Selker, E.U.; Yoder, O. Organization of ribosomal RNA genes in the fungus Cochliobolus heterostrophus. Curr. Genet. 1988, 14, 573–582. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Z.; Huang, D.W.; Kutty, G.; Ishihara, M.; Wang, H.; Abouelleil, A.; Bishop, L.; Davey, E.; Deng, R.; et al. Genome analysis of three Pneumocystis species reveals adaptation mechanisms to life exclusively in mammalian hosts. Nat. Commun. 2016, 7, 10740. [Google Scholar] [CrossRef]
- Ma, L.-J.; Ibrahim, A.S.; Skory, C.; Grabherr, M.G.; Burger, G.; Butler, M.; Elias, M.; Idnurm, A.; Lang, B.F.; Sone, T.; et al. Genomic Analysis of the Basal Lineage Fungus Rhizopus oryzae Reveals a Whole-Genome Duplication. PLoS Genet. 2009, 5, e1000549. [Google Scholar] [CrossRef]
- Lofgren, L.A.; Uehling, J.K.; Branco, S.; Bruns, T.D.; Martin, F.; Kennedy, P.G. Genome-based estimates of fungal rDNA copy number variation across phylogenetic scales and ecological lifestyles. Mol. Ecol. 2019, 28, 721–730. [Google Scholar] [CrossRef]
- Smith, A.W.; Collis, K.; Ramsden, M.; Fox, H.M.; Peberdy, J.F. Chromosome rearrangements in improved cephalosporin C-producing strains of Acremonium chrysogenum. Curr. Genet. 1991, 19, 235–237. [Google Scholar] [CrossRef]
- Suzuki, T.; Miyamae, Y.; Ishida, I. Variation of colony morphology and chromosomal rearrangement in Candida tropicalis pK233. Microbiology 1991, 137, 161–167. [Google Scholar] [CrossRef][Green Version]
- Olarte, R.A.; Menke, J.; Zhang, Y.; Sullivan, S.; Slot, J.C.; Huang, Y.; Badalamenti, J.P.; Quandt, A.C.; Spatafora, J.W.; Bushley, K.E. Chromosome rearrangements shape the diversification of secondary metabolism in the cyclosporin producing fungus Tolypocladium inflatum. BMC Genom. 2019, 20, 120. [Google Scholar] [CrossRef]
- Rajeh, A.; Lv, J.; Lin, Z. Heterogeneous rates of genome rearrangement contributed to the disparity of species richness in Ascomycota. BMC Genom. 2018, 19, 282. [Google Scholar] [CrossRef]
- Sipos, G.; Prasanna, A.N.; Walter, M.C.; O’Connor, E.; Bálint, B.; Krizsán, K.; Kiss, B.; Hess, J.; Varga, T.; Slot, J. Genome expansion and lineage-specific genetic innovations in the forest pathogenic fungi Armillaria. Nat. Ecol. Evol. 2017, 1, 1931–1941. [Google Scholar] [CrossRef]
- Daboussi, M.-J.; Capy, P. Transposable elements in filamentous fungi. Annu. Rev. Microbiol. 2003, 57, 275–299. [Google Scholar] [CrossRef]
- de la Chaux, N.; Wagner, A. BEL/Pao retrotransposons in metazoan genomes. BMC Evol. Biol. 2011, 11, 154. [Google Scholar] [CrossRef]
- Piednoël, M.; Carrete-Vega, G.; Renner, S.S. Characterization of the LTR retrotransposon repertoire of a plant clade of six diploid and one tetraploid species. Plant J. 2013, 75, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Galagan, J.E.; Henn, M.R.; Ma, L.-J.; Cuomo, C.A.; Birren, B. Genomics of the fungal kingdom: Insights into eukaryotic biology. Genome Res. 2005, 15, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, G.F.; Jacobi, V.; Plourde, K.V.; Bernier, L. Stress-induced mobility of OPHIO1 and OPHIO2, DNA transposons of the Dutch elm disease fungi. Fungal Genet. Biol. 2008, 45, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Spanu, P.D. The genomics of obligate (and nonobligate) biotrophs. Annu. Rev. Phytopathol. 2012, 50, 91–109. [Google Scholar] [CrossRef]
- Biémont, C. A brief history of the status of transposable elements: From junk DNA to major players in evolution. Genetics 2010, 186, 1085–1093. [Google Scholar] [CrossRef]
- Croll, D.; McDonald, B.A. The accessory genome as a cradle for adaptive evolution in pathogens. PLoS Pathog. 2012, 8, e1002608. [Google Scholar] [CrossRef]
- Selker, E.U. Repeat-induced gene silencing in fungi. Adv. Genet. 2002, 46, 439–450. [Google Scholar]
- Gladyshev, E. Repeat-induced point mutation and other genome defense mechanisms in fungi. In The Fungal Kingdom; ASM Press: Washington, DC, USA, 2017; pp. 687–699. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1128/9781555819583.ch33 (accessed on 11 January 2021).
