DdaCrz1, a C2H2-Type Transcription Factor, Regulates Growth, Conidiation, and Stress Resistance in the Nematode-Trapping Fungus Drechslerella dactyloides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
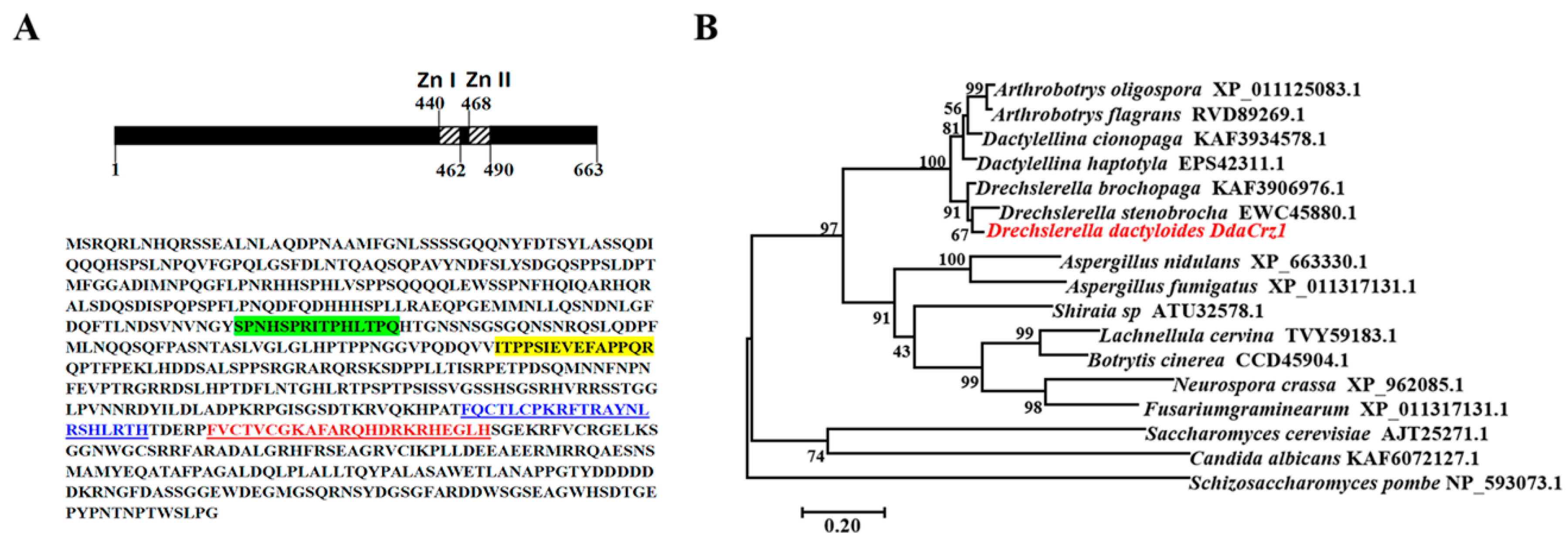
2.2. Sequence and Phylogenetic Analysis of DdaCrz1
2.3. Gene Knockout of DdaCrz1
2.4. Complementation of the DdaCrz1 Mutant
2.5. Comparison of Mycelial Growth and Conidial Production
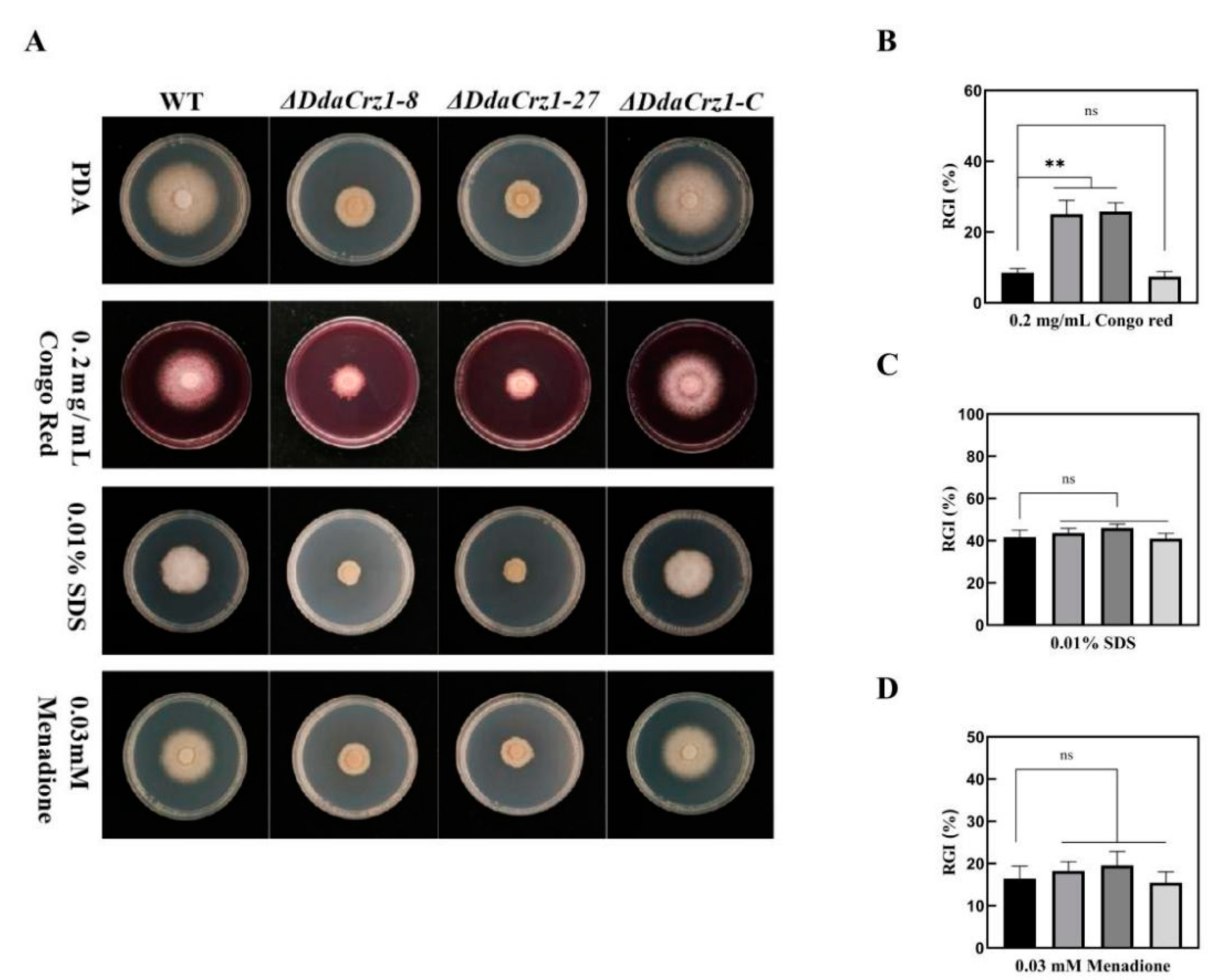
2.6. Analyses of Mycelial Growth under Different Stresses
2.7. Constricting Ring Formation and Inflation after Introducing Nematodes
2.8. RNA Extraction
2.9. Real-Time PCR (RT-PCR) Analysis
2.10. Statistical Analysis
3. Results
3.1. Identification of the Crz1 Gene of D. dactyloides
3.2. DdaCrz1 Disruption and Complementation
3.3. Deletion of DdaCrz1 Decreased Vegetative Growth and Conidiation
3.4. Disruption of DdaCrz1 Decreased Cell Wall Integrity
3.5. Involvement of DdaCrz1 in Responding to Osmotic and Metal Ion Stresses
3.6. Trap Formation and Ring Cell Inflation Affected by the Loss of DdaCrz1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Creamer, T.P. Calcineurin. Cell. Commun. Signal 2020, 18, 137. [Google Scholar] [CrossRef] [PubMed]
- Espeso, E.A. The CRaZy Calcium Cycle. Adv. Exp. Med. Biol. 2016, 892, 169–186. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Hou, Y.; Liu, W.; Lu, C.; Wang, W.; Sun, S. Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryot. Cell 2015, 14, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Rusnak, F.; Mertz, P. Calcineurin: Form and function. Physiol. Rev. 2000, 80, 1483–1521. [Google Scholar] [CrossRef] [PubMed]
- Thewes, S. Calcineurin-Crz1 signaling in lower eukaryotes. Eukaryot. Cell 2014, 13, 694–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, D.; Means, A.R. Calmodulin: A prototypical calcium sensor. Trends Cell. Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef]
- Berridge, M.; Bootman, M.; Roderick, H. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell. Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [Green Version]
- Dickman, M.B.; Yarden, O. Serine/Threonine Protein Kinases and Phosphatases in Filamentious Fungi. Fungal. Genet. Biol. 1999, 26, 99–117. [Google Scholar] [CrossRef] [Green Version]
