Development of a Monoclonal Antibody and a Serodiagnostic Lateral-Flow Device Specific to Rhizopus arrhizus (Syn. R. oryzae), the Principal Global Agent of Mucormycosis in Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Fungal Culture
2.3. Production of Hybridomas and Screening by ELISA
2.4. Determination of Ig Class and Sub-Cloning Procedure
2.5. Production of Rabbit Antiserum
2.6. Antibody Purification and Enzyme Conjugation
2.7. Antibody Specificity Tests
2.8. Epitope Characterisation by Heat and Periodate Oxidation
2.9. Polyacrylamide Gel Electrophoresis and Western Blotting
2.10. Competitive Lateral-Flow Device
2.11. LFD Specificity and Sensitivity
2.12. LFD Serum and Bronchoalveolar Lavage Fluid Tests
2.12.1. Spiked Serum
2.12.2. Spiked BALf
2.13. Statistical Analysis
3. Results
3.1. Production of Hybridomas and mAb Isotyping
3.2. Antibody Specificities
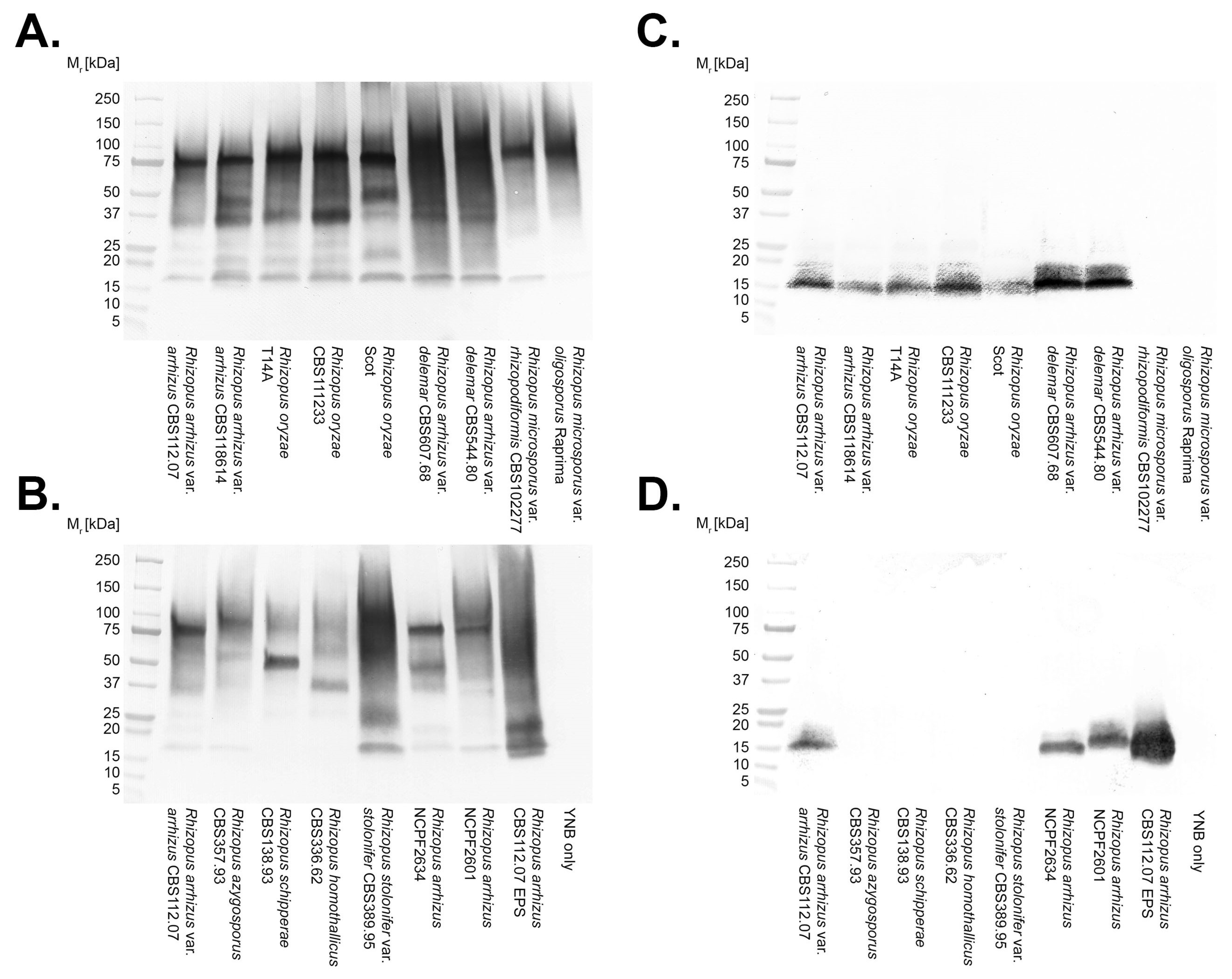
3.3. Epitope Characterisation
3.4. Lateral-Flow Device
3.4.1. Specificity and Sensitivity
3.4.2. LFD Serum and Bronchoalveolar Lavage Tests
4. Discussion
5. Trademark
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thornton, C.R. Detection of the ‘big five’ mold killers of humans: Aspergillus, Fusarium, Lomentospora, Scedosporium and Mucormycetes. Adv. Appl. Microbiol. 2020, 110, 1–61. [Google Scholar] [PubMed]
- Alvarez, E.; Sutton, D.A.; Cano, J.; Fothergill, W.W.; Stchigel, A.; Rinaldi, M.G.; Guarro, J. The spectrum of zygomycete species identified in clinically significant specimens in the United States. J. Clin. Microbiol. 2009, 47, 1650–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonifaz, A.; Tirado-Sánchez, A.; Calderón, L.; Romero-Cabello, R.; Kassack, J.; Ponce, R.M.; Mena, C.; Stchigel, A.; Cano, J.; Guarro, J. Mucormycosis in children: A study of 22 cases in a Mexican hospital. Mycoses 2014, 57, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, A.; Das, A.; Sharma, A.; Panda, N.; Das, S.; Gupta, K.L.; Sakhuja, V. Ten years’ experience in Zygomycosis at a tertiary care centre in India. J. Infect. 2001, 42, 261–266. [Google Scholar] [CrossRef]
- Diwakar, J.; Sammadar, A.; Konar, S.K.; Bhat, M.D.; Manuel, E.; Veenakumari, H.B.; Nandeesh, B.N.; Parveen, A.; Hajira, S.N.; Srinivas, D.; et al. First report of COVID-19-assciated rhino-orbital-cerebral mucormycosis in paediatric patients with type 1 diabetes mellitus. J. Med. Mycol. 2021, 31, 101203. [Google Scholar] [CrossRef]
- Francis, J.R.; Villanueva, P.; Bryant, P.; Blyth, C.C. Mucormycosis in children: Review and recommendations for management. J. Pediatr. Infect. Dis. Soc. 2018, 7, 159–164. [Google Scholar] [CrossRef]
- Prakash, H.; Chakrabarti, A. Global epidemiology of Mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Prakash, H.; Chakrabarti, A. Epidemiology of mucormycosis in India. Microorganisms 2021, 9, 523. [Google Scholar] [CrossRef]
- Prakash, H.; Ghosh, A.K.; Rudramurthy, S.M.; Singh, P.; Xess, I.; Savio, J.; Pamidimukkala, U.; Jillwin, J.; Varma, S.; Das, A.; et al. A prospective multicentre study on mucormycosis in India: Epidemiology, diagnosis, and treatment. Med. Mycol. 2019, 57, 395–402. [Google Scholar] [CrossRef]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [Green Version]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, G.; Cardoso, M.; Escada, P. Black fungus: Paranasal mucormycosis. BMJ 2021, 374, n1705. [Google Scholar] [CrossRef]
- Antoniadou, A. Outbreaks of zygomycosis in hospitals. Clin. Microbiol. Infect. 2009, 15, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Chikley, A.; Ben-Ami, R.; Kontoyiannis, D.P. Mucormycosis of the central nervous system. J. Fungi 2019, 5, 59. [Google Scholar] [CrossRef] [Green Version]
- Corzo-León, D.E.; Chora-Hernández, L.D.; Rodríguez-Zulueta, A.P.; Walsh, T.J. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: Epidemiology, diagnosis, and outcomes of reported cases. Med. Mycol. 2018, 56, 29–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Cuhna, M.A.; Nery, A.F.; Lima, F.P.; Diniz Junor, J.; Maciel Neto, J.; Calado, N.B.; Luz, K.G.; Milan, E.P. Rhinocerebral zygomycosis in a diabetic patient. Rev. Soc. Bras. Med. Trop. 2011, 44, 257–259. [Google Scholar]