- Cambareri, E.B.; Jensen, B.C.; Schabtach, E.; Selker, E.U. Repeat-induced GC to AT mutations in Neurospora. Science 1989, 244, 1571–1575. [Google Scholar] [CrossRef]
- Braumann, I.; Van Den Berg, M.; Kempken, F. Repeat induced point mutation in two asexual fungi, Aspergillus niger and Penicillium chrysogenum. Curr. Genet. 2008, 53, 287–297. [Google Scholar] [CrossRef]
- Van Wyk, S.; Wingfield, B.D.; De Vos, L.; Van der Merwe, N.A.; Steenkamp, E.T. Genome-wide analyses of repeat-induced point mutations in the Ascomycota. Front. Microbiol. 2021, 11, 3625. [Google Scholar] [CrossRef]
- Leslie, J.; Summerell, B. The Fusarium Laboratory Manual; Leslie, J.F., Summerell., B.A., Eds.; Blackwell: Hoboken, NJ, USA, 2006; pp. 1–388. [Google Scholar]
- Xu, C.; Zhang, R.; Sun, G.; Gleason, M.L. Comparative Genome Analysis Reveals Adaptation to the Ectophytic Lifestyle of Sooty Blotch and Flyspeck Fungi. Genome Biol. Evol. 2017, 9, 3137–3151. [Google Scholar] [CrossRef][Green Version]
- Tran, H.S.; You, M.P.; Khan, T.N.; Barbetti, M.J. Relative Host Resistance to Black Spot Disease in Field Pea (Pisum sativum) is Determined by Individual Pathogens. Plant Dis. 2015, 99, 580–587. [Google Scholar] [CrossRef]
- Badet, T.; Oggenfuss, U.; Abraham, L.; McDonald, B.A.; Croll, D. A 19-isolate reference-quality global pangenome for the fungal wheat pathogen Zymoseptoria tritici. BMC Biol. 2020, 18, 12. [Google Scholar] [CrossRef]
- Baumann, G. Untersuchungen zur Biologie van Mycosphaerella pinodes (Berk. et. Blox.) Stone. Kühn-Archiv 1953, 67, 305–383. [Google Scholar]
- Bowen, J.K.; Lewis, B.G.; Matthews, P. Discovery of the teleomorph of Phoma medicaginis var. pinodella in culture. Mycol. Res. 1997, 101, 80–84. [Google Scholar] [CrossRef]
- Woudenberg, J.H.; De Gruyter, J.; Crous, P.W.; ZWIERS, L.H. Analysis of the mating-type loci of co-occurring and phylogenetically related species of Ascochyta and Phoma. Mol. Plant Pathol. 2012, 13, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-H.; Arie, T.; Kaneko, I.; Yoder, O.; Turgeon, B.G. Molecular organization of mating type loci in heterothallic, homothallic, and asexual Gibberella/Fusarium species. Fungal Genet. Biol. 2000, 31, 7–20. [Google Scholar] [CrossRef]
- Debuchy, R.; Turgeon, B. Mating-type structure, evolution, and function in Euascomycetes. In Growth, Differentiation and Sexuality; Springer: Berlin/Heidelberg, Germany, 2006; pp. 293–323. [Google Scholar]
- Trapero-Casas, A.; Kaiser, W. Development of Didymella rabiei, the teleomorph of Ascochyta rabiei, on chickpea straw. Phytopathology 1992, 82, 1261–1266. [Google Scholar] [CrossRef]
- Wilson, A.D.; Kaiser, W. Cytology and genetics of sexual incompatibility in Didymella rabiei. Mycologia 1995, 87, 795–804. [Google Scholar] [CrossRef]
