Isavuconazole Treatment of Spinal Cord Invasive Aspergillosis Guided by Cerebrospinal Fluid (1,3)-β-d-Glucan Levels in a Patient with Low Interferon-Gamma and Ulcerative Colitis
Abstract
1. Introduction
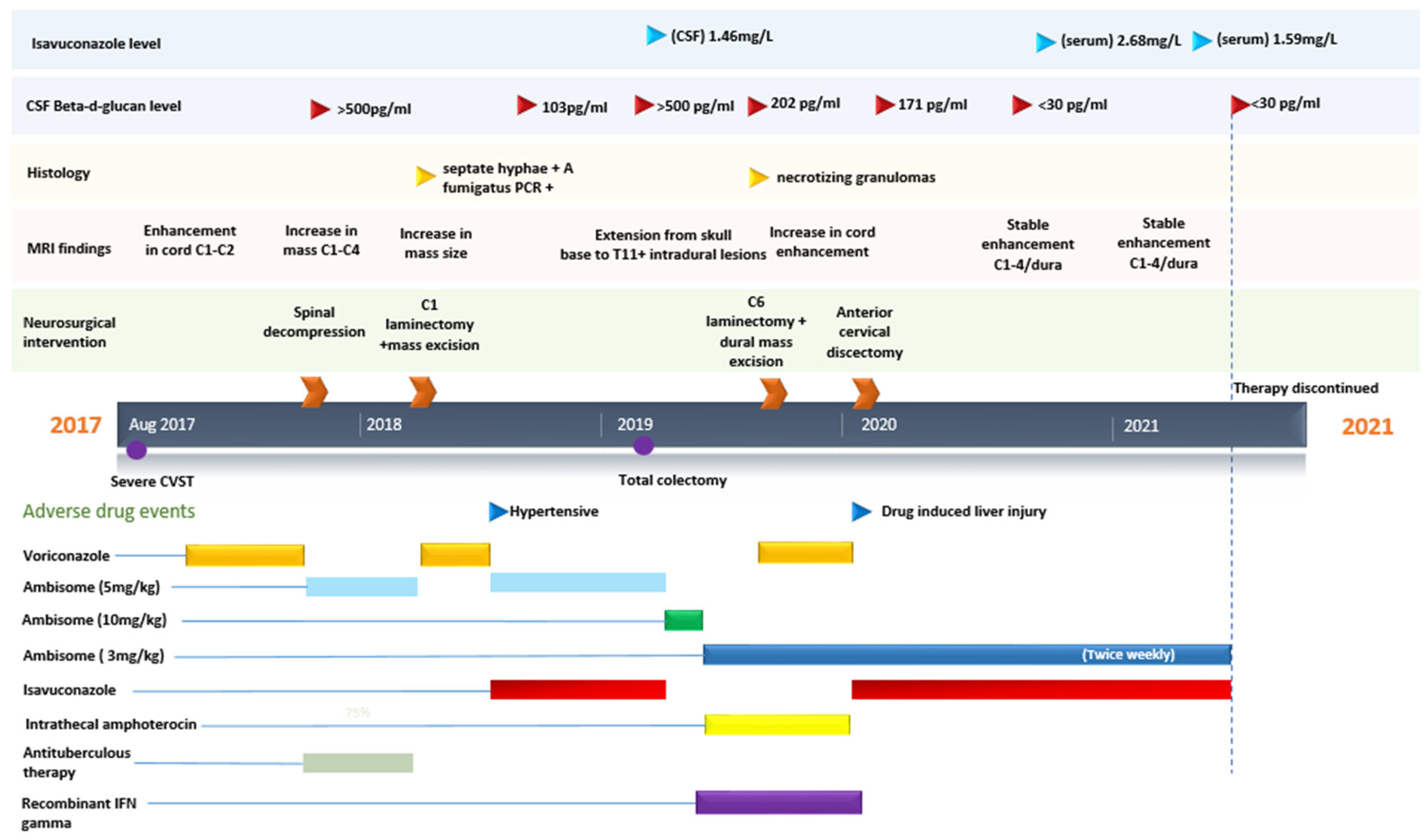
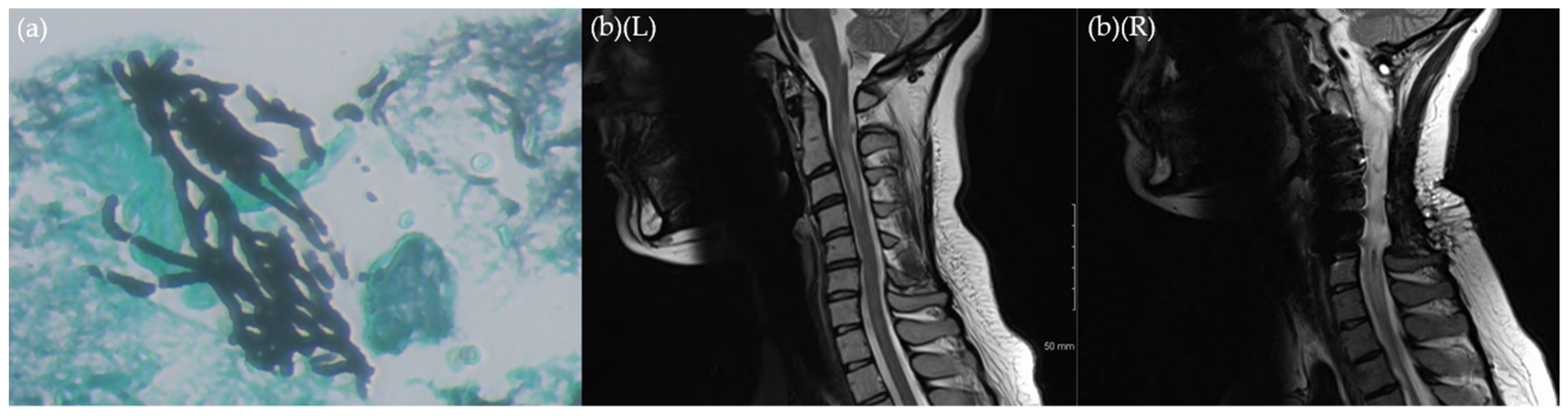
2. Case
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meena, D.S.; Kumar, D.; Bohra, G.K.; Kumar, G. Clinical manifestations, diagnosis, and treatment outcome of CNS aspergillosis: A systematic review of 235 cases. Infect. Dis. Now 2021, 51, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Hong, S.C.; Kim, H.J.; Chi, J.G.; Han, M.H.; Choi, K.S.; Han, D.H. Cerebral aspergillosis in immunologically competent patients. Surg. Neurol. 1993, 40, 326–331. [Google Scholar] [CrossRef]
- Chen, S.; Pu, J.L.; Yu, J.; Zhang, J.M. Multiple Aspergillus cerebellar abscesses in a middle-aged female: Case report and literature review. Int. J. Med. Sci. 2011, 8, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Benchaib, N.; Zagdanski, A.M.; Hocqueloux, L.; Rili, M.; Molina, J.M.; De Kerviler, E. Cerebral and spinal cord involvement resulting from invasive aspergillosis. Eur. Radiol. 2002, 12, 147–150. [Google Scholar] [CrossRef] [PubMed]
- McCaslin, A.F.; Lall, R.R.; Wong, A.P.; Lall, R.R.; Sugrue, P.A.; Koski, T.R. Thoracic spinal cord intramedullary aspergillus invasion and abscess. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2015, 22, 404–406. [Google Scholar] [CrossRef]
- Gunaratne, P.S.; Wijeyaratne, C.N.; Seneviratne, H.R. Aspergillus Meningitis in Sri Lanka—A Post-Tsunami Effect? N. Engl. J. Med. 2007, 356, 754–756. [Google Scholar] [CrossRef]
- Genzen, J.R.; Kenney, B. Central nervous system Aspergillus infection after epidural analgesia: Diagnosis, therapeutic challenges, and literature review. Diagn. Microbiol. Infect. Dis. 2009, 65, 312–318. [Google Scholar] [CrossRef][Green Version]
- Kainer, M.A.; Reagan, D.R.; Nguyen, D.B.; Wiese, A.; Wise, M.E.; Ward, J.; Park, B.J.; Kanago, M.L.; Baumblatt, J.; Schaefer, M.K.; et al. Fungal Infections Associated with Contaminated Methylprednisolone in Tennessee. N. Engl. J. Med. 2012, 367, 2194–2203. [Google Scholar] [CrossRef]
