Antibiotic-Induced Treatments Reveal Stress-Responsive Gene Expression in the Endangered Lichen Lobaria pulmonaria
Abstract
1. Introduction
2. Methods
2.1. Study Species
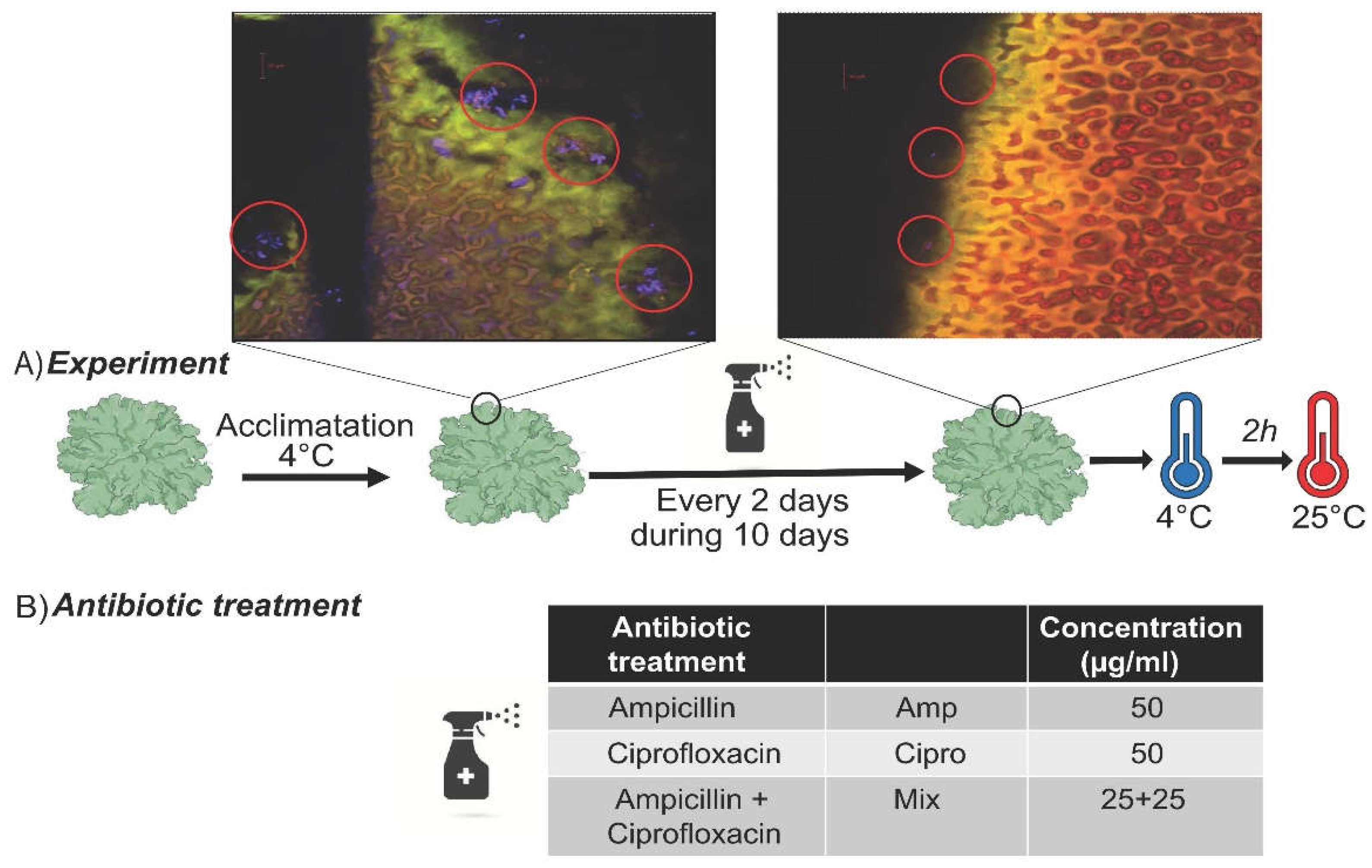
2.2. Differential Visualization of Bacteria on Lichen Surfaces
2.3. Antibiotic Treatments
2.4. RNA Extraction and DNase Digestion
2.5. RNA Sequencing
2.6. Data Analysis
2.7. Differential Expression Analysis
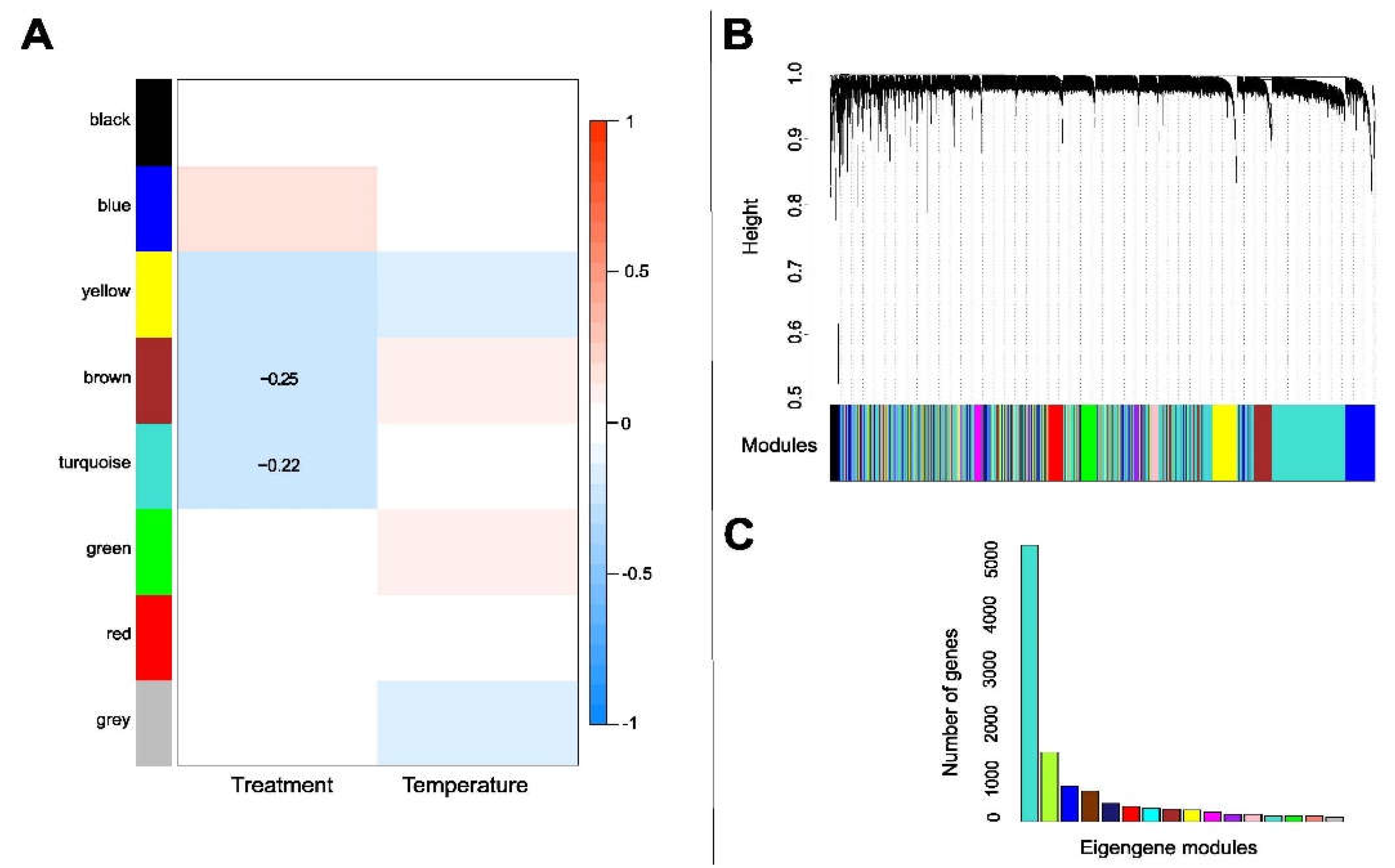
2.8. Weighted Gene Co-Expression Network Analysis
2.9. Functional Enrichment Analysis
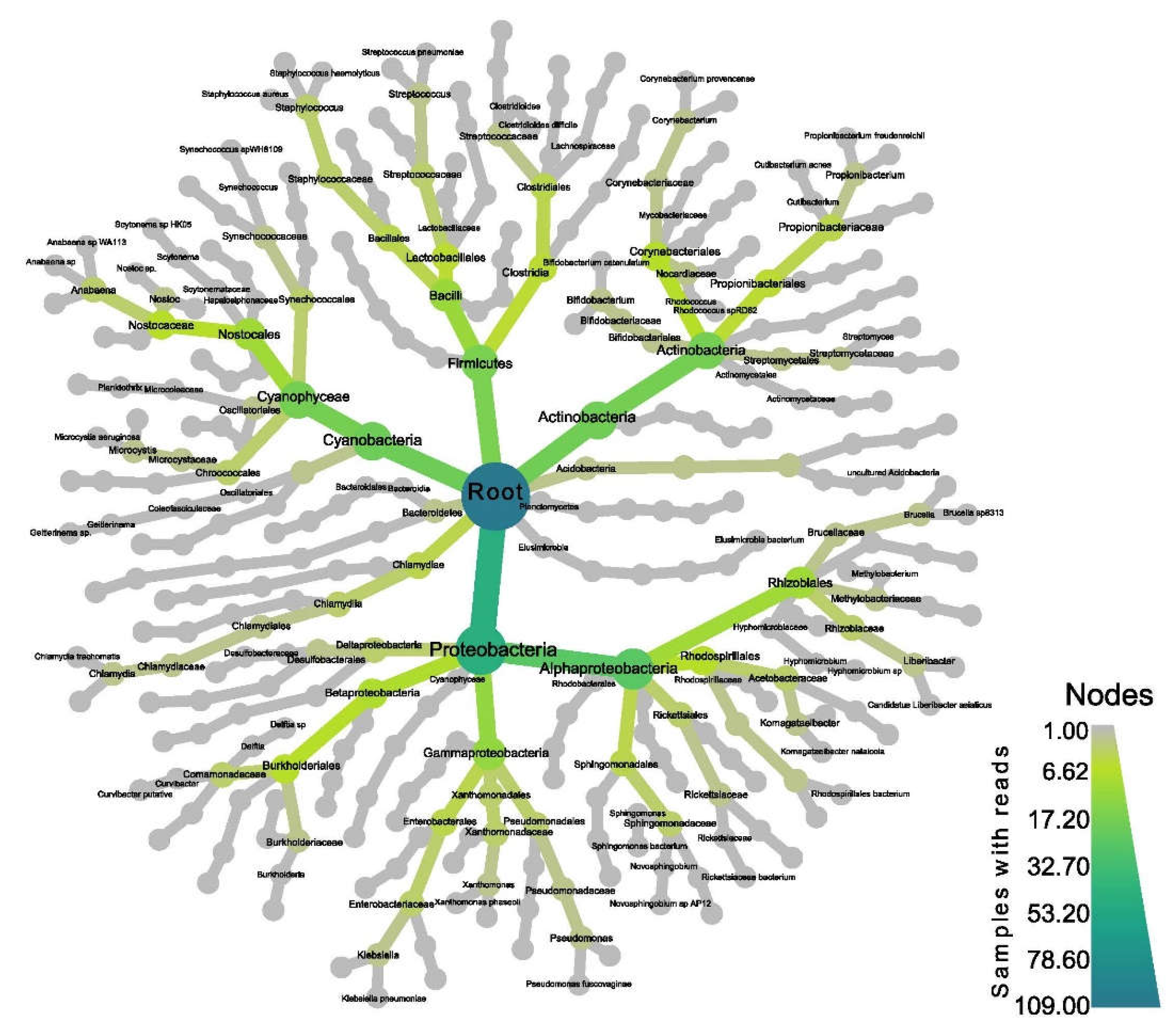
2.10. Analysis of Bacterial Communities
3. Results
3.1. Summary Statistics and Overview of RNA-Seq
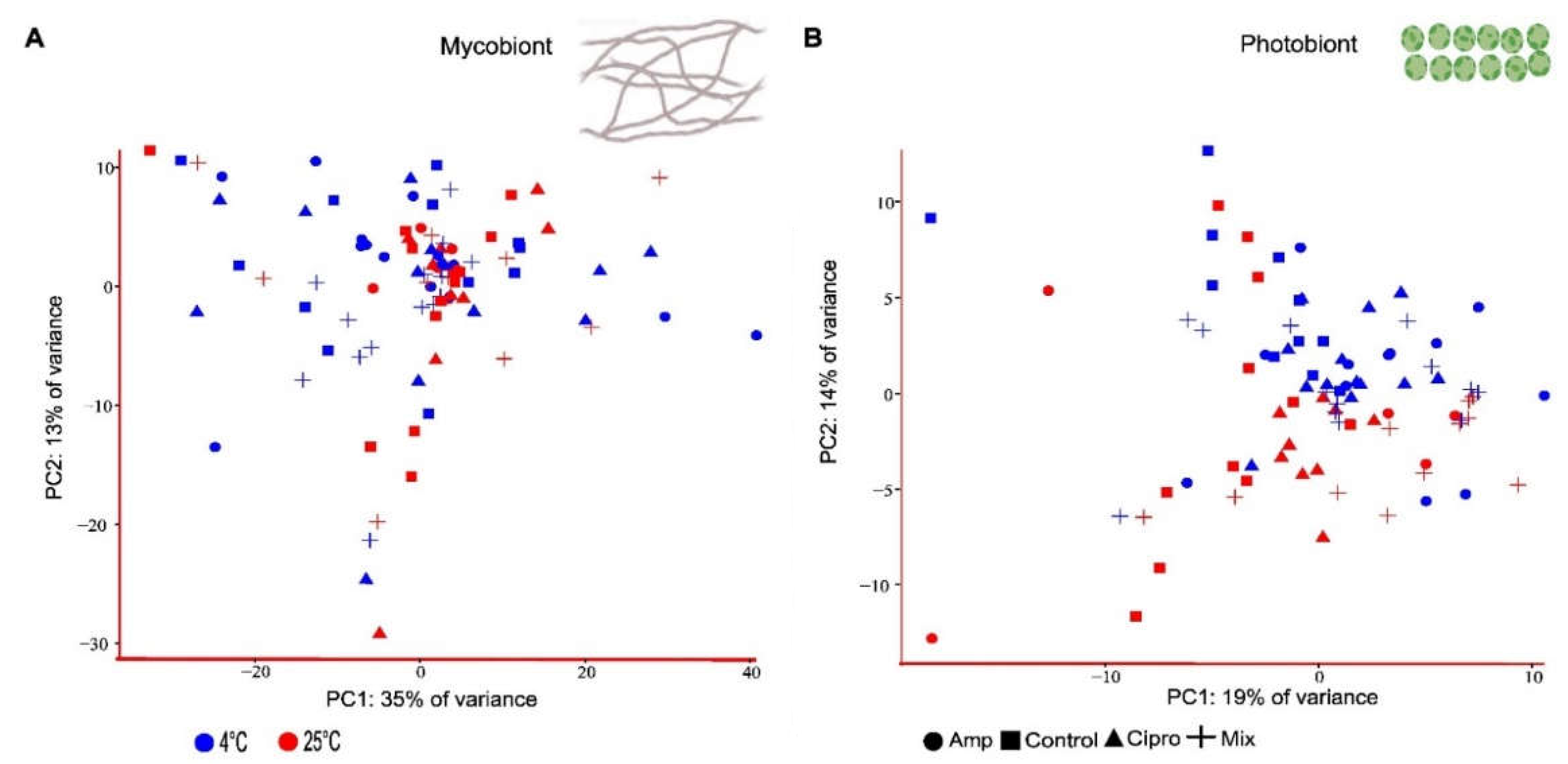
3.2. Overall Expression Patterns in Mycobiont and Photobiont Transcripts
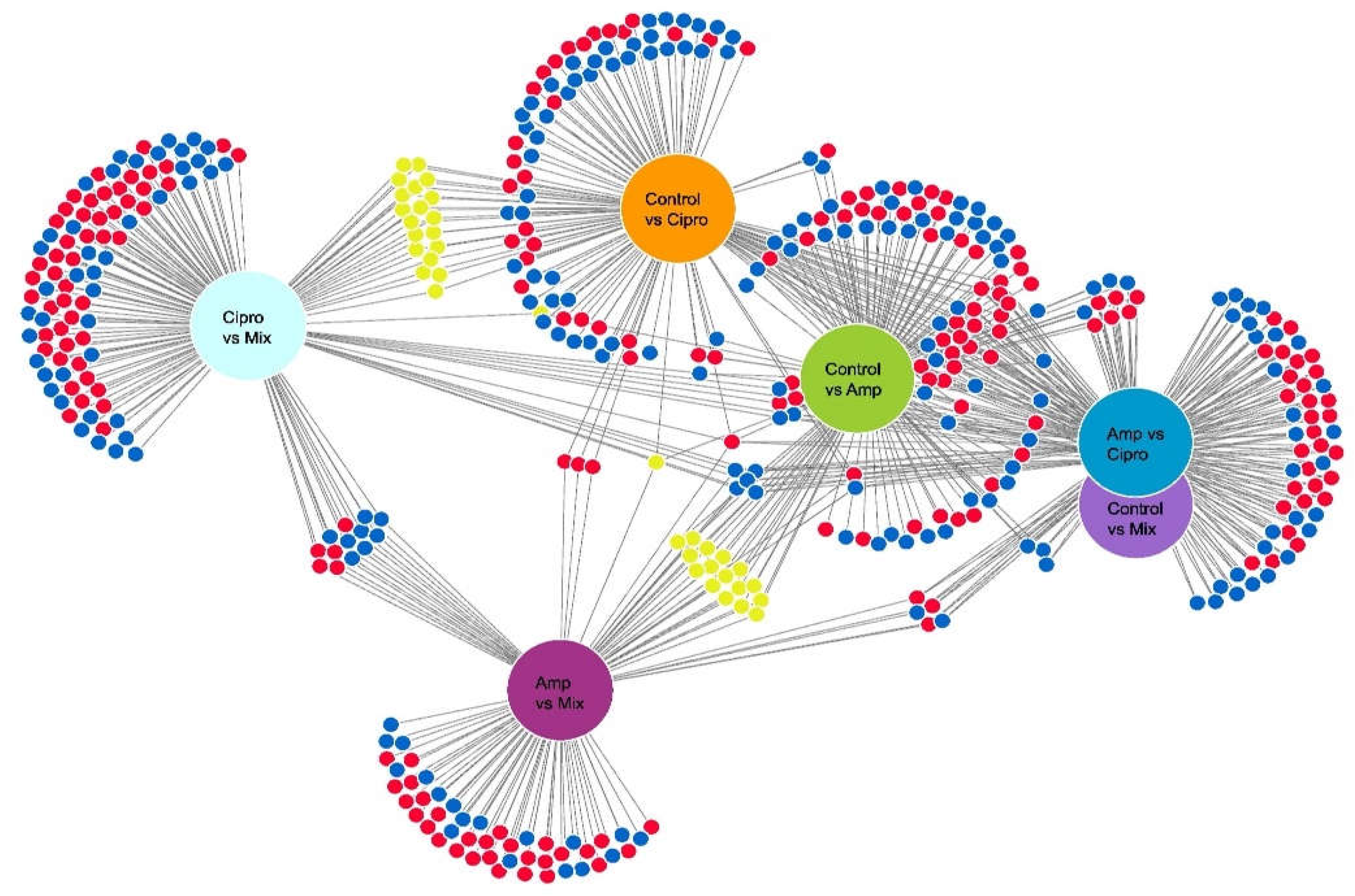
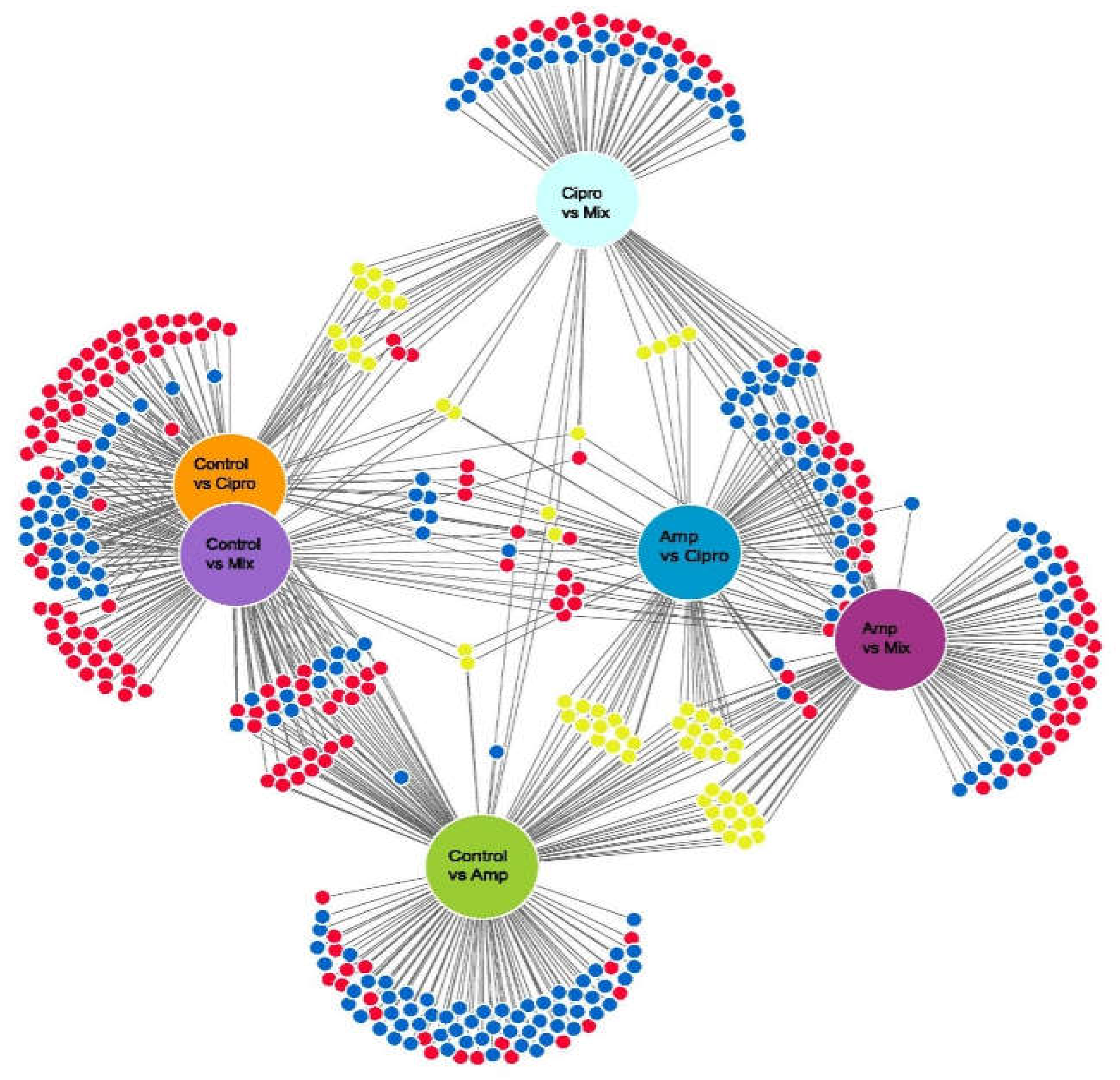
3.3. Mycobiont Differential Gene Expression in Pairwise Comparisons of Antibiotic Treatments
3.4. Mycobiont Differential Gene Expression Associated with Temperature Changes, Resulting from Pairwise Differential Expression Comparisons
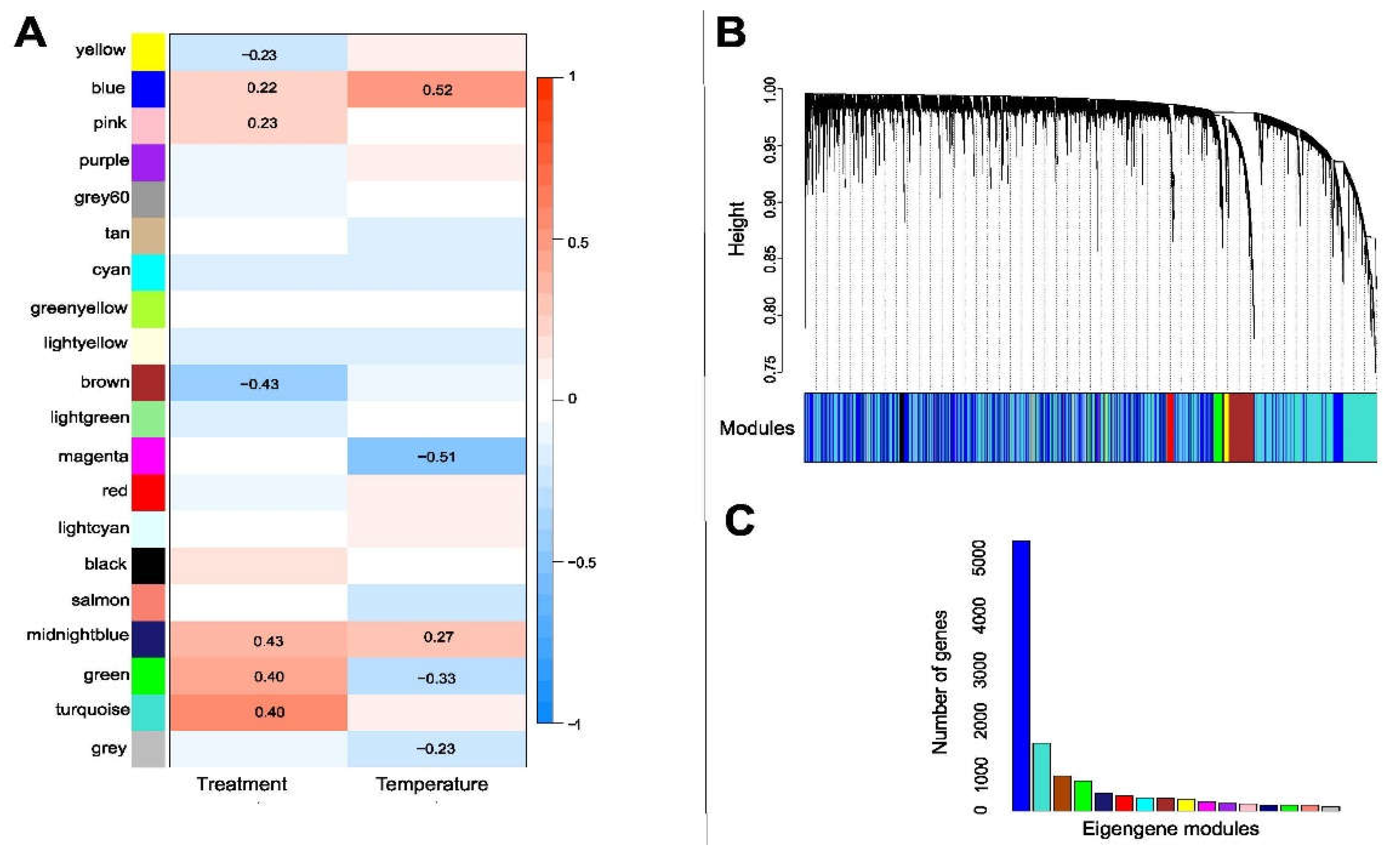
3.5. Gene Clusters Associated with Thermal and Antibiotic Treatments in the Mycobiont
3.6. Photobiont Differential Gene Expression Associated with Antibiotic Treatment and Temperature Change, Resulting from Pairwise Comparisons
3.7. Gene Clusters Associated with Antibiotic Treatment and Temperature Change in the Photobiont
3.8. Microbiome
4. Discussion
4.1. Differential Gene Expression in Response to Microbiome Manipulations of L. pulmonaria
4.2. Differential Expression Associated with Changes in Temperature in L. pulmonaria
4.3. Individuals Treated with Different Antibiotics and a Temperature Increase Reveal Plastic Gene Expression Responses at the Whole-Transcriptome Level in L. pulmonaria
4.4. Microbiome
4.5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goga, M.; Baláž, M.; Daneu, N.; Elečko, J.; Tkáčiková, L.; Marcinčinová, M.; Bačkor, M. Biological activity of selected lichens and lichen-based Ag nanoparticles prepared by a green solid-state mechanochemical approach. Mater. Sci. Eng. 2021, 119, 111640. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; He, J. Effect of antibiotics in the environment on microbial populations. Appl. Microbiol. Biotechnol. 2010, 87, 925–945. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Beck, I.C. Effects of sulfonamide and tetracycline antibiotics on soil microbial activity and microbial biomass. Chemosphere 2005, 59, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Kotzerke, A.; Sharma, S.; Schauss, K.; Heuer, H.; Thiele-Bruhn, S.; Smalla, K.; Wilke, B.M.; Schloter, M. Alterations in soil microbial activity and N-transformation processes due to sulfadiazine loads in pig-manure. Environ. Pollut. 2008, 153, 315–321. [Google Scholar] [CrossRef]
- Oliveira, R.; McDonough, S.; Ladewig, J.C.L.; Soares, A.M.V.M.; Nogueira, A.J.A.; Domingues, I. Effects of oxytetracycline and amoxicillin on development and biomarkers activities of zebrafish (Danio rerio). Environ. Toxicol. Pharm. 2013, 36, 903–912. [Google Scholar] [CrossRef]