- Barve, M.; Arie, T.; Salimath, S.; Muehlbauer, F.; Peever, T. Cloning and characterization of the mating type (MAT) locus from Ascochyta rabiei (teleomorph: Didymella rabiei) and a MAT phylogeny of legume-associated Ascochyta spp. Fungal Genet. Biol. 2003, 39, 151–167. [Google Scholar] [CrossRef]
- Friedman, R.; Hughes, A.L. The temporal distribution of gene duplication events in a set of highly conserved human gene families. Mol. Biol. Evol. 2003, 20, 154–161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hughes, A.L. The evolution of functionally novel proteins after gene duplication. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1994, 256, 119–124. [Google Scholar]
- Ohno, S. The enormous diversity in genome sizes of fish as a reflection of nature’s extensive experiments with gene duplication. Trans. Am. Fish. Soc. 1970, 99, 120–130. [Google Scholar] [CrossRef]
- Lynch, M.; Conery, J.S. The evolutionary fate and consequences of duplicate genes. Science 2000, 290, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, H.; Wang, C.; Xu, J.-R. Correction: Comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi. BMC Genom. 2014, 15, 6. [Google Scholar] [CrossRef]
- Kaur, J. Chemistry and biological activity of lignin from pea (Pisum sativum L.) peel. Chemistry 2017, 102824589. Available online: https://www.semanticscholar.org/paper/Chemistry-and-biological-activity-of-lignin-from-Kaur/c7a341e64ef3fbafa9a8deaf2995a55e86a93116 (accessed on 11 January 2021).
- Talbott, L.D.; Ray, P.M. Molecular size and separability features of pea cell wall polysaccharides: Implications for models of primary wall structure. Plant Physiol. 1992, 98, 357–368. [Google Scholar] [CrossRef]
- Keirnan, E.C.; Davidson, J.A.; Correll, R.L.; Scott, E.S. Host range investigation of Phoma koolunga, a causal agent of ascochyta blight of field pea. Australas. Plant Pathol. 2020, 49, 707–719. [Google Scholar] [CrossRef]
- Le May, C.; Guibert, M.; Baranger, A.; Tivoli, B. A wide range of cultivated legume species act as alternative hosts for the pea aschochyta blight fungus, D idymella pinodes. Plant Pathol. 2014, 63, 877–887. [Google Scholar] [CrossRef]
- Barilli, E.; Cobos, M.J.; Rubiales, D. Clarification on Host Range of Didymella pinodes the Causal Agent of Pea Ascochyta Blight. Front. Plant Sci. 2016, 7, 592. [Google Scholar] [CrossRef]
- Kinsey, G. IMI Descriptions of Fungi and Bacteria. Biology 2002, 151, 88439022. [Google Scholar]
- García-Pajón, C.; Collado, I.G. Secondary metabolites isolated from Colletotrichum species. Nat. Prod. Rep. 2003, 20, 426–431. [Google Scholar] [CrossRef]
- Rao, S.; Nandineni, M.R. Genome sequencing and comparative genomics reveal a repertoire of putative pathogenicity genes in chilli anthracnose fungus Colletotrichum truncatum. PLoS ONE 2017, 12, e0183567. [Google Scholar] [CrossRef]
