Respiratory Epithelial Cells: More Than Just a Physical Barrier to Fungal Infections
Abstract
1. Introduction
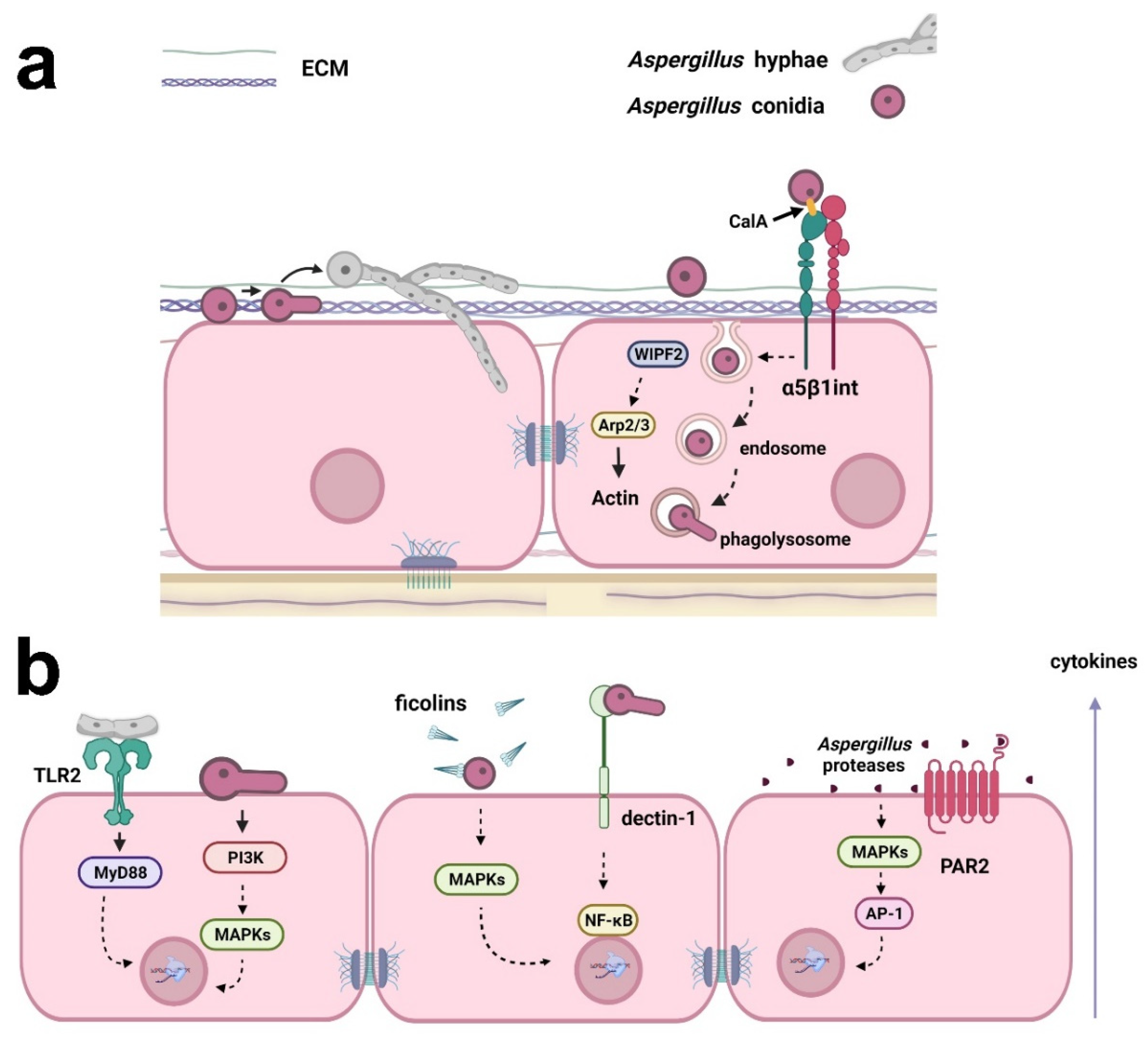
2. Aspergillus
3. Paracoccidioides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hewitt, R.J.; Lloyd, C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.D.; Wypych, T.P. Cellular and functional heterogeneity of the airway eithelium. Mucosal Immunol. 2021, 14, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Juárez, M.M.; Kolls, J.K.; Evans, S.E. Lung epithelial cells: Therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol. 2018, 11, 21–34. [Google Scholar] [CrossRef]
- de Waal, A.M.; Hiemstra, P.S.; Ottenhoff, T.H.; Joosten, S.A.; van der Does, A.M. Lung epithelial cells interact with immune cells and bacteria to shape the microenvironment in Tuberculosis. Thorax 2022, 77, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Fromentin, M.; Ricard, J.-D.; Roux, D. Lung microbiome in critically ill patients. Life 2021, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.E.B.; Kao, S.C.; Barnes, D.J.; Wong, K.K.H.; Scolyer, R.A.; Cooper, W.A.; Kohonen-Corish, M.R.J. The emerging role of the lung microbiome and its importance in non-small cell lung cancer diagnosis and treatment. Lung Cancer 2022, 165, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, R.; Lloyd, C.M.; Molyneaux, P.L. Respiratory microbiome and epithelial interactions shape immunity in the lungs. Immunology 2020, 160, 171–182. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, FUNK-0035-2016. [Google Scholar] [CrossRef]
- Gaffi. Available online: https://gaffi.org/ (accessed on 25 March 2022).
- Miller, A.S.; Wilmott, R.W. 31—The pulmonary mycoses. In Kendig’s Disorders of the Respiratory Tract in Children, 9th ed.; Wilmott, R.W., Deterding, R., Li, A., Ratjen, F., Sly, P., Zar, H.J., Bush, A., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 507–527.e3. ISBN 9780323448871. [Google Scholar]
- Salzer, H.J.F.; Burchard, G.; Cornely, O.A.; Lange, C.; Rolling, T.; Schmiedel, S.; Libman, M.; Capone, D.; Le, T.; Dalcolmo, M.P.; et al. Diagnosis and management of systemic endemic mycoses causing pulmonary disease. Respiration 2018, 96, 283–301. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary Aspergillosis: The 2020 ECMM/ISHAM Consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Griffiths, J.; Lopes Colombo, A.; Denning, D.W. The case for Paracoccidioidomycosis to be accepted as a neglected tropical (fungal) disease. PLoS Negl. Trop. Dis. 2019, 13, e0007195. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ghazzi, N.; Moreno-Velásquez, S.; Seidel, C.; Thomson, D.; Denning, D.W.; Read, N.D.; Bowyer, P.; Gago, S. Characterisation of Aspergillus fumigatus endocytic trafficking within airway epithelial cells using high-resolution automated Quantitative confocal microscopy. J. Fungi 2021, 7, 454. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.-P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.F.; Nyga, R.; Vanderbeke, L.; Jacobs, C.; Ergün, M.; Buil, J.B.; van Dijk, K.; Altenburg, J.; Bouman, C.S.C.; van der Spoel, H.I.; et al. Multinational observational cohort study of COVID-19–associated pulmonary Aspergillosis. Emerg. Infect. Dis. 2021, 27, 2892–2898. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Gaziano, R.; Doldo, E.; Marino, D.; Falconi, M.; Iacovelli, F.; Tagliaferri, D.; Pacello, L.; Bianchi, L.; Lanna, C.; et al. Antifungal effect of all-trans retinoic acid against Aspergillus fumigatus in vitro and in a pulmonary Aspergillosis in vivo Model. Antimicrob. Agents Chemother. 2021, 65, e01874-20. [Google Scholar] [CrossRef]
- Cosio, T.; Gaziano, R.; Zuccari, G.; Costanza, G.; Grelli, S.; Di Francesco, P.; Bianchi, L.; Campione, E. Retinoids in fungal infections: From bench to bedside. Pharmaceuticals 2021, 14, 962. [Google Scholar] [CrossRef]
- Ammendolia, D.A.; Bement, W.M.; Brumell, J.H. Plasma membrane integrity: Implications for health and disease. BMC Biol. 2021, 19, 71. [Google Scholar] [CrossRef]
- DeHart, D.J.; Agwu, D.E.; Julian, N.C.; Washburn, R.G. Binding and germination of Aspergillus fumigatus conidia on cultured A549 Pneumocytes. J. Infect. Dis. 1997, 175, 146–150. [Google Scholar] [CrossRef]
- Fernandes, J.; Hamidi, F.; Leborgne, R.; Beau, R.; Castier, Y.; Mordant, P.; Boukkerou, A.; Latgé, J.P.; Pretolani, M. Penetration of the human pulmonary epithelium by Aspergillus fumigatus hyphae. J. Infect. Dis. 2018, 218, 1306–1313. [Google Scholar] [CrossRef]
- Croft, C.A.; Culibrk, L.; Moore, M.M.; Tebbutt, S.J. Interactions of Aspergillus fumigatus conidia with airway epithelial cells: A critical review. Front. Microbiol. 2016, 7, 472. [Google Scholar] [CrossRef]
- Kerr, S.C.; Fischer, G.J.; Sinha, M.; McCabe, O.; Palmer, J.M.; Choera, T.; Lim, F.Y.; Wimmerova, M.; Carrington, S.D.; Yuan, S.; et al. FleA Expression in Aspergillus fumigatus is recognized by fucosylated structures on mucins and macrophages to prevent lung infection. PLoS Pathog. 2016, 12, e1005555. [Google Scholar] [CrossRef]
- Yan, T.; Han, J.; Yu, X. E-cadherin mediates adhesion of Aspergillus fumigatus to non-small cell lung cancer cells. Tumour Biol. 2016, 37, 15593–15599. [Google Scholar] [CrossRef] [PubMed]
- Gravelat, F.N.; Beauvais, A.; Liu, H.; Lee, M.J.; Snarr, B.D.; Chen, D.; Xu, W.; Kravtsov, I.; Hoareau, C.M.Q.; Vanier, G.; et al. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal β-glucan from the immune system. PLoS Pathog. 2013, 9, e1003575. [Google Scholar] [CrossRef]
