Abstract
N-chlorotaurine (NCT), the N-chloro derivative of the amino acid taurine, is a long-lived oxidant produced by stimulated human leucocytes. NCT has antimicrobial activities which are generally enhanced in the presence of organic material. The aim of this study was to investigate fungicidal effects of NCT and conventional antiseptics against Candida isolated from vulvovaginal candidiasis (VVC). Chlorhexidine (CHX, 1.6%), octenidine dihydrochloride (OCT, 0.08%), povidone iodine (PVP-I, 8%), boric acid (8%), and NCT (0.1% (5.5 mM)) were evaluated against forty-four Candida isolates, according to European Standard methods, at 30, 60, 90, and 120 min and 24 h in the presence of skim milk as an organic material. CHX, OCT, and PVP-I showed rapid fungicidal activity against all Candida isolates with 5–6 log10 reduction of viable counts after 30 min, whereas boric acid and NCT needed 1 h against Candida albicans and 2 h against non-albicans Candida for a significant 3 log10 reduction. NCT showed fungicidal activity (defined as ≥4 log10 reduction) against C. albicans within 90 min and C. non–albicans within 24 h. Based upon all presently available data, including our results, NCT could be used as a new agent for treatment of local fungal infections such as VVC.
1. Introduction
The vaginal microbiota is a dynamic healthy ecosystem and this ecosystem is defined by an acidic environment (pH less than 4.5) which is unsuitable for most bacteria, fungi, and viruses. Loss of the acidic environment may cause vaginal infections. Some vaginal infections are symptomatic and some of the most common symptoms may be discharge, itching, or odor. The most common vaginal infections are vulvovaginal candidiasis (VVC), bacterial vaginosis, and trichomoniasis, which is a sexually transmitted infection [1,2]. VVC usually is caused by Candida albicans but can also be caused by non–albicans Candida species, especially Candida glabrata [3].
More than half of all sexually mature women suffer at least one episode of VVC, and 40–45% of them have two or more episodes [4]. Based on clinical presentation, microbiology, host factors, and response to treatment, VVC can be classified as uncomplicated or complicated. Three or more symptomatic episodes of VVC in <1 year affecting <5% of women are defined as recurrent VVC that may be either idiopathic or secondary (related to frequent antibiotic use, diabetes, or other underlying host factors) [2].
Despite considerable patient morbidity, options for the treatment of VVC and recurrent VVC (particularly in the setting of azole resistance) are limited. Antifungal agents used for the treatment of VVC are orally and topically available imidazoles (e.g., clotrimazole, butoconazole, miconazole), triazoles (e.g., fluconazole, itraconazole), and polyenes (e.g., nystatin and amphotericin B) [2,3]. Azole drugs, such as fluconazole, have clinically been widely used for treatment of VVC and recurrent VVC, and although fluconazole is a primary therapeutic option for the treatment of these infections, increased use and misuse has resulted in azole resistance among Candida spp. isolated from vulvovaginitis patients. Recent studies showed that fluconazole resistance among these isolates may correlate with CDR1 gene overexpression [5,6].
Alternative treatments for vaginal infections include antiseptic agents, such as boric acid, chlorhexidine (CHX), octenidine dihydrochloride (OCT), and povidone iodine (PVP-I), each with different mechanisms of action. They are safe if administered at appropriate concentrations. Most of the vaginal antiseptics are available in the form of a vaginal douche [1]. These agents have some advantages compared to antifungal drugs, such as (1) they have faster fungicidal activity, (2) their resistance development is much less frequent or absent because of their multitarget mechanism of action, (3) they can also be used for mixed vaginal infections due to their broad antimicrobial spectrum, and (4) they can be used for pre- and postoperative prophylaxis [7].
Boric acid is an inorganic acid which is known for its antibacterial, antifungal, and antiviral activity and its antiseptic and astringent characteristics, and it has been used for decades to treat vulvovaginal and ear infections. It has become a first-line alternative to azoles in the context of resistance, and 600 mg of boric acid administered vaginally once daily in a gelatin capsule for three weeks is currently recommended by the US Centers for Disease Control and Prevention (CDC) for treatment of recurrent C. non-albicans VVC [2,8,9]. Using it as a topical powder can potentially help to control fungal growth, relieve itching and inflammation, and speed up the healing process [8].
CHX is a bisbiguanide antiseptic and preservative with a broad spectrum of antibacterial and antifungal activity used especially in the prevention and treatment of oral mucosal infections. It was found that 0.25–0.5% of CHX vaginal douches demonstrated efficacy against bacterial and fungal vaginitis [10,11].