- Takano, K.; Tatlisumak, T.; Bergmann, A.G.; Gibson, D.G., III; Fisher, M. Reproducibility and reliability of middle cerebral artery occlusion using a silicone-coated suture (Koizumi) in rats. J. Neurol. Sci. 1997, 153, 8–11. [Google Scholar] [CrossRef]
- Islam, M.S.; Haque, M.S.; Islam, M.M.; Emdad, E.M.; Halim, A.; Hossen, Q.M.M.; Hossain, M.Z.; Ahmed, B.; Rahim, S.; Rahman, M.S. Tools to kill: Genome of one of the most destructive plant pathogenic fungi Macrophomina phaseolina. BMC Genom. 2012, 13, 493. [Google Scholar] [CrossRef]
- De Wit, P.J.; Van Der Burgt, A.; Ökmen, B.; Stergiopoulos, I.; Abd-Elsalam, K.A.; Aerts, A.L.; Bahkali, A.H.; Beenen, H.G.; Chettri, P.; Cox, M.P. The genomes of the fungal plant pathogens Cladosporium fulvum and Dothistroma septosporum reveal adaptation to different hosts and lifestyles but also signatures of common ancestry. PLoS Genet. 2012, 8, e1003088. [Google Scholar] [CrossRef]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef]
- Ding, W.; Liu, W.-Q.; Jia, Y.; Li, Y.; Van Der Donk, W.A.; Zhang, Q. Biosynthetic investigation of phomopsins reveals a widespread pathway for ribosomal natural products in Ascomycetes. Proc. Natl. Acad. Sci. USA 2016, 113, 3521–3526. [Google Scholar] [CrossRef]
- Duquesne, S.; Destoumieux-Garzón, D.; Peduzzi, J.; Rebuffat, S. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat. Prod. Rep. 2007, 24, 708–734. [Google Scholar] [CrossRef]
- Drider, D.; Rebuffat, S. Prokaryotic Antimicrobial Peptides: From Genes to Applications; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Li, Y.; Rebuffat, S. The manifold roles of microbial ribosomal peptide-based natural products in physiology and ecology. J. Biol. Chem. 2020, 295, 34–54. [Google Scholar] [CrossRef]
- Kessler, S.C.; Chooi, Y.-H. Out for a RiPP: Challenges and advances in genome mining of ribosomal peptides from fungi. Natural Product Reports 2022, 39, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.L.; Christenson, J.K.; Wackett, L.P. Biosynthesis and chemical diversity of β-lactone natural products. Nat. Prod. Rep. 2019, 36, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Singh, S.K.; Riyaz-Ul-Hassan, S.; Mitchell, A.M.; Geary, B.; Sears, J. An endophytic/pathogenic Phoma sp. from creosote bush producing biologically active volatile compounds having fuel potential. FEMS Microbiol. Lett. 2011, 320, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Le May, C.; Ney, B.; Lemarchand, E.; Schoeny, A.; Tivoli, B. Effect of pea plant architecture on spatiotemporal epidemic development of ascochyta blight (Mycosphaerella pinodes) in the field. Plant Pathol. 2009, 58, 332–343. [Google Scholar] [CrossRef]
- Song, N.; Geng, Y.; Li, X. The mitochondrial genome of the phytopathogenic fungus Bipolaris sorokiniana and the utility of mitochondrial genome to infer phylogeny of Dothideomycetes. Front. Microbiol. 2020, 11, 863. [Google Scholar] [CrossRef]