- Ruhnke, M.; Kofla, G.; Otto, K.; Schwartz, S. CNS Aspergillosis. CNS Drugs 2007, 21, 659–676. [Google Scholar] [CrossRef]
- Pongbhaesaj, P.; Dejthevaporn, C.; Tunlayadechanont, S.; Witoonpanich, R.; Sungkanuparph, S.; Vibhagool, A. Aspergillosis of the central nervous system: A catastrophic opportunistic infection. Southeast Asian J. Trop. Med. Public Health 2004, 35, 119–125. [Google Scholar]
- Schwartz, S.; Ruhnke, M.; Ribaud, P.; Corey, L.; Driscoll, T.; Cornely, O.A.; Schuler, U.; Lutsar, I.; Troke, P.; Thiel, E. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood 2005, 106, 2641–2645. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Kodan, P.; Mittal, A.; Singh, G.; Netto, G.; Ramteke, P.; Malla, S.; Kumar, R.; Kumar, T.P.; Singh, K.; et al. Role of Voriconazole in the Management of Invasive Central Nervous System Aspergillosis: A Case Series from a Tertiary Care Centre in India. J. Fungi 2020, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.H. Central Nervous System Infections Due to Aspergillus and Other Hyaline Molds. J. Fungi 2019, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Theel, E.S.; Doern, C.D. β-d-glucan testing is important for diagnosis of invasive fungal infections. J. Clin. Microbiol. 2013, 51, 3478–3483. [Google Scholar] [CrossRef]
- Davis, C.; Wheat, L.J.; Myint, T.; Boulware, D.R.; Bahr, N.C. Efficacy of Cerebrospinal Fluid Beta-d-Glucan Diagnostic Testing for Fungal Meningitis: A Systematic Review. J. Clin. Microbiol. 2020, 58, e02094-19. [Google Scholar] [CrossRef]
- Salvatore, C.M.; Chen, T.K.; Toussi, S.S.; DeLaMora, P.; Petraitiene, R.; Finkelman, M.A.; Walsh, T.J. (1→3)-β-d-Glucan in Cerebrospinal Fluid as a Biomarker for Candida and Aspergillus Infections of the Central Nervous System in Pediatric Patients. J. Pediatr. Infect. Dis. Soc. 2016, 5, 277–286. [Google Scholar] [CrossRef]
- Chen, T.K.; Groncy, P.K.; Javahery, R.; Chai, R.Y.; Nagpala, P.; Finkelman, M.; Petraitiene, R.; Walsh, T.J. Successful treatment of Aspergillus ventriculitis through voriconazole adaptive pharmacotherapy, immunomodulation, and therapeutic monitoring of cerebrospinal fluid (1→3)-β-D-glucan. Med. Mycol. 2017, 55, 109–117. [Google Scholar] [CrossRef]
