How Has the Aspergillosis Case Fatality Rate Changed over the Last Two Decades in Spain?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Source
2.2. Selection of Aspergillosis Patients
2.3. Data Analysis
2.4. Ethics Statement
3. Results
Multivariate Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denning, D.W. Invasive aspergillosis. Clin. Infect. Dis. 1998, 26, 781–805. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Nosotti, M.; Tarsia, P.; Morlacchi, L.C. Infections after lung transplantation. J. Thorac. Dis. 2018, 10, 3849–3868. [Google Scholar] [CrossRef]
- Rahi, M.S.; Jindal, V.; Pednekar, P.; Parekh, J.; Gunasekaran, K.; Sharma, S.; Stender, M.; Jaiyesimi, I.A. Fungal infections in hematopoietic stem-cell transplant patients: A review of epidemiology, diagnosis, and management. Ther. Adv. Infect. Dis. 2021, 8, 20499361211039050. [Google Scholar] [CrossRef]
- Lai, C.C.; Yu, W.L. COVID-19 associated with pulmonary aspergillosis: A literature review. J. Microbiol. Immunol. Infect. 2021, 54, 46–53. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Janssen, N.A.F.; Nyga, R.; Vanderbeke, L.; Jacobs, C.; Ergün, M.; Buil, J.B.; Van Dijk, K.; Altenburg, J.; Bouman, C.S.C.; Van der Spoel, H.I.; et al. Multinational Observational Cohort Study of COVID-19-Associated Pulmonary Aspergillosis 1. Emerg. Infect. Dis. 2021, 27, 2892–2898. [Google Scholar] [CrossRef]
- Gangneux, J.P.; Dannaoui, E.; Fekkar, A.; Luyt, C.E.; Botterel, F.; De Prost, N.; Tadié, J.M.; Reizine, F.; Houzé, S.; Timsit, J.F.; et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: The French multicentre MYCOVID study. Lancet Respir. Med. 2022, 10, 180–190. [Google Scholar] [CrossRef]
- Neofytos, D.; Fishman, J.A.; Horn, D.; Anaissie, E.; Chang, C.H.; Olyaei, A.; Pfaller, M.; Steinbach, W.J.; Webster, K.M.; Marr, K.A. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl. Infect. Dis. 2010, 12, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Neofytos, D.; Chatzis, O.; Nasioudis, D.; Janke, E.B.; Lecompte, T.D.; Garzoni, C.; Berger, C.; Cussini, A.; Boggian, K.; Khanna, N.; et al. Epidemiology, risk factors and outcomes of invasive aspergillosis in solid organ transplant recipients in the Swiss Transplant Cohort Study. Transpl. Infect. Dis. 2018, 20, e12898. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Lto, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Tudela, J.L.; Alastruey-Izquierdo, A.; Gago, S.; Cuenca-Estrella, M.; León, C.; Miro, J.M.; Boluda, A.N.; Camps, I.R.; Sole, A.; Denning, D.W. Burden of serious fungal infections in Spain. Clin. Microbiol. Infect. 2015, 21, 183–189. [Google Scholar] [CrossRef]
- González-Garcia, P.; Alonso-Sardón, M.; López-Bernus, A.; Carbonell, C.; Romero-Alegría, Á.; Muro, A.; Galindo-Pérez, I.; Muñoz-Bellido, J.L.; Pardo-Lledias, J.; Belhassen-García, M. Epidemiology of aspergillosis in hospitalised Spanish patients—A 21-year retrospective study. Mycoses 2021, 64, 520–527. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Nathanson, B.H.; Harrington, R.; Spalding, J.R.; Shorr, A.F. Epidemiology and Outcomes of Hospitalizations with Invasive Aspergillosis in the United States, 2009–2013. Clin. Infect. Dis. 2018, 67, 727–735. [Google Scholar] [CrossRef]
- Cornillet, A.; Camus, C.; Nimubona, S.; Gandemer, V.; Tattevin, P.; Belleguic, C.; Chevrier, S.; Meunier, C.; Lebert, C.; Aupée, M.; et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: A 6-year survey. Clin. Infect. Dis. 2006, 43, 577–584. [Google Scholar] [CrossRef]
- Garbino, J.; Fluckiger, U.; Elzi, L.; Imhof, A.; Bille, J.; Zimmerli, S. Survey of aspergillosis in non-neutropenic patients in Swiss teaching hospitals. Clin. Microbiol. Infect. 2011, 17, 1366–1371. [Google Scholar] [CrossRef]
- Meersseman, W.; Wilmer, A. Invasive aspergillosis in a medical ICU: The spectrum of disease in 89 nonhaematology patients. Crit. Care 2004, 8, P228. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Kochanek, M.; Shimabukuro-Vornhagen, A.; Cornely, O.A. Intensive care management of influenza-associated pulmonary aspergillosis. Clin. Microbiol. Infect. 2019, 25, 1501–1509. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Friedman, D.Z.P.; Zapernick, L.; Dingle, T.C.; Lee, N.; Sligl, W.; Zelyas, N.; Smith, S.W. High Rates of Influenza-Associated Invasive Pulmonary Aspergillosis May Not Be Universal: A Retrospective Cohort Study from Alberta, Canada. Clin. Infect. Dis. 2020, 71, 1760–1763. [Google Scholar] [CrossRef]
