Novel Treatment Approach for Aspergilloses by Targeting Germination
Abstract
:1. Introduction
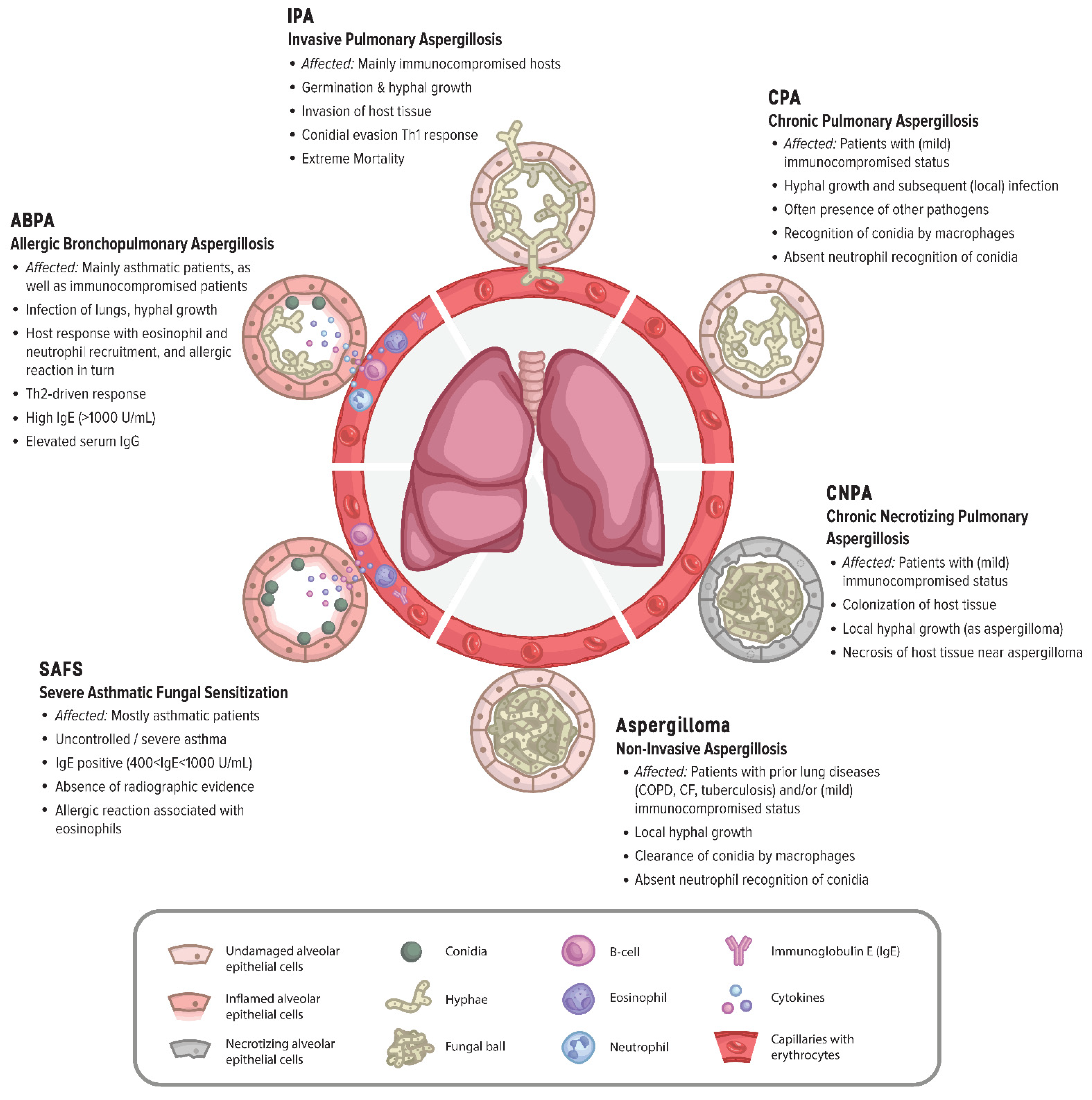
2. Host Susceptibility—Immune Clearance and Evasion of the Immune System
2.1. Pathogenicity of Aspergilli through Resilience and Dispersal
2.2. Normal Host Clearance of Aspergillus
2.3. Interactions between Hosts and Aspergillus Conidia
2.4. Abnormal Host Immune Responses to Aspergilli
3. Current and Past Treatments for Aspergillosis and the Rising Azole Resistance
3.1. Mechanisms of Azole Resistance
| Antifungal Drug | Drug Target | Year Dispensed | Resistance Status | Source(s) |
|---|---|---|---|---|
| Amphotericin B (AmB) | Sterols in the membrane of Aspergilli. Increased permeability and inhibition of ATPase proton pumps | 1958 (re-introduction in 1990s with lipid-based AmB) | Yes, but uncommon | [19,62] |
| Itraconazole | CYP450 | 1992 | Yes | [121] |
| Caspofungin | Synthesis of cell wall component 1,3-β-d-glucan | 2001 | [121,125] | |
| Voriconazole | CYP450 | 2002 | Yes | [121,123] |
| Micafungin | Synthesis of fungal cell wall component β-1,3-glucan | 2005 | Yes | [126] |
| Posaconazole | CYP450 | 2006 | Yes | [124] |
| Anidulafungin | Synthesis of fungal cell wall component β-1,3-glucan | 2006 | Yes | [146] |
| Isavuconazole | CYP450 | 2015 | Yes | [131,132,133] |
3.2. The Rise and Challenge of Azole Resistance
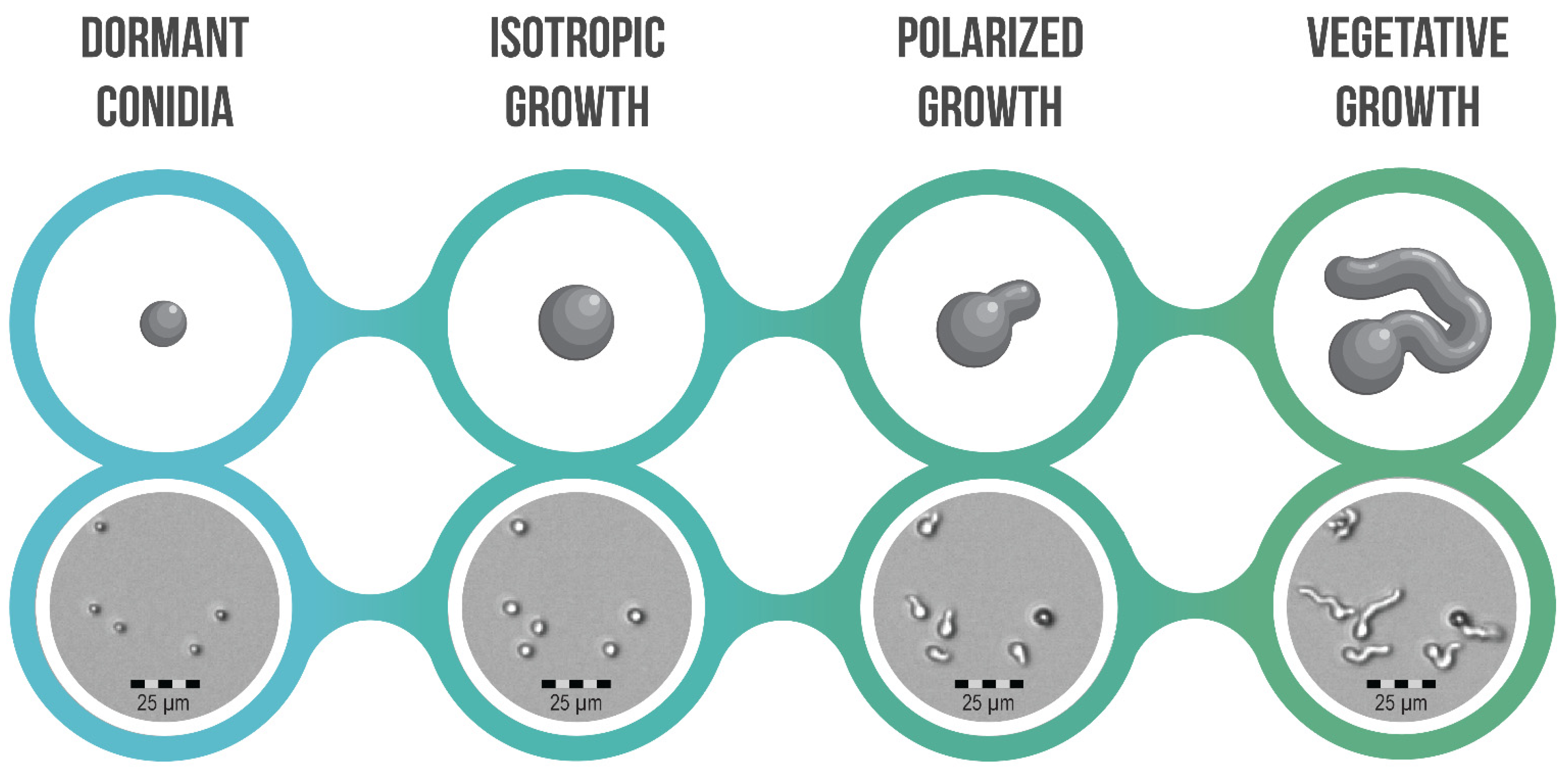
4. Conidial Germination and Its Different Morphotypes
Transitions and Differences between Morphotypes; How Are They Facilitated?