- Galgiani, J.N.; Ampel, N.M.; Blair, J.E.; Catanzaro, A.; Geertsma, F.; Hoover, S.E.; Johnson, R.H.; Kusne, S.; Lisse, J.; Macdonald, J.D.; et al. 2016 Infectious Diseases Society of America (IDSA) Clinical Practice Guideline for the Treatment of Coccidioidomycosis. Clin. Infect. Dis. 2016, 63, e112–e146. [Google Scholar] [CrossRef]
- Stevens, D.A.; Zhang, Y.; Finkelman, M.A.; Pappagianis, D.; Clemons, K.V.; Martinez, M. Cerebrospinal Fluid (1,3)-β-d-glucan Testing Is Useful in Diagnosis of Coccidioidal Meningitis. J. Clin. Microbiol. 2016, 54, 2707–2710. [Google Scholar] [CrossRef]
- Lyons, J.L.; Thakur, K.T.; Lee, R.; Watkins, T.; Pardo, C.A.; Carson, K.A.; Markley, B.; Finkelman, M.A.; Marr, K.A.; Roos, K.L.; et al. Utility of Measuring (1,3)-β-d-Glucan in Cerebrospinal Fluid for Diagnosis of Fungal Central Nervous System Infection. J. Clin. Microbiol. 2015, 53, 319–322. [Google Scholar] [CrossRef]
- Antinori, S.; Corbellino, M.; Meroni, L.; Resta, F.; Sollima, S.; Tonolini, M.; Tortorano, A.M.; Milazzo, L.; Bello, L.; Furfaro, E.; et al. Aspergillus meningitis: A rare clinical manifestation of central nervous system aspergillosis. Case report and review of 92 cases. J. Infect. 2013, 66, 218–238. [Google Scholar] [CrossRef] [PubMed]
- Morgand, M.; Rammaert, B.; Poirée, S.; Bougnoux, M.-E.; Tran, H.; Kania, R.; Chrétien, F.; Jouvion, G.; Lortholary, O. Chronic Invasive Aspergillus Sinusitis and Otitis with Meningeal Extension Successfully Treated with Voriconazole. Antimicrob. Agents Chemother. 2015, 59, 7857–7861. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.; Kontoyiannis, D.P.; Cornely, O.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. Lancet 2015, 387, 760–769. [Google Scholar] [CrossRef]
- Rouzaud, C.; Jullien, V.; Herbrecht, A.; Palmier, B.; Lapusan, S.; Morgand, M.; Guéry, R.; Dureault, A.; Danion, F.; Puget, S.; et al. Isavuconazole Diffusion in Infected Human Brain. Antimicrob. Agents Chemother. 2019, 63, e02474-18. [Google Scholar] [CrossRef]
- Schwartz, S.; Cornely, O.A.; Hamed, K.; Marty, F.M.; Maertens, J.; Rahav, G.; Herbrecht, R.; Heinz, W.J. Isavuconazole for the treatment of patients with invasive fungal diseases involving the central nervous system. Med. Mycol. 2019, 58, 417–424. [Google Scholar] [CrossRef]
