A Systematic Review to Assess the Relationship between Disseminated Cerebral Aspergillosis, Leukemias and Lymphomas, and Their Respective Therapeutics
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selections
2.2. Eligibility Criteria
2.3. Data Abstraction
2.4. Assessment of Study Quality
2.5. Data Synthesis and Analysis
3. Results
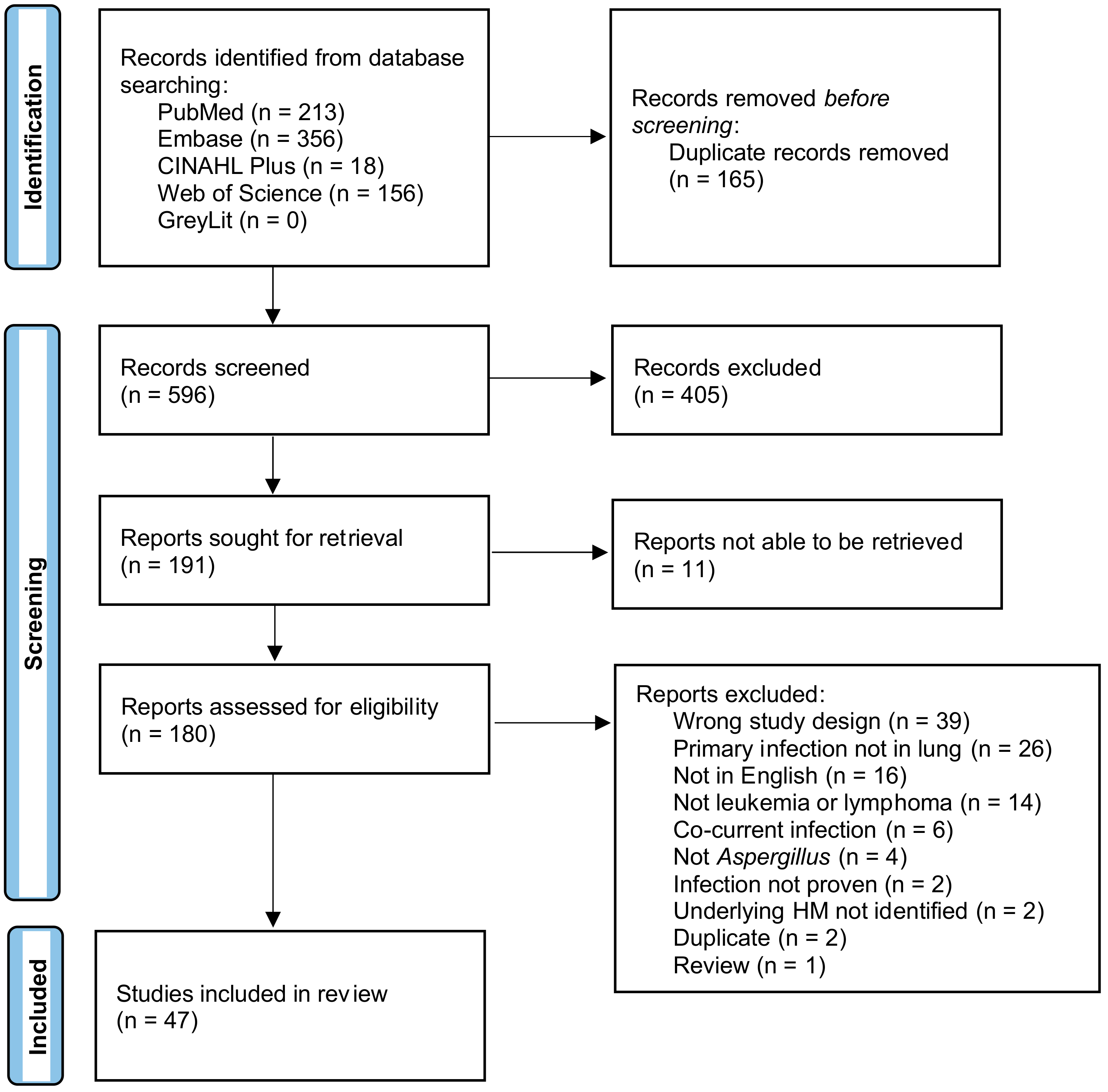
3.1. Search Results
3.2. Quality Appraisal
3.3. Demographic Characteristics
3.4. Underlying Disease
3.5. Prevalence of Known Risk Factors for IPA in the IPA + CA Population
3.6. Prophylactic Anti-Fungal Treatment
3.7. Treatment
3.8. Species
3.9. Mortality
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleinschmidt-DeMasters, B.K. Central nervous system aspergillosis: A 20-year retrospective series. Hum. Pathol. 2002, 33, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A.; Tovar-Torres, M.P.; Hingwe, A.; Cheema, F.; Welch, V.L.; Ford, K.D. The Changing Epidemiology of Invasive Aspergillosis in the Non-Traditional Host: Risk Factors and Outcomes. Pulm. Crit. Care Med. 2016, 1, 67–71. [Google Scholar] [CrossRef]
- Hessel Carvalho-Dias, V.M.; Sola, C.B.; Cunha, C.A.; Shimakura, S.E.; Pasquini, R.; Queiroz-Telles, F.D. Invasive Aspergillosis in Hematopoietic Stem Cell Transplant Recipients: A Retrospective Analysis. Braz. J. Infect. Dis. 2008, 12, 385–389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Segal, B.H. Aspergillosis. N. Engl. J. Med. 2009, 360, 1870–1884. [Google Scholar] [CrossRef] [PubMed]
- Deigendesch, N.; Nunez, J.C.; Stenzel, W. Parasitic and fungal infections. Handb. Clin. Neurol. 2018, 145, 245–262. [Google Scholar]
- Henao-Martínez, A.F.; Vela-Duarte, D. Chapter 13—Cryptococcal Meningitis and Other Opportunistic Fungal Infections of the Central Nervous System: Epidemiology, Pathogenesis, Diagnosis, and Treatment; Elsevier Inc.: Amsterdam, Netherlands, 2018; pp. 261–278. [Google Scholar]
- Patterson, T.F.; Kirkpatrick, W.R.; White, M.; Hiemenz, J.W.; Wingard, J.R.; Dupont, B.; Rinaldi, M.G.; Stevens, D.A.; Graybill, J.R. Invasive Aspergillosis Disease Spectrum, Treatment Practices, and Outcomes. Medicine 2000, 79, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Boes, B.; Bashir, R.; Boes, C.; Hahn, F.; McConnell, J.R.; McComb, R. Central Nervous System Aspergillosis: Analysis of 26 Patients. J. Neuroimaging 1994, 4, 123–129. [Google Scholar] [CrossRef]
- Economides, M.P.; Ballester, L.Y.; Kumar, V.A.; Jiang, Y.; Tarrand, J.; Prieto, V.; Torres, H.A.; Kontoyiannis, D.P. Invasive mold infections of the central nervous system in patients with hematologic cancer or stem cell transplantation (2000–2016): Uncommon, with improved survival but still deadly often. J. Infect. 2017, 75, 572–580. [Google Scholar] [CrossRef]
- Cadena, J.; Thompson, R.G.R.; Patterson, T.F. Invasive Aspergillosis: Current Strategies for Diagnosis and Management. Infect. Dis. Clin. N. Am. 2016, 30, 125–142. [Google Scholar] [CrossRef]
- Lin, S.-J.; Schranz, J.; Teutsch, S.M. Aspergillosis Case-Fatality Rate: Systematic Review of the Literature. Clin. Infect. Dis. 2001, 32, 358. [Google Scholar] [CrossRef]
- Pagano, L.; Ricci, P.; Montillo, M.; Cenacchi, A.; Nosari, A.; Tonso, A.; Cudillo, L.; Chierichini, A.; Savignano, C.; Buelli, M.; et al. Localization of aspergillosis to the central nervous system among patients with acute leukemia: Report of 14 cases. Gruppo Italiano Malattie Ematologiche dell’Adulto Infection Program. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1996, 23, 628. [Google Scholar] [CrossRef][Green Version]
- Baddley, J.W.; Andes, D.R.; Marr, K.A.; Kontoyiannis, D.P.; Alexander, B.D.; Kauffman, C.A.; Oster, R.A.; Anaissie, E.J.; Walsh, T.J.; Schuster, M.G.; et al. Factors Associated with Mortality in Transplant Patients with Invasive Aspergillosis. Clin. Infect. Dis. 2010, 50, 1559–1567. [Google Scholar] [CrossRef]