5. Proposed Targets for Novel Approach to Aspergillosis Treatments through Targeting Conidial Germination
5.1. To Target the Host or the Spores?
5.2. Possible Targets for Novel Antifungals That Hinder Germination
5.2.1. Targeting Dormant Conidia
5.2.2. Hindering the Breaking of Dormancy
5.2.3. Targeting Cell Wall Remodeling That Facilitates Isotropic Growth
5.2.4. Targeting Polarized Growth
| Category | Potential Target | Description | Which Aspergillus | Source |
|---|---|---|---|---|
| Other | AcuM, AcuK | Key transcription factors associated with gluconeogenesis and acquisition of iron. | A. fumigatus | [217] |
| CrhB, CrhC | Associated with swelling, germ tube formation and branching. Expressed mostly between t = 1 h and t = 6 h. | A. niger | [218] | |
| FacB | Transcription factor that is associated with acetate metabolism. | A. fumigatus | [219] | |
| HbxB | Key transcription factor, associated with repressed transcription of genes associated with β-glucan degradation. | A. nidulans | [220] | |
| MybA | Transcription factor that affects conidial viability. | A. fumigatus | [196,221] | |
| RlmA | Transcription factor that regulates mycotoxin production in conidia, as well as cell wall remodeling and synthesis, in particular, chitin. Associated with the fungal burden in lungs in vivo (mice). | A. fumigatus | [68,194] | |
| TreB | Trehalase, breaks down trehalose during germination. | A. niger | [222,223] | |
| Hypoxia | Cox5b, CycA, Afu3g06190, Afu1g1078, Gel4, and Rip1 | Most upregulated genes during hypoxia found in vitro with A549 cells. | A. fumigatus | [203,205] |
| Electron transport chain: complexes III and IV are essential for adaptation under hypoxic growth. | A. fumigatus | [224] | ||
| SrbA | Transcription factor in the family of sterol regulatory element-binding proteins (SREBPs). Regulator of cell wall polarity and sterol-associated genes. Involved in iron sensing and adaptation to hypoxia. | A. fumigatus | [68,205,213,214] | |
| Oxidative damage | CatA, Cat2, Sod3 | Superoxide dismutase and catalases, associated with protection from reactive oxygen species and oxidative damage in vitro with A549 cells. | A. fumigatus | [203] |
| ThiJ/Pfp1 family protein (AFUA_3G01210) | Within the Thil/Pfp1 family. Possibly associated with defense against reactive oxygen species due to similarity to YDR33C in yeast. | A. fumigatus | [195] | |
| Stress response | AtfA-D | bZip transcription factors, associated with regulation of osmotic and cell wall stress. All four interact with MAPK Saka in conditions that lacked stress. | A. fumigatus | [208] |
| DprA, DprB, Scf1 | Highly upregulated in vitro with A549 cells. | A. fumigatus | [203] | |
| MsbA | Associated functions are within the cell wall integrity pathway, cell wall morphogenesis, and sensor/signaling. A homolog of MSB2 in C. albicans, S. cerevisiae, and A. nidulans, functional as an external sensor and important for virulence. | A. fumigatus | [225] | |
| Downregulated after breaking dormancy | Cat2, MirD, Sdh2, SidA, SidC, SidD, SidF | Associated with iron acquisition. Downregulated in vitro with A549 cells. Only cat2 sidA, sidD, and mirD were found to be downregulated in vivo [68]. | A. fumigatus | [203] |
| GpgA | GPCR-γ subunit associated with gliotoxin production. A loss of function mutant showed severely delayed and impaired germination, with reduced structures in the maximum 65% germinated conidia. | A. fumigatus | [42,181,203] | |
| SltA | Downregulated as a response to nutrient deficiencies during growth in vivo (mice, IPA model). | A. fumigatus | [68] |
5.2.5. Other Anti-Germination Targets
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vallabhaneni, S.; Mody, R.K.; Walker, T.; Chiller, T. The Global Burden of Fungal Diseases. Infect. Dis. Clin. N. Am. 2016, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gago, S.; Denning, D.W.; Bowyer, P. Pathophysiological Aspects of Aspergillus Colonization in Disease. Med. Mycol. 2018, 57, S219–S227. [Google Scholar] [CrossRef]
- Cadena, J.; Thompson, G.R.; Patterson, T.F. Invasive Aspergillosis Current Strategies for Diagnosis and Management. Infect. Dis. Clin. N. Am. 2016, 30, 125–142. [Google Scholar] [CrossRef]
- Denning, D.W.; Park, S.; Lass-Florl, C.; Fraczek, M.G.; Kirwan, M.; Gore, R.; Smith, J.; Bueid, A.; Moore, C.B.; Bowyer, P.; et al. High-Frequency Triazole Resistance Found In Nonculturable Aspergillus Fumigatus from Lungs of Patients with Chronic Fungal Disease. Clin. Infect. Dis. 2011, 52, 1123–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denning, D.W.; Page, I.D.; Chakaya, J.; Jabeen, K.; Jude, C.M.; Cornet, M.; Alastruey-Izquierdo, A.; Bongomin, F.; Bowyer, P.; Chakrabarti, A.; et al. Case Definition of Chronic Pulmonary Aspergillosis in Resource-Constrained Settings. Emerg. Infect. Dis. J. 2018, 24, e171312. [Google Scholar] [CrossRef]
- Dagenais, T.R.T.; Keller, N.P. Pathogenesis of Aspergillus Fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon-Chung, K.J.; Sugui, J.A. Aspergillus Fumigatus—What Makes the Species a Ubiquitous Human Fungal Pathogen? PLoS Pathog. 2013, 9, e1003743. [Google Scholar] [CrossRef]
- Negri, C.E.; Gonçalves, S.S.; Xafranski, H.; Bergamasco, M.D.; Aquino, V.R.; Castro, P.T.O.; Colombo, A.L. Cryptic and Rare Aspergillus Species in Brazil: Prevalence in Clinical Samples and In Vitro Susceptibility to Triazoles. J. Clin. Microbiol. 2014, 52, 3633–3640. [Google Scholar] [CrossRef] [Green Version]
- Pinto, E.; Monteiro, C.; Maia, M.; Faria, M.A.; Lopes, V.; Lameiras, C.; Pinheiro, D. Aspergillus Species and Antifungals Susceptibility in Clinical Setting in the North of Portugal: Cryptic Species and Emerging Azoles Resistance in A. Fumigatus. Front. Microbiol. 2018, 9, 1656. [Google Scholar] [CrossRef] [Green Version]
- Rozaliyani, A.; Abdullah, A.; Setianingrum, F.; Sjamsuridzal, W.; Wahyuningsih, R.; Bowolaksono, A.; Fatril, A.E.; Adawiyah, R.; Tugiran, M.; Syam, R.; et al. Unravelling the Molecular Identification and Antifungal Susceptibility Profiles of Aspergillus Spp. Isolated from Chronic Pulmonary Aspergillosis Patients in Jakarta, Indonesia: The Emergence of Cryptic Species. J. Fungi 2022, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.; Tang, J.Y.M.; Ye, H.; Xing, F.; Lo, S.K.F.; Xiao, C.; Han, L.; Wu, A.K.L.; Ngan, A.H.Y.; Law, K.; et al. Rare/Cryptic Aspergillus Species Infections and Importance of Antifungal Susceptibility Testing. Mycoses 2020, 63, 1283–1298. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, W.; Liang, T.; Tan, J.; Liu, W.; Sun, Y.; Wang, Q.; Xu, H.; Li, L.; Zhou, Y.; et al. A 20-Year Antifungal Susceptibility Surveillance (from 1999 to 2019) for Aspergillus spp. and Proposed Epidemiological Cutoff Values for Aspergillus Fumigatus and Aspergillus Flavus: A Study in a Tertiary Hospital in China. Front. Microbiol. 2021, 12, 680884. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Magan, N. Aspergillus Species and Mycotoxins: Occurrence and Importance in Major Food Commodities. Curr. Opin. Food Sci. 2018, 23, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A. Characteristics, Occurrence, Detection and Detoxification of Aflatoxins in Foods and Feeds. Foods 2020, 9, 644. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, F. Global Burden of Aflatoxin-Induced Hepatocellular Carcinoma: A Risk Assessment. Environ. Health Persp. 2010, 118, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smela, M.E.; Hamm, M.L.; Henderson, P.T.; Harris, C.M.; Harris, T.M.; Essigmann, J.M. The Aflatoxin B1 Formamidopyrimidine Adduct Plays a Major Role in Causing the Types of Mutations Observed in Human Hepatocellular Carcinoma. Proc. Natl. Acad. Sci. USA 2002, 99, 6655–6660. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.S.W.; Aimanianda, V. Host Soluble Mediators: Defying the Immunological Inertness of Aspergillus Fumigatus Conidia. J. Fungi 2017, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Latgé, J.-P. Aspergillus Fumigatus and Aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310–350. [Google Scholar] [CrossRef] [Green Version]
- Ellena, V.; Sauer, M.; Steiger, M.G. The Fungal Sexual Revolution Continues: Discovery of Sexual Development in Members of the Genus Aspergillus and Its Consequences. Fungal Biol. Biotechnol. 2020, 7, 17. [Google Scholar] [CrossRef]
- Krijgsheld, P.; Bleichrodt, R.; van Veluw, G.J.; Wang, F.; Müller, W.H.; Dijksterhuis, J.; Wösten, H.A.B. Development in Aspergillus. Stud. Mycol. 2013, 74, 1–29. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, C.M.; Fuller, H.T.; Dyer, P.S. Discovery of a Sexual Cycle in the Opportunistic Fungal Pathogen Aspergillus Fumigatus. Nature 2009, 457, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Ni, M.; Li, W.; Shertz, C.; Heitman, J. The Evolution of Sex: A Perspective from the Fungal Kingdom. Microbiol. Mol. Biol. Rev. 2010, 74, 298–340. [Google Scholar] [CrossRef] [Green Version]
- Ojeda-López, M.; Chen, W.; Eagle, C.E.; Gutiérrez, G.; Jia, W.L.; Swilaiman, S.S.; Huang, Z.; Park, H.-S.; Yu, J.-H.; Cánovas, D.; et al. Evolution of Asexual and Sexual Reproduction in the Aspergilli. Stud. Mycol. 2018, 91, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Simões, D.; Caetano, L.A.; Veríssimo, C.; Viegas, C.; Sabino, R. Aspergillus Collected in Specific Indoor Settings: Their Molecular Identification and Susceptibility Pattern. Int. J. Environ. Health Rev. 2019, 31, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, M.; Schaffner, A. Host Defense Mechanism in Aspergillus Fumigatus Infections. Contrib. Microbiol. 1999, 2, 57–68. [Google Scholar] [CrossRef]
- Baltussen, T.J.H.; Zoll, J.; Verweij, P.E.; Melchers, W.J.G. Molecular Mechanisms of Conidial Germination in Aspergillus spp. Microbiol. Mol. Biol. Rev. 2019, 84. [Google Scholar] [CrossRef]
- Ijadpanahsaravi, M.; Punt, M.; Wösten, H.A.B.; Teertstra, W.R. Minimal Nutrient Requirements for Induction of Germination of Aspergillus Niger Conidia. Fungal Biol. 2021, 125, 231–238. [Google Scholar] [CrossRef]
- van Eijk, M.; Boerefijn, S.; Cen, L.; Rosa, M.; Morren, M.J.H.; van der Ent, C.K.; Kraak, B.; Dijksterhuis, J.; Valdes, I.D.; Haagsman, H.P.; et al. Cathelicidin-Inspired Antimicrobial Peptides as Novel Antifungal Compounds. Med. Mycol. 2020, 58, 1073–1084. [Google Scholar] [CrossRef] [Green Version]