- Yonar, M.E.; Yonar, S.M.; Silici, S. Protective effect of propolis against oxidative stress and immunosuppression induced by oxytetracycline in rainbow trout (Oncorhynchus mykiss, W.). Fish Shellfish Immunol. 2011, 31, 318–325. [Google Scholar] [CrossRef]
- Yonar, M.E. The effect of lycopene on oxytetracycline-induced oxidative stress and immunosuppression in rainbow trout (Oncorhynchus mykiss, W.). Fish Shellfish Immunol. 2012, 30, 994–1001. [Google Scholar] [CrossRef]
- Gnanasoundari, M.; Pari, L. Impact of naringenin on oxytetracycline-mediated oxidative damage in kidney of rats. Ren. Fail. 2006, 28, 599–605. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K.I. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lee, J.; Ji, K.; Takeda, S.; Choi, K. Potentials and mechanisms of genotoxicity of six pharmaceuticals frequently detected in freshwater environment. Toxicol. Lett. 2012, 211, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Botelho, R.G.; Christofoletti, C.A.; Correia, J.E.; Ansoar, Y.; Olinda, R.A.; Tornisielo, V.L. Genotoxic responses of juvenile tilapia (Oreochromis niloticus) exposed to florfenicol and oxytetracycline. Chemosphere 2015, 132, 206–212. [Google Scholar] [CrossRef]
- Rodrigues, S.; Antunes, S.C.; Correia, A.T.; Nunes, B. Rain-bow trout (Oncorhynchus mykiss) pro-oxidant and genotoxic responses following acute and chronic exposure to the antibiotic oxytetracycline. Ecotoxicology 2017, 26, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Bai, Y.; Chen, Z.; Mo, J.; Li, Q.; Sun, H.; Zhang, Q. Transcriptomic analysis suggests the inhibition of DNA damage repair in green alga Raphidocelis subcapitata exposed to roxithromycin. Ecotoxicol. Environ. Saf. 2020, 201, 110737. [Google Scholar] [CrossRef]
- Guo, J.; Peng, J.; Lei, Y.; Kanerva, M.; Li, Q.; Song, J.; Guo, J.; Sun, H. Comparison of oxidative stress induced by clarithromycin in two freshwater microalgae Raphidocelis subcapitata and Chlorella vulgaris. Aquat. Toxicol. 2020, 219, 105376. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Hill, R.; Doblin, M.; Ralph, P.J. Microbial consortia increase thermal tolerance of corals. Mar. Biol. 2012, 159, 1763–1771. [Google Scholar] [CrossRef]
- Dedeine, F.; Vavre, F.; Fleury, F.; Loppin, B.; Hochberg, M.E.; Boulétreau, M. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc. Natl. Acad. Sci. USA 2001, 98, 6247–6252. [Google Scholar] [CrossRef]
- Zchori-Fein, E.; Borad, C.; Harari, A.R. Oogenesis in the date stone beetle, Coccotrypes dactyliperda, depends on symbiotic bacteria. Physiol. Entomol. 2006, 31, 164–169. [Google Scholar] [CrossRef]
- Nash, T.H., III. Lichen Biology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008; p. 486. [Google Scholar] [CrossRef]
- Grube, M.; Cardinale, M.; de Castro, J.V.; Müller, H.; Berg, G. Species-specific structural and functional diversity of bacterial communities in lichen symbioses. ISME J. 2009, 3, 1105–1115. [Google Scholar] [CrossRef]
- Grube, M.; Cernava, T.; Soh, J.; Fuchs, S.; Aschenbrenner, I.; Lassek, C.; Wegner, U.; Becher, D.; Riedel, K.; Sensen, C.W.; et al. Exploring functional contexts of symbiotic sustain within lichen-associated bacteria by comparative omics. ISME J. 2015, 9, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L.; Grube, M. Lichens redefined as complex ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef]