- Kourkoumpetis, T.K.; Desalermos, A.; Muhammed, M.; Mylonakis, E. Central nervous system aspergillosis: A series of 14 cases from a general hospital and review of 123 cases from the literature. Medicine 2012, 91, 328–336. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Lau, S.K.; Wong, S.C.; To, K.K.; So, S.Y.; Leung, S.S.; Chan, S.M.; Pang, C.M.; Xiao, C.; Hung, I.F.; et al. A 10-year study reveals clinical and laboratory evidence for the ‘semi-invasive’ properties of chronic pulmonary aspergillosis. Emerg. Microbes Infect. 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi. 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Hori, A.; Kami, M.; Kishi, Y.; Machida, U.; Matsumura, T.; Kashima, T. Clinical significance of extra-pulmonary involvement of invasive aspergillosis: A retrospective autopsy-based study of 107 patients. J. Hosp. Infect. 2002, 50, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Subira, M.; Martino, R.; Franquet, T.; Puzo, C.; Altés, A.; Sureda, A.; Brunet, S.; Sierra, J. Invasive pulmonary aspergillosis in patients with hematologic malignancies: Survival and prognostic factors. Haematologica 2002, 87, 528–534. [Google Scholar] [PubMed]
- Ramos Fernández, V.; Prieto Rodríguez, M.; Paradís Alós, A.; López Chulía, F.; Salom Fúster, J.V.; Vera-Sempere, F.J. Angio-invasive disseminated aspergillosis: Autopsy diagnosis in leukemic patients. An. Med. Interna 1993, 10, 337–340. [Google Scholar]
- Chierichini, A.; Monardo, F.; Anaclerico, B.; Anticoli Borza, P.; Bongarzoni, V.; Campagna, D.; Cedrone, M.; Fenu, S.; Norata, M.; Paoloni, F.; et al. Autopsy Analysis On Epidemiology and Site of Involvement Of Invasive Fungal Infections (IFI) In Hematological Malignancies: A Retrospective Study at Hematologic Tertiary Care Department. Blood 2013, 122, 5589-5589. [Google Scholar] [CrossRef]
- Ueno, M.; Nakano, K.; Yoshinari, H.; Nakayamada, S.; Iwata, S.; Kubo, S.; Miyagawa, I.; Tanaka, Y. An autopsy case with cerebral hemorrhaging due to disseminated aspergillosis during glucocorticoid therapy for overlap syndrome of systemic lupus erythematosus and systemic sclerosis. Intern. Med. 2019, 58, 1023–1027. [Google Scholar] [CrossRef]
- Mylonakis, E.; Paliou, M.; Sax, P.E.; Skolnik, P.R.; Baron, M.J.; Rich, J.D. Central nervous system aspergillosis in patients with human immunodeficiency virus infection: Report of 6 cases and review. Medicine 2000, 79, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Dotis, J.; Iosifidis, E.; Roilides, E. Central nervous system aspergillosis in children: A systematic review of reported cases. Int. J. Infect. Dis. 2007, 11, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Jantunen, E.; Volin, L.; Salonen, O.; Piilonen, A.; Parkkali, T.; Anttila, V.J.; Paetau, A.; Ruutu, T. Central nervous system aspergillosis in allogeneic stem cell transplant recipients. Bone Marrow Transplant. 2003, 31, 191–196. [Google Scholar] [CrossRef]
- Schwartz, S.; Thiel, E. Cerebral aspergillosis: Tissue penetration is the key. Med. Mycol. 2009, 47 (Suppl. 1), S387–S393. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Zhang, J.T.; Chen, Y.; Huang, X.S.; Jia, W.Q.; Yu, S.Y. Cerebral aspergillosis: A retrospective analysis of eight cases. Int. J. Neurosci. 2017, 127, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Torre-Cisneros, J.; Lopez, O.L.; Kusne, S.; Martinez, A.J.; Starzl, T.E.; Simmons, R.L.; Martin, M. CNS aspergillosis in organ transplantation: A clinicopathological study. J. Neurol. Neurosurg. Psychiatry 1993, 56, 188. [Google Scholar] [CrossRef] [PubMed]
- Amanati, A.; Lotfi, M.; Masoudi, M.S.; Jafarian, H.; Ghasemi, F.; Bozorgi, H.; Badiee, P. Cerebral and pulmonary aspergillosis, treatment and diagnostic challenges of mixed breakthrough invasive fungal infections: Case report study. BMC Infect. Dis. 2020, 20, 535. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Hier, D.B.; Caplan, L.R. Aspergillosis of the central nervous system: Clinicopathological analysis of 17 patients. Ann. Neurol. 1985, 18, 574–582. [Google Scholar] [CrossRef]
- Sullivan, B.N.B.M.; O’Connell, S.S.; Pickett, K.M.; Steele, C. A Systematic Review and Meta-Analysis to Assess the Relationship between Disseminated Cerebral Aspergillosis and Therapeutics used in Leukemias and Lymphomas. Available online: https://www.crd.york.ac.uk/PROSPERO/ (accessed on 2 December 2021).