Another alternative for the treatment of resistant cases is PVP-I. It is a broad-spectrum antiseptic and has germicidal effects against Gram-positive and Gram-negative bacteria, viruses, and fungi. It has been in use for over 60 years as a topical solution, ointment, or vaginal suppository. Clinical applications of PVP-I include antisepsis of the skin, wounds, oral cavity, eyes, intrasurgical lavage, and vagina [8,12].
OCT is a cationic active compound that is well established as skin, mucous membrane, and wound antiseptic solution, and, additionally, it is recommended as an alternative in case of triclosan resistance. Moreover, OCT has broad-spectrum activity, including common pathogens of wound infections and multidrug resistant bacteria [13]. Today, it is an established antiseptic in a wide range of applications and represents an alternative to older substances such as CHX, PVP-I, or triclosan [14]. It was recently shown to be effective even at lower doses than commercially available concentrations against the multidrug-resistant yeast Candida auris, which causes significant outbreaks in hospitals and infections with high mortality [15].
N-chlorotaurine (Cl–HN–CH2–CH2–SO3-, NCT), the N-chloro derivative of the amino acid taurine, is a long-lived oxidant produced by activated human granulocytes and monocytes. Besides immune modulatory effects, NCT has bactericidal (Gram-positive and Gram-negative bacteria), virucidal (herpes simplex, adenoviruses, influenza, SARS-CoV-2 (COVID-19), respiratory syncytial virus, human immune deficiency virus), protozoocidal (amoebae, leishmaniae, and trichomonads), and also fungicidal (yeasts and molds) activities [16,17]. NCT is a mild, long-lived oxidant that can be applied to sensitive body regions as an endogenous antiseptic. The pure crystalline sodium salt of NCT (MW = 181.57 g/mol) can be chemically synthesized [18]. Due to its unspecific oxidative mechanism of action, the development of resistance is extremely unlikely and actually has not been detected in laboratory tests [16,17,18].
Although the antifungal properties of NCT are known, studies investigating its efficacy against Candida spp. are few [19,20]. Therefore, the purpose of this study was to determine the in vitro activities of NCT and conventional antiseptics against Candida isolates from patients with VVC and to compare them.
2. Materials and Methods
2.1. Test Microorganisms
Forty-four strains of Candida isolates were used for the experiment. All strains were collected from patients diagnosed with VVC at the Clinical Microbiology Laboratories of the Group Florence Nightingale Hospitals in Turkey. Twenty-nine of the strains were C. albicans, whereas fifteen of them were C. non-albicans. The total number of strains is shown in Table 1 (fluconazole resistance profiles were conducted in a previous study [21]).The yeasts were identified by VITEK 2 (BioMerieux, Craponne, France) and CHROMagar, and verified by API 20C AUX (BioMerieux, France) systems. Prior to analysis, each isolate was cultured on Sabouraud dextrose agar (SDA, Merck) plates to ensure viability. C. albicans ATCC 10231 was used as the quality control strain.

Table 1.
Total number of test strains and fluconazole resistance profiles.
2.2. Test Products
Five different antiseptics were included in the study. NCT was manufactured at the Institute of Hygiene and Medical Microbiology at the Medical University of Innsbruck, Austria [18]. NCT as a crystalline sodium salt (molecular weight 181.57 g/L) was prepared to be pharmaceutical quality using chloramine T and taurine at the Institute of Hygiene and Medical Microbiology at the Medical University of Innsbruck, Austria [18]. The used lot from 27 November 2017 was sterile and pyrogen-free with a potency of >99%. Final concentration of NCT used in experiments was 0.1% (1 mg/mL, 5.5 mM). Standard concentrations of antiseptics that were within the range of use or possible use were chosen. Commercially available products CHX (2%, Microvem, Altun Sterilizasyon ve Medikal), OCT (0.1%, Octenisept, Schülke & Mayr GmbH), and PVP-I (10%, Batiqon, Alfa Temizlik Medikal ve Sağlik. Ürünleri İmalatı) were provided from manufacturers. Boric acid was dissolved from dry powder with distillated water (10%, Merck).
2.3. Antiseptic Activity Assay
Quantitative killing tests used to assess fungicidal activity were performed as described in the European Standards with some modifications [22,23]. Yeasts were grown in SDA for 24 h. After incubation, cultures were collected into sterile physiological buffered saline (PBS), centrifuged (about 5000× g, 5–10 min), and washed with PBS. Cells were suspended in PBS until a cellular density equivalent to 1–3 × 108 cfu/mL of the McFarland standard was reached. The dilutions of the yeasts were prepared in PBS and spread on duplicate SDA and plates were incubated at 37 °C for 48 h. At the end of the incubation, emergent colonies were counted and the number of cfu/mL were determined to confirm the yeast inoculum.