- Erami, M.; Shams-Ghahfarokhi, M.; Jahanshiri, Z.; Sharif, A.; Razzaghi-Abyaneh, M. Rhinocerebral mucormycosis due to Rhizopus oryzae in a diabetic patient: A case report. J. Mycol. Med. 2013, 23, 123–129. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Guo, W.-W.; Wang, Y.-R.; Shi, W.-X.; Liu, C.; Li, D.-M.; Qiu, Y.; Shi, D.-M. Rhinocerebral mucormycosis caused by Rhizopus oryzae in a patient with acute myeloid leukemia. World J. Dermatol. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, H. From the pharynx to the brain: A case of rapidly progressing mucormycosis. Mycopathologia 2019, 184, 797–798. [Google Scholar] [CrossRef]
- Miller, R.P.; Farrugi, L.; Leask, J.; Khalsa, K.; Khanna, N.; Melia, L. Successful treatment of Rhizopus arrhizus rhino-orbital-cerebral mucormycosis with isavuconazole salvage therapy following extensive debridement. Med. Mycol. Case Rep. 2021, 32, 39–42. [Google Scholar] [CrossRef]
- Patel, A.; Kaur, H.; Xess, I.; Michael, J.S.; Savio, J.; Rudramurthy, S.; Singh, R.; Shastri, P.; Umabala, P.; Sardana, R.; et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin. Microbiol. Infect. 2020, 26, 944.e9–944.e15. [Google Scholar] [CrossRef]
- Ribes, J.A.; Vanover Sams, C.L.; Baker, D.J. Zygomycetes in human disease. Clin. Microbiol. Rev. 2000, 13, 236–301. [Google Scholar] [CrossRef]
- Walsh, T.J.; Gamaletsou, M.N.; McGinnis, M.R.; Hayden, R.T.; Kontoyiannis, D.P. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin. Infect. Dis. 2012, 54, S55–S60. [Google Scholar] [CrossRef] [PubMed]
- Meyerowitz, E.A.; Sanchez, S.; Mansour, M.I.; Triant, V.A.; Goldberg, M.B. Isolated cerebral mucormycosis in immunocompetent adults who inject drugs: Case reports and systematic review of the literature. Open Forum Infect. Dis. 2020, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Molina, K.C.; Gutman, J.A.; Scherger, S.; Lum, J.M.; Mossad, S.B.; Burgess, M.; Cheng, M.P.; Chuang, S.P.; Jacobs, S.E.; et al. Mucormycosis in hematopoietic cell transplant recipients and in patients with hematological malignancies in the era of new antifungal agents. Open Forum Infect. Dis. 2020, 8, ofaa646. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Azam, K.; Hussain, Z.; Afreen, A.; Ahmed, M. Disseminated infection with multiple cold abscesses caused by Rhizopus arrhizus in an immunocompetent girl. J. Coll. Physicians Surg. Pak. 2017, 27, 648–650. [Google Scholar]
- Albízuri-Prado, M.F.; Sánchez-Orta, A.; Rodríguez-Bandera, A.; Rodríguez-Feito, M. Primary cutaneous mucormycosis due to Rhizopus arrhizus in an 8-year-old girl. Actas Dermosifiliogr. 2018, 109, 562–564. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Chatterjee, S.S.; Das, A.; Panda, N.; Shivaprakash, M.R.; Kaur, A.; Varma, S.C.; Singhi, S.; Bhansali, A.; Sakhuja, V. Invasive zygomycosis in India: Experience in a tertiary care hospital. Postgrad. Med. J. 2009, 85, 573–581. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Kaur, H.; Savio, J.; Rudramurthy, S.M.; Patel, A.; Shastri, P.; Pamidimukkala, U.; Karthik, R.; Bhattacharya, S.; Kindo, A.J.; et al. Epidemiology and clinical outcomes of invasive mould infections in Indian intensive care units (FISF study). J. Crit. Care 2019, 15, 64–70. [Google Scholar] [CrossRef]
- Do Monte Junior, E.S.; dos Santos, M.E.L.; Ribeiro, I.B.; de Oliveira Luz, G.; Baba, E.R.; Hirsch, B.S.; Funari, M.P.; de Moura, E.G.H. Rare and fatal gastrointestinal mucormycosis (zygomycosis) in a COVID-19 patient: A case report. Clin. Endosc. 2020, 53, 746–749. [Google Scholar] [CrossRef]