- Lawrey, J.D.; Diederich, P. Lichenicolous fungi: Interactions, evolution, and biodiversity. Bryologist 2003, 106, 80–120. [Google Scholar] [CrossRef]
- Hodkinson, B.P.; Lutzoni, F. A microbiotic survey of lichen-associated bacteria reveals a new lineage from the Rhizobiales. Symbiosis 2009, 49, 163–180. [Google Scholar] [CrossRef]
- Schneider, T.; Schmid, E.; De Castro, J.V., Jr.; Cardinale, M.; Eberl, L.; Grube, M.; Berg, G.; Riedel, K. Structure and function of the symbiosis partners of the lung lichen (Lobaria pulmonaria (L.) Hoffm.) analyzed by metaproteomics. Proteomics 2011, 11, 2752–2756. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Grube, M.; Castro, J.V., Jr.; Müller, H.; Berg, G. Bacterial taxa associated with the lung lichen Lobaria pulmonaria are differentially shaped by geography and habitat. FEMS Microbiol. Lett. 2012, 329, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, I.A.; Cernava, T.; Berg, G.; Grube, M. Understanding microbial multi-species symbioses. Front Microbiol. 2016, 18, 180. [Google Scholar] [CrossRef]
- Muggia, L.; Klug, B.; Berg, G.; Grube, M. Localization of bacteria in lichens from Alpine soil crusts by fluorescence in situ hybridization. Appl. Soil Ecol. 2013, 68, 20–25. [Google Scholar] [CrossRef]
- Spribille, T.; Tuovinen, V.; Resl, P.; Vanderpool, D.; Wolinski, H.; Aime, M.C.; Schneider, K.; Stabentheiner, E.; Toome-Heller, M.; Thor, G.; et al. Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science 2016, 353, 488–492. [Google Scholar] [CrossRef]
- Cheviron, Z.A.; Whitehead, A.; Brumfield, R.T. Transcriptomic variation and plasticity in rufouscollared sparrows (Zonotrichia capensis) along an altitudinal gradient. Mol. Ecol. 2008, 17, 4556–4569. [Google Scholar] [CrossRef]
- Whitehead, A.; Triant, D.A.; Champlin, D.; Nacci, D. Comparative transcriptomics implicates mechanisms of evolved pollution tolerance in a killifish population. Mol. Ecol. 2010, 19, 5186–5203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Zhou, Q.; Zhang, X.; Wei, J. Comparative transcriptome analysis of the lichen-forming fungus Endocarpon pusillum elucidates its drought adaptation mechanisms. Sci. China Life Sci. 2015, 58, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Bayram, O.; Feussner, K.; Dumkow, M.; Herrfurth, C.; Feussner, I.; Braus, G.H. Changes of global gene expression and secondary metabolite accumulation during light-dependent Aspergillus nidulans development. Fungal Genet. Biol. 2016, 87, 30–53. [Google Scholar] [CrossRef] [PubMed]
- Steinhäuser, S.S.; Andrésson, S.; Pálsson, A.; Werth, S. Fungal and cyanobacterial gene expression in a lichen symbiosis: Effect of temperature and location. Fungal Biol. 2016, 120, 1194–1208. [Google Scholar] [CrossRef]
- Takahashi, H.; Kusuya, Y.; Hagiwara, D.; Takahashi-Nakaguchi, A.; Sakai, K.; Gonoi, T. Global gene expression reveals stress responsive genes in Aspergillus fumigatus mycelia. BMC Genom. 2017, 18, 942. [Google Scholar] [CrossRef]
- Kenkel, C.D.; Matz, M.V. Gene expression plasticity as a mechanism of coral adaptation to a variable environment. Nat. Ecol. Evol. 2016, 1, 0014. [Google Scholar] [CrossRef]