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Gaye, E.; Le Bot, A.; Talarmin, J.P.; Le Calloch, R.; Belaz, S.; Dupont, M.; Tattevin, P. Cerebral aspergillosis: An emerging opportunistic infection in patients receiving ibrutinib for chronic lymphocytic leukemia? Med. Mal. Infect. 2018, 48, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Groll, A.H.; Kurz, M.; Schneider, W.; Witt, V.; Schmidt, H.; Schneider, M.; Schwabe, D. Five-year-survey of invasive aspergillosis in a paediatric cancer centre. Epidemiology, management and long-term survival. Mycoses 1999, 42, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Anciones, C.; de Felipe, A.; de Albóniga-Chindurza, A.; Acebrón, F.; Pián, H.; Masjuán, J.; Corral, I. Acute Stroke as First Manifestation of Cerebral Aspergillosis. J. Stroke Cerebrovasc. Dis. 2018, 27, 3289–3293. [Google Scholar] [CrossRef] [PubMed]
- Hummel, M.; Spiess, B.; Kentouche, K.; Niggemann, S.; Böhm, C.; Reuter, S.; Kiehl, M.; Mörz, H.; Hehlmann, R.; Buchheidt, D. Detection of Aspergillus DNA in cerebrospinal fluid from patients with cerebral aspergillosis by a nested PCR assay. J. Clin. Microbiol. 2006, 44, 3989–3993. [Google Scholar] [CrossRef]
- Iwen, P.C.; Reed, E.C.; Armitage, J.O.; Bierman, P.J.; Kessinger, A.; Vose, J.M.; Arneson, M.A.; Winfield, B.A.; Woods, G.L. Nosocomial invasive aspergillosis in lymphoma patients treated with bone marrow or peripheral stem cell transplants. Infect. Control Hosp. Epidemiol. 1993, 14, 131–139. [Google Scholar] [CrossRef]
- Iwen, P.C.; Rupp, M.E.; Hinrichs, S.H. Invasive mold sinusitis: 17 cases in immunocompromised patients and review of the literature. Clin. Infect. Dis. 1997, 24, 1178–1184. [Google Scholar] [CrossRef]
- Kaste, S.C.; Rodriguez-Galindo, C.; Furman, W.L.; Langston, J.; Thompson, S.J. Imaging aspects of neurologic emergencies in children treated for non-CNS malignancies. Pediatr. Radiol. 2000, 30, 558–565. [Google Scholar] [CrossRef]
- Kawanami, T.; Kurita, K.; Yamakawa, M.; Omoto, E.; Kato, T. Cerebrovascular disease in acute leukemia: A clinicopathological study of 14 patients. Intern. Med. 2002, 41, 1130–1134. [Google Scholar] [CrossRef]
- Kreisel, W.; Köchling, G.; von Schilling, C.; Azemar, M.; Kurzweil, B.; Dölken, G.; Lindemann, A.; Blum, U.; Windfuhr, M.; Müller, J. Therapy of invasive aspergillosis with itraconazole: Improvement of therapeutic efficacy by early diagnosis. Mycoses 1991, 34, 385–394. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Dunleavy, K.; Roschewski, M.; Widemann, B.C.; Butman, J.A.; Schmitz, R.; Yang, Y.; Cole, D.E.; Melani, C.; Higham, C.S.; et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell 2017, 31, 833–843.e5. [Google Scholar] [CrossRef]
- Middelhof, C.A.; Loudon, W.G.; Muhonen, M.D.; Xavier, C.; Greene, C.S., Jr. Improved survival in central nervous system aspergillosis: A series of immunocompromised children with leukemia undergoing stereotactic resection of aspergillomas—Report of four cases. J. Neurosurg. 2005, 103, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Pascale, S.; Sestili, S.; Mancini, I.; Di Carlo, P.; Natale, A.; Salutari, P.; Di Bartolomeo, P. Invasive aspergillosis during consolidation chemotherapy for acute myeloid leukemia: Report of two simultaneous cases involving central nervous system. Haematologica 2015, 100, 140-140. [Google Scholar]
- Ruchlemer, R.; Ben-Ami, R.; Bar-Meir, M.; Brown, J.R.; Malphettes, M.; Mous, R.; Tonino, S.H.; Soussain, C.; Barzic, N.; Messina, J.A.; et al. Ibrutinib-associated invasive fungal diseases in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: An observational study. Mycoses 2019, 62, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Schauwvlieghe, A.F.A.D.; Bredius, R.G.M.; Verdijk, R.M.; Smiers, F.J.W.; van der Beek, M.T.; Goemans, B.F.; Zwaan, C.M.; Brüggemann, R.J.; Rijnders, B.J.A. Management of cerebral azole-resistant Aspergillus fumigatus infection: A role for intraventricular liposomal-amphotericin B. J. Glob. Antimicrob. Resist. 2020, 22, 354–357. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gucalp, R.; Llena, J.F.; Moser, F.G.; Wiernik, P.H. Cerebral infection complicating systemic aspergillosis in acute-leukemia—Clinical and radiographic presentation. J. Neuro-Oncol. 1992, 13, 91–100. [Google Scholar] [CrossRef]
- Van der Linden, J.W.M.; Snelders, E.; Kampinga, G.A.; Rijnders, B.J.; Mattsson, E.; Debets-Ossenkopp, Y.J.; Kuijper, E.J.; Van Tiel, F.H.; Melchers, W.J.; Verweij, P.E. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg. Infect. Dis. 2011, 17, 1846–1854. [Google Scholar] [CrossRef]
- Wright, J.A.; Bradfield, S.M.; Park, J.R.; Hawkins, D.S. Prolonged survival after invasive aspergillosis: A single-institution review of 11 cases. J. Pediatr. Hematol. Oncol. 2003, 25, 286–291. [Google Scholar] [CrossRef]
- Yeh, T.C.; Liu, H.C.; Wang, L.Y.; Chen, S.H.; Liang, D.C. Invasive fungal infection in children undergoing chemotherapy for cancer. Ann. Trop. Paediatr. 2007, 27, 141–147. [Google Scholar] [CrossRef]
- Zwitserloot, A.M.; Warris, A.; van’t Hek, L.G.; van Die, L.E.; Verweij, P.E.; Mavinkurve-Groothuis, A.M. Disseminated aspergillosis in an adolescent with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2008, 51, 423–426. [Google Scholar] [CrossRef]