- Gardiner, B.J.; Simpson, I.; Khuu, M.H.; Kidd, S.E.; Lo, C.H.; Jenkin, G.A. An unusual ulcer: A case of cutaneous mucormycosis caused by Rhizopus oryzae. Med. Mycol. Case Rep. 2015, 7, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Gabremariam, T.; Wiederhold, N.P.; Fothergill, A.W.; Garvey, E.P.; Hoekstra, W.J.; Schotzinger, R.P.; Patterson, T.F.; Filler, S.G.; Ibrahim, A.S. VT-1161 protects immunosuppressed mice from Rhizopus arrhizus var. arrhizus infection. Antimicrob. Agents Chemother. 2015, 59, 7815–7817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagel, S.; Ewald, C.; Doenst, T.; Sachse, S.; Roedel, J.; Pletz, M.W. Ventriculitis due to infection by Rhizopus arrhizus. Med. Mycol. Case Rep. 2015, 10, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C.-A. The epidemiology and clinical manifestations of mycormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Kanemaru, M.; Tashima, S.; Yamazaki, A.; Masuda, K.; Nagoshi, H.; Kobayashi, T.; Kuroda, J.; Hiruma, M.; Taniwaki, M.; Katoh, N. Disseminated mucormycosis due to Rhizopus oryzae diagnosed by skin biopsy. J. Dermatol. 2014, 42, 100–101. [Google Scholar] [CrossRef]
- Keisling, M.P.; Seemungal, I.A.; Mercado, D.I.; Voth, S.; Michael, A.; Gomez, B.L.; Munoz, C.; Schlecht, H.P.; Mapow, B.C.; Ownbey, R.T.; et al. Rhinocerebral mucormycosis in a patient with pre-B cell acute lymphoblastic leukaemia: PCR identifying Rhizopus oryzae from culture-negative tissue specimens. JMM Case Rep. 2014, 1, e000984. [Google Scholar] [CrossRef]
- Li, H.; Hwang, S.K.; Zhou, C.; Du, J.; Zhang, J. Gangrenous cutaneous mucormycosis caused by Rhizopus oryzae: A case report and review of primary cutaneous mucormycosis in China over past 20 years. Mycopathologia 2013, 176, 123–128. [Google Scholar] [CrossRef]
- LeMaile-Williams, M.; Burwell, L.A.; Salisbury, D.; Noble-Wang, J.; Arduino, M.; Lott, T.; Brandt, M.E.; Iiames, S.; Srinivasan, A.; Fridkin, S.K. Outbreak of cutaneous Rhizopus arrhizus infection associated with Karya ostomy bags. Clin. Infect. Dis. 2006, 43, e83–e88. [Google Scholar] [CrossRef]
- Martín, L.B.; Rodríguez, M.A.M.; Mercier, N.; Lafont, M.O.; Fernández, E.O.; de la Parte, A.R.; Estefania, M. Rhizopus arrhizus invasive infection due to self-inflicted scratch injuries in a diabetic patient with non-ketotic acidosis. Mycopathologia 2017, 182, 927–931. [Google Scholar] [CrossRef]
- Krishna, V.; Bansal, N.; Morjaria, J.; Kaul, S. COVID-19-associated pulmonary mucormycosis. J. Fungi 2022, 8, 711. [Google Scholar] [CrossRef]
- Rammaert, B.; Lanternier, F.; Zahar, J.-P.; Dannaoui, E.; Bougnoux, M.-E.; Lecuit, M.; Lortholary, O. Healthcare-associated mucormycosis. Clin. Infect. Dis. 2012, 54, S44–S54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, A.G.; Reddy, V.S.; Aparna, K.; Haqqani, R.; Jagadevapuram, K.; Gupta, S.; Fathima, K.; Tejal, M.; Muppirala, D. Zygomycosis of the scalp caused by Rhizopus oryzae presenting as kerion in an immunocompetent child. Indian J. Dermatol. 2019, 64, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lobato, E.; Ramírez-Hobak, L.; Aquino-Matus, J.E.; Ramírez-Hinojosa, J.P.; Lozano-Fernández, V.H.; Xicohtencatl-Cortes, J.; Hernández-Castro, R.; Arenas, R. Primary cutaneous mucormycosis caused by Rhizopus oryzae: A case report and review of literature. Mycopathologia 2017, 182, 387–392. [Google Scholar] [CrossRef]
- Singla, K.; Samra, T.; Bhatia, N. Primary cutaneous mucormycosis in a trauma patient with Morel-Lavallée lesion. Indian J. Crit. Care Med. 2018, 22, 375–377. [Google Scholar] [PubMed]
- Song, Y.; Qiao, J.; Giovanni, G.; Liu, G.; Yang, H.; Wu, J.; Chen, J. Mucormycosis in renal transplant recipients: Review of 174 reported cases. BMC Infect. Dis. 2017, 17, 283. [Google Scholar] [CrossRef]
- Tabarsi, P.; Khalili, N.; Pourabdollah, M.; Sharifynia, S.; Naeni, A.S.; Ghorbani, J.; Mohamadnia, A.; Abtahian, Z.; Askari, E. COVID-19 associated rhinosinusitis mucormycosis due to Rhizopus oryzae: A rare but potentially fatal infection occurring after treatment with corticosteroids. Am. J. Trp. Med. Hyg. 2021, 105, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Winkler, S.; Susani, S.; Willinger, B.; Apsner, R.; Rosenkranz, A.R.; Pötzi, R.; Berlakovich, G.A.; Pohanka, E. Gastric mucormycosis due to Rhizopus oryzae in a renal transplant recipient. J. Clin. Microbiol. 1996, 34, 2585–2587. [Google Scholar] [CrossRef] [Green Version]