- Lohman, B.K.; Stutz, W.E.; Bolnick, D.I. Gene expression stasis and plasticity following migration into a foreign environment. Mol. Ecol. 2017, 26, 4657–4670. [Google Scholar] [CrossRef]
- Lopez-Maury, L.; Marguerat, S.; Bahler, J. Tuning gene expression to changing environments: From rapid responses to evolutionary adaptation. Nat. Rev. Genet. 2008, 9, 583–593. [Google Scholar] [CrossRef]
- Kraft, M.; Scheidegger, C.; Werth, S. Stressed out: The effects of heat stress and parasitism on gene expression of the lichen-forming fungus Lobaria pulmonaria. Lichenologist 2022, 54, 71–83. [Google Scholar] [CrossRef]
- Chavarria-Pizarro, T.; Resl, P.; Janjic, A.; Werth, S. Gene expression responses to thermal shifts in the endangered lichen Lobaria pulmonaria. Mol. Ecol. 2021, 31, 839–858. [Google Scholar] [CrossRef]
- Werth, S.; Wagner, H.H.; Holderegger, R.; Kalwij, J.M.; Scheidegger, C. Effect of disturbances on the genetic diversity of an old-forest associated lichen. Mol. Ecol. 2006, 15, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Škaloud, P.; Friedl, T.; Hallmann, C.; Beck, A.; Grande, F.D. Taxonomic revision and species delimitation of coccoid green algae currently assigned to the genus Dictyochloropsis (Trebouxiophyceae, Chlorophyta). J. Phycol. 2016, 52, 599–617. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, C.; Scheidegger, C. New morphological aspects of cephalodium formation in the lichen Lobaria pulmonaria (Lecanorales, Ascomycota). Lichenologist 2013, 45, 77–87. [Google Scholar] [CrossRef]
- Aschenbrenner, I.A.; Cardinale, M.; Berg, G.; Grube, M. Microbial cargo: Do bacteria on symbiotic propagules reinforce the microbiome of lichens? Environ. Microbiol. 2014, 16, 3743–3752. [Google Scholar] [CrossRef] [PubMed]
- Erlacher, A.; Cernava, T.; Cardinale, M.; Soh, J.; Sensen, C.W.; Grube, M.; Berg, G. Rhizobiales as functional and endosymbiontic members in the lichen symbiosis of Lobaria pulmonaria. Front. Microbiol. 2015, 6, 53. [Google Scholar] [CrossRef]
- Cardinale, M.; De Castro, J.V., Jr.; Mã¼Ller, H.; Berg, G.; Grube, M. In situ analysis of the bacterial community associated with the reindeer lichen Cladonia arbuscula reveals predominance of Alphaproteobacteria. FEMS Microbiol. Ecol. 2008, 66, 63–71. [Google Scholar] [CrossRef]
- Alquéres, S.; Meneses, C.; Rouws, L.; Rothballer, M.; Baldani, I.; Schmid, M.; Hartmann, A. The bacterial superoxide dismutase and glutathione reductase are crucial for endophytic colonization of rice roots by Gluconacetobacter diazotrophicus PAL5. MPMI 2013, 26, 937–945. [Google Scholar] [CrossRef]
- Amann, R.I.; Krumholz, L.; Stahl, D.A. Fluorescent-oligonucleotide probing of whole cells for determinative and environmental studies in microbiology. J. Bacteriol. 1990, 172, 762–770. [Google Scholar] [CrossRef]
- Daims, H.; Bruhl, A.; Amann, R.I.; Schleifer, K.-H.; Wagner, M. The domain specific probe EUB-338 is insufficient for the detection of all bacteria: Development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 1999, 22, 434–444. [Google Scholar] [CrossRef]