- Yuan, X.-L.; Cao, M.; Shen, G.-M.; Zhang, H.-B.; Du, Y.-M.; Zhang, Z.-F.; Li, Q.; Gao, J.-M.; Xue, L.; Wang, Z.-P. Characterization of Nuclear and Mitochondrial Genomes of Two Tobacco Endophytic Fungi Leptosphaerulina chartarum and Curvularia trifolii and Their Contributions to Phylogenetic Implications in the Pleosporales. Int. J. Mol. Sci. 2020, 21, 2461. [Google Scholar] [CrossRef]
- Joardar, V.; Abrams, N.F.; Hostetler, J.; Paukstelis, P.J.; Pakala, S.; Pakala, S.B.; Zafar, N.; Abolude, O.O.; Payne, G.; Andrianopoulos, A. Sequencing of mitochondrial genomes of nine Aspergillus and Penicillium species identifies mobile introns and accessory genes as main sources of genome size variability. BMC Genom. 2012, 13, 698. [Google Scholar] [CrossRef]
- Sandor, S.; Zhang, Y.; Xu, J. Fungal mitochondrial genomes and genetic polymorphisms. Appl. Microbiol. Biotechnol. 2018, 102, 9433–9448. [Google Scholar] [CrossRef]
- Megarioti, A.H.; Kouvelis, V.N. The coevolution of fungal mitochondrial introns and their homing endonucleases (GIY-YIG and LAGLIDADG). Genome Biol. Evol. 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, L.; Cai, Y.; Tao, W.; Zhang, Y.-J.; Huang, B. Mitochondrial genome of the entomophthoroid fungus Conidiobolus heterosporus provides insights into evolution of basal fungi. Appl. Microbiol. Biotechnol. 2019, 103, 1379–1391. [Google Scholar] [CrossRef]
- Cummings, D.J.; McNally, K.L.; Domenico, J.M.; Matsuura, E.T. The complete DNA sequence of the mitochondrial genome of Podospora anserina. Curr. Genet. 1990, 17, 375–402. [Google Scholar] [CrossRef]
- Foury, F.; Roganti, T.; Lecrenier, N.; Purnelle, B. The complete sequence of the mitochondrial genome of Saccharomyces cerevisiae. FEBS Lett. 1998, 440, 325–331. [Google Scholar] [CrossRef]
- Field, D.J.; Sommerfield, A.; Saville, B.J.; Collins, R.A. A group II intron in the Neurospora mitochondrial col gene: Nucleotide sequence and implications for splicing and molecular evolution. Nucleic Acids Res. 1989, 17, 9087–9099. [Google Scholar] [CrossRef]
- Nadimi, M.; Beaudet, D.; Forget, L.; Hijri, M.; Lang, B.F. Group I intron–mediated trans-splicing in mitochondria of gigaspora rosea and a robust phylogenetic affiliation of arbuscular mycorrhizal fungi with mortierellales. Mol. Biol. Evol. 2012, 29, 2199–2210. [Google Scholar] [CrossRef]
- Burger, G.; Yan, Y.; Javadi, P.; Lang, B.F. Group I-intron trans-splicing and mRNA editing in the mitochondria of placozoan animals. Trends Genet. 2009, 25, 381–386. [Google Scholar] [CrossRef]
- Grewe, F.; Viehoever, P.; Weisshaar, B.; Knoop, V. A trans-splicing group I intron and tRNA-hyperediting in the mitochondrial genome of the lycophyte Isoetes engelmannii. Nucleic Acids Res. 2009, 37, 5093–5104. [Google Scholar] [CrossRef]
- Pombert, J.-F.; Keeling, P.J. The mitochondrial genome of the entomoparasitic green alga Helicosporidium. PLoS ONE 2010, 5, e8954. [Google Scholar] [CrossRef]