- Ahmadikia, K.; Hashemi, S.J.; Khodavaisy, S.; Getso, M.I.; Alijani, H.; Badali, H.; Mirhendi, H.; Salehi, M.; Tabari, A.; Ardehali, M.M.; et al. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: A case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses 2021, 64, 798–808. [Google Scholar] [CrossRef]
- Arana, C.; Ramírez, R.E.C.; Xipell, M.; Casals, J.; Moreno, A.; Herrera, S.; Bodro, M.; Cofan, F.; Diekmann, F.; Esforzado, N. Mucormycosis associated with COVID-19 in two kidney transplant patients. Transpl. Infect. Dis. 2021, 23, e13652. [Google Scholar] [CrossRef]
- Buil, J.B.; van Zanten, A.R.H.; Bentvelsen, R.G.; Rijpstra, T.A.; Goorhuis, B.; van der Voort, S.; Wammes, L.J.; Janson, J.A.; Melchers, M.; Heusinkveld, M.; et al. Case series of four secondary mucormycosis infections in COVID-19 patients, the Netherlands, December 2020 to May 2021. Euro. Serveill. 2021, 26, 2100510. [Google Scholar] [CrossRef]
- Ghosh, A.; Sarkar, A.; Paul, P.; Patel, P. The rise in cases of mucormycosis, candidiasis and aspergillosis amidst COVID-19. Fungal Biol. Rev. 2021, 38, 67–91. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, W.F.; Abdelazim, M.H.; Eldsoky, I.; Ibrahim, A.A.; Alsobky, M.E.; Zafan, E.; Hasan, A. The impact of COVID-19 outbreak on the incidence of acute invasive fungal rhinosinusitis. Am. J. Otolaryngol. 2021, 42, 103080. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Chang, K.-M.; Berlinrut, I.; Wallach, F. Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient—Case report and review of the literature. J. Med. Mycol. 2021, 31, 101125. [Google Scholar] [CrossRef] [PubMed]
- Maini, A.; Tomar, G.; Khanna, D.; Kini, Y.; Mehta, H.; Bhagyasree, V. Sino-orbital mucormycosis in a COVID-19 patient: A case report. Int. J. Surg. Case Rep. 2021, 82, 105957. [Google Scholar] [CrossRef] [PubMed]
- Pasero, D.; Sanna, S.; Liperi, C.; Piredda, D.; Branca, G.P.; Casadio, L.; Simeo, R.; Buselli, A.; Rizzo, D.; Bussu, F. A challenging complication following SARS-CoV-2 infection: A case report of pulmonary mucormycosis. Infection 2021, 49, 1055–1060. [Google Scholar] [CrossRef]
- Revannavar, S.M.; Supriya, P.S.; Samaga, L.; Vineeth, V.K. COVID-19 triggering mucormycosis in a susceptible patient: A new phenomenon in the developing world? BMJ Case Rep. 2021, 14, e241663. [Google Scholar] [CrossRef]
- Salazar, F.; Bignell, E.; Brown, G.D.; Cook, P.C.; Warris, A. Pathogenesis of respiratory viral and fungal coinfections. Clin. Microbiol. Rev. 2022, 35, e00094-21. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Alhumaid, S.; Alshukairi, A.N.; Temsah, M.-H.; Barry, M.; Al Mutair, A.; Rabaan, A.A.; Al-Omari, A.; Tirupathi, R.; AlQahtani, M.; et al. COVID-19 and mucormycosis superinfection: The perfect storm. Infection 2021, 49, 833–853. [Google Scholar] [CrossRef]
- Banerjee, I.; Robinson, J.; Asim, M.; Sathian, B.; Banerjee, I. Mucormycosis and COVID-19 an epidemic in a pandemic. Nepal J. Epidemiol. 2021, 11, 1034–1039. [Google Scholar] [CrossRef]
- Chander, J.; Kaur, M.; Singla, N.; Punia, R.P.S.; Singhal, S.K.; Attri, A.K.; Alastruey-Izquierdo, A.; Stchigel, A.M.; Cano-Lira, J.F.; Guarro, J. Mucormycosis: Battle with the deadly enemy over a five-year period in India. J. Fungi 2018, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Garg, D.; Muthu, V.; Sehgal, I.S.; Ramachandran, R.; Kaur, H.; Bhalla, A.; Puri, G.D.; Chakrabarti, A.; Agarwal, R. Coronavirus disease (COVID-19) associated mucormycosis (CAM): Case report and systematic review of literature. Mycopathologia 2021, 186, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Badri, M.; Safari, S.; Hemmat, N. A case report of rhino-facial mucormycosis in a non-diabetic patient with COVID-19: A systematic review of literature and current update. BMC Infect. Dis. 2021, 21, 906. [Google Scholar] [CrossRef] [PubMed]