- Janjic, A.; Wange, L.E.; Bagnoli, J.W.; Geuder, J.; Nguyen, P.; Richter, D.; Vieth, B.; Vick, B.; Jeremias, I.; Ziegenhain, C.; et al. Prime-seq, efficient and powerful bulk RNA sequencing. Genome Biol. 2022, 23, 88. [Google Scholar] [CrossRef]
- Parekh, S.; Ziegenhain, C.; Vieth, B.; Enard, W.; Hellmann, I. zUMIs—A fast and flexible pipeline to process RNA sequencing data with UMIs. GigaScience 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence alignment/map (SAM) format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Salzberg, S.L. TopHat-Fusion: An algorithm for discovery of novel fusion transcripts. Genome Biol. 2011, 12, R72. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Research 2015, 4, 1521. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Sun, L.; Dong, S.; Ge, Y.; Fonseca, J.P.; Robinson, Z.T.; Mysore, K.; Mehta, P. DiVenn: An interactive and integrated web-based visualization tool for comparing gene lists. Front. Genet. 2019, 10, 421. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Buchfink, B.; Reuter, C.; Drost, H. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 2021, 18, 366–368. [Google Scholar] [CrossRef]
- Huson, D.H.; Beier, S.; Flade, I.; Gorska, A.; El-Hadidi, H.; Mitra, S.; Ruscheweyh, H.; Tappu, R. MEGAN community edition–interactive exploration and analysis of large-scale microbiome sequencing data. PLoS Comput. Biol. 2016, 12, e1004957. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Breitwieser, F.P.; Thielen, P.; Salzberg, S.L. Bracken: Estimating species abundance in metagenomics data. PeerJ Comput. Sci. 2017, 3, e104. [Google Scholar] [CrossRef]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Foster, Z.S.L.; Sharpton, T.J.; Grünwald, N.J. Metacoder: An R package for visualization and manipulation of community taxonomic diversity data. PLoS Comput. Biol. 2017, 13, e1005404. [Google Scholar] [CrossRef]
- Edlund, A.; Ek, K.; Breitholtz, M.; Gorokhova, E. Antibiotic-induced change of bacterial communities associated with the copepod Nitocra spinipes. PLoS ONE 2012, 7, e33107. [Google Scholar] [CrossRef]
- Mills, E.; Shechtman, K.; Loya, Y.; Rosenberg, E. Bacteria appear to play important roles in both causing and preventing the bleaching of the coral Oculina patagonica. Mar. Ecol. Prog. Ser. 2013, 489, 155–162. [Google Scholar] [CrossRef]
- Glasl, B.; Herndl, G.J.; Frade, P.R. The microbiome of coral surface mucus has a key role in mediating holobiont health and survival upon disturbance. ISME J. 2016, 10, 2280–2292. [Google Scholar] [CrossRef]
- Kenkel, C.; Meyer, E.; Matz, M.V. Evidence for a host role in thermotolerance divergence between populations of the mustard hill coral (Porites astreoides) from different reef environments. Mol. Ecol. 2013, 22, 4335–4348. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lanetty, M.; Harii, S.; Hoegh-Guldberg, O. Early molecular responses of coral larvae to hyperthermal stress. Mol. Ecol. 2009, 18, 5101–5114. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology 2004, 14, 53R–62R. [Google Scholar] [CrossRef]
- Antonyuk, L.P.; Evseeva, N.V. Wheat lectin as a factor in plant microbial communication and a stress response protein. Microbiology 2006, 75, 470–475. [Google Scholar] [CrossRef]