- Salzer, H.J.F.; Heyckendorf, J.; Kalsdorf, B.; Rolling, T.; Lange, C. Characterization of Patients with Chronic Pulmonary Aspergillosis according to the New ESCMID/ERS/ECMM and IDSA Guidelines. Mycoses 2017, 60, 136–142. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.; Pleuvry, A.; Cole, D. Global Burden of Chronic Pulmonary Aspergillosis as a Sequel to Pulmonary Tuberculosis. Bull. World Health Organ. 2011, 89, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Armstead, J.; Morris, J.; Denning, D.W. Multi-Country Estimate of Different Manifestations of Aspergillosis in Cystic Fibrosis. PLoS ONE 2014, 9, e98502. [Google Scholar] [CrossRef]
- Jones, A.M.; Horsley, A.; Denning, D.W. What Is the Importance of Classifying Aspergillus Disease in Cystic Fibrosis Patients? Expert Rev. Resp. Med. 2014, 8, 389–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, J.; Brunel, S.F.; Warris, A. Aspergillus Infections in Cystic Fibrosis. J. Infect. 2016, 72, S50–S55. [Google Scholar] [CrossRef] [PubMed]
- Sabino, R.; Ferreira, J.A.G.; Moss, R.B.; Valente, J.; Veríssimo, C.; Carolino, E.; Clemons, K.V.; Everson, C.; Banaei, N.; Penner, J.; et al. Molecular Epidemiology of Aspergillus Collected from Cystic Fibrosis Patients. J. Cyst. Fibros. 2015, 14, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Currie, A.J.; Main, E.T.; Wilson, H.M.; Armstrong-James, D.; Warris, A. CFTR Modulators Dampen Aspergillus-Induced Reactive Oxygen Species Production by Cystic Fibrosis Phagocytes. Front. Cell. Infect. Microbiol. 2020, 10, 372. [Google Scholar] [CrossRef]
- Saunders, R.V.; Modha, D.E.; Claydon, A.; Gaillard, E.A. Chronic Aspergillus Fumigatus Colonization of the Pediatric Cystic Fibrosis Airway Is Common and May Be Associated with a More Rapid Decline in Lung Function. Med. Mycol. 2016, 54, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Oosthuizen, J.L.; Gomez, P.; Ruan, J.; Hackett, T.L.; Moore, M.M.; Knight, D.A.; Tebbutt, S.J. Dual Organism Transcriptomics of Airway Epithelial Cells Interacting with Conidia of Aspergillus Fumigatus. PLoS ONE 2011, 6, e20527. [Google Scholar] [CrossRef] [Green Version]
- Seidel, C.; Moreno-Velásquez, S.D.; Ben-Ghazzi, N.; Gago, S.; Read, N.D.; Bowyer, P. Phagolysosomal Survival Enables Non-Lytic Hyphal Escape and Ramification Through Lung Epithelium During Aspergillus Fumigatus Infection. Front. Microbiol. 2020, 11, 1955. [Google Scholar] [CrossRef]
- Paterson, D.L.; Singh, N. Invasive Aspergillosis in Transplant Recipients. Medicine 1999, 78, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.D.; Ayubi, T.; Chung, D.; Tubau-Juni, N.; Leber, A.; Dang, H.X.; Karyala, S.; Hontecillas, R.; Lawrence, C.B.; Cramer, R.A.; et al. Modulation of Immune Signaling and Metabolism Highlights Host and Fungal Transcriptional Responses in Mouse Models of Invasive Pulmonary Aspergillosis. Sci. Rep. 2017, 7, 17096. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Cuenca-Estrella, M. Changes in the Epidemiological Landscape of Invasive Mould Infections and Disease. J. Antimicrob. Chemoth. 2017, 72, i5–i11. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Anaissie, E. Fungal Infections in Hematopoietic Stem Cell Transplantation and Solid-Organ Transplantation—Focus on Aspergillosis. Clin. Chest Med. 2009, 30, 295–306. [Google Scholar] [CrossRef]
- Herbrecht, R.; Bories, P.; Moulin, J.; Ledoux, M.; Letscher-Bru, V. Risk Stratification for Invasive Aspergillosis in Immunocompromised Patients. Ann. N. Y. Acad. Sci. 2012, 1272, 23–30. [Google Scholar] [CrossRef]
- Mühlemann, K.; Wenger, C.; Zenhäusern, R.; Täuber, M.G. Risk Factors for Invasive Aspergillosis in Neutropenic Patients with Hematologic Malignancies. Leukemia 2005, 19, 545–550. [Google Scholar] [CrossRef]
- Kosmidis, C.; Denning, D.W. The Clinical Spectrum of Pulmonary Aspergillosis. Thorax 2015, 70, 270. [Google Scholar] [CrossRef] [Green Version]
- Gomez, P.; Hackett, T.L.; Moore, M.M.; Knight, D.A.; Tebbutt, S.J. Functional Genomics of Human Bronchial Epithelial Cells Directly Interacting with Conidia of Aspergillus Fumigatus. BMC Genom. 2010, 11, 358. [Google Scholar] [CrossRef] [Green Version]
- Allard, J.B.; Poynter, M.E.; Marr, K.A.; Cohn, L.; Rincon, M.; Whittaker, L.A. Aspergillus Fumigatus Generates an Enhanced Th2-Biased Immune Response in Mice with Defective Cystic Fibrosis Transmembrane Conductance Regulator. J. Immunol. 2006, 177, 5186–5194. [Google Scholar] [CrossRef] [Green Version]
- Ueki, S.; Hebisawa, A.; Kitani, M.; Asano, K.; Neves, J.S. Allergic Bronchopulmonary Aspergillosis–A Luminal Hypereosinophilic Disease With Extracellular Trap Cell Death. Front. Immunol. 2018, 9, 2346. [Google Scholar] [CrossRef]
- Bigot, J.; Guillot, L.; Guitard, J.; Ruffin, M.; Corvol, H.; Balloy, V.; Hennequin, C. Bronchial Epithelial Cells on the Front Line to Fight Lung Infection-Causing Aspergillus Fumigatus. Front. Immunol. 2020, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Wasylnka, J.A.; Moore, M.M. Aspergillus Fumigatus Conidia Survive and Germinate in Acidic Organelles of A549 Epithelial Cells. J. Cell Sci. 2003, 116, 1579–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akoumianaki, T.; Kyrmizi, I.; Valsecchi, I.; Gresnigt, M.S.; Samonis, G.; Drakos, E.; Boumpas, D.; Muszkieta, L.; Prevost, M.-C.; Kontoyiannis, D.P.; et al. Aspergillus Cell Wall Melanin Blocks LC3-Associated Phagocytosis to Promote Pathogenicity. Cell Host Microbe 2016, 19, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thywißen, A.; Heinekamp, T.; Dahse, H.-M.; Schmaler-Ripcke, J.; Nietzsche, S.; Zipfel, P.F.; Brakhage, A.A. Conidial Dihydroxynaphthalene Melanin of the Human Pathogenic Fungus Aspergillus Fumigatus Interferes with the Host Endocytosis Pathway. Front. Microbiol. 2011, 2, 96. [Google Scholar] [CrossRef] [Green Version]
- Cowley, A.C.; Thornton, D.J.; Denning, D.W.; Horsley, A. Aspergillosis and the Role of Mucins in Cystic Fibrosis. Pediatr. Pulm. 2017, 52, 548–555. [Google Scholar] [CrossRef]
- Treacy, K.; Tunney, M.; Elborn, J.S.; Bradley, J.M. Mucociliary Clearance in Cystic Fibrosis: Physiology and Pharmacological Treatments. Paediatr. Child Health 2011, 21, 425–430. [Google Scholar] [CrossRef]
- Grazziutti, M.L.; Rex, J.H.; Cowart, R.E.; Anaissie, E.J.; Ford, A.; Savary, C.A. Aspergillus Fumigatus Conidia Induce a Th1-Type Cytokine Response. J. Infect. Dis. 1997, 176, 1579–1583. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.-C.; Yu, W.-L. COVID-19 Associated with Pulmonary Aspergillosis: A Literature Review. J. Microbiol. Immunol. Infect. 2020, 54, 46–53. [Google Scholar] [CrossRef]
- Kakamad, F.H.; Mahmood, S.O.; Rahim, H.M.; Abdulla, B.A.; Abdullah, H.O.; Othman, S.; Mohammed, S.H.; Kakamad, S.H.; Mustafa, S.M.; Salih, A.M. Post COVID-19 Invasive Pulmonary Aspergillosis: A Case Report. Int. J. Surg. Case Rep. 2021, 82, 105865. [Google Scholar] [CrossRef]
- Armstrong-James, D.; Youngs, J.; Bicanic, T.; Abdolrasouli, A.; Denning, D.W.; Johnson, E.; Mehra, V.; Pagliuca, T.; Patel, B.; Rhodes, J.; et al. Confronting and Mitigating the Risk of COVID-19 Associated Pulmonary Aspergillosis. Eur. Respir. J. 2020, 56, 2002554. [Google Scholar] [CrossRef]
- Rutsaert, L.; Steinfort, N.; Hunsel, T.V.; Bomans, P.; Naesens, R.; Mertes, H.; Dits, H.; Regenmortel, N.V. COVID-19-Associated Invasive Pulmonary Aspergillosis. Ann. Intensive Care 2020, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Andes, D.R. Antifungal Agents Spectrum of Activity, Pharmacology, and Clinical Indications. Infect. Dis. Clin. N. Am. 2016, 30, 51–83. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.G.P.; Slabbers, L.; de Jong, C.; Melchers, W.J.G.; Hagen, F.; Verweij, P.E.; Merkus, P.; Meis, J.F.; Dutch Cystic Fibrosis Fungal Collection Consortium. Prevalence and Diversity of Filamentous Fungi in the Airways of Cystic Fibrosis Patients—A Dutch, Multicentre Study. J. Cyst. Fibros. 2019, 18, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Lestrade, P.P.A.; Meis, J.F.; Melchers, W.J.G.; Verweij, P.E. Triazole Resistance in Aspergillus Fumigatus: Recent Insights and Challenges for Patient Management. Clin. Microbiol. Infect. 2019, 25, 799–806. [Google Scholar] [CrossRef]
- Verweij, P.E.; Chowdhary, A.; Melchers, W.J.G.; Meis, J.F. Azole Resistance in Aspergillus Fumigatus: Can We Retain the Clinical Use of Mold-Active Antifungal Azoles? Clin. Infect. Dis. 2016, 62, 362–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, J.I.P.; Fava, V.M.; Kerkaert, J.D.; Subramanian, A.S.; Gravelat, F.N.; Lehoux, M.; Howell, P.L.; Cramer, R.A.; Sheppard, D.C. Reducing Aspergillus Fumigatus Virulence through Targeted Dysregulation of the Conidiation Pathway. Mbio 2020, 11, e03202-19. [Google Scholar] [CrossRef] [Green Version]
- Bahn, Y.-S. Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets. PLoS Pathog. 2015, 11, e1004936. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Xu, W.; Bruno, V.M.; Phan, Q.T.; Solis, N.V.; Woolford, C.A.; Ehrlich, R.L.; Shetty, A.C.; McCraken, C.; Lin, J.; et al. Determining Aspergillus Fumigatus Transcription Factor Expression and Function during Invasion of the Mammalian Lung. PLoS Pathog. 2021, 17, e1009235. [Google Scholar] [CrossRef]
- Hagiwara, D.; Suzuki, S.; Kamei, K.; Gonoi, T.; Kawamoto, S. The Role of AtfA and HOG MAPK Pathway in Stress Tolerance in Conidia of Aspergillus Fumigatus. Fungal Genet. Biol. 2014, 73, 138–149. [Google Scholar] [CrossRef]
- Dai, J.; Chen, Y.; Jiang, F. Allicin Reduces Inflammation by Regulating ROS/NLRP3 and Autophagy in the Context of A. Fumigatus Infection in Mice. Gene 2020, 762, 145042. [Google Scholar] [CrossRef]
- Baltussen, T.J.H.; Coolen, J.P.M.; Verweij, P.E.; Dijksterhuis, J.; Melchers, W.J.G. Identifying Conserved Generic Aspergillus Spp. Co-Expressed Gene Modules Associated with Germination Using Cross-Platform and Cross-Species Transcriptomics. J. Fungi 2021, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Seagle, E.E.; Williams, S.L.; Chiller, T.M. Recent Trends in the Epidemiology of Fungal Infections. Infect. Dis. Clin. N. Am. 2021, 35, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, S.; Ricci, E.; Migliorati, G.; Gentili, M.; Riccardi, C. How Glucocorticoids Affect the Neutrophil Life. Int. J. Mol. Sci. 2018, 19, 4090. [Google Scholar] [CrossRef] [Green Version]