- Wandroo, F.; Stableforth, P.; Hasan, Y. Aspergillus brain abscess in a patient with acute myeloid leukaemia successfully treated with voriconazole. Clin. Lab. Haematol. 2006, 28, 130–133. [Google Scholar] [CrossRef]
- Trigg, M.E.; Menezes, A.H.; Giller, R.; Lanza, L.; Smith, R.J.; Sato, Y.; Peters, C.; Altman, A. Combined anti-fungal therapy and surgical resection as treatment of disseminated aspergillosis of the lung and brain following BMT. Bone Marrow Transplant. 1993, 11, 493–496. [Google Scholar] [PubMed]
- Tracy, S.L.; McGinnis, M.R.; Peacock, J.E., Jr.; Cohen, M.S.; Walker, D.H. Disseminated infection by Aspergillus terreus. Am. J. Clin. Pathol. 1983, 80, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Tattevin, P.; Bruneel, F.; Lellouche, F.; de Broucker, T.; Chevret, S.; Wolff, M.; Régnier, B. Successful treatment of brain aspergillosis with voriconazole. Clin. Microbiol. Infect. 2004, 10, 928–931. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schamroth Pravda, M.; Pravda, N.S.; Lishner, M. The Muddied Waters of Ibrutinib Therapy. Acta Haematol. 2019, 141, 209–213. [Google Scholar] [CrossRef]
- Sancho, J.M.; Ribera, J.M.; Rosell, A.; Munoz, C.; Feliu, E. Unusual invasive bronchial aspergillosis in a patient with acute lymphoblastic leukemia. Haematologica 1997, 82, 701–702. [Google Scholar]
- Sakata, N.; Okano, M.; Masako, R.; Tanaka, A.; Yamashita, Y.; Karasuno, T.; Imadome, K.I.; Okada, M.; Sugimoto, K. Donor-derived myelodysplastic syndrome after allogeneic stem cell transplantation in a family with germline GATA2 mutation. Int. J. Hematol. 2021, 113, 290–296. [Google Scholar] [CrossRef]
- Prakash, G.; Thulkar, S.; Arava, S.K.; Bakhshi, S. Cerebral aspergillus infection in pediatric acute lymphoblastic leukemia induction therapy. Indian J. Med. Paediatr. Oncol. 2012, 33, 236–238. [Google Scholar] [CrossRef]
- Pongbhaesaj, P.; Dejthevaporn, C.; Tunlayadechanont, S.; Witoonpanich, R.; Sungkanuparph, S.; Vibhagool, A. Aspergillosis of the central nervous system: A catastrophic opportunistic infection. Southeast Asian J. Trop. Med. Public Health 2004, 35, 119–125. [Google Scholar]
- Peng, H.L.; Yi, Y.F.; Shen, X.H.; Yin, Y.F.; Zhang, G.S. Dramatic response to itraconazole in central nervous system aspergillosis complicating acute promyelocytic leukemia. Infect. Dis. 2015, 47, 104–106. [Google Scholar] [CrossRef]
- Nov, A.A.; Cromwell, L.D. Computed tomography of neuraxis aspergillosis. J. Comput. Assist. Tomogr. 1984, 8, 413–415. [Google Scholar] [CrossRef]
- Mori, T.; Matsumura, M.; Yamada, K.; Irie, S.; Oshimi, K.; Suda, K.; Oguri, T.; Ichinoe, M. Systemic aspergillosis caused by an aflatoxin-producing strain of Aspergillus flavus. Med. Mycol. 1998, 36, 107–112. [Google Scholar] [CrossRef][Green Version]
- Marbello, L.; Nosari, A.; Carrafiello, G.; Anghilieri, M.; Cesana, C.; Cafro, A.M.; D’Avanzo, G.; Morra, E. Successful treatment with voriconazole of cerebral aspergillosis in an hematologic patient. Haematologica 2003, 88, Ecr05. [Google Scholar]
- Mahlknecht, U.; von Lintig, F.; Mertelsmann, R.; Lindemann, A.; Lübbert, M. Successful treatment of disseminated central nervous aspergillosis in a patient with acute myeloblastic leukemia. Leuk Lymphoma 1997, 27, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Kurdow, R.; Boehle, A.S.; Ankermann, T.; Schniewind, B.; Dohrmann, P. Case report: Successful interdisciplinary treatment of cerebrally disseminated invasive pulmonal aspergillosis in a child with acute myeloid leukemia. J. Pediatr. Surg. 2005, 40, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Park, I.S.; Kim, E.Y.; Lee, J.S.; Lim, J.H.; Lee, M.H.; Kim, C.S.; Lee, H.J.; Yi, H.G. Disseminated invasive aspergillosis with multiple brain abscess after allogeneic hematopoietic stem cell transplantation treated successfully with voriconazole and neurosurgical intervention. Infect. Chemother. 2012, 44, 395–398. [Google Scholar] [CrossRef]
- Henze, G.; Aldenhoff, P.; Stephani, U.; Grosse, G.; Kazner, E.; Staib, F. Successful treatment of pulmonary and cerebral aspergillosis in an immunosuppressed child. Eur. J. Pediatr. 1982, 138, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Benchaib, N.; Zagdanski, A.M.; Hocqueloux, L.; Rili, M.; Molina, J.M.; de Kerviler, E. Cerebral and spinal cord involvement resulting from invasive aspergillosis. Eur. Radiol. 2002, 12, 147–150. [Google Scholar] [CrossRef]
- Flatt, T.; Neville, K.; Lewing, K.; Dalal, J. Successful treatment of fanconi anemia and T-cell acute lymphoblastic leukemia. Case Rep. Hematol. 2012, 2012, 396395. [Google Scholar] [CrossRef]
- Faisal, M.S.; Shaikh, H.; Khattab, A.; Albrethsen, M.; Fazal, S. Cerebral aspergillosis in a patient on ibrutinib therapy—A predisposition not to overlook. J. Oncol. Pharm. Pract. 2019, 25, 1486–1490. [Google Scholar] [CrossRef]
- Eichenberger, E.M.; Saullo, J.; Brander, D.; Wang, S.H.; Perfect, J.R.; Messina, J.A. A case of CNS aspergillosis in a patient with chronic lymphocytic leukemia on first-line ibrutinib therapy. Med. Mycol. Case Rep. 2020, 27, 17–21. [Google Scholar] [CrossRef]
- De Leonardis, F.; Novielli, C.; Giannico, B.; Mariggio, M.A.; Castagnola, E.; Santoro, N. Isavuconazole Treatment of Cerebral and Pulmonary Aspergillosis in a Pediatric Patient With Acute Lymphoblastic Leukemia: Case Report and Review of Literature. J. Pediatr. Hematol. Oncol. 2020, 42, E469–E471. [Google Scholar] [CrossRef] [PubMed]