- Heidari, A.; Quinlan, M.; Benjamin, D.J.; Laurence, B.; Mu, A.; Ngai, T.; Hoffman, W.J.; Cohen, S.H.; McHardy, I.; Johnson, R.; et al. Isavuconazole in the Treatment of Coccidioidal Meningitis. Antimicrob. Agents Chemother. 2019, 63, e02232-18. [Google Scholar] [CrossRef]
- Guest, J.M.; Singh, P.K.; Revankar, S.G.; Chandrasekar, P.H.; Kumar, A. Isavuconazole for Treatment of Experimental Fungal Endophthalmitis Caused by Aspergillus fumigatus. Antimicrob. Agents Chemother. 2018, 62, e01537-18. [Google Scholar] [CrossRef]
- De Leonardis, F.; Novielli, C.; Giannico, B.; Mariggiò, M.A.; Castagnola, E.; Santoro, N. Isavuconazole Treatment of Cerebral and Pulmonary Aspergillosis in a Pediatric Patient with Acute Lymphoblastic Leukemia: Case Report and Review of Literature. J. Pediatr. Hematol. 2019, 42, e469–e471. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Regan, S.; O’Kelly, B.; Reidy, P.; O’Toole, A.; Caird, J.; McNally, C.; McConkey, S.; De Barra, E. Isavuconazole Treatment of Spinal Cord Invasive Aspergillosis Guided by Cerebrospinal Fluid (1,3)-β-d-Glucan Levels in a Patient with Low Interferon-Gamma and Ulcerative Colitis. J. Fungi 2022, 8, 557. https://doi.org/10.3390/jof8060557
O’Regan S, O’Kelly B, Reidy P, O’Toole A, Caird J, McNally C, McConkey S, De Barra E. Isavuconazole Treatment of Spinal Cord Invasive Aspergillosis Guided by Cerebrospinal Fluid (1,3)-β-d-Glucan Levels in a Patient with Low Interferon-Gamma and Ulcerative Colitis. Journal of Fungi. 2022; 8(6):557. https://doi.org/10.3390/jof8060557
Chicago/Turabian StyleO’Regan, Siobhan, Brendan O’Kelly, Paul Reidy, Aoibhlinn O’Toole, John Caird, Cora McNally, Samuel McConkey, and Eoghan De Barra. 2022. "Isavuconazole Treatment of Spinal Cord Invasive Aspergillosis Guided by Cerebrospinal Fluid (1,3)-β-d-Glucan Levels in a Patient with Low Interferon-Gamma and Ulcerative Colitis" Journal of Fungi 8, no. 6: 557. https://doi.org/10.3390/jof8060557
APA StyleO’Regan, S., O’Kelly, B., Reidy, P., O’Toole, A., Caird, J., McNally, C., McConkey, S., & De Barra, E. (2022). Isavuconazole Treatment of Spinal Cord Invasive Aspergillosis Guided by Cerebrospinal Fluid (1,3)-β-d-Glucan Levels in a Patient with Low Interferon-Gamma and Ulcerative Colitis. Journal of Fungi, 8(6), 557. https://doi.org/10.3390/jof8060557