- Guerini, D. Calcineurin: Not just a simple protein phosphatase. Biochem. Biophys. Res. Commun. 1997, 235, 271–275. [Google Scholar] [CrossRef]
- Stathopoulos, A.M.; Cyert, M.S. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Devel. 1997, 11, 3432–3444. [Google Scholar] [CrossRef] [Green Version]
- Cyert, M.S. Calcineurin signaling in Saccharomyces cerevisiae: How yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 2003, 311, 1143–1150. [Google Scholar] [CrossRef]
- Ruiz, A.; Yenush, L.; Arino, J. Regulation of ENA1 Na+-ATPase Gene Expression by the Ppz1 Protein Phosphatase Is Mediated by the Calcineurin Pathway. Eukaryot. Cell 2003, 2, 937–948. [Google Scholar] [PubMed] [Green Version]
- Onyewu, C.; Wormley, F.L., Jr.; Perfect, J.R.; Heitman, J. The calcineurin target, Crz1, functions in azole tolerance but is not required for virulence of Candida albicans. Infect. Immun. 2004, 72, 7330–7333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bader, T.; Schröppel, K.; Bentink, S.; Agabian, N.; Köhler, G.; Morschhäuser, J. Role of calcineurin in stress resistance, morphogenesis, and virulence of a Candida albicans wild-type strain. Infect. Immun. 2006, 74, 4366–4369. [Google Scholar] [CrossRef] [Green Version]
- Munro, C.A.; Selvaggini, S.; de Bruijn, I.; Walker, L.; Lenardon, M.D.; Gerssen, B.; Milne, S.; Brown, A.J.; Gow, N.A. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 2007, 63, 1399–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.; de Larrinoa, I.F. Functional characterization of the Candida albicans CRZ1 gene encoding a calcineurin-regulated transcription factor. Curr. Genet. 2005, 48, 88–100. [Google Scholar] [CrossRef]
- Schumacher, J.; de Larrinoa, I.F.; Tudzynski, B. Calcineurin-responsive zinc finger transcription factor CRZ1 of Botrytis cinerea is required for growth, development, and full virulence on bean plants. Eukaryot. Cell 2008, 7, 584–601. [Google Scholar] [CrossRef] [Green Version]
- Soriani, F.M.; Malavazi, I.; da Silva Ferreira, M.E.; Savoldi, M.; Von Zeska Kress, M.R.; de Souza Goldman, M.H.; Loss, O.; Bignell, E.; Goldman, G.H. Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Mol. Microbiol. 2008, 67, 1274–1291. [Google Scholar] [CrossRef]