Initially, suspensions of 1–3 × 108 cfu/mL of yeast were prepared, but after the dilutions (adding skim milk and the test antiseptics, see below) 1–3 × 107 cfu/mL was the final fungal concentration in the samples. An amount of 1 mL of yeast inoculum was added to 1 mL of 10% skim milk (Merck) as organic substances and incubated for 2 min at room temperature. Then, 8 mL of antiseptic solution was added to the mixture and incubated for 30, 60, 90 and 120 min and 24 h at 37 °C and pH 7. Final concentrations of antiseptics were 0.1% NCT, 1.6% CHX, 0.08% OCT, 8% PVP-I, and 8% boric acid. After incubation, 1 mL of suspension was transferred into 8 mL of neutralizer solution and 1 mL of distillated water. The neutralizer solution contained lecithin 3 g/L, polysorbate-80 30 g/L, sodium thiosulfate 5 g/L, and L-histidine 1 g/L, and the pH was adjusted to 7.0. After 5 min of neutralization process, 1 mL of the solution was transferred to plates in duplicate and melted SDA was poured onto the plate. Plates were incubated for 48 h at 30 °C to count yeast colonies and calculate the number of living yeasts. A reduction in cfu/mL by least 4 log10 was determined as fungicidal activity.
2.4. Validation Procedures
The efficacy of inactivation of test agents and absence of antimicrobial effects of the neutralizer were evaluated according to the European Standard Methods to validate the test results [23].
2.5. Statistics
The data are presented as mean values and standard deviations (SD) or standard error of the mean (SEM) of independent experiments with 29 strains of C. albicans and 15 strains of C. non-albicans. The Kruskal–Wallis test was used to test for a difference between the test and control groups. A p-value of <0.05 was considered significant for all tests. Calculations were performed with the GraphPad Prism 8.0.1 software (GraphPad, Inc., La Jolla, CA, USA).
To gain an improved survey on the microbicidal activity of the different antiseptics against C. albicans and C. non-albicans, the recently introduced integral method was used, which transforms the whole killing curve (log10 CFU/mL versus time) into one value of “Bactericidal Activity (BA, log10 CFU ml−1 min−1)” [24]. The method allows an expanded statistical analysis, particularly between killing curves with small differences. One-way analysis of variance (ANOVA) and Tukey’s multiple comparison test were used to test for a difference between BA values of the antiseptics.
3. Results
A total of forty-four strains of Candida isolates were collected from patients with VVC. Among these strains, 29 were identified as C. albicans, whereas there were 8 C. glabrata, 2 Candida tropicalis, 2 Candida kefyr, 1 Candida krusei, 1 Candida famata, and 1 Candida lusitaniae that were identified (Table 1).
The quantitative killing assays with five antiseptics against Candida isolates were determined according to the European Standards. The percentages of yeast strains reduced by ≥4 log10 after the respective incubation time are summarized in Figure 1 and Figure 2. According to these results, 0.08% OCT and 1.6% CHX were the most effective agents against C. albicans within 30 min. PVP-I (8%) and 0.1% NCT killed all C. albicans isolates at 60 and 90 min, respectively, by ≥4 log10. Among the antiseptics, only 8% boric acid did not lead to a ≥4 log10 reduction against all isolates after 24 h (Figure 1), but had a little lower activity against one isolate each of C. albicans and C. non-albicans with a log10 reduction of 3.60 and 3.52, respectively.
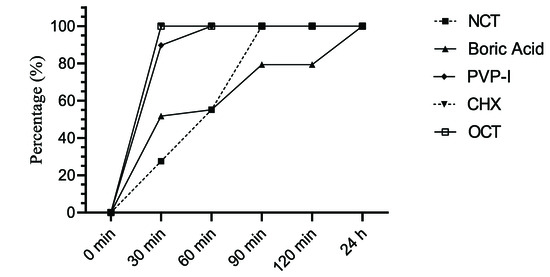
Figure 1.
Percentage of C. albicans strains (n = 29) reduced by ≥4 log10 by antiseptics after indicated incubation periods.
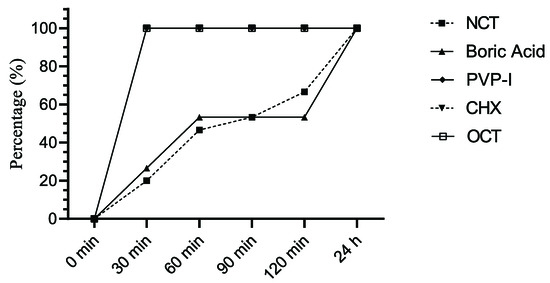
Figure 2.