- Arastehfar, A.; Carvalho, A.; Houbraken, J.; Lombardi, L.; Garcia-Rubio, R.; Jenks, J.D.; Rivero-Menendez, O.; Aljohani, R.; Jacobsen, I.D.; Berman, J.; et al. Aspergillus Fumigatus and Aspergillosis: From Basics to Clinics. Stud. Mycol. 2021, 100, 100115. [Google Scholar] [CrossRef]
- Denning, D.W.; Chakrabarti, A. Pulmonary and Sinus Fungal Diseases in Non-Immunocompromised Patients. Lancet Infect. Dis. 2017, 17, e357–e366. [Google Scholar] [CrossRef]
- O’Neill, S.; Brault, J.; Stasia, M.-J.; Knaus, U.G. Genetic Disorders Coupled to ROS Deficiency. Redox Biol. 2015, 6, 135–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballard, E.; Zoll, J.; Melchers, W.J.G.; Brown, A.J.P.; Warris, A.; Verweij, P.E. Raw Genome Sequence Data for 13 Isogenic Aspergillus Fumigatus Strains Isolated over a 2 Year Period from a Patient with Chronic Granulomatous Disease. Data Brief 2019, 25, 104021. [Google Scholar] [CrossRef]
- Saijo, S.; Iwakura, Y. Dectin-1 and Dectin-2 in Innate Immunity against Fungi. Int. Immunol. 2011, 23, 467–472. [Google Scholar] [CrossRef]
- Danion, F.; Aimanianda, V.; Bayry, J.; Duréault, A.; Wong, S.S.W.; Bougnoux, M.-E.; Tcherakian, C.; Alyanakian, M.-A.; Guegan, H.; Puel, A.; et al. Aspergillus Fumigatus Infection in Humans With STAT3-Deficiency Is Associated With Defective Interferon-Gamma and Th17 Responses. Front. Immunol. 2020, 11, 38. [Google Scholar] [CrossRef] [Green Version]
- van de Veerdonk, F.L.; Gresnigt, M.S.; Romani, L.; Netea, M.G.; Latgé, J.-P. Aspergillus Fumigatus Morphology and Dynamic Host Interactions. Nat. Rev. Microbiol. 2017, 15, 661–674. [Google Scholar] [CrossRef]
- Bennett, J.W. An Overview of the Genus Aspergillus. In Aspergillus: Molecular Biology and Genomics; Caister Academic Press: Poole, UK, 2010; pp. 1–17. [Google Scholar]
- Rhodes, J.C. Aspergillus Fumigatus: Growth and Virulence. Med. Mycol. 2006, 44, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Voltersen, V.; Blango, M.G.; Herrmann, S.; Schmidt, F.; Heinekamp, T.; Strassburger, M.; Krüger, T.; Bacher, P.; Lother, J.; Weiss, E.; et al. Proteome Analysis Reveals the Conidial Surface Protein CcpA Essential for Virulence of the Pathogenic Fungus Aspergillus Fumigatus. Mbio 2018, 9, e01557-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobar, N.; Ordonez, S.R.; Wösten, H.A.B.; Haas, P.-J.A.; de Cock, H.; Haagsman, H.P. Hide, Keep Quiet, and Keep Low: Properties That Make Aspergillus Fumigatus a Successful Lung Pathogen. Front. Microbiol. 2016, 7, 438. [Google Scholar] [CrossRef] [PubMed]
- Carrion, S.D.J.; Leal, S.M.; Ghannoum, M.A.; Aimanianda, V.; Latgé, J.-P.; Pearlman, E. The RodA Hydrophobin on Aspergillus Fumigatus Spores Masks Dectin-1– and Dectin-2–Dependent Responses and Enhances Fungal Survival In Vivo. J. Immunol. 2013, 191, 2581–2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aimanianda, V.; Bayry, J.; Bozza, S.; Kniemeyer, O.; Perruccio, K.; Elluru, S.R.; Clavaud, C.; Paris, S.; Brakhage, A.A.; Kaveri, S.V.; et al. Surface Hydrophobin Prevents Immune Recognition of Airborne Fungal Spores. Nature 2009, 460, 1117–1121. [Google Scholar] [CrossRef]
- Valsecchi, I.; Lai, J.I.; Stephen-Victor, E.; Pillé, A.; Beaussart, A.; Lo, V.; Pham, C.L.L.; Aimanianda, V.; Kwan, A.H.; Duchateau, M.; et al. Assembly and Disassembly of Aspergillus Fumigatus Conidial Rodlets. Cell Surf. 2019, 5, 100023. [Google Scholar] [CrossRef]
- Valsecchi, I.; Dupres, V.; Stephen-Victor, E.; Guijarro, J.I.; Gibbons, J.; Beau, R.; Bayry, J.; Coppee, J.-Y.; Lafont, F.; Latgé, J.-P.; et al. Role of Hydrophobins in Aspergillus Fumigatus. J. Fungi 2017, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Pihet, M.; Vandeputte, P.; Tronchin, G.; Renier, G.; Saulnier, P.; Georgeault, S.; Mallet, R.; Chabasse, D.; Symoens, F.; Bouchara, J.-P. Melanin Is an Essential Component for the Integrity of the Cell Wall of Aspergillus Fumigatus Conidia. BMC Microbiol. 2009, 9, 177. [Google Scholar] [CrossRef]
- Fontaine, T.; Beauvais, A.; Loussert, C.; Thevenard, B.; Fulgsang, C.C.; Ohno, N.; Clavaud, C.; Prevost, M.-C.; Latgé, J.-P. Cell Wall A1-3glucans Induce the Aggregation of Germinating Conidia of Aspergillus Fumigatus. Fungal Genet. Biol. 2010, 47, 707–712. [Google Scholar] [CrossRef]
- Chakraborty, A.; Fernando, L.D.; Fang, W.; Widanage, M.C.D.; Wei, P.; Jin, C.; Fontaine, T.; Latgé, J.-P.; Wang, T. A Molecular Vision of Fungal Cell Wall Organization by Functional Genomics and Solid-State NMR. Nat. Commun. 2021, 12, 6346. [Google Scholar] [CrossRef]
- Rambach, G.; Blum, G.; Latgé, J.-P.; Fontaine, T.; Heinekamp, T.; Hagleitner, M.; Jeckström, H.; Weigel, G.; Würtinger, P.; Pfaller, K.; et al. Identification of Aspergillus Fumigatus Surface Components That Mediate Interaction of Conidia and Hyphae With Human Platelets. J. Infect. Dis. 2015, 212, 1140–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinekamp, T.; Thywißen, A.; Macheleidt, J.; Keller, S.; Valiante, V.; Brakhage, A.A. Aspergillus Fumigatus Melanins: Interference with the Host Endocytosis Pathway and Impact on Virulence. Front. Microbiol. 2013, 3, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunde, M.; Kwan, A.H.Y.; Templeton, M.D.; Beever, R.E.; Mackay, J.P. Structural Analysis of Hydrophobins. Micron 2008, 39, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Stappers, M.H.T.; Clark, A.E.; Aimanianda, V.; Bidula, S.; Reid, D.M.; Asamaphan, P.; Hardison, S.E.; Dambuza, I.M.; Valsecchi, I.; Kerscher, B.; et al. Recognition of DHN-Melanin by a C-Type Lectin Receptor Is Required for Immunity to Aspergillus. Nature 2018, 555, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Keizer, E.M.; Wösten, H.A.B.; Cock, H. de EphA2-Dependent Internalization of A. Fumigatus Conidia in A549 Lung Cells Is Modulated by DHN-Melanin. Front. Microbiol. 2020, 11, 534118. [Google Scholar] [CrossRef]
- Hagiwara, D.; Takahashi, H.; Kusuya, Y.; Kawamoto, S.; Kamei, K.; Gonoi, T. Comparative Transcriptome Analysis Revealing Dormant Conidia and Germination Associated Genes in Aspergillus Species: An Essential Role for AtfA in Conidial Dormancy. BMC Genom. 2016, 17, 358. [Google Scholar] [CrossRef] [Green Version]
- Sugui, J.A.; Kim, H.S.; Zarember, K.A.; Chang, Y.C.; Gallin, J.I.; Nierman, W.C.; Kwon-Chung, K.J. Genes Differentially Expressed in Conidia and Hyphae of Aspergillus Fumigatus upon Exposure to Human Neutrophils. PLoS ONE 2008, 3, e2655. [Google Scholar] [CrossRef]
- Dolan, S.K.; O’Keeffe, G.; Jones, G.W.; Doyle, S. Resistance Is Not Futile: Gliotoxin Biosynthesis, Functionality and Utility. Trends Microbiol. 2015, 23, 419–428. [Google Scholar] [CrossRef]
- Becker, K.L.; Gresnigt, M.S.; Smeekens, S.P.; Jacobs, C.W.; Magis-Escurra, C.; Jaeger, M.; Wang, X.; Lubbers, R.; Oosting, M.; Joosten, L.A.B.; et al. Pattern Recognition Pathways Leading to a Th2 Cytokine Bias in Allergic Bronchopulmonary Aspergillosis Patients. Clin. Exp. Allergy 2015, 45, 423–437. [Google Scholar] [CrossRef]
- O’Sullivan, J.A.; Bochner, B.S. Eosinophils and Eosinophil-Associated Diseases: An Update. J. Allergy Clin. Immunol. 2018, 141, 505–517. [Google Scholar] [CrossRef] [Green Version]
- Curran, A.K.; Hava, D.L. Allergic Diseases Caused by Aspergillus Species in Patients with Cystic Fibrosis. Antibiotics 2021, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Eickmeier, O.; Zissler, U.M.; Wittschorek, J.; Unger, F.; Schmitt-Grohé, S.; Schubert, R.; Herrmann, E.; Zielen, S. Clinical Relevance of Aspergillus Fumigatus Sensitization in Cystic Fibrosis. Clin. Exp. Allergy 2020, 50, 325–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouzani, M.; Ok, M.; McCormick, A.; Ebel, F.; Kurzai, O.; Morton, C.O.; Einsele, H.; Loeffler, J. Human NK Cells Display Important Antifungal Activity against Aspergillus Fumigatus, Which Is Directly Mediated by IFN-γ Release. J. Immunol. 2011, 187, 1369–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, M.I.; Catalan-Dibene, J.; Zlotnik, A. B Cells Responses and Cytokine Production Are Regulated by Their Immune Microenvironment. Cytokine 2015, 74, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Miki, M.; Ohara, Y.; Tsujino, K.; Kawasaki, T.; Kuge, T.; Yamamoto, Y.; Matsuki, T.; Miki, K.; Kida, H. Pulmonary Eosinophilia May Indicate Onset Stage of Allergic Bronchopulmonary Aspergillosis. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. 2021, 17, 118. [Google Scholar] [CrossRef]
- Patterson, K.; Strek, M.E. Allergic Bronchopulmonary Aspergillosis. Proc. Am. Thorac. Soc. 2010, 7, 237–244. [Google Scholar] [CrossRef]
- Cortez, K.J.; Lyman, C.A.; Kottilil, S.; Kim, H.S.; Roilides, E.; Yang, J.; Fullmer, B.; Lempicki, R.; Walsh, T.J. Functional Genomics of Innate Host Defense Molecules in Normal Human Monocytes in Response to Aspergillus Fumigatus. Infect. Immunol. 2006, 74, 2353–2365. [Google Scholar] [CrossRef] [Green Version]
- Doran, E.; Cai, F.; Holweg, C.T.J.; Wong, K.; Brumm, J.; Arron, J.R. Interleukin-13 in Asthma and Other Eosinophilic Disorders. Front. Med. 2017, 4, 139. [Google Scholar] [CrossRef]
- Roufosse, F. Targeting the Interleukin-5 Pathway for Treatment of Eosinophilic Conditions Other than Asthma. Front. Med. 2018, 5, 49. [Google Scholar] [CrossRef] [Green Version]
- Maleki, M.; Mortezaee, V.; Hassanzad, M.; Mahdaviani, S.A.; Poorabdollah, M.; Mehrian, P.; Behnampour, N.; Mirenayat, M.S.; Abastabar, M.; Tavakoli, M.; et al. Prevalence of Allergic Bronchopulmonary Aspergillosis in Cystic Fibrosis Patients Using Two Different Diagnostic Criteria. Eur. Ann. Allergy Clin. Immunol. 2020, 52, 74. [Google Scholar] [CrossRef]
- Müller, C.; Braag, S.A.; Herlihy, J.-D.; Wasserfall, C.H.; Chesrown, S.E.; Nick, H.S.; Atkinson, M.A.; Flotte, T.R. Enhanced IgE Allergic Response to Aspergillus Fumigatus in CFTR−/− Mice. Lab. Investig. 2006, 86, 130–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, R.; Chakrabarti, A.; Shah, A.; Gupta, D.; Meis, J.F.; Guleria, R.; Moss, R.; Denning, D.W.; Group, A. Complicating asthma I. working Allergic Bronchopulmonary Aspergillosis: Review of Literature and Proposal of New Diagnostic and Classification Criteria. Clin. Exp. Allergy 2013, 43, 850–873. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; Gaggar, A.; Bruscia, E.; Hector, A.; Marcos, V.; Jung, A.; Greene, C.; McElvaney, G.; Mall, M.; Döring, G. Innate Immunity in Cystic Fibrosis Lung Disease. J. Cyst. Fibros. 2012, 11, 363–382. [Google Scholar] [CrossRef] [Green Version]