- Hecht, J.; Grewe, F.; Knoop, V. Extreme RNA editing in coding islands and abundant microsatellites in repeat sequences of Selaginella moellendorffii mitochondria: The root of frequent plant mtDNA recombination in early tracheophytes. Genome Biol. Evol. 2011, 3, 344–358. [Google Scholar] [CrossRef]
- Pelin, A.; Pombert, J.F.; Salvioli, A.; Bonen, L.; Bonfante, P.; Corradi, N. The mitochondrial genome of the arbuscular mycorrhizal fungus Gigaspora margarita reveals two unsuspected trans-splicing events of group I introns. New Phytol. 2012, 194, 836–845. [Google Scholar] [CrossRef]
- Pombert, J.-F.; Otis, C.; Turmel, M.; Lemieux, C. The mitochondrial genome of the prasinophyte Prasinoderma coloniale reveals two trans-spliced group I introns in the large subunit rRNA gene. PLoS ONE 2013, 8, e84325. [Google Scholar] [CrossRef] [PubMed]
- Kamikawa, R.; Shiratori, T.; Ishida, K.-I.; Miyashita, H.; Roger, A.J. Group II intron-mediated trans-splicing in the gene-rich mitochondrial genome of an enigmatic eukaryote, Diphylleia rotans. Genome Biol. Evol. 2016, 8, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, P.L.; De-Paula, R.B.; Araújo, D.S.; Tomé, L.M.R.; Mendes-Pereira, T.; Rodrigues, W.F.C.; Del-Bem, L.-E.; Aguiar, E.R.; Góes-Neto, A. Global characterization of fungal mitogenomes: New insights on genomic diversity and dynamism of coding genes and accessory elements. Front. Microbiol. 2021, 12, 787283. [Google Scholar] [CrossRef] [PubMed]
- Beaudet, D.; Nadimi, M.; Iffis, B.; Hijri, M. Rapid mitochondrial genome evolution through invasion of mobile elements in two closely related species of arbuscular mycorrhizal fungi. PLoS ONE 2013, 8, e60768. [Google Scholar] [CrossRef] [PubMed]
- Handa, H. Linear plasmids in plant mitochondria: Peaceful coexistences or malicious invasions? Mitochondrion 2008, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]

| Isolates | No. of Contigs/SCAFFOLD | Largest Contig/Scaffold (Mb) | Total Length (Mb) | Scaffold N50 | GC (%) | BUSCO (%) |
|---|---|---|---|---|---|---|
| Isolate1Pk ** | 34 | 7.2 | 55.9 | 3.8 | 44.8 | 99.2 |
| Isolate2Pk ** | 29 | 7.8 | 56.5 | 4.1 | 44.8 | 99.6 |
| Isolate22Pk | 270 | 2.5 | 58.7 | 1.0 | 44.6 | 96.5 |
| Isolate32Pk | 81 | 3.0 | 57.5 | 1.6 | 44.5 | 95.6 |
| Isolate36Pk | 27 | 8.0 | 56.1 | 4.3 | 44. 8 | 97.6 |
| Isolate42Pk | 40 | 8.0 | 55.3 | 3.4 | 44.7 | 97.5 |
| Isolate18Ppll | 16 | 6.0 | 37.2 | 2.6 | 50.9 | 96.4 |
| Isolate27Ppll | 26 | 4.3 | 35.2 | 2.4 | 51.6 | 96.4 |
| Isolate58Ppll | 48 | 3.7 | 37.9 | 2.2 | 50.7 | 97.0 |
| Isolate72Ppll | 12 | 7.7 | 34.7 | 3.4 | 51.7 | 93.9 |
| Isolate104Ppll | 42 | 4.5 | 37.5 | 2.0 | 51.6 | 96.0 |
| Isolate113Ppll | 45 | 3.0 | 37.6 | 1.7 | 51.5 | 96.6 |
| Isolate3Pp ** | 16 | 6.3 | 34.6 | 3.7 | 52.6 | 99.1 |