- Wauters, J.; Baar, I.; Meersseman, P.; Meersseman, W.; Dams, K.; De Paep, R.; Lagrou, K.; Wilmer, A.; Jorens, P.; Hermans, G. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: A retrospective study. Intensive Care Med. 2012, 38, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; Kallenbach, J.; Ruff, P.; Zaltzman, M.; Abramowitz, J.; Zwi, S. Invasive pulmonary aspergillosis complicating influenza A pneumonia in a previously healthy patient. Chest 1985, 87, 691–693. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rijnders, B.J.A.; Schauwvlieghe, A.F.A.D.; Wauters, J. Influenza-Associated Pulmonary Aspergillosis: A Local or Global Lethal Combination? Clin. Infect. Dis. 2020, 71, 1764–1767. [Google Scholar] [CrossRef] [PubMed]
- Peláez-García de la Rasilla, T.; González-Jiménez, I.; Fernández-Arroyo, A.; Roldán, A.; Carretero-Ares, J.L.; García-Clemente, M.; Telenti-Asensio, M.; García-Prieto, E.; Martínez-Suarez, M.; Vázquez-Valdés, F.; et al. COVID-19 Associated Pulmonary Aspergillosis (CAPA): Hospital or Home Environment as a Source of Life-Threatening Aspergillus fumigatus Infection? J. Fungi 2022, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- CDC. Principles of Epidemiology in Public Health Practice. An Introduction to Applied Epidemiology and Biostatistics, 3rd ed. Available online: https://stacks.cdc.gov/gsearch?ref=docDetails&related_series=Self-studycourse (accessed on 18 March 2022).
- Lin, S.J.; Schranz, J.; Teutsch, S.M. Aspergillosis case-fatality rate: Systematic review of the literature. Clin. Infect. Dis. 2001, 32, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Bakri, F.; Young, P.; Hahn, T.; Ball, D.; Baer, M.R.; Wetzler, M.; Slack, J.L.; Loud, P.; Czuczman, M.; et al. Invasive filamentous fungal infections in allogeneic hematopoietic stem cell transplant recipients after recovery from neutropenia: Clinical, radiologic, and pathologic characteristics. Mycopathologia 2005, 159, 181–188. [Google Scholar] [CrossRef]
- Tejeda, M.I.; Salso, S.; Barberán, J. Invasive pulmonary aspergillosis in non-neutropenic patients. Rev. Esp. Quimioter. 2016, 29 (Suppl. 1), 56–58. [Google Scholar]