- Yiallouros, P.K.; Matthaiou, A.Μ.; Anagnostopoulou, P.; Kouis, P.; Libik, M.; Adamidi, T.; Eleftheriou, A.; Demetriou, A.; Ioannou, P.; Tanteles, G.A.; et al. Demographic Characteristics, Clinical and Laboratory Features, and the Distribution of Pathogenic Variants in the CFTR Gene in the Cypriot Cystic Fibrosis (CF) Population Demonstrate the Utility of a National CF Patient Registry. Orphanet J. Rare Dis. 2021, 16, 409. [Google Scholar] [CrossRef]
- Blohmke, C.J.; Victor, R.E.; Hirschfeld, A.F.; Elias, I.M.; Hancock, D.G.; Lane, C.R.; Davidson, A.G.F.; Wilcox, P.G.; Smith, K.D.; Overhage, J.; et al. Innate Immunity Mediated by TLR5 as a Novel Antiinflammatory Target for Cystic Fibrosis Lung Disease. J. Immunol. 2008, 180, 7764–7773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bercusson, A.; Jarvis, G.; Shah, A. CF Fungal Disease in the Age of CFTR Modulators. Mycopathologia 2021, 186, 655–664. [Google Scholar] [CrossRef]
- Boyle, M.P.; Boeck, K.D. A New Era in the Treatment of Cystic Fibrosis: Correction of the Underlying CFTR Defect. Lancet Respir. Med. 2013, 1, 158–163. [Google Scholar] [CrossRef]
- Rang, C.; Kotsinbos, T.; Wilson, J. Overview of CFTR Modulators and Gene Therapy. In Cystic Fibrosis—Heterogeneity and Personalized Treatment; Wat, D., Nazareth, D., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78984-146-6. [Google Scholar]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty Years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef]
- Diekema, D.J.; Messer, S.A.; Hollis, R.J.; Jones, R.N.; Pfaller, M.A. Activities of Caspofungin, Itraconazole, Posaconazole, Ravuconazole, Voriconazole, and Amphotericin B against 448 Recent Clinical Isolates of Filamentous Fungi. J. Clin. Microbiol. 2003, 41, 3623–3626. [Google Scholar] [CrossRef] [Green Version]
- Verweij, P.E.; Dorsthorst, D.T.A.T.; Rijs, A.J.M.M.; Vries-Hospers, H.G.D.; Meis, J.F.G.M. Nationwide Survey of In Vitro Activities of Itraconazole and Voriconazole against Clinical Aspergillus Fumigatus Isolates Cultured between 1945 and 1998. J. Clin. Microbiol. 2002, 40, 2648–2650. [Google Scholar] [CrossRef] [Green Version]
- Saravolatz, L.D.; Johnson, L.B.; Kauffman, C.A. Voriconazole: A New Triazole Antifungal Agent. Clin. Infect. Dis. 2003, 36, 630–637. [Google Scholar] [CrossRef] [Green Version]
- Nagappan, V.; Deresinski, S. Posaconazole: A Broad-Spectrum Triazole Antifungal Agent. Clin. Infect. Dis. 2007, 45, 1610–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, J.C.; Hicks, P.S.; Kurtz, M.B.; Rosen, H.; Schmatz, D.M.; Liberator, P.A.; Douglas, C.M. The Antifungal Echinocandin Caspofungin Acetate Kills Growing Cells of Aspergillus Fumigatus In Vitro. Antimicrob. Agents Chemother. 2002, 46, 3001–3012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denning, D.W.; Marr, K.A.; Lau, W.M.; Facklam, D.P.; Ratanatharathorn, V.; Becker, C.; Ullmann, A.J.; Seibel, N.L.; Flynn, P.M.; van Burik, J.-A.H.; et al. Micafungin (FK463), Alone or in Combination with Other Systemic Antifungal Agents, for the Treatment of Acute Invasive Aspergillosis. J. Infect. 2006, 53, 337–349. [Google Scholar] [CrossRef]
- Vahedi-Shahandashti, R.; Lass-Flörl, C. Novel Antifungal Agents and Their Activity against Aspergillus Species. J. Fungi 2020, 6, 213. [Google Scholar] [CrossRef]
- Lima, S.L.; Colombo, A.L.; Junior, J.N.D.A. Fungal Cell Wall: Emerging Antifungals and Drug Resistance. Front. Microbiol. 2019, 10, 2573. [Google Scholar] [CrossRef] [Green Version]
- Verweij, P.E.; Snelders, E.; Kema, G.H.; Mellado, E.; Melchers, W.J. Azole Resistance in Aspergillus Fumigatus: A Side-Effect of Environmental Fungicide Use? Lancet Infect. Dis. 2009, 9, 789–795. [Google Scholar] [CrossRef]
- Snelders, E.; van der Lee, H.A.L.; Kuijpers, J.; Rijs, A.J.M.M.; Varga, J.; Samson, R.A.; Mellado, E.; Donders, A.R.T.; Melchers, W.J.G.; Verweij, P.E. Emergence of Azole Resistance in Aspergillus Fumigatus and Spread of a Single Resistance Mechanism. PLoS Med. 2008, 5, e219. [Google Scholar] [CrossRef]
- Warn, P.A.; Sharp, A.; Denning, D.W. In Vitro Activity of a New Triazole BAL4815, the Active Component of BAL8557 (the Water-Soluble Prodrug), against Aspergillus spp. J. Antimicrob. Chemoth. 2006, 57, 135–138. [Google Scholar] [CrossRef]
- Pettit, N.N.; Carver, P.L. Isavuconazole. Ann. Pharmacother. 2015, 49, 825–842. [Google Scholar] [CrossRef]
- Ellsworth, M.; Ostrosky-Zeichner, L. Isavuconazole: Mechanism of Action, Clinical Efficacy, and Resistance. J. Fungi 2020, 6, 324. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.J.; Cerar, D.; Anderson, M.J.; Albarrag, A.; Fisher, M.C.; Pasqualotto, A.C.; Laverdiere, M.; Arendrup, M.C.; Perlin, D.S.; Denning, D.W. Frequency and Evolution of Azole Resistance in Aspergillus Fumigatus Associated with Treatment Failure1. Emerg. Infect. Dis. 2009, 15, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Guerra, T.M.; Mellado, E.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. A Point Mutation in the 14α-Sterol Demethylase Gene Cyp51A Contributes to Itraconazole Resistance in Aspergillus Fumigatus. Antimicrob. Agents Chemother. 2003, 47, 1120–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellado, E.; Garcia-Effron, G.; Alcazar-Fuoli, L.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Substitutions at Methionine 220 in the 14α-Sterol Demethylase (Cyp51A) of Aspergillus Fumigatus Are Responsible for Resistance In Vitro to Azole Antifungal Drugs. Antimicrob. Agents Chemother. 2004, 48, 2747–2750. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Jimenez, I.; Lucio, J.; Amich, J.; Cuesta, I.; Arroyo, R.S.; Alcazar-Fuoli, L.; Mellado, E. A Cyp51B Mutation Contributes to Azole Resistance in Aspergillus Fumigatus. J. Fungi 2020, 6, 315. [Google Scholar] [CrossRef]
- Ballard, E.; Weber, J.; Melchers, W.J.G.; Tammireddy, S.; Whitfield, P.D.; Brakhage, A.A.; Brown, A.J.P.; Verweij, P.E.; Warris, A. Recreation of In-Host Acquired Single Nucleotide Polymorphisms by CRISPR-Cas9 Reveals an Uncharacterised Gene Playing a Role in Aspergillus Fumigatus Azole Resistance via a Non-Cyp51A Mediated Resistance Mechanism. Fungal Genet. Biol. 2019, 130, 98–106. [Google Scholar] [CrossRef]
- Sharma, C.; Nelson-Sathi, S.; Singh, A.; Pillai, M.R.; Chowdhary, A. Genomic Perspective of Triazole Resistance in Clinical and Environmental Aspergillus Fumigatus Isolates without Cyp51A Mutations. Fungal Genet. Biol. 2019, 132, 103265. [Google Scholar] [CrossRef]
- Furukawa, T.; van Rhijn, N.; Fraczek, M.; Gsaller, F.; Davies, E.; Carr, P.; Gago, S.; Fortune-Grant, R.; Rahman, S.; Gilsenan, J.M.; et al. The Negative Cofactor 2 Complex Is a Key Regulator of Drug Resistance in Aspergillus Fumigatus. Nat. Commun. 2020, 11, 427. [Google Scholar] [CrossRef] [Green Version]
- Snelders, E.; Karawajczyk, A.; Verhoeven, R.J.A.; Venselaar, H.; Schaftenaar, G.; Verweij, P.E.; Melchers, W.J.G. The Structure–Function Relationship of the Aspergillus Fumigatus Cyp51A L98H Conversion by Site-Directed Mutagenesis: The Mechanism of L98H Azole Resistance. Fungal Genet. Biol. 2011, 48, 1062–1070. [Google Scholar] [CrossRef]
- Lelièvre, L.; Groh, M.; Angebault, C.; Maherault, A.-C.; Didier, E.; Bougnoux, M.-E. Azole Resistant Aspergillus Fumigatus: An Emerging Problem. Méd. Mal. Infect. 2013, 43, 139–145. [Google Scholar] [CrossRef]
- Valsecchi, I.; Mellado, E.; Beau, R.; Raj, S.; Latgé, J.-P. Fitness Studies of Azole-Resistant Strains of Aspergillus Fumigatus. Antimicrob. Agents Chemother. 2015, 59, 7866–7869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natesan, S.K.; Wu, W.; Cutright, J.L.; Chandrasekar, P.H. In Vitro–In Vivo Correlation of Voriconazole Resistance Due to G448S Mutation (Cyp51A Gene) in Aspergillus Fumigatus. Diagn. Microbiol. Infect. Dis. 2012, 74, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.; Abdolrasouli, A.; Dunne, K.; Sewell, T.R.; Zhang, Y.; Ballard, E.; Brackin, A.P.; van Rhijn, N.; Chown, H.; Tsitsopoulou, A.; et al. Population Genomics Confirms Acquisition of Drug-Resistant Aspergillus Fumigatus Infection by Humans from the Environment. Nat. Microbiol. 2022, 7, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Sabol, K.; Gumbo, T. Anidulafungin in the Treatment of Invasive Fungal Infections. Ther. Clin. Risk Manag. 2008, 4, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Hodiamont, C.J.; Dolman, K.M.; Berge, I.J.M.T.; Melchers, W.J.G.; Verweij, P.E.; Pajkrt, D. Multiple-Azole-Resistant Aspergillus Fumigatus Osteomyelitis in a Patient with Chronic Granulomatous Disease Successfully Treated with Long-Term Oral Posaconazole and Surgery. Med. Mycol. 2009, 47, 217–220. [Google Scholar] [CrossRef] [Green Version]
- van der Linden, J.W.M.; Camps, S.M.T.; Kampinga, G.A.; Arends, J.P.A.; Debets-Ossenkopp, Y.J.; Haas, P.J.A.; Rijnders, B.J.A.; Kuijper, E.J.; van Tiel, F.H.; Varga, J.; et al. Aspergillosis Due to Voriconazole Highly Resistant Aspergillus Fumigatus and Recovery of Genetically Related Resistant Isolates From Domiciles. Clin. Infect. Dis. 2013, 57, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Bromley, M.J.; van Muijlwijk, G.; Fraczek, M.G.; Robson, G.; Verweij, P.E.; Denning, D.W.; Bowyer, P. Occurrence of Azole-Resistant Species of Aspergillus in the UK Environment. J. Glob. Antimicrob. Resist. 2014, 2, 276–279. [Google Scholar] [CrossRef]
- Prigitano, A.; Esposto, M.C.; Romanò, L.; Auxilia, F.; Tortorano, A.M. Azole-Resistant Aspergillus Fumigatus in the Italian Environment. J. Glob. Antimicrob. Resist. 2019, 16, 220–224. [Google Scholar] [CrossRef]
- Chowdhary, A.; Kathuria, S.; Xu, J.; Meis, J.F. Emergence of Azole-Resistant Aspergillus Fumigatus Strains Due to Agricultural Azole Use Creates an Increasing Threat to Human Health. PLoS Pathog. 2013, 9, e1003633. [Google Scholar] [CrossRef]
- Schoustra, S.E.; Debets, A.J.M.; Rijs, A.J.M.M.; Zhang, J.; Snelders, E.; Leendertse, P.C.; Melchers, W.J.G.; Rietveld, A.G.; Zwaan, B.J.; Verweij, P.E. Environmental Hotspots for Azole Resistance Selection of Aspergillus Fumigatus, the Netherlands. Emerg. Infect. Dis. 2019, 25, 1347–1353. [Google Scholar] [CrossRef] [Green Version]