- Choi, J.; Kim, Y.; Kim, S.; Park, J.; Lee, Y.H. MoCRZ1, a gene encoding a calcineurin-responsive transcription factor, regulates fungal growth and pathogenicity of Magnaporthe oryzae. Fungal. Genet. Biol. 2009, 46, 243–254. [Google Scholar] [CrossRef]
- Su, H.; Zhao, Y.; Zhou, J.; Feng, H.; Jiang, D.; Zhang, K.Q.; Yang, J. Trapping devices of nematode-trapping fungi: Formation, evolution, and genomic perspectives. Biol. Rev. Camb. Philos. Soc. 2017, 92, 357–368. [Google Scholar] [CrossRef]
- Yang, C.T.; Vidal-Diez de Ulzurrun, G.; Gonçalves, A.P.; Lin, H.C.; Chang, C.W.; Huang, T.Y.; Chen, S.A.; Lai, C.K.; Tsai, I.J.; Schroeder, F.C.; et al. Natural diversity in the predatory behavior facilitates the establishment of a robust model strain for nematode-trapping fungi. Proc. Natl. Acad. Sci. USA 2020, 117, 6762–6770. [Google Scholar] [CrossRef] [PubMed]
- Nordbring-Hertz, B. Nematophagous fungi: Strategies for nematode exploitation and for survival. Microbiol. Sci. 1988, 5, 108–116. [Google Scholar] [PubMed]
- Dijksterhuis, J.; Veenhuis, M.; Harder, W.; Nordbring-Hertz, B. Nematophagous fungi: Physiological aspects and structure-function relationships. Adv. Microb. Physiol. 1994, 36, 111–143. [Google Scholar] [PubMed]
- Zhen, Z.; Zhang, G.; Yang, L.; Ma, N.; Li, Q.; Ma, Y.; Niu, X.; Zhang, K.Q.; Yang, J. Characterization and functional analysis of calcium/calmodulin- dependent protein kinases (CaMKs) in the nematode-trapping fungus Arthrobotrys oligospora. Appl. Microbiol. Biotechnol. 2019, 103, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, C.; Hussain, M.; Chen, J.; Xiang, M.; Liu, X. Role of low-affinity calcium system member Fig1 homologous proteins in conidiation and trap-formation of nematode-trapping fungus Arthrobotrys oligospora. Sci. Rep. 2019, 9, 4440. [Google Scholar] [CrossRef]
- Chen, T.H.; Hsu, C.S.; Tsai, P.J.; Lin, H.N.S. Heterotrimeric G-protein and signal transduction in the nematode-trapping fungus Arthrobotrys dactyloides. Planta 2001, 212, 858–863. [Google Scholar] [CrossRef]
- Pui-Jen, T.; Tu, J.; Tsung-Hsien, C. Cloning of a Ca2+/calmodulin-dependent protein kinase gene from the filamentous fungus Arthrobotrys dactyloides. FEMS Microbiol. Lett. 2002, 212, 7–13. [Google Scholar]