- Muthu, V.; Rudramurthy, S.M.; Chakrabarti, A.; Agarwal, R. Epidemiology and pathophysiology of COVID-19-associated mucormycosis: India versus the rest of the world. Mycopathologia 2021, 186, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Nehara, H.R.; Puri, I.; Kumar, V.; IH, S.; Bishnoi, B.R.; Sirohi, P. Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: Case series from the north-western part of India. Indian J. Med. Microbiol. 2021, 39, 380–383. [Google Scholar] [CrossRef]
- Patel, A.; Agarwal, R.; Rudramurthy, M.R.; Shevkani, M.; Xess, I.; Sharma, R.; Savio, J.; Sethuraman, N.; Madan, S.; Shastri, P.; et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg. Infect. Dis. 2021, 27, 2349–2359. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Hoenigl, M.; Meis, J.F.; Cornely, O.A.; Muthu, V.; Gangneux, J.P.; Perfect, J.; Chakrabarti, A.; ECMM and ISHAM. ECMM/ISHAM recommendations for clinical management of COVID-19 associated mucormycosis in low- and middle-income countries. Mycoses 2021, 64, 1028–1037. [Google Scholar] [CrossRef]
- Sen, M.; Honavar, S.G.; Bansal, R.; Sengupta, S.; Rao, R.; Kim, U.; Sharma, M.; Sachdev, M.; Grover, A.K.; Surve, A.; et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India—Collaborative OPAI-IJO study on mucormycosis in COVID-19 (COSMIC), Report 1. Indian J. Ophthalmol. 2021, 69, 1670–1692. [Google Scholar]
- Dilek, A.; Ozaras, R.; Ozkaya, S.; Sunbul, M.; Sen, E.I.; Leblebicioglu, H. COVID-19-associated mucormycosis: Case report and systematic review. Travel Med. Infect Dis. 2021, 44, 102148. [Google Scholar] [CrossRef]
- Skiada, A.; Pavlaes, I.; Drogari-Apiranthitou, M. Epidemiology and diagnosis of mucormycosis: An update. J. Fungi 2020, 6, 265. [Google Scholar] [CrossRef]
- Katragkou, A.; Walsh, T.J.; Roilides, E. Why is mucormycosis more difficult to cure than more common mycoses? Clin. Microbiol. Infect. 2014, 20, 74–81. [Google Scholar]
- Lackner, N.; Posch, W.; Lass-Flörl, C. Microbiological and molecular diagnosis of mucormycosis: From old to new. Microorganisms 2021, 9, 1518. [Google Scholar] [CrossRef] [PubMed]
- Weiss, Z.F.; Leon, A.; Koo, S. The evolving landscape of fungal diagnostics, current and emerging microbiological approaches. J. Fungi 2021, 7, 127. [Google Scholar] [CrossRef]
- Osaigbovo, I.I.; Bongomin, F. Point of care tests for invasive fungal infections: A blue print for increasing availability in Africa. Ther. Adv. Infect. Dis. 2021, 8, 20499361211034266. [Google Scholar] [CrossRef]
- Dolatabadi, S.; Kolecka, A.; Versteeg, M.; de Hoog, S.G.; Boekhout, T. Differentiation of clinically relevant mucorales Rhizopus microsporus and R. arrhizus by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). J. Med. Microbiol. 2015, 64, 694–701. [Google Scholar] [CrossRef] [Green Version]
- Padhye, A.A.; Ajello, L. Simple method of inducing sporulation by Apophysomyces elegans and Saksenaea vasiformis. J. Clin. Microbiol. 1988, 26, 1861–1863. [Google Scholar] [CrossRef] [Green Version]
- Thornton, C.R. Immunological Methods for Fungi. In Molecular and Cellular Biology of Filamentous Fungi: A Practical Approach, 1st ed.; Talbot, N.J., Ed.; Oxford University Press: Oxford, UK, 2001; pp. 227–256. [Google Scholar]
- Jensen, H.E.; Aalbæk, B.; Schønheyder, H. Immunohistochemical identification of aetiological agents of systemic bovine zygomycosis. J. Comp. Path. 1994, 110, 65–77. [Google Scholar] [CrossRef]
- Jensen, H.E.; Aalbæk, B.; Lind, P.; Krogh, H.V. Immunohistochemical diagnosis of systemic bovine zygomycosis by murine monoclonal antibodies. Vet. Pathol. 1996, 33, 176–183. [Google Scholar] [CrossRef]
- Jensen, H.E.; Salonen, J.; Ekfors, T.O. The use of immunohistochemistry to improve sensitivity and specificity in the diagnosis of systemic mycoses in patients with haematological malignancies. J. Pathol. 1997, 181, 100–105. [Google Scholar] [CrossRef]