- Snelders, E.; Camps, S.M.T.; Karawajczyk, A.; Schaftenaar, G.; Kema, G.H.J.; van der Lee, H.A.; Klaassen, C.H.; Melchers, W.J.G.; Verweij, P.E. Triazole Fungicides Can Induce Cross-Resistance to Medical Triazoles in Aspergillus Fumigatus. PLoS ONE 2012, 7, e31801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, S.; Chazli, Y.E.; Babu, A.F.; Coste, A.T. Azole Resistance in Aspergillus Fumigatus: A Consequence of Antifungal Use in Agriculture? Front. Microbiol. 2017, 8, 1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, C.; Hagen, F.; Moroti, R.; Meis, J.F.; Chowdhary, A. Triazole-Resistant Aspergillus Fumigatus Harbouring G54 Mutation: Is It de Novo or Environmentally Acquired? J. Glob. Antimicrob. Resist. 2015, 3, 69–74. [Google Scholar] [CrossRef]
- Vaezi, A.; Fakhim, H.; Javidnia, J.; Khodavaisy, S.; Abtahian, Z.; Vojoodi, M.; Nourbakhsh, F.; Badali, H. Pesticide Behavior in Paddy Fields and Development of Azole-Resistant Aspergillus Fumigatus: Should We Be Concerned? J. Mycol. Méd. 2018, 28, 59–64. [Google Scholar] [CrossRef]
- Zhang, J.; Snelders, E.; Zwaan, B.J.; Schoustra, S.E.; Meis, J.F.; van Dijk, K.; Hagen, F.; van der Beek, M.T.; Kampinga, G.A.; Zoll, J.; et al. A Novel Environmental Azole Resistance Mutation in Aspergillus Fumigatus and a Possible Role of Sexual Reproduction in Its Emergence. Mbio 2017, 8, e00791-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballard, E.; Melchers, W.J.G.; Zoll, J.; Brown, A.J.P.; Verweij, P.E.; Warris, A. In-Host Microevolution of Aspergillus Fumigatus: A Phenotypic and Genotypic Analysis. Fungal Genet. Biol. 2018, 113, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zoll, J.; Engel, T.; van den Heuvel, J.; Verweij, P.E.; Debets, A.J.M. The Medical Triazole Voriconazole Can Select for Tandem Repeat Variations in Azole-Resistant Aspergillus Fumigatus Harboring TR34/L98H Via Asexual Reproduction. J. Fungi 2020, 6, 277. [Google Scholar] [CrossRef]
- van der Linden, J.W.M.; Snelders, E.; Kampinga, G.A.; Rijnders, B.J.A.; Mattsson, E.; Debets-Ossenkopp, Y.J.; Tiel, F.H.V.; Melchers, W.J.G.; Verweij, P.E.; van der Linden, J.W.M.; et al. Clinical Implications of Azole Resistance in Aspergillus Fumigatus, the Netherlands, 2007–2009. Emerg. Infect. Dis. 2011, 17, 1846–1854. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.-C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and Related Genera (Eurotiales): An Overview of Families, Genera, Subgenera, Sections, Series and Species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef]
- Al-Bader, N.; Vanier, G.; Liu, H.; Gravelat, F.N.; Urb, M.; Hoareau, C.M.-Q.; Campoli, P.; Chabot, J.; Filler, S.G.; Sheppard, D.C. Role of Trehalose Biosynthesis in Aspergillus Fumigatus Development, Stress Response, and Virulence. Infect. Immunol. 2010, 78, 3007–3018. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, T.T.; Wösten, H.A.B.; Dijksterhuis, J. Chapter Two Fungal Spores for Dispersion in Space and Time. Adv. Appl. Microbiol. 2013, 85, 43–91. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, S.; Chaveroche, M.-K.; van Dijck, P.; de Vries, R.; Ruijter, G.; Thevelein, J.; d’Enfert, C. Trehalose Is Required for the Acquisition of Tolerance to a Variety of Stresses in the Filamentous Fungus Aspergillus Nidulans. Microbiology 2001, 147, 1851–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.E.; Celia, B.N.; Bensasson, D.; Momany, M. Sporulation Environment Drives Phenotypic Variation in the Pathogen Aspergillus Fumigatus. G3 Genes Genomes Genet. 2021, 11, jkab208. [Google Scholar] [CrossRef]
- Wang, F.; Sethiya, P.; Hu, X.; Guo, S.; Chen, Y.; Li, A.; Tan, K.; Wong, K.H. Transcription in Fungal Conidia before Dormancy Produces Phenotypically Variable Conidia That Maximize Survival in Different Environments. Nat. Microbiol. 2021, 6, 1066–1081. [Google Scholar] [CrossRef]
- Danion, F.; van Rhijn, N.; Dufour, A.C.; Legendre, R.; Sismeiro, O.; Varet, H.; Olivo-Marin, J.-C.; Mouyna, I.; Chamilos, G.; Bromley, M.; et al. Aspergillus Fumigatus, One Uninucleate Species with Disparate Offspring. J. Fungi 2021, 7, 30. [Google Scholar] [CrossRef]
- Bleichrodt, R.-J.; Foster, P.; Howell, G.; Latgé, J.-P.; Read, N.D. Cell Wall Composition Heterogeneity between Single Cells in Aspergillus Fumigatus Leads to Heterogeneous Behavior during Antifungal Treatment and Phagocytosis. Mbio 2020, 11, e03015-19. [Google Scholar] [CrossRef]
- Margalit, A.; Kavanagh, K. The Innate Immune Response to Aspergillus Fumigatus at the Alveolar Surface. FEMS Microbiol. Rev. 2015, 39, 670–687. [Google Scholar] [CrossRef] [Green Version]
- Van Leeuwen, M.R.; Krijgsheld, P.; Bleichrodt, R.; Menke, H.; Stam, H.; Stark, J.; Wösten, H.A.B.; Dijksterhuis, J. Germination of Conidia of Aspergillus Niger Is Accompanied by Major Changes in RNA Profiles. Stud. Mycol. 2013, 74, 59–70. [Google Scholar] [CrossRef]
- Bonazzi, D.; Julien, J.-D.; Romao, M.; Seddiki, R.; Piel, M.; Boudaoud, A.; Minc, N. Symmetry Breaking in Spore Germination Relies on an Interplay between Polar Cap Stability and Spore Wall Mechanics. Dev. Cell 2014, 28, 534–546. [Google Scholar] [CrossRef] [Green Version]
- Fredborg, M.; Andersen, K.R.; Jørgensen, E.; Droce, A.; Olesen, T.; Jensen, B.B.; Rosenvinge, F.S.; Sondergaard, T.E. Real-Time Optical Antimicrobial Susceptibility Testing. J. Clin. Microbiol. 2013, 51, 2047–2053. [Google Scholar] [CrossRef] [Green Version]
- Baltussen, T.J.H.; Coolen, J.P.M.; Zoll, J.; Verweij, P.E.; Melchers, W.J.G. Gene Co-Expression Analysis Identifies Gene Clusters Associated with Isotropic and Polarized Growth in Aspergillus Fumigatus Conidia. Fungal Genet. Biol. 2018, 116, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Novodvorska, M.; Hayer, K.; Pullan, S.T.; Wilson, R.; Blythe, M.J.; Stam, H.; Stratford, M.; Archer, D.B. Transcriptional Landscape of Aspergillus Nigerat Breaking of Conidial Dormancy Revealed by RNA-Sequencing. BMC Genom. 2013, 14, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Enfert, C.; Fontaine, T. Molecular Characterization of the Aspergillus Nidulans TreA Gene Encoding an Acid Trehalase Required for Growth on Trehalose. Mol. Microbiol. 1997, 24, 203–216. [Google Scholar] [CrossRef] [PubMed]
- D’Enfert, C.; Bonini, B.M.; Zapella, P.D.A.; Fontaine, T.; Silva, A.M.D.; Terenzi, H.F. Neutral Trehalases Catalyse Intracellular Trehalose Breakdown in the Filamentous Fungi Aspergillus Nidulans and Neurospora Crassa. Mol. Microbiol. 1999, 32, 471–483. [Google Scholar] [CrossRef]
- Hagiwara, D.; Sakai, K.; Suzuki, S.; Umemura, M.; Nogawa, T.; Kato, N.; Osada, H.; Watanabe, A.; Kawamoto, S.; Gonoi, T.; et al. Temperature during Conidiation Affects Stress Tolerance, Pigmentation, and Trypacidin Accumulation in the Conidia of the Airborne Pathogen Aspergillus Fumigatus. PLoS ONE 2017, 12, e0177050. [Google Scholar] [CrossRef]
- Hayer, K.; Stratford, M.; Archer, D.B. Germination of Aspergillus Niger Conidia Is Triggered by Nitrogen Compounds Related to L-Amino Acids. Appl. Environ. Microb. 2014, 80, 6046–6053. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.-H.; Chae, K.-S.; Han, D.-M.; Jahng, K.-Y. The GanB Gα-Protein Negatively Regulates Asexual Sporulation and Plays a Positive Role in Conidial Germination in Aspergillus Nidulans. Genetics 2004, 167, 1305–1315. [Google Scholar] [CrossRef] [Green Version]
- Liebmann, B.; Gattung, S.; Jahn, B.; Brakhage, A.A. CAMP Signaling in Aspergillus Fumigatus Is Involved in the Regulation of the Virulence Gene PksP and in Defense against Killing by Macrophages. Mol. Genet. Genom. 2003, 269, 420–435. [Google Scholar] [CrossRef]
- Shin, K.-S.; Kwon, N.-J.; Yu, J.-H. Gβγ-Mediated Growth and Developmental Control in Aspergillus Fumigatus. Curr. Genet. 2009, 55, 631. [Google Scholar] [CrossRef]
- Kerr, S.C.; Fischer, G.J.; Sinha, M.; McCabe, O.; Palmer, J.M.; Choera, T.; Lim, F.Y.; Wimmerova, M.; Carrington, S.D.; Yuan, S.; et al. FleA Expression in Aspergillus Fumigatus Is Recognized by Fucosylated Structures on Mucins and Macrophages to Prevent Lung Infection. PLoS Pathog. 2016, 12, e1005555. [Google Scholar] [CrossRef] [Green Version]
- Houser, J.; Komarek, J.; Kostlanova, N.; Cioci, G.; Varrot, A.; Kerr, S.C.; Lahmann, M.; Balloy, V.; Fahy, J.V.; Chignard, M.; et al. A Soluble Fucose-Specific Lectin from Aspergillus Fumigatus Conidia—Structure, Specificity and Possible Role in Fungal Pathogenicity. PLoS ONE 2013, 8, e83077. [Google Scholar] [CrossRef]
- Sakai, K.; Hiemori, K.; Tateno, H.; Hirabayashi, J.; Gonoi, T. Fucose-Specific Lectin of Aspergillus Fumigatus: Binding Properties and Effects on Immune Response Stimulation. Med. Mycol. 2018, 57, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Marti, L.; Varrot, A.; Guillot, L.; Guitard, J.; Hennequin, C.; Imberty, A.; Corvol, H.; Chignard, M.; Balloy, V. Human Bronchial Epithelial Cells Inhibit Aspergillus Fumigatus Germination of Extracellular Conidia via FleA Recognition. Sci. Rep. 2018, 8, 15699. [Google Scholar] [CrossRef]
- Lehot, V.; Brissonnet, Y.; Dussouy, C.; Brument, S.; Cabanettes, A.; Gillon, E.; Deniaud, D.; Varrot, A.; Pape, P.L.; Gouin, S.G. Multivalent Fucosides with Nanomolar Affinity for the Aspergillus Fumigatus Lectin FleA Prevent Spore Adhesion to Pneumocytes. Chem. Eur. J. 2018, 24, 19243–19249. [Google Scholar] [CrossRef]
- Dussouy, C.; Lalys, P.-A.; Cabanettes, A.; Lehot, V.; Deniaud, D.; Gillon, E.; Balloy, V.; Varrot, A.; Gouin, S.G. Hexavalent Thiofucosides to Probe the Role of the Aspergillus Fumigatus Lectin FleA in Fungal Pathogenicity. Org. Biomol. Chem. 2021, 19, 3234–3240. [Google Scholar] [CrossRef]
- Suh, M.-J.; Fedorova, N.D.; Cagas, S.E.; Hastings, S.; Fleischmann, R.D.; Peterson, S.N.; Perlin, D.S.; Nierman, W.C.; Pieper, R.; Momany, M. Development Stage-Specific Proteomic Profiling Uncovers Small, Lineage Specific Proteins Most Abundant in the Aspergillus Fumigatus Conidial Proteome. Proteome Sci. 2012, 10, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, M.C.; Fabri, J.H.T.M.; de Godoy, K.F.; de Castro, P.A.; Hori, J.I.; da Cunha, A.F.; Arentshorst, M.; Ram, A.F.J.; van den Hondel, C.A.M.J.J.; Goldman, G.H.; et al. Aspergillus Fumigatus MADS-Box Transcription Factor RlmA Is Required for Regulation of the Cell Wall Integrity and Virulence. G3 Genes Genomes Genet. 2016, 6, 2983–3002. [Google Scholar] [CrossRef] [Green Version]
- Gago, S.; Overton, N.L.D.; Ben-Ghazzi, N.; Novak-Frazer, L.; Read, N.D.; Denning, D.W.; Bowyer, P. Lung Colonization by Aspergillus Fumigatus Is Controlled by ZNF77. Nat. Commun. 2018, 9, 3835. [Google Scholar] [CrossRef]