- Bouchara, J.P.; Sanchez, M.; Chevailler, A.; Marot-Leblond, A.; Lissitzky, J.C.; Tronchin, G.; Chabasse, D. Sialic acid-dependent recognition of laminin and fibrinogen by Aspergillus fumigatus conidia. Infect. Immun. 1997, 65, 2717–2724. [Google Scholar] [CrossRef] [PubMed]
- Warwas, M.L.; Watson, J.N.; Bennet, A.J.; Moore, M.M. Structure and role of sialic acids on the surface of Aspergillus fumigatus conidiospores. Glycobiology 2007, 17, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lee, M.J.; Solis, N.V.; Phan, Q.T.; Swidergall, M.; Ralph, B.; Ibrahim, A.S.; Sheppard, D.C.; Filler, S.G. Aspergillus fumigatus CalA binds to integrin α5β1 and mediates host cell invasion. Nat. Microbiol. 2016, 2, 16211. [Google Scholar] [CrossRef]
- Culibrk, L.; Croft, C.A.; Toor, A.; Yang, S.J.; Singhera, G.K.; Dorscheid, D.R.; Moore, M.M.; Tebbutt, S.J. Phagocytosis of Aspergillus fumigatus by human bronchial epithelial cells is mediated by the Arp2/3 complex and WIPF2. Front. Cell. Infect. Microbiol. 2019, 9, 16. [Google Scholar] [CrossRef]
- Bertuzzi, M.; Hayes, G.; Icheoku, U.; van Rhijn, N.; Denning, D.; Osherov, N.; Bignell, E. Anti-Aspergillus activities of the respiratory epithelium in health and disease. J. Fungi 2018, 4, 8. [Google Scholar] [CrossRef]
- Shlezinger, N.; Hohl, T.M. Mitochondrial reactive oxygen species enhance alveolar macrophage activity against Aspergillus fumigatus but are dispensable for host protection. mSphere 2021, 6, e0026021. [Google Scholar] [CrossRef]
- Feldman, M.B.; Dutko, R.A.; Wood, M.A.; Ward, R.A.; Leung, H.M.; Snow, R.F.; De La Flor, D.J.; Yonker, L.M.; Reedy, J.L.; Tearney, G.J.; et al. Aspergillus fumigatus cell wall promotes apical airway epithelial recruitment of human neutrophils. Infect. Immun. 2020, 88, e00813-19. [Google Scholar] [CrossRef]
- Sheng, R.; Zhong, X.; Yang, Z.; Wang, X. The role of CARD9 deficiency in neutrophils. Mediat. Inflamm. 2021, 6643603. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, C.S.; Dubey, L.K.; Colmorten, K.B.; Moeller, J.B.; Hammond, M.A.; Nielsen, O.; Schlosser, A.; Templeton, S.P.; Sorensen, G.L.; Holmskov, U. FIBCD1 binds Aspergillus fumigatus and regulates lung epithelial response to cell wall components. Front. Immunol. 2018, 9, 1967. [Google Scholar] [CrossRef] [PubMed]