- Hammond, E.E.; McDonald, C.S.; Vestbo, J.; Denning, D.W. The global impact of Aspergillus infection on COPD. BMC Pulm. Med. 2020, 20, 241. [Google Scholar] [CrossRef]
- Xu, H.; Li, L.; Huang, W.J.; Wang, L.X.; Li, W.F.; Yuan, W.F. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: A case control study from China. Clin. Microbiol. Infect. 2012, 18, 403–408. [Google Scholar] [CrossRef]
- Guinea, J.; Torres-Narbona, M.; Gijón, P.; Muñoz, P.; Pozo, F.; Peláez, T.; de Miguel, J.; Bouza, E. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: Incidence, risk factors, and outcome. Clin. Microbiol. Infect. 2010, 16, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Valerio, M.; Álvarez-Uría, A.; Olmedo, M.; Veintimilla, C.; Padilla, B.; De la Villa, S.; Guinea, J.; Escribano, P.; Ruiz-Serrano, M.J.; et al. Invasive pulmonary aspergillosis in the COVID-19 era: An expected new entity. Mycoses 2021, 64, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Ergün, M.; Brüggemann, R.J.M.; Alanio, A.; Dellière, S.; van Arkel, A.; Bentvelsen, R.G.; Rijpstra, T.; van der Sar-Van der Brugge, S.; Lagrou, K.; Janssen, N.A.F.; et al. Aspergillus test profiles and mortality in critically ill COVID-19 patients. J. Clin. Microbiol. 2021, 59, e01229-21. [Google Scholar] [CrossRef] [PubMed]

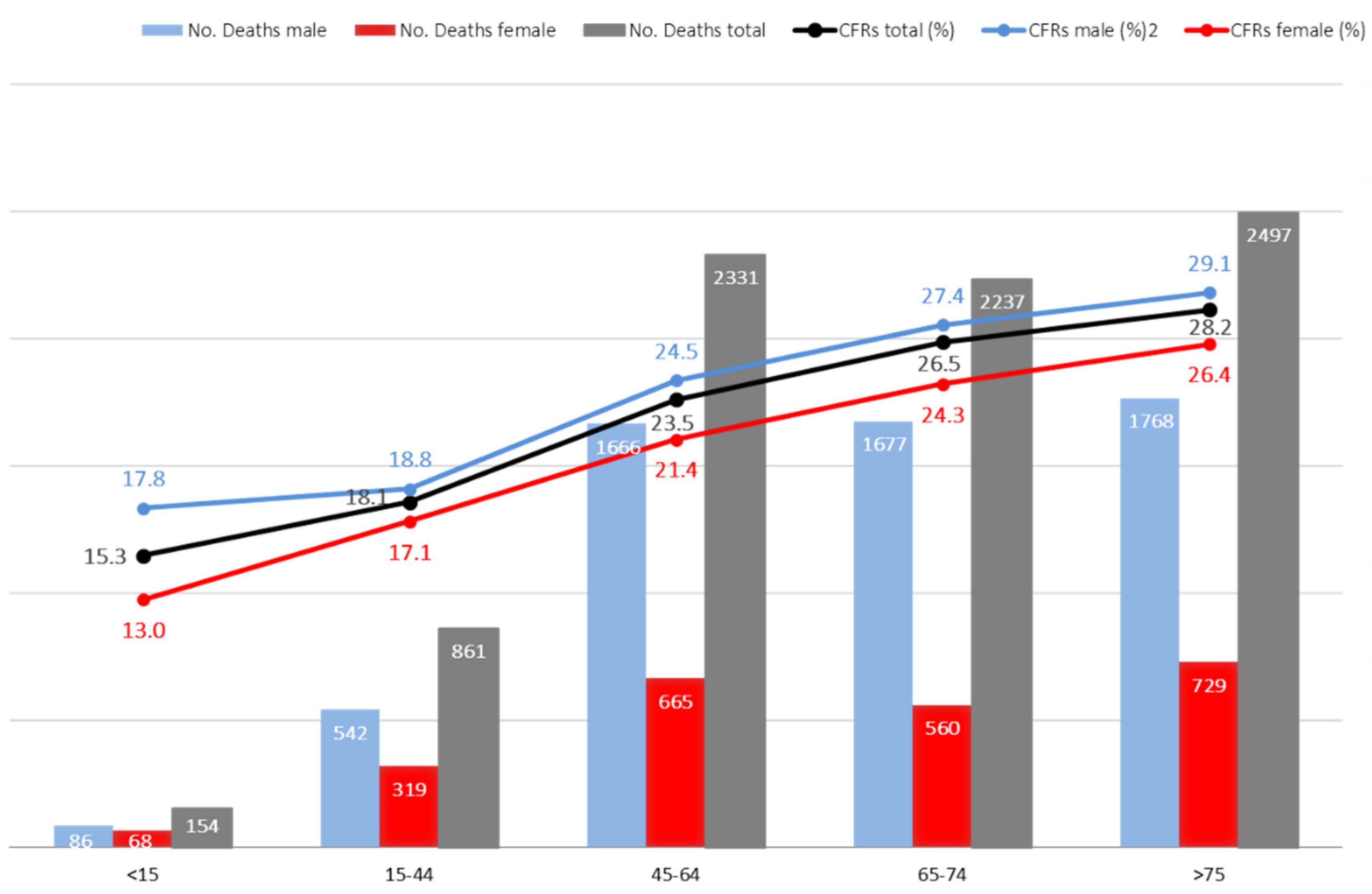
| TOTAL, n (%) N = 32,960 Cases (100%) | Deaths, n (%) N1 = 8080 (24.5%) | Survivors, n (%) N2 = 24,880 Cases (75.5%) | p-Value | OR (95% CI) | |
|---|---|---|---|---|---|
| Age, mean ± SD | 61.1 ± 19.1 | 64.1 ± 17.2 | 60.2 ± 19.6 | <0.001 | |
| <15 years | 1009 (3.1) | 154 (1.9) | 855 (3.4) | <0.001 * | |
| 15–44 years | 4753 (14.4) | 861 (10.7) | 3892 (15.6) | ||
| 45–64 years | 9921 (30.1) | 2331 (28.8) | 7590 (30.5) | ||
| 65–74 years | 8437 (25.6) | 2237 (27.7) | 6200 (24.9) | ||
| ≥75 years | 8840 (26.8) | 2497 (30.9) | 6343 (25.5) | ||
| Gender | |||||
| Male | 22,383 (67.9) | 5739 (71.0) | 16,643 (66.9) | <0.001 * | 1.2 (1.1–1.3) |
| Female | 10,577 (32.1) | 2341 (29.0) | 8236 (33.1) | ||
| Diagnosis causing hospitalization | |||||