- Potenza, L.; Vallerini, D.; Barozzi, P.; Riva, G.; Gilioli, A.; Forghieri, F.; Candoni, A.; Cesaro, S.; Quadrelli, C.; Maertens, J.; et al. Mucorales-specific T cells in patients with hematologic malignancies. PLoS ONE 2016, 11, e0149108. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Oinuma, K.-I.; Niki, M.; Yamagoe, S.; Miyazaki, Y.; Asai, K.; Yamada, K.; Hirata, K.; Kaneko, Y.; Kakeya, H. Identification of a novel Rhizopus-specific antigen by screening with a signal sequence trap and evaluation as a possible diagnostic marker of mucormycosis. Med. Mycol. 2017, 55, 713–719. [Google Scholar] [CrossRef] [Green Version]
- Shibata, W.; Niki, M.; Sato, K.; Fujimoto, H.; Yamada, K.; Watanabe, T.; Miyazaki, Y.; Asai, K.; Obata, Y.; Tachibana, T.; et al. Detection of Rhizopus-specific antigen in human and murine serum and bronchoalveolar lavage. Med. Mycol. 2020, 58, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Burnham-Marusich, A.R.; Hubbard, B.; Kvam, A.J.; Gates-Hollingsworth, M.; Green, H.R.; Soukup, E.; Limper, A.H.; Kozel, T.R. Conservation of mannan synthesis in fungi of the zygomycota and ascomycota reveals a broad diagnostic target. mSphere 2018, 3, e00094-18. [Google Scholar] [CrossRef] [Green Version]
- Orne, C.; Burnham-Marusich, A.; Baldin, C.; Gebremariam, T.; Ibrahim, A.; Kvam, A.; Kozel, T. Cell wall fucomannan is a biomarker for diagnosis of invasive murine mucormycosis. In Proceedings of the 28th ECCMID, Madrid, Spain, 21–24 April 2018. [Google Scholar]
- De Almeida Júnior, J.N.; Ibrahim, K.Y.; Del Negro, G.M.B.; Bezerra, E.D.; Neto, A.N.D.; Batista, M.V.; Siciliano, R.F.; Giudice, M.C.; Motta, A.L.; Rossi, F.; et al. Rhizopus arrhizus and Fusarium solani concomitant infection in an immunocompromised host. Mycopathologia 2015, 181, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Hinojosa, J.P.; Medrano-Ahumada, S.; Arenas, R.; Bravo-Escobar, A.; Paraguirre-Martínez, S.; Xicohtencatl-Cortes, J.; Martínez-Herrera, E.; Hernández-Castro, R. Fungal invasive co-infection due to Aspergillus fumigatus and Rhizopus arrhizus: A rhino-orbital presentation. J. Fungi 2021, 7, 1096. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.K.; Ghazarian, Z.; Cendrowski, K.D.; Persichino, J.G. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med. Mycol. Case Rep. 2021, 32, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Luckowitsch, M.; Rudolph, H.; Bochennek, K.; Porto, L.; Lehrnbecher, T. Central nervous system mold infections in children with hematological malignancies: Advances in diagnosis and management and treatment. J. Fungi 2021, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Meena, S.; Singh, G.; Pandey, M.; Xess, I. Exophiala jeanselmei and Rhizopus oryzae co-infection post renal transplant. J. Clin. Diagn. Res. 2019, 13, DD01–DD03. [Google Scholar] [CrossRef]
- Arndt, S.; Aschendorff, A.; Echternach, M.; Daemmrich, T.D.; Maier, W. Rhino-orbital-cerebral mucormycosis and aspergillosis: Differential diagnosis and treatment. Eur. Arch. Otorhinolaryngol. 2009, 266, 71–76. [Google Scholar] [CrossRef]
- Lass-Flörl, C.; Samardzic, E.; Knoll, M. Serology anno 2021-fungal infections: From invasive to chronic. Clin. Microbiol. Infect. 2021, 27, P1230–P1241. [Google Scholar] [CrossRef]
- Skiada, A.; Lanternier, F.; Groll, A.H.; Pagano, L.; Zimmerli, S.; Herbrecht, R.; Lortholary, O.; Petrikkos, G.L. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: Guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica 2013, 98, 492–504. [Google Scholar] [CrossRef] [Green Version]
- Dichtl, K.; Seybold, U.; Ormanns, S.; Horns, H.; Wagener, J. Evaluation of a novel Aspergillus antigen enzyme-linked immunosorbent assay. J. Clin. Microbiol. 2019, 57, e00136-19. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.P.; Heaney, J.L.J.; Shemar, M.; Baldwin, D.; Griffin, A.E.; Oldridge, E.; Goodall, M.; Afzal, Z.; Plant, T.; Cobbold, M.; et al. Development of a rapid and quantitative lateral flow assay for the simultaneous measurement of serum and immunoglobulin free light chains (FLC): From inception of a new near-patient FLC screening tool. Clin. Chem. Lab. Med. 2017, 55, 424–434. [Google Scholar] [CrossRef] [Green Version]