- Damaj, G.; Ivanov, V.; Le Brigard, B.; D’Incan, E.; Doglio, M.F.; Bilger, K.; Faucher, C.; Vey, N.; Gastaut, J.A. Rapid improvement of disseminated aspergillosis with caspofungin/voriconazole combination in an adult leukemic patient. Ann. Hematol. 2004, 83, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Beresford, R.; Dolot, V.; Foo, H. Cranial aspergillosis in a patient receiving ibrutinib for chronic lymphocytic leukemia. Med. Mycol. Case Rep. 2019, 24, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Athanassiadou, F.; Tragiannidis, A.; Kourti, M.; Papageorgiou, T.; Velegraki, A.; Kalogera, A. Treatment of disseminated aspergillosis with voriconazole/liposomal amphotericin B in a child with leukemia. Pediatr. Blood Cancer 2005, 45, 1003–1004. [Google Scholar] [CrossRef]
- Palmisani, E.; Barco, S.; Cangemi, G.; Moroni, C.; Dufour, C.; Castagnola, E. Need of voriconazole high dosages, with documented cerebrospinal fluid penetration, for treatment of cerebral aspergillosis in a 6-month-old leukaemic girl. J. Chemother. 2017, 29, 42–44. [Google Scholar] [CrossRef]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Fianchi, L.; Martino, B.; Pastore, D.; Picardi, M.; Bonini, A.; Chierichini, A.; et al. The epidemiology of fungal infections in patients with hematologic malignancies: The SEIFEM-2004 study. Haematologica 2006, 91, 1068–1075. [Google Scholar]
- Nicolle, M.-C.; Bénet, T.; Thiebaut, A.; Bienvenu, A.-L.; Voirin, N.; Duclos, A.; Sobh, M.; Cannas, G.; Thomas, X.; Nicolini, F.-E.; et al. Invasive aspergillosis in patients with hematologic malignancies: Incidence and description of 127 cases enrolled in a single institution prospective survey from 2004 to 2009. Haematologica 2011, 96, 1685. [Google Scholar] [CrossRef]
- Pardo, E.; Lemiale, V.; Mokart, D.; Stoclin, A.; Moreau, A.-S.; Kerhuel, L.; Calvet, L.; Valade, S.; De Jong, A.; Darmon, M.; et al. Invasive pulmonary aspergillosis in critically ill patients with hematological malignancies. Intensive Care Med. 2019, 45, 1732–1741. [Google Scholar] [CrossRef]
- Heo, S.T.; Tatara, A.M.; Jimenez-Ortigosa, C.; Jiang, Y.; Lewis, R.E.; Tarrand, J.; Tverdek, F.; Albert, N.D.; Verweij, P.E.; Meis, J.F.G.M.; et al. Changes in In Vitro Susceptibility Patterns of Aspergillus to Triazoles and Correlation With Aspergillosis Outcome in a Tertiary Care Cancer Center, 1999–2015. Clin. Infect. Dis. 2017, 65, 216–225. [Google Scholar] [CrossRef]
- Li, J.; Smith, A.; Crouch, S.; Oliver, S.; Roman, E. Estimating the prevalence of hematological malignancies and precursor conditions using data from Haematological Malignancy Research Network (HMRN). Cancer Causes Control 2016, 27, 1019–1026. [Google Scholar] [CrossRef][Green Version]
- Smith, A.; Howell, D.; Patmore, R.; Jack, A.; Roman, E. Incidence of haematological malignancy by sub-type: A report from the Haematological Malignancy Research Network. Br. J. Cancer 2011, 105, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Bow, E.J.; Loewen, R.; Cheang, M.S.; Schacter, B. Invasive Fungal Disease in Adults Undergoing Remission-Induction Therapy for Acute Myeloid Leukemia: The Pathogenetic Role of the Antileukemic Regimen. Clin. Infect. Dis. 1995, 21, 361–369. [Google Scholar] [CrossRef]
- Barkati, S.M.D.; Dufresne, S.F.M.D.; Bélanger, S.; Vadnais, B.M.; Bergeron, J.M.D.; Labbé, A.C.M.D.; Laverdière, M.M.D. Incidence of invasive aspergillosis following remission–induction chemotherapy for acute leukemia: A retrospective cohort study in a single Canadian tertiary care centre. CMAJ Open 2014, 2, E86–E93. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lin, C.; Ho, C.-L.; Lin, J.-C. Primary Fungal Prophylaxis in Hematological Malignancy: A Network Meta-Analysis of Randomized Controlled Trials. Antimicrob. Agents Chemother. 2018, 62, e00355-18. [Google Scholar] [CrossRef] [PubMed]
- Facchinelli, D.; Marchesini, G.; Nadali, G.; Pagano, L. Invasive Fungal Infections in Patients with Chronic Lymphoproliferative Disorders in the Era of Target Drugs. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018063. [Google Scholar]
- Teh, B.W.; Chui, W.; Handunnetti, S.; Tam, C.; Worth, L.J.; Thursky, K.A.; Slavin, M.A. High rates of proven invasive fungal disease with the use of ibrutinib monotherapy for relapsed or refractory chronic lymphocytic leukemia. Leuk. Lymphoma 2019, 60, 1572–1575. [Google Scholar] [CrossRef]
- Sharman, J.P.; Mato, A.R.; Keating, M.J. Ibrutinib for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 1592. [Google Scholar]
- Zhang, H.; Jiang, N.; Lin, X.; Wanggou, S.; Olson, J.J.; Li, X. Invasive sphenoid sinus aspergillosis mimicking sellar tumor: A report of 4 cases and systematic literature review. Chin. Neurosurg. J. 2020, 6, 10. [Google Scholar] [CrossRef]
- Creuzet, E.; Nourrisson, C.; Chaleteix, C.; Poirier, P.; Moniot, M. Cerebral aspergillosis in a patient on ibrutinib therapy. Br. J. Haematol. 2021, 193, 1025-1025. [Google Scholar] [CrossRef]
- Cummins, K.C.; Cheng, M.P.; Kubiak, D.W.; Davids, M.S.; Marty, F.M.; Issa, N.C. Isavuconazole for the treatment of invasive fungal disease in patients receiving ibrutinib. Leuk. Lymphoma 2019, 60, 527–530. [Google Scholar] [CrossRef]