Percentage of C. non-albicans strains (n = 15) reduced by ≥4 log10 by antiseptics after indicated incubation periods.
OCT, CHX, and PVP-I were more effective antiseptic agents against C. glabrata and other C. non–albicans within 30 min than NCT and boric acid, which needed 24 h to kill the yeasts to the detection limit. (Figure 2).
The killing curves are shown in Figure 3 and Figure 4. The rapid decrease in viable fungal counts by OCT, CHX, and PVP-I is clearly visible with no statistical difference between these three antiseptics and with no difference between C. albicans and C. non-albicans. The killing was significantly slower with boric acid and NCT (p < 0.01), which showed similar curves (p > 0.05). The activity of boric acid and NCT was slightly higher against C. albicans than against C. non-albicans. The killing curve of NCT against different strains of C. albicans was much more uniform than that of boric acid. In C. non-albicans, great differences between single strains occurred for both of these antiseptics. Except C. tropicalis strains, NCT showed quicker activity than boric acid against non-albicans strains. Against eight C. glabrata strains, both disinfectants showed similar effects overall, but different effects for the individual strains.
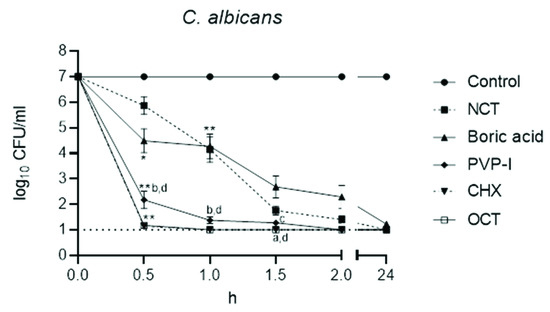
Figure 3.
Fungicidal activity of antiseptics against C. albicans (n = 29) at 37 °C and pH 7. Mean values ± standard error of the mean (SEM); * threshold p < 0.05 versus control; ** threshold p < 0.01 versus control; a p < 0.05 versus NCT; b p < 0.01 versus NCT; c p < 0.05 versus boric acid; d p < 0.01 versus boric acid; p > 0.05 between NCT and boric acid; p > 0.05 between PVP-I, CHX, OCT (Kruskal–Wallis test).
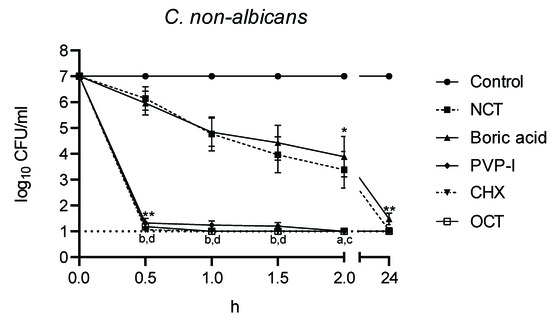
Figure 4.
Fungicidal activity of antiseptics against C. non-albicans (n = 15) at 37 °C and pH 7. Mean values ± standard error of the mean (SEM); * threshold p < 0.05 versus control; ** threshold p < 0.01 versus control; a p < 0.05 versus NCT; b p < 0.01 versus NCT; c p < 0.05 versus boric acid; d p < 0.01 versus boric acid; p > 0.05 between NCT and boric acid; p > 0.05 between PVP-I, CHX, OCT (Kruskal–Wallis test).
Validation of inactivation activity and absence of antifungal activity of the neutralizer was within the margin of error, according to the European Standards.
The killing curves were analyzed with the integral method (Table 2). Because of the large number of isolates, the small differences in the course of the killing curves expressed as BA values also turned out to be significant between all test antiseptics at their individual test concentrations used.

Table 2.
Fungicidal activity (BA values) of antiseptics.
4. Discussion
It has been estimated that 50–75% of women will experience VVC at some time in their lives [25]. Antiseptics are currently available on the market, which can be used for adjunctive treatment of VVC, such as boric acid, CHX, OCT, and PVP-I. In the present work, five different antiseptics were investigated against forty-four strains of Candida isolates which were collected from patients with VVC. CHX, OCT, and PVP-I showed rapid fungicidal activity against all Candida isolates within 30–60 min, whereas boric acid and low-concentrated NCT needed a few hours for a reduction of viable counts by ≥4 log10 steps.