- Pratt, G.W.; Fan, A.; Melakeberhan, B.; Klapperich, C.M. A competitive lateral flow assay for the detection of tenofivir. Anal. Chim. Acta 2018, 1017, 34–40. [Google Scholar] [CrossRef]
- Bever, C.S.; Adams, C.A.; Hnasko, R.M.; Cheng, L.W.; Stanker, L.H. Lateral flow immunoassay (LFIA) for the detection of lethal amatoxins in mushrooms. PLoS ONE 2020, 15, e0231781. [Google Scholar] [CrossRef]
- Lan, J.; Sun, W.; Chen, L.; Zhou, H.; Fan, Y.; Diao, X.; Wang, B.; Zhao, H. Simultaneous and rapid detection of carbofuran and 3-hydroxy-carbofuran in water samples and pesticide preparation using lateral-flow immunochromatographic assay. Food Agric. Immunol. 2020, 31, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Wakeham, A.J.; Keane, G.; Kennedy, R. Field evaluation of a competitive lateral-flow assay for detection of Alternaria brassicae in vegetable brassica crops. Plant Dis. 2016, 100, 1831–1839. [Google Scholar] [CrossRef] [Green Version]
- Walther, G.; Wagner, L.; Kurzai, O. Updates on the taxonomy of mucorales with an emphasis on clinically important taxa. J. Fungi 2019, 5, 106. [Google Scholar] [CrossRef] [Green Version]
- Andryukov, B.G. Six decades of lateral flow immunoassay: From determining metabolic markers to diagnosing COVID-19. AIMS Microbiol. 2020, 6, 280–304. [Google Scholar] [CrossRef]
- Misulka, M.; Furfaro, E.; Bassetti, M. The importance of quantitative results of a lateral flow Aspergillus assay: The significant impact on specificity. Clin. Infect. Dis. 2021, 73, e1783–e1784. [Google Scholar]
- Jenks, J.D.; Prattes, J.; Buchheidt, D.; Hoenigl, M. Reply to Mikulska et al. Clin. Infect. Dis. 2021, 73, e1784–e1785. [Google Scholar] [CrossRef]
- Jensen, H.E.; Frandsen, P.L.; Schønheydner, H. Experimental systemic bovine zygomycosis with reference to pathology and secretion of antigen in urine. J. Vet. Med. B 1993, 40, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.R. Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin. Vacc. Immunol. 2008, 15, 1095–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercier, T.; Dunbar, A.; de Kort, E.; Schauwvlieghe, A.; Reynders, M.; Guldentops, E.; Blijlevens, N.M.A.; Vonk, A.G.; Rijnders, B.; Verweij, P.E.; et al. Lateral flow assays for diagnosing invasive pulmonary aspergillosis in adult hematology patients: A comparative multicentre study. Med. Mycol. 2020, 58, 444–452. [Google Scholar] [CrossRef]
- Laternier, F.; Dannaoui, E.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Huerre, M.; Bitar, D.; Dromer, F.; Lortholary, O.; French Mycosis Study Group. A global analysis of mucormycosis in France: The RetroZygo study (2005–2007). Clin. Infect. Dis. 2012, 54, S35–S43. [Google Scholar] [CrossRef] [Green Version]
- Pana, Z.D.; Seidel, D.; Skiada, A.; Groll, A.H.; Petrikkos, G.; Cornely, O.A.; Roilides, E. Invasive mucormycosis in children: An epidemiological study in European and non-European countries based on two registries. BMC Infect. Dis. 2016, 16, 667. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, A.; Singh, R. Mucormycosis in India: Unique features. Mycoses 2014, 57, 85–90. [Google Scholar] [CrossRef]
- Hoenigl, M.; Seidel, D.; Carvalho, A.; Rudramurthy, S.M.; Arastehfar, A.; Gangneux, J.-P.; Nasir, N.; Bonifaz, A.; Araiza, J.; Klimko, N.; et al. The emergence of COVID-19 associated mucormycosis: A review of cases from 18 countries. Lancet Microbe 2022, 3, e543–e552. [Google Scholar] [CrossRef]


| Species | Isolate Number | Source 1 | ELISA 2 |
|---|---|---|---|
| Absidia glauca | 2 | CRT | 0.060 |
| Absidia spinosa | 3 | CRT | 0.044 |
| Actinomucor elegans var. kuwaitensis | 117697 | CBS | 0.062 |
| Apophysomyces elegans | 477.78 | CBS | 0.058 |
| Apophysomyces mexicanus | 136361 | CBS | 0.039 |
| Apophysomyces ossiformis | 125533 | CBS | 0.037 |
| Apophysomyces variabilis | 658.93 | CBS | 0.067 |