- Dackman, C.; Nordbring-Hertz, B. Conidial traps—A new survival structure of the nematode-trapping fungus Arthrobotrys oligospora. Mycol. Res. 1992, 96, 194–198. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Andrie, R.M.; Martinez, J.P.; Ciuffetti, L.M. Development of ToxA and ToxB promoter-driven fluorescent protein expression vectors for use in filamentous Ascomycetes. Mycologia 2005, 97, 1152–1161. [Google Scholar] [CrossRef]
- Zhang, A.; Lu, P.; Dahl-Roshak, A.M.; Paress, P.S.; Kennedy, S.; Tkacz, J.S.; An, Z. Efficient disruption of a polyketide synthase gene (pks1) required for melanin synthesis through Agrobacterium-mediated transformation of Glarea lozoyensis. Mol. Genet. Genom. 2003, 268, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.N.; Zhang, W.W.; Chen, Y.; Xiang, M.C.; Liu, X.Z. DdaSTE12 is involved in trap formation, ring inflation, conidiation, and vegetative growth in the nematode-trapping fungus Drechslerella dactyloides. Appl. Microbiol. Biotechnol. 2021, 19, 7379–7393. [Google Scholar] [CrossRef] [PubMed]
- Gawel, N.J.; Jarret, R.L. A modified CTAB DNA extraction procedure for Musa and Ipomoea. Plant Mol. Biol. Report. 1991, 9, 262–266. [Google Scholar] [CrossRef]
- Liang, L.; Liu, Z.; Liu, L.; Li, J.; Gao, H.; Yang, J.; Zhang, K.Q. The nitrate assimilation pathway is involved in the trap formation of Arthrobotrys oligospora, a nematode-trapping fungus. Fungal Genet. Biol. 2016, 92, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, J.; Ying, S.H.; Feng, M.G. Three mitogen-activated protein kinases required for cell wall integrity contribute greatly to biocontrol potential of a fungal entomopathogen. PLoS ONE 2014, 9, e87948. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, X.; Xie, M.; Zhang, G.; Yang, L.; Bai, N.; Zhao, Y.; Li, D.; Zhang, K.Q.; Yang, J. The Arf-GAP AoGlo3 regulates conidiation, endocytosis, and pathogenicity in the nematode-trapping fungus Arthrobotrys oligospora. Fungal Genet. Biol. 2020, 138, 103352. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.K.; Zhang, W.W.; Lai, Y.L.; Xiang, M.C.; Wang, X.N.; Zhang, X.L.; Liu, X.Z. Drechslerella stenobrocha genome illustrates the mechanism of constricting rings and the origin of nematode predation in fungi. BMC Genom. 