| Isolate4Pp ** | 20 | 3.2 | 34.6 | 2.0 | 52.6 | 99.0 |
| Isolate5Pp ** | 18 | 4.0 | 36.7 | 2.7 | 52.4 | 98.5 |
| Isolate87Pp | 14 | 6.2 | 34.5 | 3.8 | 52.5 | 96.6 |
| Isolate88Pp | 19 | 6.2 | 34.5 | 3.6 | 52.6 | 95.8 |
| Isolate97Pp | 23 | 4.2 | 35.5 | 2.4 | 52.6 | 95.5 |
| Augustus | Braker | GeneMarkES | RNAmmer | tRNAscan-SE | ||||
|---|---|---|---|---|---|---|---|---|
| Isolates/Species | Gene | BUSCO (%) | Gene | BUSCO (%) | Gene | BUSCO (%) | Total rRNA | tRNA |
| Isolate1Pk | 16,127 | 66.2 | 10,024 | 98.9 | 10,198 | 97.7 | 53 | 141 |
| Isolate2Pk | 14,085 | 73.1 | 9851 | 98.6 | 10,319 | 98.4 | 107 | 139 |
| Isolate22Pk | 15,571 | 60.5 | 10,165 | 84.5 | 10,284 | 89.4 | 75 | 144 |
| Isolate32Pk | 15,548 | 54.4 | 9799 | 83.9 | 9884 | 89.9 | 87 | 137 |
| Isolate36Pk | 12,737 | 69.7 | 9914 | 89.1 | 10,009 | 92.1 | 83 | 142 |
| Isolate42Pk | 9388 | 87.2 | 10,454 | 86.6 | 9717 | 89.8 | 87 | 128 |
| Isolate18Ppll | 12,582 | 89.8 | 13,117 | 90.7 | 12,954 | 91.8 | 59 | 150 |
| Isolate27Ppll | 11,090 | 90.2 | 13,014 | 85.9 | 11,503 | 92.5 | 47 | 137 |
| Isolate58Ppll | 14,906 | 70.8 | 10,897 | 88.5 | 11,518 | 92.4 | 133 | 135 |
| Isolate72Ppll | 14,156 | 63.1 | 10,746 | 80.5 | 11,056 | 88.3 | 76 | 135 |
| Isolate104Ppll | 11,854 | 88.0 | 14,406 | 91.7 | 12,249 | 90.9 | 40 | 144 |
| Isolate113Ppll | 11,594 | 88.6 | 12,217 | 91.4 | 11,988 | 91.4 | 58 | 135 |
| Isolate3Pp | 16,562 | 71.4 | 10,742 | 97.8 | 11,417 | 98.0 | 104 | 129 |
| Isolate4Pp | 11,019 | 97.2 | 10,898 | 97.8 | 11,096 | 94.6 | 55 | 130 |
| Isolate5Pp | 17,970 | 71.8 | 10,964 | 96.2 | 11,797 | 96.9 | 58 | 129 |
| Isolate87Pp | 16,693 | 65.2 | 10,872 | 87.3 | 10,953 | 90.1 | 72 | 129 |
| Isolate88Pp | 16,850 | 66.7 | 10,569 | 88.9 | 10,987 | 90.6 | 98 | 129 |
| Isolate97Pp | 11,137 | 87.7 | 12,276 | 85.2 | 11,562 | 91.2 | 54 | 132 |
| TE | P. koolunga | P. pinodes | P. pinodella |
|---|---|---|---|
| I non-LTR | 7212 | 26,401 | 62,440 |
| Mariner | 326 | 19,131 | 1092 |
| MOLLY | 164 | 4700 | 3552 |
| Gypsy | 49 | 2954 | 410 |
| Copia | 89 | 32 | 1177 |
| MarCry | 18 | 101 | 105 |
| Tad1 | 50 | 16 | 10 |
| PYGGY | 21 | 1 | 5 |
| LMR1 | 77 | 1 | 5 |
| AFUT1 | 37 | 0 | 0 |
| REALAA | 11 | 0 | 2 |
| MGR583 | 6 | 7 | 7 |
| MAGGY | 1 | 8 | 0 |
| CFT1 | 0 | 8 | 0 |
| TFO1 | 0 | 2 | 0 |
| POT2 | 0 | 0 | 2 |
| FoHeli3 | 0 | 0 | 0 |
| Total | 8062 | 53,361 | 68,806 |
| Species | Clusters | Orthologous Clusters | SCG Clusters |
|---|---|---|---|
| P. pinodella | 11,782 | 3543 | 8239 |
| P. pinodes | 11,653 | 2438 | 9215 |
| P. koolunga | 10,305 | 1885 | 8420 |
| Species | Annotation | Protein Name |
|---|---|---|
| P. pinodes | GO:0005525 | F:GTP binding |
| GO:0016705 | F:oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen | |
| GO:0004847 | F:urea carboxylase activity | |
| Q9UV10 | Heterokaryon incompatibility protein 6, OR allele | |