| Aspergillus fumigatus | Af293 | FGSC | 0.077 |
| Aspergillus flavus | 91856iii | IMI | 0.055 |
| Aspergillus nidulans | A4 | FGSC | 0.034 |
| Aspergillus niger | 102.4 | CBS | 0.033 |
| Aspergillus terreus var. terreus | 601.65 | CBS | 0.069 |
| Basidiobolus ranarum | 117.29 | CBS | 0.051 |
| Candida albicans | SC5314 | SB | 0.058 |
| Cokeromyces recurvatus | 168.59 | CBS | 0.061 |
| Conidiobolus coronatus | 110.76 | CBS | 0.071 |
| Cryptococcus neoformans | 8710 | CBS | 0.070 |
| Cunninghamella bertholletiae | 151.8 | CBS | 0.030 |
| Fusarium oxysporum | 167.3 | CBS | 0.080 |
| Fusarium solani | 224.34 | CBS | 0.055 |
| Lichtheimia corymbifera | 109940 | CBS | 0.066 |
| Lichtheimia corymbifera | 120580 | CBS | 0.047 |
| Lichtheimia hyalospora | 146576 | CBS | 0.056 |
| Lichtheimia ornata | 142195 | CBS | 0.029 |
| Lichtheimia ramosa | 112528 | CBS | 0.088 |
| Lichtheimia ramosa | 124197 | CBS | 0.049 |
| Lichtheimia ramosa | 2845 | NCPF | 0.039 |
| Lomentospora prolificans | 3.1 | CRT | 0.062 |
| Mucor circinelloides | E2A (FJ713065) | CRT | 0.081 |
| Mucor circinelloides | B5-2 (KT876701) | CRT | 0.045 |
| Mucor indicus | 120.08 | CBS | 0.071 |
| Mucor mucedo | 95 | CRT | 0.056 |
| Mucor piriformis | 169.25 | CBS | 0.070 |
| Mucor plumbeus | 96 | CRT | 0.042 |
| Mucor racemosus f. racemosus | 111557 | CBS | 0.033 |
| Mucor racemosus f. racemosus | 112382 | CBS | 0.067 |
| Mucor racemosus f. racemosus | 222.81 | CBS | 0.062 |
| Mucor racemosus f. sphaerosporus | 115.08 | CBS | 0.054 |
| Mucor ramosissimus | 135.65 | CBS | 0.051 |
| Phycomyces nitens | 133 | CRT | 0.073 |
| Rhizomucor pusillus | 120586 | CBS | 0.044 |
| Rhizomucor pusillus | 120587 | CBS | 0.081 |
| Rhizopus arrhizus | T14A | CRT | 1.355 |
| Rhizopus arrhizus | 2601 | NCPF | 1.442 |
| Rhizopus arrhizus | 2634 | NCPF | 1.392 |
| Rhizopus arrhizus var. arrhizus | 112.07 | CBS | 1.395 |
| Rhizopus arrhizus var. arrhizus | 118614 | CBS | 1.365 |
| Rhizopus arrhizus var. delemar | 544.8 | CBS | 1.466 |
| Rhizopus arrhizus var. delemar | 607.68 | CBS | 1.622 |
| Rhizopus azygosporus | 357.93 | CBS | 0.010 |
| Rhizopus homothallicus | 336.62 | CBS | 0.030 |
| Rhizopus microsporus var. oligosporus | tempeh starter (Raprima) | CRT | 0.040 |
| Rhizopus microsporus var. rhizopodiformis | 102277 | CBS | 0.013 |
| Rhizopus schipperae | 138.95 | CBS | 0.030 |
| Rhizopus oryzae | 102659 | CBS | 1.369 |
| Rhizopus oryzae | 111233 | CBS | 1.407 |
| Rhizopus oryzae | tempeh starter (Scot) | CRT | 1.225 |
| Rhizopus stolonifer var. stolonifer | 389.95 | CBS | 0.020 |
| Scedosporium apiospermum | 117467 | CBS | 0.083 |
| Scedosporium aurantiacum | 121926 | CBS | 0.066 |
| Saksenaea vasiformis | 113.96 | CBS | 0.053 |
| Syncephalastrum racemosum | 155 | CRT | 0.061 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davies, G.E.; Thornton, C.R. Development of a Monoclonal Antibody and a Serodiagnostic Lateral-Flow Device Specific to Rhizopus arrhizus (Syn. R. oryzae), the Principal Global Agent of Mucormycosis in Humans. J. Fungi 2022, 8, 756. https://doi.org/10.3390/jof8070756
Davies GE, Thornton CR. Development of a Monoclonal Antibody and a Serodiagnostic Lateral-Flow Device Specific to Rhizopus arrhizus (Syn. R. oryzae), the Principal Global Agent of Mucormycosis in Humans. Journal of Fungi. 2022; 8(7):756. https://doi.org/10.3390/jof8070756
Chicago/Turabian StyleDavies, Genna E., and Christopher R. Thornton. 2022. "Development of a Monoclonal Antibody and a Serodiagnostic Lateral-Flow Device Specific to Rhizopus arrhizus (Syn. R. oryzae), the Principal Global Agent of Mucormycosis in Humans" Journal of Fungi 8, no. 7: 756. https://doi.org/10.3390/jof8070756
APA StyleDavies, G. E., & Thornton, C. R. (2022). Development of a Monoclonal Antibody and a Serodiagnostic Lateral-Flow Device Specific to Rhizopus arrhizus (Syn. R. oryzae), the Principal Global Agent of Mucormycosis in Humans. Journal of Fungi, 8(7), 756. https://doi.org/10.3390/jof8070756