- Grommes, C.; Pastore, A.; Gavrilovic, I.; Kaley, T.; Nolan, C.; Omuro, A.M.; Wolfe, J.; Pentsova, E.; Hatzoglou, V.; Mellinghoff, I.; et al. Single-Agent Ibrutinib in Recurrent/Refractory Central Nervous System Lymphoma. Blood 2016, 128, 783-783. [Google Scholar] [CrossRef]
- Ghez, D.; Calleja, A.; Protin, C.; Baron, M.; Ledoux, M.-P.; Damaj, G.; Dupont, M.; Dreyfus, B.; Ferrant, E.; Herbaux, C.; et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood 2018, 131, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Aldoss, I.; Dadwal, S.; Zhang, J.; Tegtmeier, B.; Mei, M.; Arslan, S.; Al Malki, M.M.; Salhotra, A.; Ali, H.; Aribi, A.; et al. Invasive fungal infections in acute myeloid leukemia treated with venetoclax and hypomethylating agents. Blood Adv. 2019, 3, 4043–4049. [Google Scholar] [CrossRef] [PubMed]
- Tse, E.; Leung, R.Y.Y.; Kwong, Y.-L. Invasive fungal infections after obinutuzumab monotherapy for refractory chronic lymphocytic leukemia. Ann. Hematol. 2014, 94, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Maertens, J.; Winston, D.J.; Perfect, J.; Ullmann, A.J.; Walsh, T.J.; Helfgott, D.; Holowiecki, J.; Stockelberg, D.; Goh, Y.-T.; et al. Posaconazole vs. Fluconazole or Itraconazole Prophylaxis in Patients with Neutropenia. N. Engl. J. Med. 2007, 356, 348–359. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Lipton, J.H.; Vesole, D.H.; Chandrasekar, P.; Langston, A.; Tarantolo, S.R.; Greinix, H.; Morais de Azevedo, W.; Reddy, V.; Boparai, N.; et al. Posaconazole or Fluconazole for Prophylaxis in Severe Graft-versus-Host Disease. N. Engl. J. Med. 2007, 356, 335–347. [Google Scholar] [CrossRef]
- Halpern, A.B.; Lyman, G.H.; Walsh, T.J.; Kontoyiannis, D.P.; Walter, R.B. Primary antifungal prophylaxis during curative-intent therapy for acute myeloid leukemia. Blood 2015, 126, 2790–2797. [Google Scholar] [CrossRef]
- Dib, R.W.; Hachem, R.Y.; Chaftari, A.M.; Ghaly, F.; Jiang, Y.; Raad, I. Treating invasive aspergillosis in patients with hematologic malignancy: Diagnostic-driven approach versus empiric therapies. BMC Infect. Dis. 2018, 18, 656. [Google Scholar]
- Hoenigl, M.; Zollner-Schwetz, I.; Sill, H.; Linkesch, W.; Lass-Flörl, C.; Schnedl, W.J.; Krause, R. Epidemiology of invasive fungal infections and rationale for antifungal therapy in patients with haematological malignancies. Mycoses 2011, 54, 454–459. [Google Scholar] [CrossRef]
- Fracchiolla, N.S.; Sciumè, M.; Orofino, N.; Guidotti, F.; Grancini, A.; Cavalca, F.; Freyrie, A.; Goldaniga, M.C.; Consonni, D.; Mattiello, V.; et al. Epidemiology and treatment approaches in management of invasive fungal infections in hematological malignancies: Results from a single-centre study. PLoS ONE 2019, 14, e0216715. [Google Scholar] [CrossRef]
- Robenshtok, E.; Gafter-Gvili, A.; Goldberg, E.; Weinberger, M.; Yeshurun, M.; Leibovici, L.; Paul, M. Antifungal Prophylaxis in Cancer Patients After Chemotherapy or Hematopoietic Stem-Cell Transplantation: Systematic Review and Meta-Analysis. J. Clin. Oncol. 2007, 25, 5471–5489. [Google Scholar] [CrossRef] [PubMed]
- Ethier, M.C.; Science, M.; Beyene, J.; Briel, M.; Lehrnbecher, T.; Sung, L. Mould-active compared with fluconazole prophylaxis to prevent invasive fungal diseases in cancer patients receiving chemotherapy or haematopoietic stem-cell transplantation: A systematic review and meta-analysis of randomised controlled trials. Br. J. Cancer 2012, 106, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Herbrecht, R.; Denning, D.W.; Patterson, T.F.; Bennett, J.E.; Greene, R.E.; Oestmann, J.-W.; Kern, W.V.; Marr, K.A.; Ribaud, P.; Lortholary, O.; et al. Voriconazole versus Amphotericin B for Primary Therapy of Invasive Aspergillosis. N. Engl. J. Med. 2002, 347, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Van den Abeele, A.-M.; Bulpa, P.; Misset, B.; Meersseman, W.; Cardoso, T.; Paiva, J.-A.; Blasco-Navalpotro, M.; De Laere, E.; Dimopoulos, G.; et al. Epidemiology of invasive aspergillosis in critically ill patients: Clinical presentation, underlying conditions, and outcomes. Crit. Care 2015, 19, 7. [Google Scholar] [CrossRef]
- Denning, D.W.; Marinus, A.; Cohen, J.; Spence, D.; Herbrecht, R.; Pagano, L.; Kibbler, C.; Kcrmery, V.; Offner, F.; Cordonnier, C.; et al. An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: Diagnosis and therapeutic outcome. J. Infect. 1998, 37, 173–180. [Google Scholar] [CrossRef]
| Ref. | Country of Diagnosis | Year of Diagnosis | Patient # | Gender | Age (Years) | HM | Aspergillus Species | Outcome |
|---|---|---|---|---|---|---|---|---|
| [33] | France | 2017 | 1 | M | 75 | CLL | A. fumigatus | Survived |
| 2 | M | 65 | CLL | A. fumigatus | Survived | |||
| [34] | Germany | 1992 | 3 | M | 17 | ALL | Aspergillus spp. | Survived |
| 4 | M | 16 | ALL | A. fumigatus | Died | |||
| [35] | Spain | 2011–2017 | 5 | M | 58 | AML | Aspergillus spp. | Died |
| 6 | M | 52 | AML | Aspergillus spp. | Died | |||
| 7 | M | 56 | MM | Aspergillus spp. | Died | |||
| [36] | Germany | 2002 | 8 | F | 12 | ALL | A. fumigatus, A. flavus | Survived |
| 9 | F | 63 | AML | A. fumigatus | Died | |||
| 2003 | 10 | F | 4 | ALL | A. fumigatus | Survived | ||
| [37] | USA | 1985–1990 | 11 | NA | NA | NHL | A. flavus | Died |
| 12 | NA | NA | NHL | A. flavus | Died | |||
| 13 | NA | NA | NHL | A. flavus | Died | |||