| P. pinodella | GO:0016125 | P:sterol metabolic process |
| GO:0016114 | P:terpenoid biosynthetic process | |
| GO:0055114 | P:oxidation-reduction process | |
| GO:0005525 | F:GTP binding | |
| GO:0006520 | P:cellular amino acid metabolic process | |
| GO:0008643 | P:carbohydrate transport | |
| GO:0140041 | P:cellular detoxification of methylglyoxal | |
| P. koolunga | GO:0009405 | P:pathogenesis |
| GO:0006364 | P:rRNA processing | |
| GO:0016746 | F:transferase activity, transferring acyl groups | |
| GO:0010124 | P:phenylacetate catabolic process | |
| GO:0034517 | P:ribophagy | |
| GO:0006487 | P:protein N-linked glycosylation | |
| GO:0006810 | P:transport | |
| GO:0004022 | F:alcohol dehydrogenase (NAD) activity |
| Isolates | Species | Total Length (bp) | GC Content (%) |
|---|---|---|---|
| Isolate1Pk | P. koolunga | 73,511 | 28.9 |
| Isolate2Pk | P. koolunga | 68,282 | 28.7 |
| Isolate22Pk | P. koolunga | 68,003 | 28.9 |
| Isolate32Pk | P. koolunga | 67,924 | 28.9 |
| Isolate36Pk | P. koolunga | 67,828 | 28.9 |
| Isolate42Pk | P. koolunga | 65,622 | 28.8 |
| Isolate18Ppll | P. pinodella | 48,429 | 28.8 |
| Isolate27Ppll | P. pinodella | 50,457 | 29.4 |
| Isolate58Ppll | P. pinodella | 49,507 | 29.5 |
| Isolate72Ppll | P. pinodella | 50,405 | 29.1 |
| Isolate104Ppll | P. pinodella | 50,009 | 29.5 |
| Isolate113Ppll | P. pinodella | 50,374 | 29.5 |
| Isolate3Pp | P. pinodes | 55,213 | 29.1 |
| Isolate4Pp | P. pinodes | 56,229 | 29.2 |
| Isolate5Pp | P. pinodes | 56,330 | 29.2 |
| Isolate87Pp | P. pinodes | 55,990 | 29.4 |
| Isolate88Pp | P. pinodes | 55,567 | 29.3 |
| Isolate97Pp | P. pinodes | 55,931 | 29.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogaji, Y.O.; Lee, R.C.; Sawbridge, T.I.; Cocks, B.G.; Daetwyler, H.D.; Kaur, S. De Novo Long-Read Whole-Genome Assemblies and the Comparative Pan-Genome Analysis of Ascochyta Blight Pathogens Affecting Field Pea. J. Fungi 2022, 8, 884. https://doi.org/10.3390/jof8080884
Ogaji YO, Lee RC, Sawbridge TI, Cocks BG, Daetwyler HD, Kaur S. De Novo Long-Read Whole-Genome Assemblies and the Comparative Pan-Genome Analysis of Ascochyta Blight Pathogens Affecting Field Pea. Journal of Fungi. 2022; 8(8):884. https://doi.org/10.3390/jof8080884
Chicago/Turabian StyleOgaji, Yvonne O., Robert C. Lee, Tim I. Sawbridge, Benjamin G. Cocks, Hans D. Daetwyler, and Sukhjiwan Kaur. 2022. "De Novo Long-Read Whole-Genome Assemblies and the Comparative Pan-Genome Analysis of Ascochyta Blight Pathogens Affecting Field Pea" Journal of Fungi 8, no. 8: 884. https://doi.org/10.3390/jof8080884
APA StyleOgaji, Y. O., Lee, R. C., Sawbridge, T. I., Cocks, B. G., Daetwyler, H. D., & Kaur, S. (2022). De Novo Long-Read Whole-Genome Assemblies and the Comparative Pan-Genome Analysis of Ascochyta Blight Pathogens Affecting Field Pea. Journal of Fungi, 8(8), 884. https://doi.org/10.3390/jof8080884