- Schalper, K.A.; Carleton, M.; Zhou, M.; Chen, T.; Feng, Y.; Huang, S.-P.; Walsh, A.M.; Baxi, V.; Pandya, D.; Baradet, T.; et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat. Med. 2020, 26, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, G.; Meng, G. Pathogenic fungal infection in the lung. Front. Immunol. 2019, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Z.; Wang, S.; Zhang, C.; Han, L.; Sun, Q.; Han, X. Aspergillus fumigatus induces the release of IL-8 and MCP-1 by activating nuclear transcription through Dectin-1 and CR3 receptors in alveolar epithelial cells. Curr. Microbiol. 2021, 78, 3474–3482. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, C.; Jia, X.; Wang, S.; Wang, J.; Chen, Y.; Zhao, J.; Tian, S.; Han, X.; Han, L. Transcriptome profiles of human lung epithelial cells A549 interacting with Aspergillus fumigatus by RNA-Seq. PLoS ONE 2015, 10, e0135720. [Google Scholar] [CrossRef]
- Oya, E.; Becher, R.; Ekeren, L.; Afanou, A.K.J.; Ovrevik, J.; Holme, J.A. Pro-inflammatory responses in human bronchial epithelial cells induced by spores and hyphal fragments of common damp indoor molds. Int. J. Environ. Res. Public Health 2019, 16, 1085. [Google Scholar] [CrossRef]
- Sun, W.-K.; Lu, X.; Li, X.; Sun, Q.-Y.; Su, X.; Song, Y.; Sun, H.-M.; Shi, Y. Dectin-1 is Inducible and plays a crucial role in Aspergillus-induced innate immune responses in human bronchial epithelial cells. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2755–2764. [Google Scholar] [CrossRef]
- Balloy, V.; Sallenave, J.-M.; Wu, Y.; Touqui, L.; Latgé, J.-P.; Si-Tahar, M.; Chignard, M. Aspergillus fumigatus-induced interleukin-8 synthesis by respiratory epithelial cells is controlled by the phosphatidylinositol 3-kinase, P38 MAPK, and ERK1/2 pathways and not by the toll-like receptor-MyD88 pathway. J. Biol. Chem. 2008, 283, 30513–30521. [Google Scholar] [CrossRef]
- Oosthuizen, J.L.; Gomez, P.; Ruan, J.; Hackett, T.L.; Moore, M.M.; Knight, D.A.; Tebbutt, S.J. Dual organism transcriptomics of airway epithelial cells interacting with conidia of Aspergillus fumigatus. PLoS ONE 2011, 6, e20527. [Google Scholar] [CrossRef]
- Bellanger, A.-P.; Millon, L.; Khoufache, K.; Rivollet, D.; Bièche, I.; Laurendeau, I.; Vidaud, M.; Botterel, F.; Bretagne, S. Aspergillus fumigatus germ tube growth and not conidia ingestion induces expression of inflammatory mediator genes in the human lung epithelial cell line A549. J. Med. Microbiol. 2009, 58, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.; Namvar, S.; Gago, S.; Labram, B.; Bowyer, P.; Richardson, M.D.; Herrick, S.E. Differential proinflammatory responses to Aspergillus fumigatus by airway epithelial cells in vitro are protease dependent. J. Fungi 2021, 7, 468. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, V.; Rivera, A. First line of defense: Innate cell-mediated control of pulmonary Aspergillosis. Front. Microbiol. 2016, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Garth, J.M.; Steele, C. Innate lung defense during invasive Aspergillosis: New mechanisms. J. Innate Immun. 2017, 9, 271–280. [Google Scholar] [CrossRef]