- Miao, V.P.W.; Manoharan, S.; Snæbjarnarson, V.; Andrésson, Ó.S. Expression of lec-1, a mycobiont gene encoding a galectin-like protein in the lichen Peltigera membranacea. Symbiosis 2012, 57, 23–31. [Google Scholar] [CrossRef]
- Dasgupta, A.; Sprouse, R.O.; French, S.; Aprikian, P.; Hontz, R.; Juedes, S.A.; Smith, J.S.; Beyer, A.L.; Auble, D.T. Regulation of rRNA synthesis by TATA-binding protein-associated factor Mot1. Mol. Cell Biol. 2007, 27, 2886–2896. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Easton, D.B.; Subjeck, J.R. Large mammalian hsp70 family proteins, hsp110 and grp170, and their roles in biology and cancer therapy. Protein Rev. 2007, 7, 178–205. [Google Scholar] [CrossRef]
- Doyle, S.M.; Wickner, S. Hsp104 and ClpB: Protein disaggregating machines. Trends Biochem. Sci. 2009, 34, 40–48. [Google Scholar] [CrossRef]
- Bahn, Y.-S.; Xue, C.; Idnurm, A.; Rutherford, J.C.; Heitman, J.E.; Cardenas, M. Sensing the environment: Lessons from fungi. Nat. Rev. Microbiol. 2007, 5, 57–69. [Google Scholar] [CrossRef]
- Brown, S.M.; Campbell, L.T.; Lodge, J.K. Cryptococcus neoformans, a fungus under stress. Curr. Opin. Microbiol. 2007, 10, 320–325. [Google Scholar] [CrossRef]
- Alonso, A.; Rojo, F.; Martínez, J.L. Environmental and clinical isolates of Pseudomonas aeruginosa show pathogenic and biodegradative properties irrespective of their origin. Environ. Microbiol. 1999, 1, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lehr, N.; Trail, F.; Townsend, J.P. Differential impact of nutrition on developmental and metabolic gene expression during fruiting body development in Neurospora crassa. Fungal Genet. Biol. 2012, 49, 405–413. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barabote, R.D.; Thekkiniath, J.; Strauss, R.E.; Vediyappan, G.; Fralick, J.A.; Francisco, M.J.S. Xenobiotic efflux in bacteria and fungi: A genomics update. Adv. Enzymol. Relat. Areas Mol. Biol. 2011, 77, 237–306. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lemieux, M.J.; Song, J.; Auer, M.; Wang, D.N. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science 2003, 301, 616–620. [Google Scholar] [CrossRef]
- Roohparvar, R.; Mehrabi, R.; Van Nistelrooy, J.G.M.; Zwiers, L.-H.; De Waard, M.A. The drug transporter MgMfs1 can modulate sensitivity of field strains of the fungal wheat pathogen Mycosphaerella graminicola to the strobilurin fungicide trifloxystrobin. Pest Manag. Sci. 2008, 64, 685–693. [Google Scholar] [CrossRef]
- Rogers, P.D.; Barker, K.S. Genome-wide expression profile analysis reveals coordinately regulated genes associated with stepwise acquisition of azole resistance in Candida albicans clinical isolates. Antimicrob. Agents Chemother. 2003, 47, 1220–1227. [Google Scholar] [CrossRef]
- Holmes, A.R.; Lin, Y.-H.; Niimi, K.; Lamping, E.; Keniya, M.; Niimi, M.; Tanabe, K.; Monk, B.C.; Cannon, R.D. ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazoleresistant Candida albicans clinical isolates. Antimicrob. Agents Chemother. 2008, 52, 3851–3862. [Google Scholar] [CrossRef]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef]
- Mao, Y.; Yu, Y.; Ma, Z.; Li, H.; Yu, W.; Cao, L.; He, Q. Azithromycin induces dual effects on microalgae: Roles of photosynthetic damage and oxidative stress. Ecotoxicol. Environ. Saf. 2021, 222, 112496. [Google Scholar] [CrossRef]