CHX is a bisbiguanide antiseptic with a broad spectrum of antimicrobial activity and many in vitro and in vivo studies have shown that CHX is very effective against Candida infections, including vaginal infections [10]. Additionally, it has been shown that the use of 2% CHX gluconate as a vaginal preoperative preparation was safe and was not associated with increased vaginal irritation or allergic reactions [26]. Previous studies have shown that CHX is effective against vaginal infections of either bacterial or fungal origin, both in vitro and in vivo mouse and rat vaginitis infection models [10,11].
OCT and PVP-I have broad spectrum activity and are used as skin antiseptics against common pathogens. Many researchers have shown that OCT and PVP-I have antimicrobial activity against various yeasts including Candida, which are isolated from VVC [27,28,29]. Consistent with these results, we found that CHX, OCT, and PVP-I were fungicidal against a variety of Candida isolates in the present work.
It is known that boric acid is a safe, alternative, economic option for women with recurrent and chronic symptoms of VVC even when conventional treatment fails [30]. De Seta et al. [31] showed that 5% boric acid was fungicidal against C. albicans strains, and it also interfered with the development of biofilm and hyphal transformation after 24 h of incubation. Our results also showed that it was effective against all Candida strains at 8% concentration within 24 h.
NCT has killing activity against bacteria, fungi, viruses, and parasites, and the 1% aqueous solution can be used as an antiseptic and applied to the eye, skin ulcerations, urinary bladder, outer ear canal, nasal and paranasal sinuses, oral cavity, and lower airways via inhalation [16,32]. According to our results, 0.1% NCT showed fungicidal activity with statistical significance and about a 3 log10 reduction in viable counts against C. albicans isolates within 60 min and C. non-albicans within 2 h.
The antimicrobial properties of NCT have been demonstrated by many researchers. A detailed investigation of the activity of 1.0% NCT against Candida species was provided by Nagl et al. [20]. According to this work, viable counts of C. albicans, C. krusei, C. dubliniensis, and C. tropicalis were reduced significantly by 1–3 log10 within 1–2 h at 37 °C in phosphate buffered saline at pH 7.2. C. glabrata was found the most resistant species with a decrease of 2 log10 after 4 h. Consistent with these results, NCT showed lower fungicidal activity against C. non-albicans (especially C. tropicalis) than C. albicans. Since entrance of the active chlorine of NCT to the cytosol is necessary for the killing of microorganisms [33], it must be assumed that penetration of the substance into C. glabrata is prolonged compared to the other Candida species.
Of note, in the present study a low concentration of NCT, namely 0.1%, was tested in skim milk and revealed approximately similar killing activity as 1.0% in PBS [18]. This is surprising at first view since chlorine consumption by reducing substances in skim milk will decrease the activity. However, it can be explained by an overcompensation of this consumption by chlorine transfer from NCT to amino compounds in the milk with the formation of corresponding chloramines in equilibrium (transchlorination, transhalogenation) [34,35]. Particularly, the formation of monochloramine (NH2Cl) from NCT and ammonium is important, since this compound is more lipophilic and penetrates pathogens better than NCT with clear enhancement of killing activity against bacteria and, particularly, fungi [36,37].
Therefore, transhalogenation significantly increases the microbicidal activity of NCT in the presence of organic matter. Gruber et al. [19] showed that at a concentration of 1% NCT killed bacterial and fungal spores within 10 min and 15 min, respectively, in artificial sputum medium, which mimics the composition of cystic fibrosis mucus. The BA values for fungicidal activity of 1.0% NCT in this study came to 0.71 for C. albicans and to 0.37–0.50 for molds. This means that NCT at the clinically preferred concentration of 1% may have similar microbicidal activity as CHX, OCT, and PVP-I in the presence of organic matter occurring in body fluids and exudates. Although the skim milk used in our study was insufficient to imitate body fluids, it has been shown in previous studies that enhancement by body fluids is a specific advantage of NCT since other antiseptics used in human medicine up to date lose activity by organic matter [17]. In acidic milieu, the killing activity of NCT becomes higher, but the effect is more pronounced in bacteria than in fungi [38,39]. Furthermore, one of the main advantages of NCT over other antiseptics is that NCT is a natural antiseptic with exceptional tolerability by the human mucosa [16]. A further important fact for VVC may be that NCT is active against long-term biofilms of up to several months, irrespective of both single and mixed biofilms of bacteria and C. albicans [40]. Secretory aspartyl proteinases are one of the major virulence factors of C. albicans that have been suggested to play a role in vaginitis [41]. Moreover, secretory aspartyl proteinases of C. albicans are downregulated by sublethal concentrations of NCT [20]. These properties of NCT should be considered for adjunctive treatment of Candida vaginitis. Although NCT may be used for short-term antisepsis before surgery, extensive studies are needed to evaluate the effect of long-term use of NCT as an antiseptic on the vaginal mucosa, vaginal microbiota, and, especially, vaginal lactobacilli.