- Jensen, K.; Lund, K.P.; Christensen, K.B.; Holm, A.T.; Dubey, L.K.; Moeller, J.B.; Jepsen, C.S.; Schlosser, A.; Galgóczy, L.; Thiel, S.; et al. M-ficolin is present in Aspergillus fumigatus infected lung and modulates epithelial cell immune responses elicited by fungal cell wall polysaccharides. Virulence 2017, 8, 1870–1879. [Google Scholar] [CrossRef] [PubMed]
- Bidula, S.; Sexton, D.W.; Yates, M.; Abdolrasouli, A.; Shah, A.; Wallis, R.; Reed, A.; Armstrong-James, D.; Schelenz, S. H-ficolin binds Aspergillus fumigatus leading to activation of the lectin complement pathway and modulation of lung epithelial immune responses. Immunology 2015, 146, 281–291. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, S.-H. TGF-β/SMAD4 mediated ucp2 downregulation contributes to Aspergillus protease-induced inflammation in primary bronchial epithelial cells. Redox Biol. 2018, 18, 104–113. [Google Scholar] [CrossRef]
- Namvar, S.; Labram, B.; Rowley, J.; Herrick, S. Aspergillus fumigatus—Host interactions mediating airway wall remodelling in asthma. J. Fungi 2022, 8, 159. [Google Scholar] [CrossRef]
- Namvar, S.; Warn, P.; Farnell, E.; Bromley, M.; Fraczek, M.; Bowyer, P.; Herrick, S. Aspergillus fumigatus proteases, Asp f 5 and Asp f 13, are essential for airway inflammation and remodelling in a murine inhalation model. Clin. Exp. Allergy 2015, 45, 982–993. [Google Scholar] [CrossRef]
- Shen, H.D.; Lin, W.L.; Tam, M.F.; Chou, H.; Wang, C.W.; Tsai, J.J.; Wang, S.R.; Han, S.H. Identification of vacuolar serine proteinase as a major allergen of Aspergillus fumigatus by immunoblotting and N-Terminal amino acid sequence analysis. Clin. Exp. Allergy 2001, 31, 295–302. [Google Scholar] [CrossRef]
- Deckers, J.; De Bosscher, K.; Lambrecht, B.N.; Hammad, H. Interplay between barrier epithelial cells and dendritic cells in allergic sensitization through the lung and the skin. Immunol. Rev. 2017, 278, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Dietz, C.J.; Sun, H.; Yao, W.C.; Citardi, M.J.; Corry, D.B.; Luong, A.U. Aspergillus fumigatus induction of IL-33 expression in chronic rhinosinusitis is PAR2-dependent. Laryngoscope 2019, 129, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Liebana-Jordan, M.; Brotons, B.; Falcon-Perez, J.M.; Gonzalez, E. Extracellular vesicles in the fungi kingdom. Int. J. Mol. Sci. 2021, 22, 7221. [Google Scholar] [CrossRef]