| Principal diagnosis | 6574 (19.9) | 1496 (18.5) | 5078 (20.4) | <0.001 * | 1.1 (1.0–1.2) |
| Secondary diagnosis | 26,386 (80.1) | 6584 (81.5) | 19,802 (79.6) | ||
| Process type | |||||
| Medical | 21,728 (65.9) | 4724 (58.5) | 17,004 (68.3) | <0.001 * | 0.6 (0.6–0.7) |
| Surgical | 4474 (13.6) | 1368 (16.9) | 3105 (12.5) | ||
| Unclassified | 6758 (20.5) | 1987 (24.6) | 4771 (19.2) | ||
| Hospital stays (days), mean ± SD | 26.8 ± 26.9 | 32.2 ± 29.4 | 25.0± 25.8 | <0.001 ** |
| No. Cases | No. Deaths | CFR (Percent) | ||
|---|---|---|---|---|
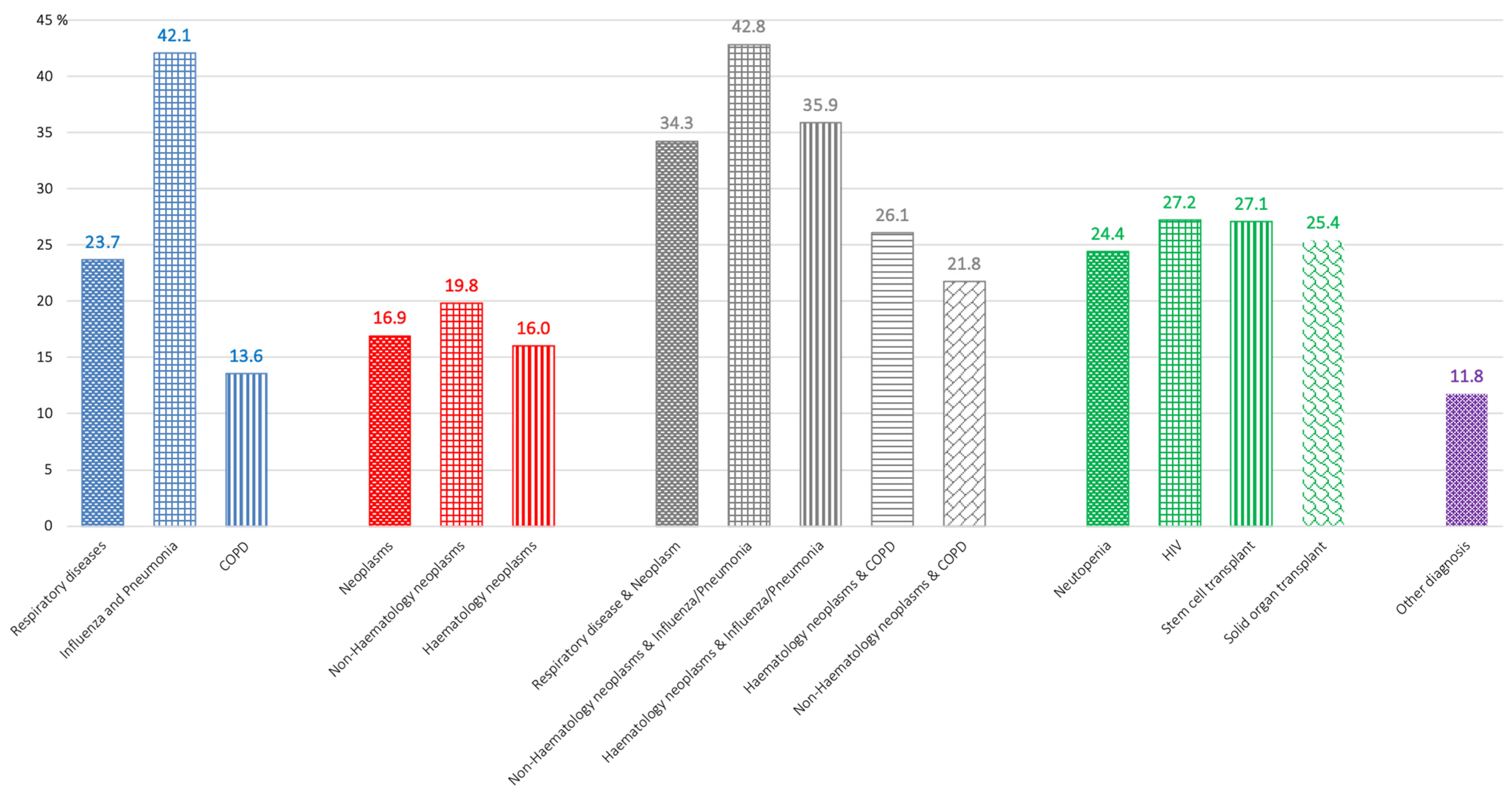
| Solid organ transplant | 232 | 59 | 25.4 | |
| Stem cell transplantation | 632 | 171 | 27.1 | |
| HIV | 944 | 257 | 27.2 | |
| Neutropenia | 2668 | 650 | 24.4 | |
| Respiratory disease | 19,118 | 4533 | 23.7 | |
| Chronic obstructive pulmonary disease (COPD) | 9101 | 1233 | 13.5 | |
| Influenza/ Pneumonia | 2999 | 1262 | 42.1 | |
| COPD & Influenza/Pneumonia | 3297 | 1069 | 32.5 | |
| Other respiratory disease | 3721 | 969 | 26.0 | |
| Neoplasms | 3368 | 570 | 16.9 | |
| Hematology neoplasms | 2477 | 396 | 16.0 | |
| Non-Hematology neoplasms | 727 | 144 | 19.8 | |
| Hematology & Non-Hematology neoplasms | 164 | 30 | 18.3 | |
| Neoplasms & respiratory disease | 7761 | 2658 | 34.2 | |
| Hematology neoplasms & | COPD | 472 | 123 | 26.1 |
| Influenza and Pneumonia | 2250 | 807 | 35.9 | |
| COPD & Influenza and Pneumonia | 263 | 96 | 36.5 | |
| Other respiratory disease | 1319 | 498 | 37,7 | |
| Non-Hematology neoplasms & | COPD | 1144 | 249 | 21.8 |
| Influenza and Pneumonia | 789 | 338 | 42.8 | |
| COPD & Influenza and Pneumonia | 448 | 156 | 34.8 | |
| Other respiratory disease | 799 | 302 | 37.8 | |
| Hematology & Non-Hematology neoplasms & | COPD | 39 | 7 | 17.9 |
| Influenza and Pneumonia | 137 | 46 | 33.6 | |
| COPD & Influenza and Pneumonia | 30 | 8 | 26.7 | |
| Other respiratory disease | 71 | 28 | 39.4 | |
| Other diagnosis | 2713 | 319 | 11.8 | |
| Critical Care Unit * | 1981 | 1872 | 94.5 | |
| Patient with respiratory disease | 1299 | 1224 | 94.2 | |
| Patient with neoplasms | 39 | 36 | 92.3 | |
| Patient with neoplasms & respiratory disease | 572 | 551 | 96.3 | |
| Patient with other diagnosis | 71 | 61 | 85.9 | |