Because of the widespread use of fluconazole, resistance to this drug is reported in both C. albicans and non-albicans species. According to the results of another study by our group [21] it was found that out of forty-four studied Candida strains, four (two C. glabrata, one C. albicans) were characterized as susceptible dose-dependent, and two (C. glabrata) were found to be resistant to fluconazole. Due to the unspecific oxidizing/chlorinating mechanism of action of active chlorine compounds and confirmed previous studies against different kinds of pathogens, none of the studied Candida strains showed resistance to NCT [37,39,42,43]. Therefore, the use of NCT as an alternative antiseptic is also important in the treatment of VVC caused by fluconazole-resistant Candida strains.
5. Conclusions
Since the treatment of VVC is difficult and challenging for both patients and physicians, alternative treatments for these infections, including antiseptics, draw attention. CHX, OCT, PVP-I, and boric acid have been used for years, including for vaginal infections. Based upon all presently available data, including our results, NCT may be a good candidate to be as an endogenous antiseptic with specific advantages and efficacy against Candida species isolated from VVC. However, studies on the tolerance of human vaginal epithelium and vaginal microbiota to NCT are needed, especially for long-term use as an antiseptic.
Author Contributions
Conceptualization, M.H. and M.N.; methodology, M.H. and O.O.; formal analysis, M.H., F.N.Y. and M.N.; resources, M.N.; data curation, M.N.; writing—original draft preparation, M.H. and F.N.Y.; writing—review and editing, O.O. and M.N.; project administration, M.H.; funding acquisition, M.H. and M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parra Linares, A.M.; Amaya-Guio, J.; Grillo-Ardila, C.F.; Toro Cubides, A.M. Antiseptics and disinfectants for the treatment of vaginal discharge in non-pregnant women. Cochrane Database Syst. Rev. 2019, 11, CD013467. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Sexually Transmitted Infections Treatment Guidelines, Vulvovaginal Candidiasis. Available online: https://www.cdc.gov/std/treatment-guidelines/candidiasis.htm (accessed on 21 February 2021).
- Zhang, J.Y.; Liu, J.H.; Liu, F.D.; Xia, Y.H.; Wang, J.; Liu, X.; Zhang, Z.Q.; Zhu, N.; Yan-Yan Ying, Y.; Huang, X.T. Vulvovaginal candidiasis: Species distribution, fluconazole resistance and drug efflux pump gene overexpression. Mycoses 2014, 57, 584–591. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sobel, J.D.; White, T.C. A Combination Fluorescence Assay Demonstrates Increased Efflux Pump Activity as a Resistance Mechanism in Azole-Resistant Vaginal Candida albicans Isolates. Antimicrob. Agents Chemother. 2016, 60, 5858–5866. [Google Scholar] [CrossRef] [Green Version]
- Mendling, W.; Weissenbacher, E.R.; Gerber, S.; Prasauskas, V.; Grob, P. Use of locally delivered dequalinium chloride in the treatment of vaginal infections: A review. Arch. Gynecol. Obstet. 2016, 293, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Farr, A.; Effendy, I.; Frey Tirri, B.; Hof, H.; Mayser, P.; Petricevic, L.; Ruhnke, M.; Schaller, M.; Schaefer, A.P.A.; Sustr, V.; et al. Guideline: Vulvovaginal candidosis (AWMF 015/072, level S2k). Mycoses 2021, 64, 583–602. [Google Scholar] [CrossRef]
- Powell, A.; Ghanem, K.G.; Rogers, L.; Zinalabedini, A.; Brotman, R.; Zenilman, J.; Tuddenham, S. Clinicians’ use of intravaginal boric acid maintenance therapy for recurrent vulvovaginal candidiasis and bacterial vaginosis. Sex. Transm. Dis. 2019, 46, 810–812. [Google Scholar] [CrossRef]