| [38] | USA | 1985–1994 | 14 | F | 36 | NHL | A. flavus | Died |
| 15 | M | 38 | NHL | A. flavus | Died | |||
| 16 | M | 16 | ALL | A. fumigatus | Died | |||
| [39] | USA | 1997–1999 | 17 | M | 16 | ALL | Aspergillus spp. | Died |
| 18 | M | 6 | AML | Aspergillus spp. | Survived | |||
| [40] | Japan | 1995 | 19 | F | 71 | AML | Aspergillus spp. | Died |
| 1978–1995 | 20 | F | 71 | AML | Aspergillus spp. | Died | ||
| 21 | F | 57 | ALL | Aspergillus spp. | Died | |||
| [41] | Germany | 1988 | 22 | M | 49 | ALL | Aspergillus spp. | Survived |
| 1989 | 23 | F | 23 | AML | Aspergillus spp. | Died | ||
| [42] | USA | 2014–2016 | 24 | M | 65 | NHL | A. fumigatus | Died |
| 25 | F | 87 | NHL | A. fumigatus | Died | |||
| 26 | M | 49 | NHL | A. fumigatus | Survived | |||
| [43] | USA | 2001 | 27 | F | 6 | AML | Aspergillus spp. | Survived |
| 28 | M | 6 | AML | Aspergillus spp. | Survived | |||
| [44] | Italy | 2015 | 29 | F | 65 | AML | Aspergillus spp. | Died |
| 30 | F | 60 | AML | Aspergillus spp. | Survived | |||
| [45] | Unknown | 2014–2017 | 31 | M | 67 | CLL | Aspergillus spp. | Died |
| 32 | M | 71 | CLL | Aspergillus spp. | Died | |||
| [46] | Netherlands | 2019 | 33 | F | 18 | ALL | A. fumigatus | Survived |
| 34 | F | 15 | ALL | A. fumigatus | Survived | |||
| [47] | USA | 1982–1990 | 35 | F | 22 | AML | A. flavus | Died |
| 36 | F | 31 | ALL | Aspergillus spp. | Died | |||
| 37 | F | 57 | AML | Aspergillus spp. | Died | |||
| 38 | F | 32 | ALL | Aspergillus spp. | Died | |||
| 39 | F | 20 | AML | A. flavus | Died | |||
| 40 | F | 21 | ALL | Aspergillus spp. | Died | |||
| [48] | Netherlands | 2007–2009 | 41 | F | 13 | NHL | A. fumigatus | Died |
| 2007–2010 | 42 | M | 60 | AML | A. fumigatus | Survived | ||
| [29] | USA | 1956–1985 | 43 | M | 60 | AML | Aspergillus spp. | Survived |
| 44 | F | 62 | AML | Aspergillus spp. | Survived | |||
| 45 | M | 59 | NHL | Aspergillus spp. | Died | |||
| 46 | M | 14 | ALL | Aspergillus spp. | Died | |||
| [49] | USA | 1995–2002 | 47 | NA | 10 | AML | A. flavus | Died |
| [50] | Taiwan | 1987–2005 | 48 | M | 11 | AML | Aspergillus spp. | Survived |
| [51] | Netherlands | 2007 | 49 | F | 16 | ALL | A. fumigatus | Survived |
| [52] | United Kingdom | 2006 | 50 | M | 34 | AML | A. fumigatus | Survived |
| [53] | USA | 1991 | 51 | F | 6 | ALL | A. fumigatus | Survived |
| [54] | USA | 1981 | 52 | M | 23 | AML | A. terreus | Died |
| [55] | France | 1994–1995 | 53 | M | 61 | AML | A. fumigatus | Died |
| [56] | Israel | 2018 | 54 | M | 37 | NHL | A. fumigatus | Died |
| [57] | Spain | 1997 | 55 | M | 43 | ALL | Aspergillus spp. | Died |
| [58] | Japan | 2017 | 56 | M | 15 | AML | Aspergillus spp. | Died |
| [59] | India | 2011 | 57 | M | 14 | ALL | Aspergillus spp. | Died |
| [60] | Thailand | 1991–2000 | 58 | F | 36 | ALL | A. fumigatus | Died |
| [61] | China | 2012 | 59 | M | 53 | AML | Aspergillus spp. | Survived |
| [62] | USA | Before 1987 | 60 | F | 32 | AML | Aspergillus spp. | Survived |
| [63] | Japan | 1995 | 61 | M | 41 | AML | A. flavus | Died |
| [64] | Italy | 2000 | 62 | F | 53 | CLL | A. flavus | Survived |
| [65] | Germany | 1996 | 63 | F | 62 | AML | Aspergillus spp. | Survived |
| [66] | Germany | 2003 | 64 | F | 9 | AML | A. fumigatus | Survived |
| [67] | Korea | 2011 | 65 | F | 31 | AML | Aspergillus spp. | Survived |
| [68] | Germany | 1980 | 66 | M | 12 | ALL | A. fumigatus | Survived |
| [69] | France | 1999 | 67 | F | 30 | CML | A. fumigatus | Died |
| [70] | USA | 2009 | 68 | M | 17 | ALL | Aspergillus spp. | Survived |
| [71] | USA | 2015 | 69 | M | 76 | CLL | A. fumigatus | Survived |
| [72] | USA | 2017 | 70 | M | 62 | CLL | A. fumigatus | Survived |
| [73] | Italy | 2017 | 71 | F | 3 | ALL | Aspergillus spp. | Survived |
| [74] | France | 2002 | 72 | M | 57 | AML | Aspergillus spp. | Survived |
| [75] | Australia | 2018 | 73 | M | 66 | CLL | A. felis | Survived |
| [76] | Greece | 2004 | 74 | M | 2 | ALL | A. fumigatus | Survived |
| [28] | Iran | 2018 | 75 | M | 1.5 | ALL | A. fumigatus, A. niger | Died |
| [77] | Italy | 2015 | 76 | F | 0.5 | ALL | Aspergillus spp. | Survived |
| Age of all Patients (N a = 73) (Mean ± SD (Years)) | 32.5 ± 21.9 |
|---|---|
| % (n b) | |
| <18 years | 34.25% (25) |
| ≥18, <50 years | 27.40% (20) |
| ≥50 years | 38.36% (28) |
| Identify as male (N = 72) | 54.17% (39) |
| % (n a) | |
|---|---|
| Underlying HM patient subgroup | (N b = 76) |
| AML | 39.47% (30) |
| ALL | 32.89% (25) |
| CML | 1.32% (1) |
| CLL | 10.53% (8) |
| NHL | 14.47% (11) |
| MM | 1.32% (1) |
| Neutropenic (N = 51) | 78.43% (40) |
| Immunosuppressive therapies | |
| Chemotherapy (N = 67) | 88.06% (59) |
| Phase (N = 23) | |
| Induction | 73.91% (17) |
| Consolidation | 26.09% (6) |
| Type + regimen (N = 27) | |
| Mono therapy | 18.52% (5) |
| Multi therapy | 81.48% (22) |
| Regimen includes Cytarabine | 66.66% (18) |
| Regimen includes Daunorubicin | 40.74% (11) |
| Regimen includes Vincristine | 33.33% (9) |
| SCT (N = 58) | 36.21% (21) |
| Allogenic | 78.57% (11) |
| Autologous | 21.43% (3) |
| Corticosteroids (N = 54) | 61.11% (33) |
| Type (N = 21) | |
| Prednisone | 61.90% (13) |
| Dexamethasone | 38.10% (8) |
| Prophylactic anti-fungal | |
| Yes (N = 48) | 47.91% (23) |
| Type + Regimen (N = 13) | |
| AmBc | 61.54% (8) |
| Fluconazole | 23.08% (3) |
| Itraconazole | 7.69% (1) |