| Transplant Unit | 468 | 39 | 8.3 | |
| TOTAL | 32,960 | 8080 | 24.5 | |
| Dependent Variable: Exitus Letalis | ||||
|---|---|---|---|---|
| Independent Variables | Sig. | Exp(B) | 95% CI EXP(B) | |
| Lower | Upper | |||
| Sex | 0.000 | 1.243 | 1.167 | 1.325 |
| Age | 0.000 | 1.882 | 1.766 | 2.006 |
| Hematology neoplasms | 0.000 | 1.490 | 1.380 | 1.608 |
| Non-Hematology neoplasms | 0.000 | 1.508 | 1.393 | 1.633 |
| Influenza/Pneumonia | 0.000 | 2.133 | 2.012 | 2.262 |
| COPD | 0.000 | 0.660 | 0.619 | 0.705 |
| HIV | 0.000 | 1.357 | 1.143 | 1.610 |
| Neutropenia | 0.051 | 0.789 | 0.705 | 0.884 |
| ICU | 0.000 | 68.228 | 55.742 | 83.510 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-García, P.; Alonso-Sardón, M.; Rodríguez-Alonso, B.; Almeida, H.; Romero-Alegría, Á.; Vega-Rodríguez, V.-J.; López-Bernús, A.; Muñoz-Bellido, J.L.; Muro, A.; Pardo-Lledías, J.; et al. How Has the Aspergillosis Case Fatality Rate Changed over the Last Two Decades in Spain? J. Fungi 2022, 8, 576. https://doi.org/10.3390/jof8060576
González-García P, Alonso-Sardón M, Rodríguez-Alonso B, Almeida H, Romero-Alegría Á, Vega-Rodríguez V-J, López-Bernús A, Muñoz-Bellido JL, Muro A, Pardo-Lledías J, et al. How Has the Aspergillosis Case Fatality Rate Changed over the Last Two Decades in Spain? Journal of Fungi. 2022; 8(6):576. https://doi.org/10.3390/jof8060576
Chicago/Turabian StyleGonzález-García, Pablo, Montserrat Alonso-Sardón, Beatriz Rodríguez-Alonso, Hugo Almeida, Ángela Romero-Alegría, Víctor-José Vega-Rodríguez, Amparo López-Bernús, Juan Luis Muñoz-Bellido, Antonio Muro, Javier Pardo-Lledías, and et al. 2022. "How Has the Aspergillosis Case Fatality Rate Changed over the Last Two Decades in Spain?" Journal of Fungi 8, no. 6: 576. https://doi.org/10.3390/jof8060576
APA StyleGonzález-García, P., Alonso-Sardón, M., Rodríguez-Alonso, B., Almeida, H., Romero-Alegría, Á., Vega-Rodríguez, V.-J., López-Bernús, A., Muñoz-Bellido, J. L., Muro, A., Pardo-Lledías, J., & Belhassen-García, M. (2022). How Has the Aspergillosis Case Fatality Rate Changed over the Last Two Decades in Spain? Journal of Fungi, 8(6), 576. https://doi.org/10.3390/jof8060576