2014, 15, 114. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Feldman, L. A rapid TRIzol-based two-step method for DNA-free RNA extraction from Arabidopsis siliques and dry seeds. Biotechnol. J. 2010, 5, 183–186. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Luan, S.; Wang, C. Calcium signaling mechanisms across Kingdoms. Annu. Rev. Cell. Dev. Biol. 2021, 37, 311–340. [Google Scholar] [CrossRef]
- Cunningham, K.W.; Fink, G.R. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell. Biol. 1994, 124, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, H.; Saltsman, K.; Gasch, A.P.; Li, H.X.; Ogawa, N.; Botstein, D.; Brown, P.O.; Cyert, M.S. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 2002, 277, 31079–33108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhao, Q.; Liu, K.; Zhang, Z.; Wang, Y.; Zheng, X. MgCRZ1, a transcription factor of Magnaporthe grisea, controls growth, development and is involved in full virulence. FEMS Microbiol. Lett. 2009, 293, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Fan, Y.; Xiang, M.; Kang, S.; Wang, S.; Liu, X. DdaCrz1, a C2H2-Type Transcription Factor, Regulates Growth, Conidiation, and Stress Resistance in the Nematode-Trapping Fungus Drechslerella dactyloides. J. Fungi 2022, 8, 750. https://doi.org/10.3390/jof8070750
Zhao X, Fan Y, Xiang M, Kang S, Wang S, Liu X. DdaCrz1, a C2H2-Type Transcription Factor, Regulates Growth, Conidiation, and Stress Resistance in the Nematode-Trapping Fungus Drechslerella dactyloides. Journal of Fungi. 2022; 8(7):750. https://doi.org/10.3390/jof8070750
Chicago/Turabian StyleZhao, Xiaozhou, Yani Fan, Meichun Xiang, Seogchan Kang, Shunxian Wang, and Xingzhong Liu. 2022. "DdaCrz1, a C2H2-Type Transcription Factor, Regulates Growth, Conidiation, and Stress Resistance in the Nematode-Trapping Fungus Drechslerella dactyloides" Journal of Fungi 8, no. 7: 750. https://doi.org/10.3390/jof8070750
APA StyleZhao, X., Fan, Y., Xiang, M., Kang, S., Wang, S., & Liu, X. (2022). DdaCrz1, a C2H2-Type Transcription Factor, Regulates Growth, Conidiation, and Stress Resistance in the Nematode-Trapping Fungus Drechslerella dactyloides. Journal of Fungi, 8(7), 750. https://doi.org/10.3390/jof8070750