- Molteni, B.; D’Antuono, A.; Bandini, P.; Sintini, G.; Barcellona, E.; Agnello, A.; Milani, M. Efficacy and tolerability of a new chlorhexidine-based vaginal gel in vaginal infections. Curr. Med. Res. Opin. 2004, 20, 849–853. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, Y.; Chen, M.; Hu, C.; Chen, Y. Functional synergy of antimicrobial peptides and chlorhexidine acetate against gram-negative/gram-positive bacteria and a fungus in vitro and in vivo. Infect. Drug Resist. 2019, 12, 3227–3239. [Google Scholar] [CrossRef] [Green Version]
- Tan, E.L.; Johari, N.H. Comparative in vitro evaluation of the antimicrobial activities of povidone-iodine and other commercially available antiseptics against clinically relevant pathogens. GMS Hyg. Infect. Control. 2021, 16. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Kostek, M.; Drozłowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Dołęgowska, B. The antibacterial activity of lavender essential oil alone and in combination with octenidine dihydrochloride against MRSA strains. Molecules 2020, 25, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hübner, N.O.; Siebert, J.; Kramer, A. Octenidine dihydrochloride, a modern antiseptic for skin, mucous membranes and wounds. Skin Pharmacol. Physiol. 2010, 23, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Ponnachan, P.; Vinod, V.; Pullanhi, U.; Varma, P.; Singh, S.; Biswas, R.; Kumar, A. Antifungal activity of octenidine dihydrochloride and ultraviolet-C light against multidrug-resistant Candida auris. J. Hosp. Infect. 2019, 102, 120–124. [Google Scholar] [CrossRef]
- Gottardi, W.; Nagl, M. N-chlorotaurine, a natural antiseptic with outstanding tolerability. J. Antimicrob Chemother. 2010, 65, 399–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagl, M.; Arnitz, R.; Lackner, M. N-chlorotaurine, a promising future candidate for topical therapy of fungal infections. Mycopathologia 2018, 183, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Gottardi, W.; Nagl, M. Chemical properties of N-chlorotaurine sodium, a key compound in the human defence system. Arch. Pharm. Med. Chem. 2002, 335, 411–421. [Google Scholar] [CrossRef]
- Gruber, M.; Moser, I.; Nagl, M.; Lackner, M. Bactericidal and fungicidal activity of N-chlorotaurine is enhanced in cystic fibrosis sputum medium. Antimicrob. Agents Chemother. 2017, 61, e02527-16. [Google Scholar] [CrossRef] [Green Version]
- Nagl, M.; Gruber, A.; Fuchs, A.; Lell, C.P.; Lemberger, E.M.; Borg-Von Zepelin, M.; Würzner, R. Impact of N-chlorotaurine on viability and production of secreted aspartyl proteinases of Candida spp. Antimicrob. Agents Chemother. 2002, 46, 1996–1999. [Google Scholar] [CrossRef] [Green Version]
- Hacioglu, M.; Guzel, C.B.; Savage, P.B.; Tan, A.S.B. Antifungal susceptibilities, in vitro production of virulence factors and activities of ceragenins against Candida spp. isolated from vulvovaginal candidiasis. Med. Mycol. 2019, 57, 291–299. [Google Scholar] [CrossRef]
- Birteksoz Tan, A.S.; Dosler, S.; Otuk, G. Determination and comparison of the antimicrobial efficacy of alcohol based hand hygiene products. J. Pharm. Istanbul Univ. 2015, 45, 139–152. [Google Scholar]
- European Standard EN 1275; Chemical Ddisinfectants and Antiseptics-Quantitative Suspension Test for Evaluation of Fungicidal or Yeasticidal Activity of Chemical Disinfectants and Antiseptics—Test Method and Requirements (Phase 1). European Committee for Standardization: Brussels, Belgium, 2005.