- Doni, A.; Parente, R.; Laface, I.; Magrini, E.; Cunha, C.; Colombo, F.S.; Lacerda, J.F.; Campos, A.; Mapelli, S.N.; Petroni, F.; et al. Serum Amyloid P Component Is an Essential Element of Resistance against Aspergillus Fumigatus. Nat. Commun. 2021, 12, 3739. [Google Scholar] [CrossRef]
- Clark, H.R.; Powell, A.B.; Simmons, K.A.; Ayubi, T.; Kale, S.D. Endocytic Markers Associated with the Internalization and Processing of Aspergillus Fumigatus Conidia by BEAS-2B Cells. Msphere 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- De Assis, L.J.; Ries, L.N.A.; Savoldi, M.; Dinamarco, T.M.; Goldman, G.H.; Brown, N.A. Multiple Phosphatases Regulate Carbon Source-Dependent Germination and Primary Metabolism in Aspergillus Nidulans. G3 Genes Genomes Genet. 2015, 5, 857–872. [Google Scholar] [CrossRef] [Green Version]
- Rocha, M.C.; Fabri, J.H.T.M.; Simões, I.T.; Silva-Rocha, R.; Hagiwara, D.; da Cunha, A.F.; Goldman, G.H.; Cánovas, D.; Malavazi, I. The Cell Wall Integrity Pathway Contributes to the Early Stages of Aspergillus Fumigatus Asexual Development. Appl. Environ. Microb. 2020, 86, e02347-19. [Google Scholar] [CrossRef] [PubMed]
- Teutschbein, J.; Albrecht, D.; Pötsch, M.; Guthke, R.; Aimanianda, V.; Clavaud, C.; Latgé, J.-P.; Brakhage, A.A.; Kniemeyer, O. Proteome Profiling and Functional Classification of Intracellular Proteins from Conidia of the Human-Pathogenic Mold Aspergillus Fumigatus. J. Proteome Res. 2010, 9, 3427–3442. [Google Scholar] [CrossRef] [PubMed]
- Bayram, Ö.S.; Latgé, J.P.; Bayram, Ö. MybA, a New Player Driving Survival of the Conidium of the Human Pathogen Aspergillus Fumigatus. Curr. Genet. 2018, 64, 141–146. [Google Scholar] [CrossRef]
- Campione, E.; Gaziano, R.; Doldo, E.; Marino, D.; Falconi, M.; Iacovelli, F.; Tagliaferri, D.; Pacello, L.; Bianchi, L.; Lanna, C.; et al. Antifungal Effect of All-Trans Retinoic Acid against Aspergillus Fumigatus In Vitro and in a Pulmonary Aspergillosis In Vivo Model. Antimicrob. Agents Chemother. 2021, 65, e01874-20. [Google Scholar] [CrossRef]
- Cosio, T.; Gaziano, R.; Zuccari, G.; Costanza, G.; Grelli, S.; Francesco, P.D.; Bianchi, L.; Campione, E. Retinoids in Fungal Infections: From Bench to Bedside. Pharmaceuticals 2021, 14, 962. [Google Scholar] [CrossRef]
- Lamoth, F.; Juvvadi, P.R.; Fortwendel, J.R.; Steinbach, W.J. Heat Shock Protein 90 Is Required for Conidiation and Cell Wall Integrity in Aspergillus Fumigatus. Eukaryot. Cell 2012, 11, 1324–1332. [Google Scholar] [CrossRef] [Green Version]
- Irmer, H.; Tarazona, S.; Sasse, C.; Olbermann, P.; Loeffler, J.; Krappmann, S.; Conesa, A.; Braus, G.H. RNAseq Analysis of Aspergillus Fumigatus in Blood Reveals a Just Wait and See Resting Stage Behavior. BMC Genom. 2015, 16, 640. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Li, S.; Kaminskyj, S. An Amylase-Like Protein, AmyD, Is the Major Negative Regulator for α-Glucan Synthesis in Aspergillus Nidulans during the Asexual Life Cycle. Int. J. Mol. Sci. 2017, 18, 695. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Alarcón, D.; Balloy, V.; Bouchara, J.-P.; Pieters, R.J.; Varrot, A. Biochemical and Structural Studies of Target Lectin SapL1 from the Emerging Opportunistic Microfungus Scedosporium Apiospermum. Sci. Rep. 2021, 11, 16109. [Google Scholar] [CrossRef]
- Escobar, N.; Valdes, I.D.; Keizer, E.M.; Ordonez, S.R.; Ohm, R.A.; Wösten, H.A.B.; de Cock, H. Expression Profile Analysis Reveals That Aspergillus Fumigatus but Not Aspergillus Niger Makes Type II Epithelial Lung Cells Less Immunological Alert. BMC Genom. 2018, 19, 534. [Google Scholar] [CrossRef]
- Son, Y.-E.; Park, H.-S. Genome Wide Analysis Reveals the Role of VadA in Stress Response, Germination, and Sterigmatocystin Production in Aspergillus Nidulans Conidia. Microorganisms 2020, 8, 1319. [Google Scholar] [CrossRef]
- Willger, S.D.; Puttikamonkul, S.; Kim, K.-H.; Burritt, J.B.; Grahl, N.; Metzler, L.J.; Barbuch, R.; Bard, M.; Lawrence, C.B.; Cramer, R.A. A Sterol-Regulatory Element Binding Protein Is Required for Cell Polarity, Hypoxia Adaptation, Azole Drug Resistance, and Virulence in Aspergillus Fumigatus. PLoS Pathog. 2008, 4, e1000200. [Google Scholar] [CrossRef] [Green Version]
- Oda, K.; Bignell, E.; Kang, S.E.; Momany, M. Transcript Levels of the Aspergillus Fumigatus Cdc42 Module, Polarisome, and Septin Genes Show Little Change from Dormancy to Polarity Establishment. Med. Mycol. 2016, 55, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Chabane, S.; Sarfati, J.; Ibrahim-Granet, O.; Du, C.; Schmidt, C.; Mouyna, I.; Prevost, M.-C.; Calderone, R.; Latgé, J.-P. Glycosylphosphatidylinositol-Anchored Ecm33p Influences Conidial Cell Wall Biosynthesis in Aspergillus Fumigatus. Appl. Environ. Microb. 2006, 72, 3259–3267. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.P.; Horta, M.A.C.; Goldman, G.H. Genetic Interactions Between Aspergillus Fumigatus Basic Leucine Zipper (BZIP) Transcription Factors AtfA, AtfB, AtfC, and AtfD. Front. Fungal Biol. 2021, 2, 632048. [Google Scholar] [CrossRef]
- Mouyna, I.; Dellière, S.; Beauvais, A.; Gravelat, F.; Snarr, B.; Lehoux, M.; Zacharias, C.; Sun, Y.; Carrion, S.D.J.; Pearlman, E.; et al. What Are the Functions of Chitin Deacetylases in Aspergillus Fumigatus? Front. Cell Infect. Microbiol. 2020, 10, 28. [Google Scholar] [CrossRef]
- Levery, S.B.; Momany, M.; Lindsey, R.; Toledo, M.S.; Shayman, J.A.; Fuller, M.; Brooks, K.; Doong, R.L.; Straus, A.H.; Takahashi, H.K. Disruption of the Glucosylceramide Biosynthetic Pathway in Aspergillus Nidulans and Aspergillus Fumigatus by Inhibitors of UDP-Glc:Ceramide Glucosyltransferase Strongly Affects Spore Germination, Cell Cycle, and Hyphal Growth. FEBS Lett. 2002, 525, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Bao, D.; Yuen, G.; Harris, S.D.; Calvo, A.M. BasA Regulates Cell Wall Organization and Asexual/Sexual Sporulation Ratio in Aspergillus Nidulans. Genetics 2007, 176, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Du, L.; Yuen, G.; Harris, S.D. Distinct Ceramide Synthases Regulate Polarized Growth in the Filamentous Fungus Aspergillus Nidulans. Mol. Biol. Cell 2006, 17, 1218–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gsaller, F.; Hortschansky, P.; Furukawa, T.; Carr, P.D.; Rash, B.; Capilla, J.; Müller, C.; Bracher, F.; Bowyer, P.; Haas, H.; et al. Sterol Biosynthesis and Azole Tolerance Is Governed by the Opposing Actions of SrbA and the CCAAT Binding Complex. PLoS Pathog. 2016, 12, e1005775. [Google Scholar] [CrossRef]
- Chung, D.; Barker, B.M.; Carey, C.C.; Merriman, B.; Werner, E.R.; Lechner, B.E.; Dhingra, S.; Cheng, C.; Xu, W.; Blosser, S.J.; et al. ChIP-Seq and In Vivo Transcriptome Analyses of the Aspergillus Fumigatus SREBP SrbA Reveals a New Regulator of the Fungal Hypoxia Response and Virulence. PLoS Pathog. 2014, 10, e1004487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gastebois, A.; Fontaine, T.; Latgé, J.-P.; Mouyna, I. β(1-3)Glucanosyltransferase Gel4p Is Essential for Aspergillus Fumigatus. Eukaryot. Cell 2010, 9, 1294–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouyna, I.; Fontaine, T.; Vai, M.; Monod, M.; Fonzi, W.A.; Diaquin, M.; Popolo, L.; Hartland, R.P.; Latgé, J.-P. Glycosylphosphatidylinositol-Anchored Glucanosyltransferases Play an Active Role in the Biosynthesis of the Fungal Cell Wall. J. Biol. Chem. 2000, 275, 14882–14889. [Google Scholar] [CrossRef] [Green Version]
- Pongpom, M.; Liu, H.; Xu, W.; Snarr, B.D.; Sheppard, D.C.; Mitchell, A.P.; Filler, S.G. Divergent Targets of Aspergillus Fumigatus AcuK and AcuM Transcription Factors during Growth In Vitro versus Invasive Disease. Infect. Immun. 2015, 83, 923–933. [Google Scholar] [CrossRef] [Green Version]
- Van Leeuwe, T.M.; Wattjes, J.; Niehues, A.; Forn-Cuní, G.; Geoffrion, N.; Mélida, H.; Arentshorst, M.; Molina, A.; Tsang, A.; Meijer, A.H.; et al. A Seven-Membered Cell Wall Related Transglycosylase Gene Family in Aspergillus Niger Is Relevant for Cell Wall Integrity in Cell Wall Mutants with Reduced α-Glucan or Galactomannan. Cell Surf. 2020, 6, 100039. [Google Scholar] [CrossRef]
- Ries, L.N.A.; de Castro, P.A.; Silva, L.P.; Valero, C.; Reis, T.F.D.; Saborano, R.; Duarte, I.F.; Persinoti, G.F.; Steenwyk, J.L.; Rokas, A.; et al. Aspergillus Fumigatus Acetate Utilisation Impacts Virulence Traits and Pathogenicity. bioRxiv 2021, 12, e01682-21. [Google Scholar] [CrossRef]
- Son, S.-H.; Lee, M.-K.; Son, Y.-E.; Park, H.-S. HbxB Is a Key Regulator for Stress Response and β-Glucan Biogenesis in Aspergillus Nidulans. Microorganisms 2021, 9, 144. [Google Scholar] [CrossRef]
- Valsecchi, I.; Sarikaya-Bayram, Ö.; Hoi, J.W.S.; Muszkieta, L.; Gibbons, J.; Prevost, M.; Mallet, A.; Krijnse-Locker, J.; Ibrahim-Granet, O.; Mouyna, I.; et al. MybA, a Transcription Factor Involved in Conidiation and Conidial Viability of the Human Pathogen Aspergillus Fumigatus. Mol. Microbiol. 2017, 105, 880–900. [Google Scholar] [CrossRef] [Green Version]
- Perfect, J.R.; Tenor, J.L.; Miao, Y.; Brennan, R.G. Trehalose Pathway as an Antifungal Target. Virulence 2016, 8, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Svanström, Å.; Melin, P. Intracellular Trehalase Activity Is Required for Development, Germination and Heat-Stress Resistance of Aspergillus Niger Conidia. Res. Microbiol. 2013, 164, 91–99. [Google Scholar] [CrossRef]
- Kroll, K.; Pähtz, V.; Hillmann, F.; Vaknin, Y.; Schmidt-Heck, W.; Roth, M.; Jacobsen, I.D.; Osherov, N.; Brakhage, A.A.; Kniemeyer, O. Identification of Hypoxia-Inducible Target Genes of Aspergillus Fumigatus by Transcriptome Analysis Reveals Cellular Respiration as an Important Contributor to Hypoxic Survival. Eukaryot. Cell 2014, 13, 1241–1253. [Google Scholar] [CrossRef] [Green Version]
- Gurgel, I.L.D.S.; Jorge, K.T.D.O.S.; Malacco, N.L.S.D.O.; Souza, J.A.M.; Rocha, M.C.; Fernandes, M.F.; Martins, F.R.B.; Malavazi, I.; Teixeira, M.M.; Soriani, F.M. The Aspergillus Fumigatus Mucin MsbA Regulates the Cell Wall Integrity Pathway and Controls Recognition of the Fungus by the Immune System. Msphere 2019, 4, e00350-19. [Google Scholar] [CrossRef] [Green Version]
- Donadieu, J.; Fenneteau, O.; Beaupain, B.; Mahlaoui, N.; Chantelot, C.B. Congenital Neutropenia: Diagnosis, Molecular Bases and Patient Management. Orphanet. J. Rare Dis. 2011, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Liyanage, G.; Gonapaladeniya, M.; Dissanayake, T. Invasive Candidiasis Associated with Adenovirus Pneumonia. Case Rep. Pediatrics 2021, 2021, 9905474. [Google Scholar] [CrossRef]