- Hofmann, G.E. Patterns of Hsp gene expression in ectothermic marine organisms on small to large biogeographic scales. Integr. Comp. Biol. 2005, 45, 247–255. [Google Scholar] [CrossRef]
- Gracey, A.Y.; Chaney, M.L.; Boomhower, J.P.; Tyburczy, W.R.; Connor, K.; Somero, G.N. Rhythms of gene expression in a fluctuating intertidal environment. Curr. Biol. 2008, 18, 1501–1507. [Google Scholar] [CrossRef]
- Lockwood, B.L.; Sanders, J.G.; Somero, G.N. Transcriptomic responses to heat stress in invasive and native blue mussels (genus Mytilus): Molecular correlates of invasive success. J. Exp. Biol. 2010, 213, 3548–3558. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.; Atkin, O.; Bonser, S.; Davidson, A.; Finnegan, E.; Mathesius, U.; Poot, P.; Purugganan, M.; Richards, C.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.-Y.; Kaplan, F.; Lee, K.-J.; Guy, C.L. Acquired tolerance to temperature extremes. Trends Plant Sci. 2003, 8, 179–187. [Google Scholar] [CrossRef]
- Sun, W.; van Montagu, M.; Verbruggen, N. Small heat shock proteins and stress tolerance in plants. Biochim. Biophys. Acta (BBA)—Gene Struct. Expr. 2002, 1577, 1–9. [Google Scholar] [CrossRef]
- Wu, C. Heat shock transcription factors: Structure and regulation. Annu. Rev. Cell Dev. Biol. 1995, 11, 441–469. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Hu, G.; Han, B. Genome-wide survey and expression profiling of heat shock proteins and heat shock factors revealed overlapped and stress specific response under abiotic stresses in rice. Plant Sci. 2009, 176, 583–590. [Google Scholar] [CrossRef]
- Hu, W.; Hu, G.; Han, B. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding competent state. EMBO J. 1997, 16, 659–671. [Google Scholar] [CrossRef]
- Lee, G.J.; Roseman, A.M.; Saibil, H.R.; Vierling, E. Molecular characterization of rice hsp101: Complementation of yeast hsp104 mutation by disaggregation of protein granules and differential expression in indica and japonica rice types. Plant Mol. Biol. 2003, 51, 543–553. [Google Scholar] [CrossRef]
- Pratt, W.B.; Toft, D.O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 2003, 228, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Matsuba, S.; Funatsuki, H.; Kawaguchi, K.; Saruyama, H.; Tanida, M.; Sato, Y. Over-expression of a small heat shock protein, sHSP17.7, confers both heat tolerance and UV-B resistance to rice plants. Mol. Breed. 2004, 13, 165–175. [Google Scholar] [CrossRef]
- Thao, N.P.; Chen, L.; Nakashima, A.; Hara, S.-I.; Umemura, K.; Takahashi, A.; Shirasu, K.; Kawasaki, T.; Shimamoto, K. RAR1 and HSP90 form a complex with Rac/Rop GTPase and function2 in innate-immune responses in rice. Plant Cell 2007, 19, 4035–4045. [Google Scholar] [CrossRef] [PubMed]
- Baniwal, S.K.; Chan, K.Y.; Scharf, K.-D.; Nover, L. Role of heat stress transcription factor HsfA5 as specific repressor of HsfA4. J. Biol. Chem. 2007, 282, 3605–3613. [Google Scholar] [CrossRef]
- Sato, Y.; Yokoya, S. Enhanced tolerance to drought stress in transgenic rice plants overexpressing a small heat-shock protein, sHSP17.7. Plant Cell Rep. 2008, 27, 329–334. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Kristensen, T.N.; Loeschcke, V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 2003, 6, 1025–1037. [Google Scholar] [CrossRef]