- Gottardi, W.; Pfleider, J.; Nagl, M. The integral method, a new approach to quantify bactericidal activity. J. Microbiol. Methods. 2015, 115, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, Y.; Zhang, T.; Xue, Y.; Xu, H.; An, R. Risk Factors of vulvovaginal candidiasis among women of reproductive age in Xi’an: A cross-sectional study. Biomed. Res. Int. 2018, 2018, 9703754. [Google Scholar] [CrossRef] [Green Version]
- Al-Niaimi, A.; Rice, L.W.; Shitanshu, U.; Garvens, B.; Fitzgerald, M.; Zerbel, S.; Safdar, N. Safety and tolerability of chlorhexidine gluconate (2%) as a vaginal operative preparation in patients undergoing gynecologic surgery. Am. J. Infect. Control. 2016, 44, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Friese, K.; Neumann, G.; Siebert, J. Topical antiseptics as an alternative in the treatment of acute vulvovaginal candidosis. Arch. Gynecol. Obstet. 2003, 268, 194–197. [Google Scholar] [CrossRef]
- Moore, G.; Schelenz, S.; Borman, A.M.; Johnson, E.M.; Brown, C.S. Yeasticidal activity of chemical disinfectants and antiseptics against Candida auris. J. Hosp. Infect. 2017, 97, 371–375. [Google Scholar] [CrossRef]
- Théraud, M.; Bédouin, Y.; Guiguen, C.; Gangneux, J.P. Efficacy of antiseptics and disinfectants on clinical and environmental yeast isolates in planktonic and biofilm conditions. J. Med. Microbiol. 2004, 53, 1013–1018. [Google Scholar] [CrossRef] [Green Version]
- Iavazzo, C.; Gkegkes, I.D.; Zarkada, I.M.; Falagas, M.E. Boric acid for recurrent vulvovaginal candidiasis: The clinical evidence. J. Womens Health 2011, 20, 1245–1255. [Google Scholar] [CrossRef]
- De Seta, F.; Schmidt, M.; Vu, B.; Essmann, M.; Larsen, B. Antifungal mechanisms supporting boric acid therapy of Candida vaginitis. J. Antimicrob Chemother. 2009, 63, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Arnitz, R.; Stein, M.; Bauer, P.; Lanthaler, B.; Jamnig, H.; Scholl-Bürgi, S.; Stempfl-Al-Jazrawi, K.; Ulmer, H.; Baumgartner, B.; Embacher, S.; et al. Tolerability of inhaled N-chlorotaurine in humans—A double-blind randomized phase I clinical study. Ther. Adv. Resp. Dis. 2018, 12, 1753466618778955. [Google Scholar] [CrossRef]
- Gottardi, W.; Nagl, M. Chlorine covers on living bacteria: The initial step in antimicrobial action of active chlorine compounds. J. Antimicrob. Chemother. 2005, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Grisham, M.B.; Jefferson, M.M.; Melton, D.F.; Thomas, E.L. Chlorination of endogenous amines by isolated neutrophils. Ammonia-dependent bactericidal, cytotoxic, and cytolytic activities of the chloramines. J. Biol. Chem. 1984, 259, 10404–10413. [Google Scholar] [CrossRef]
- Thomas, E.L.; Grisham, M.B.; Jefferson, M.M. Preparation and characterization of chloramines. Methods Enzymol. 1986, 132, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, W.N.; Arnitz, R.; Nagl, M. N-chlorotaurine and ammonium chloride: An antiseptic preparation with strong bactericidal activity. Int. J. Pharm. 2007, 335, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Lackner, M.; Binder, U.; Reindl, M.; Gönül, B.; Fankhauser, H.; Mair, C.; Nagl, M. N-chlorotaurine exhibits fungicidal activity against therapy-refractory Scedosporium species and Lomentospora prolificans. Antimicrob. Agents Chemother. 2015, 59, 6454–6462. [Google Scholar] [CrossRef] [Green Version]
- Nagl, M.; Gottardi, W. In vitro experiments on the bactericidal action of N-chlorotaurine. Hyg. Med. 1992, 17, 431–439. [Google Scholar]
- Nagl, M.; Lass-Flörl, C.; Neher, A.; Gunkel, A.R.; Gottardi, W. Enhanced fungicidal activity of N-chlorotaurine in nasal secretion. J. Antimicrob. Chemother 2001, 47, 871–874. [Google Scholar] [CrossRef]
- Grimus, V.; Coraça-Huber, D.C.; Steixner, S.J.M.; Nagl, M. Activity of N-chlorotaurine against long-term biofilms of bacteria and yeasts. Antibiotics 2021, 10, 891. [Google Scholar] [CrossRef]
- Pericolini, E.; Gabrielli, E.; Amacker, M.; Kasper, L.; Roselletti, E.; Luciano, E.; Sabbatini, S.; Kaeser, M.; Moser, C.; Hube, B.; et al. Secretory aspartyl proteinases cause vaginitis and can mediate vaginitis caused by Candida albicans in mice. mBio 2015, 6, e00724-15. [Google Scholar] [CrossRef] [Green Version]
- Anich, C.; Orth-Höller, D.; Lackner, M.; Nagl, M. Microbicidal activity of N-chlorotaurine against multiresistant nosocomial bacteria. J. Appl. Microbiol. 2021, 131, 1742–1748. [Google Scholar] [CrossRef]
- Lackner, M.; Rössler, A.; Volland, A.; Stadtmüller, M.; Müllauer, B.; Banki, Z.; Ströhle, J.; Luttick, A.; Fenner, J.; Stoiber, H.; et al. N-chlorotaurine, a novel inhaled virucidal antiseptic is highly active against respiratory viruses including SARS-CoV-2 (COVID-19) in vitro. Emerg. Microbes Infection. 2022, 11, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).