- Levine, A.M.; Karim, R.; Mack, W.; Gravink, D.J.; Anastos, K.; Young, M.; Cohen, M.; Newman, M.; Augenbraun, M.; Gange, S.; et al. Neutropenia in Human Immunodeficiency Virus Infection: Data From the Women’s Interagency HIV Study. Arch. Intern. Med. 2006, 166, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Moses, A.; Nelson, J.; Bagby, G.C. The Influence of Human Immunodeficiency Virus-1 on Hematopoiesis. Blood 1998, 91, 1479–1495. [Google Scholar] [CrossRef] [Green Version]
- Lemieux, P.; Grégoire, J.-P.; Thibeault, R.; Bergeron, L. Higher Risk of Neutropenia Associated With Piperacillin-Tazobactam Compared With Ticarcillin-Clavulanate in Children. Clin. Infect. Dis. 2015, 60, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Andrès, E.; Villalba, N.L.; Zulfiqar, A.-A.; Serraj, K.; Mourot-Cottet, R.; Gottenberg, J.-E. State of Art of Idiosyncratic Drug-Induced Neutropenia or Agranulocytosis, with a Focus on Biotherapies. J Clin Med. 2019, 8, 1351. [Google Scholar] [CrossRef] [Green Version]
- Lipton, A. Chronic Idiopathic Neutropenia: Treatment With Corticosteroids and Mercaptopurine. Arch. Intern. Med. 1969, 123, 694–700. [Google Scholar] [CrossRef]
- Uys, A.; Rapoport, B.L.; Anderson, R. Febrile Neutropenia: A Prospective Study to Validate the Multinational Association of Supportive Care of Cancer (MASCC) Risk-Index Score. Support. Care Cancer 2004, 12, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Scott, S. Identification of Cancer Patients at High Risk of Febrile Neutropenia. Am. J. Health-Syst. Pharm. 2002, 59, S16–S19. [Google Scholar] [CrossRef] [PubMed]
- Rivera, E.; Erder, M.H.; Fridman, M.; Frye, D.; Hortobagyi, G.N. First-Cycle Absolute Neutrophil Count Can Be Used to Improve Chemotherapy-Dose Delivery and Reduce the Risk of Febrile Neutropenia in Patients Receiving Adjuvant Therapy: A Validation Study. Breast Cancer Res. 2003, 5, R114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delsing, C.E.; Gresnigt, M.S.; Leentjens, J.; Preijers, F.; Frager, F.A.; Kox, M.; Monneret, G.; Venet, F.; Bleeker-Rovers, C.P.; van de Veerdonk, F.L.; et al. Interferon-Gamma as Adjunctive Immunotherapy for Invasive Fungal Infections: A Case Series. BMC Infect. Dis 2014, 14, 166. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.H.T.; Maher, S.G.; Young, H.A. Clinical Use of Interferon-γ. Ann. N. Y. Acad. Sci. 2009, 1182, 69–79. [Google Scholar] [CrossRef]
- O’Brien, T.J.; Figueroa, W.; Welch, M. Decreased Efficacy of Antimicrobial Agents in a Polymicrobial Environment. ISME J. 2022, 16, 1694–1704. [Google Scholar] [CrossRef]
- Buil, J.B.; Meijer, E.F.J.; Denning, D.W.; Verweij, P.E.; Meis, J.F. Burden of Serious Fungal Infections in the Netherlands. Mycoses 2020, 63, 625–631. [Google Scholar] [CrossRef]
- Cortez, K.J.; Roilides, E.; Quiroz-Telles, F.; Meletiadis, J.; Antachopoulos, C.; Knudsen, T.; Buchanan, W.; Milanovich, J.; Sutton, D.A.; Fothergill, A.; et al. Infections Caused by Scedosporium spp. Clin. Microbiol. Rev. 2008, 21, 157–197. [Google Scholar] [CrossRef] [Green Version]
- Latif, A.-L.; Newcombe, A.; Li, S.; Gilroy, K.; Robertson, N.A.; Lei, X.; Stewart, H.J.S.; Cole, J.; Terradas, M.T.; Rishi, L.; et al. BRD4-Mediated Repression of P53 Is a Target for Combination Therapy in AML. Nat. Commun. 2021, 12, 241. [Google Scholar] [CrossRef]
- Nishimura, R.; Okumura, Y.; Arima, N. Trastuzumab Monotherapy versus Combination Therapy for Treating Recurrent Breast Cancer: Time to Progression and Survival. Breast Cancer 2008, 15, 57–64. [Google Scholar] [CrossRef]
- Wang, J.; Su, S.; Li, J.; Li, Y. Efficacy and Safety of Camrelizumab Monotherapy and Combination Therapy for Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 695512. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Wolfensberger, A.; Nemeth, J.; Schreiber, P.W.; Sax, H.; Kuster, S.P. Monotherapy versus Combination Therapy for Multidrug-Resistant Gram-Negative Infections: Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 15290. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.E.; Crawford, L.C.; Halliday, C.L. Antifungal Susceptibility Testing and Identification. Infect. Dis. Clin. N. Am. 2021, 35, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Henneberg, S.; Hasenberg, A.; Maurer, A.; Neumann, F.; Bornemann, L.; Gonzalez-Menendez, I.; Kraus, A.; Hasenberg, M.; Thornton, C.R.; Pichler, B.J.; et al. Antibody-Guided In Vivo Imaging of Aspergillus Fumigatus Lung Infections during Antifungal Azole Treatment. Nat. Commun. 2021, 12, 1707. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein–Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2018, 47, gky1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Germination Morphotype to Disrupt | Potential Target | Description | Which Aspergillus | Source |
|---|---|---|---|---|
| Dormant conidia | Arp1, Arp2, Ayg1 | Associated with melanin biosynthesis (black-cluster Baltussen et al., 2018). Expression was found to be exclusively high in dormant conidia. | A. fumigatus | [173,195] |
| Aspf3, Aspf8 | Cell surface-associated proteins can be recognized as allergen by host’s immune system. | A. fumigatus | [97] | |
| AtfA | Key transcription factor present in dormant conidia that negatively regulates calA and calB, which are involved in breaking of dormancy. | A. fumigatus | [97,196] | |
| CatA, ConJ, Fhk1 | Genes that are upregulated in an AtfA-dependent manner. CatA is a spore-specific catalase. ConJ has an unknown function in A. fumigatus. Fhk1 is a transcription factor that regulates the CLB2 cluster of genes in the G2/M phase of the cell cycle, associated with cell growth, mitosis, and cytokinesis. | A. fumigatus | [97] | |
| CatA, Cat2, Cat3 | Catalases that protect dormant conidia against oxidative stress. | A. fumigatus | [97,195] | |
| CcpA | Associated with stress resistance in vitro with cells. | A. fumigatus | [83] | |
| Cyp4 | Peptidyl-prolyl cis-trans isomerase. | A. fumigatus | [83] | |
| DprA, DprB, DprC | Dehydrin-like proteins involved in stress–response of dormant conidia, upregulated in an AtfA-dependent manner. | A. fumigatus | [83] | |
| Hsp90 | Heat-shock protein, associated with temperature stress. | A. fumigatus | [83,166,197,198,199] | |
| RpL3 | Ribosomal protein L3. | A. fumigatus | [83] | |
| Involved in alcohol fermentation (pyruvate decarboxylase and alcohol dehydrogenase). | A. fumigatus | [174,195] | ||
| Breaking of Dormancy | Ace2 | Transcription factor for Swi5, regulates germination, pigment production, and virulence. Tightly regulated, upregulated at t = 0.5 h, downregulated at t = 2.5 h. | A. fumigatus | [200] |
| AmyD | Key regulator associated with α-glucan synthesis and cell wall remodeling. | A. nidulans | [201] | |
| AreA, NirA | Transcription activators that respond to nitrogen. Found to be germination triggers and for nitrogen uptake | A. fumigatus | [68,178] | |
| CalA, CalB | Thaumatin-like protein, associated with triggering breaking of dormancy. Negatively regulated by AtfA. | A. fumigatus | [97] | |
| CreA (An02g03830), (An02g03540) | Fermentation/ Glycolysis: creA is a catabolite repressor. | [174] | ||
| FleA | Recognizes and binds receptors, plays a role in attachment/adhesion to epithelial cells, as well as recognition by host’s immune system. | A. fumigatus and S. apiospermum (as SapL1) | [182,183,184,185,186,187,202] | |
| PmaA, (An11g04370), (An01g10190), (An04g02550), (An08g08720) | Mitochondria/Respiration. | A. fumigatus | [174] | |
| -Translation initiation factor CpcC -Transcription factor CpcA -Neutral amino acid transporters (An16g05880, An04g09420, An17g00860) -Transporter proteins (An11g00450), (An03g05590) | Nitrogen metabolism. | A. fumigatus | [174] | |
| TCA cycle. | A. fumigatus | [174] | ||
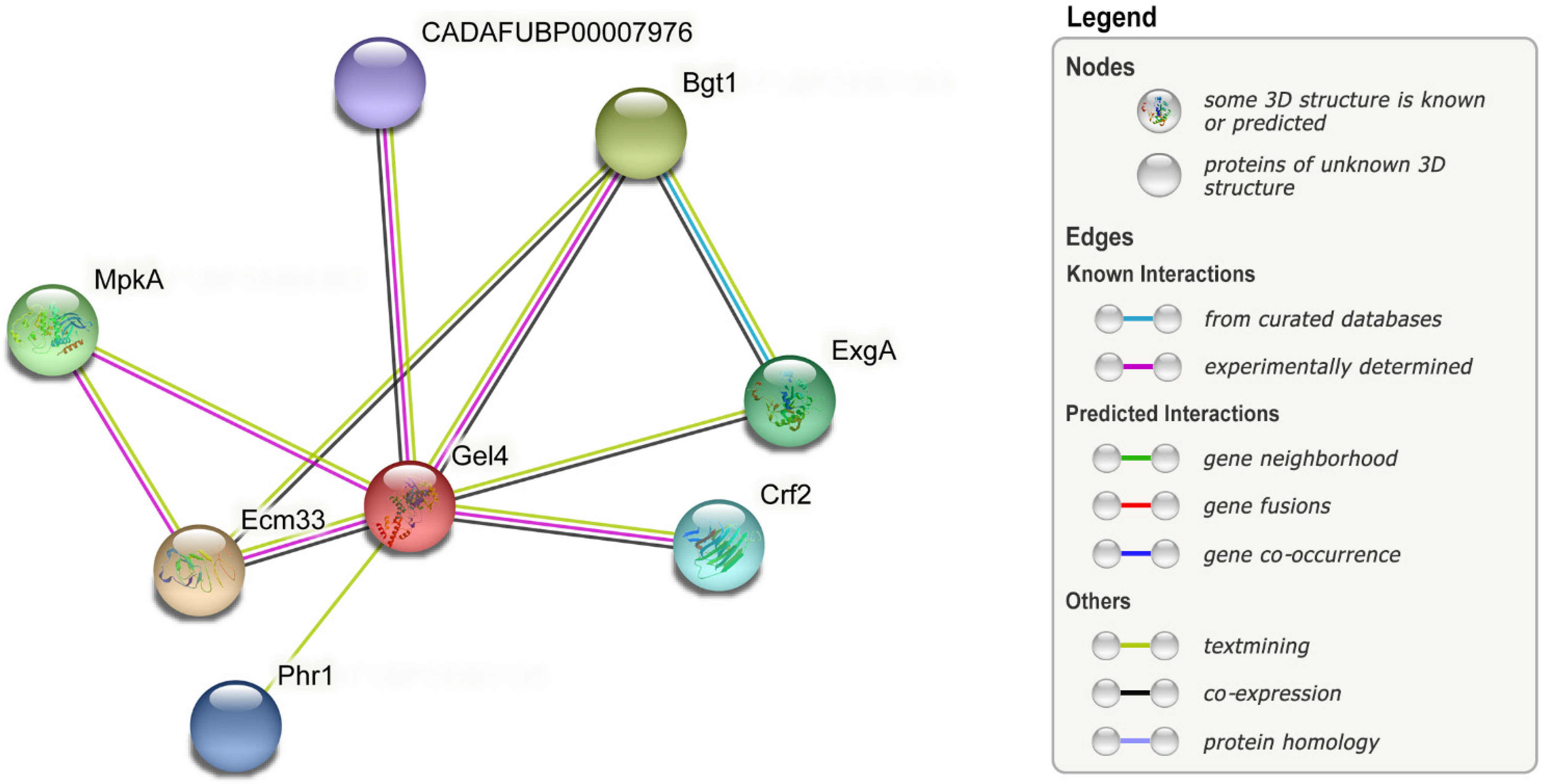
| Isotropic growth | Gel1, Gel4 | Gel family, important for cell wall remodeling. Linking and elongating of β-1,3-glucans. | A. fumigatus | [173,203] |
| VadA | Transcription factor involved in regulation of genes associated with polysaccharide metabolism, cell wall, and stress. | A. nidulans | [204] | |
| Polarized growth | Bisque4 module | Associated with cellular growth, includes genes such as sun1 (involved in modification of β-1,3-glucan), sidA (essential for the primary step of siderophore biosynthesis), GEL family genes (gel2, gel3, gel5), and chitin synthase genes. | A. fumigatus | [170,173] |
| ChiA1 | Class III chitinase, associated with conidial stress, upregulated in hypoxic conditions. | A. fumigatus | [205,206] | |
| Ecm33 | GPI-anchored protein associated with cell wall biosynthesis, stress resistance, and evasion of host’s immune system. | A. fumigatus | [27,207] | |
| Sienna3 module | Associated with regulation of the cell cycle and DNA processing -mitotic metaphase plate congression -assembly of the midzone of the mitotic spindle -nucleation of microtubules by the spindle pole body. | A. fumigatus | [173] | |
| SrbA | Transcription factor in the family of sterol regulatory element-binding proteins (SREBPs). Regulator of cell wall polarity and essential for outgrowth of germ tubes. | A. fumigatus | [171,205] | |
| Sod3 | Sod3 is an allergenic putative manganese superoxide dismutase, associated with reactive oxygen defense. | A. fumigatus | [203] | |
| Trr1 | Putative thioredoxin reductase. | A. fumigatus | [203] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verburg, K.; van Neer, J.; Duca, M.; de Cock, H. Novel Treatment Approach for Aspergilloses by Targeting Germination. J. Fungi 2022, 8, 758. https://doi.org/10.3390/jof8080758
Verburg K, van Neer J, Duca M, de Cock H. Novel Treatment Approach for Aspergilloses by Targeting Germination. Journal of Fungi. 2022; 8(8):758. https://doi.org/10.3390/jof8080758
Chicago/Turabian StyleVerburg, Kim, Jacq van Neer, Margherita Duca, and Hans de Cock. 2022. "Novel Treatment Approach for Aspergilloses by Targeting Germination" Journal of Fungi 8, no. 8: 758. https://doi.org/10.3390/jof8080758
APA StyleVerburg, K., van Neer, J., Duca, M., & de Cock, H. (2022). Novel Treatment Approach for Aspergilloses by Targeting Germination. Journal of Fungi, 8(8), 758. https://doi.org/10.3390/jof8080758