- Sharp, V.; Brown, B.E.; Miller, D.J. Heat shock protein (hsp 70) expression in the tropical reef coral Goniopora djiboutiensis. J. Therm. Biol. 1997, 22, 11–19. [Google Scholar] [CrossRef]
- Meyer, E.; Aglyamova, G.V.; Matz, M.V. Profiling gene expression responses of coral larvae (Acropora millepora) to elevated temperature and settlement inducers using a novel RNA-Seq procedure. Mol. Ecol. 2011, 20, 3599–3616. [Google Scholar] [CrossRef]
- Cernava, T.; Müller, H.; Aschenbrenner, I.A.; Grube, M.; Berg, G. Analyzing the antagonistic potential of the lichen microbiome against pathogens by bridging metagenomic with culture studies. Front. Microbiol. 2015, 6, 620. [Google Scholar] [CrossRef]
- Cernava, T.; Erlacher, A.; Aschenbrenner, I.A.; Krug, L.; Lassek, C.; Riedel, K.; Grube, M.; Berg, G. Deciphering functional diversification within the lichen microbiota by meta-omics. Microbiome 2017, 5, 82. [Google Scholar] [CrossRef]
- Eymann, C.; Lassek, C.; Wegner, U.; Bernhardt, J.; Fritsch, O.A.; Fuchs, S.; Otto, A.; Albrecht, D.; Schiefelbein, U.; Cernava, T.; et al. Symbiotic interplay of fungi, algae, and bacteria within the lung lichen Lobaria pulmonaria (L.) Hoffm. as assessed by state-of-the-art metaproteomics. J. Proteome Res. 2017, 16, 2160–2173. [Google Scholar] [CrossRef] [PubMed]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic tolerance facilitates the evolution of resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Fridman, O.; Goldberg, A.; Ronin, I.; Shoresh, N.; Balaban, N.Q. Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature 2014, 513, 418–421. [Google Scholar] [CrossRef] [PubMed]

| Observed | Chao1 | ACE | Shannon | Simpson | InvSimpson | |
|---|---|---|---|---|---|---|
| Parameter | 7 | 7 | 7 | 7 | 7 | 7 |
| Statistic | 7.31 | 7.32 | 7.60 | 5.83 | 3.58 | 3.58 |
| p-value | 0.39 | 0.39 | 0.36 | 0.56 | 0.82 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavarria-Pizarro, T.; Resl, P.; Kuhl-Nagel, T.; Janjic, A.; Fernandez Mendoza, F.; Werth, S. Antibiotic-Induced Treatments Reveal Stress-Responsive Gene Expression in the Endangered Lichen Lobaria pulmonaria. J. Fungi 2022, 8, 625. https://doi.org/10.3390/jof8060625
Chavarria-Pizarro T, Resl P, Kuhl-Nagel T, Janjic A, Fernandez Mendoza F, Werth S. Antibiotic-Induced Treatments Reveal Stress-Responsive Gene Expression in the Endangered Lichen Lobaria pulmonaria. Journal of Fungi. 2022; 8(6):625. https://doi.org/10.3390/jof8060625
Chicago/Turabian StyleChavarria-Pizarro, Tania, Philipp Resl, Theresa Kuhl-Nagel, Aleksandar Janjic, Fernando Fernandez Mendoza, and Silke Werth. 2022. "Antibiotic-Induced Treatments Reveal Stress-Responsive Gene Expression in the Endangered Lichen Lobaria pulmonaria" Journal of Fungi 8, no. 6: 625. https://doi.org/10.3390/jof8060625
APA StyleChavarria-Pizarro, T., Resl, P., Kuhl-Nagel, T., Janjic, A., Fernandez Mendoza, F., & Werth, S. (2022). Antibiotic-Induced Treatments Reveal Stress-Responsive Gene Expression in the Endangered Lichen Lobaria pulmonaria. Journal of Fungi, 8(6), 625. https://doi.org/10.3390/jof8060625