| AmB + fluconazole | 7.69% (1) |
| Chemotherapy | Corticosteroids | SCT | Anti-Fungal Prophylaxis | |
|---|---|---|---|---|
| HM | (N a = 59) | (N = 33) | (N = 21) | (N = 23) |
| % (n b) | ||||
| AML (N = 30) | 73.33% (22) | 30% (10) | 30% (10) | 23.33% (7) |
| ALL (N = 25) | 88% (22) | 48% (12) | 12% (3) | 28% (7) |
| CML (N = 1) | 100% (1) | 100% (1) | 100% (1) | - |
| CLL (N = 8) | 37.5% (3) | 50% (4) | - | 33.33% (2) |
| NHL (N = 11) | 90.91% (10) | 45.45% (5) | 54.55% (6) | 54.55% (6) |
| MM (N = 1) | 100% (1) | 100% (1) | 100% (1) | 100% (1) |
| % (n a) | |
|---|---|
| Chemotherapy, SCT | N b = 49 |
| yes, yes | 28.57% (14) |
| yes, no | 57.14% (28) |
| no, yes | 2.04% (1) |
| no, no | 12.24% (6) |
| Corticosteroid, SCT | N = 39 |
| yes, yes | 23.08% (9) |
| yes, no | 30.77% (12) |
| no, yes | 5.13% (2) |
| no, no | 41.03% (16) |
| Chemotherapy, Corticosteroid | N = 52 |
| yes, yes | 53.85% (28) |
| yes, no | 30.77% (16) |
| no, yes | 7.69% (4) |
| no, no | 7.69% (4) |
| % (n a) | |
|---|---|
| chemotherapy, anti-fungal prophylaxis | N b = 46 |
| yes, yes | 45.65% (21) |
| yes, no | 39.13% (18) |
| no, yes | 2.17% (1) |
| no, no | 13.04% (6) |
| corticosteroid, anti-fungal prophylaxis | N = 39 |
| yes, yes | 25.64% (10) |
| yes, no | 28.21% (11) |
| no, yes | 15.38% (6) |
| no, no | 30.77% (12) |
| SCT, anti-fungal prophylaxis | N = 44 |
| yes, yes | 27.27% (12) |
| yes, no | 9.09% (4) |
| no, yes | 22.73% (10) |
| no, no | 40.91% (18) |
| % (n a) | |
|---|---|
| Antifungal therapy (N b = 69) | 91.30% (63) |
| Type of therapy (N = 61) | |
| Mono therapy | 31.15% (19) |
| Multiple therapy | 68.84% (42) |
| Regimen (N = 61) | |
| Included AmB | 80.33% (49) |
| DAmB | 75.51% (37) |
| L-AmB | 24.49% (12) |
| Included Voriconazole | 50.82% (31) |
| Included Caspofungin | 18.03% (11) |
| Included Itraconazole | 16.39% (10) |
| Included Flucytosine | 13.11% (8) |
| Included Posaconazole | 6.55% (4) |
| Voriconazole + AmBc | 24.59% (15) |
| Voriconazole + Caspofunginc | 4.92% (3) |
| Voriconazole + AmB + Caspofunginc | 9.84% (6) |
| Other | 11.48% (7) |
| Type not disclosed | 7.35% (5) |
| Surgery (N = 58) | 65.38% (38) |
| Patients (N a = 39) | Survived | Died | |
|---|---|---|---|
| % (n b) | |||
| Aspergillus fumigatus | 64.10% (25) | 60.00% (15) | 40.00% (10) |
| A. fumigatus + A. flavusc | 2.56% (1) | 100% (1) | |
| A. fumigatus + A. nigerd | 2.56% (1) | 100% (1) | |
| Aspergillus flavus | 25.64% (10) | 10.00% (1) | 90.00% (9) |
| Aspergillus terreus | 2.56% (1) | 100% (1) | |
| Aspergillus felis | 2.56% (1) | 100% (1) | |
| % Mortality (n a) | |
|---|---|
| Overall mortality | 53.95% (41) |
| Age | |
| <18 years (N b = 25) | 36.00% (9) |
| ≥18, <50 years (N = 20) | 70.00% (14) |
| ≥50 years (N = 39) | 53.57% (15) |
| Mortality rate according to HM | |
| AML (N = 30) | 50.00% (15) |
| ALL (N = 25) | 48.00% (12) |
| CML (N = 1) | 100.00% (1) |
| CLL (N = 8) | 25.00% (2) |
| NHL (N = 11) | 90.91% (10) |
| MM (N = 1) | 100.00% (1) |
| Mortality rate according to chemotherapy at time of IPA diagnosis | |
| Chemotherapy (N = 59) | 59.32% (35) |
| No (N = 8) | 25.00% (2) |
| Mortality rate according to SCT prior to IPA diagnosis | |
| SCT (N = 21) | 76.19% (16) |
| No (N = 37) | 35.14% (13) |
| Mortality rate according to corticosteroids at time of IPA diagnosis | |
| Steroids (N = 33) | 60.61% (20) |
| No (N = 21) | 33.33% (7) |
| Mortality rate according to prophylactic anti-fungal | |
| Anti-fungal prophylaxis (N = 23) | 73.91% (17) |
| No (N = 25) | 28.00% (7) |
| Mortality rate according to therapeutic anti-fungal | |
| Anti-fungal therapy (N = 66) | 51.52% (34) |
| No (N = 3) | 100.00% (3) |
| Mortality rate according to surgical intervention post-diagnosis | |
| Surgical intervention (N = 26) | 34.62% (9) |
| No (N = 32) | 62.50% (20) |
| Mortality rate according to therapeutic anti-fungal & surgical intervention | |
| Surgical intervention, anti-fungal therapy (N = 25) | 36.00% (9) |
| No Surgical intervention, anti-fungal therapy (N = 26) | 61.54% (16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sullivan, B.N.; Baggett, M.A.; O’Connell, S.S.; Pickett, K.M.; Steele, C. A Systematic Review to Assess the Relationship between Disseminated Cerebral Aspergillosis, Leukemias and Lymphomas, and Their Respective Therapeutics. J. Fungi 2022, 8, 722. https://doi.org/10.3390/jof8070722
Sullivan BN, Baggett MA, O’Connell SS, Pickett KM, Steele C. A Systematic Review to Assess the Relationship between Disseminated Cerebral Aspergillosis, Leukemias and Lymphomas, and Their Respective Therapeutics. Journal of Fungi. 2022; 8(7):722. https://doi.org/10.3390/jof8070722
Chicago/Turabian StyleSullivan, Brianne N., Mia A. Baggett, Samantha S. O’Connell, Keith M. Pickett, and Chad Steele. 2022. "A Systematic Review to Assess the Relationship between Disseminated Cerebral Aspergillosis, Leukemias and Lymphomas, and Their Respective Therapeutics" Journal of Fungi 8, no. 7: 722. https://doi.org/10.3390/jof8070722
APA StyleSullivan, B. N., Baggett, M. A., O’Connell, S. S., Pickett, K. M., & Steele, C. (2022). A Systematic Review to Assess the Relationship between Disseminated Cerebral Aspergillosis, Leukemias and Lymphomas, and Their Respective Therapeutics. Journal of Fungi, 8(7), 722. https://doi.org/10.3390/jof8070722