- Rizzo, J.; Rodrigues, M.L.; Janbon, G. Extracellular vesicles in fungi: Past, present, and future perspectives. Front. Cell. Infect. Microbiol. 2020, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Makarova, M.; May, R.C. Fungal extracellular vesicles in interkingdom communication. Curr. Top. Microbiol. Immunol. 2021, 432, 81–88. [Google Scholar]
- Brauer, V.S.; Pessoni, A.M.; Bitencourt, T.A.; de Paula, R.G.; de Oliveira Rocha, L.; Goldman, G.H.; Almeida, F. Extracellular vesicles from Aspergillus flavus induce M1 polarization in vitro. mSphere 2020, 5, e00190-20. [Google Scholar] [CrossRef]
- Souza, J.A.M.; Baltazar, L.D.M.; Carregal, V.M.; Gouveia-Eufrasio, L.; de Oliveira, A.G.; Dias, W.G.; Campos Rocha, M.; Rocha de Miranda, K.; Malavazi, I.; Santos, D.D.A.; et al. Characterization of Aspergillus fumigatus extracellular vesicles and their effects on macrophages and neutrophils functions. Front. Microbiol. 2019, 10, 2008. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; Queiroz-Telles, F.D.; Kono, A.S.G.; Paniago, A.M.M.; Nathan, A.; Valle, A.C.F.D.; Bagagli, E.; Benard, G.; et al. Brazilian guidelines for the clinical management of Paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2017, 50, 715–740. [Google Scholar] [CrossRef]
- Martinez, R. New trends in Paracoccidioidomycosis epidemiology. J. Fungi 2017, 3, 1. [Google Scholar] [CrossRef]
- Millington, M.A.; Nishioka, S.D.A.; Martins, S.T.; Dos Santos, Z.M.G.; de Lima Júnior, F.E.F.; Alves, R.V. Paracoccidioidomycosis: Historical approach and perspectives for implementation of surveillance and control. Epidemiol. Serv. Saude 2018, 27, e0500002. [Google Scholar]
- do Valle, A.C.F.; Marques de Macedo, P.; Almeida-Paes, R.; Romão, A.R.; dos Santos Lazéra, M.; Wanke, B. Paracoccidioidomycosis after highway construction, Rio de Janeiro, Brazil. Emerg. Infect. Dis. 2017, 23, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- Barrozo, L.V.; Benard, G.; Silva, M.E.S.; Bagagli, E.; Marques, S.A.; Mendes, R.P. First Description of a cluster of acute/subacute Paracoccidioidomycosis cases and its association with a climatic anomaly. PLoS Negl. Trop. Dis. 2010, 4, e643. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R. Epidemiology of Paracoccidioidomycosis. Rev. Inst. Med. Trop. Sao Paulo 2015, 57 (Suppl. 19), 11–20. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.D.M.; Theodoro, R.C.; Oliveira, F.F.M.D.; Machado, G.C.; Hahn, R.C.; Bagagli, E.; San-Blas, G.; Soares Felipe, M.S. Paracoccidioides lutzii Sp. Nov.: Biological and clinical implications. Med. Mycol. 2014, 52, 19–28. [Google Scholar]
- Bocca, A.L.; Amaral, A.C.; Teixeira, M.M.; Sato, P.K.; Shikanai-Yasuda, M.A.; Soares Felipe, M.S. Paracoccidioidomycosis: Eco-Epidemiology, taxonomy and clinical and therapeutic issues. Future Microbiol. 2013, 8, 1177–1191. [Google Scholar] [CrossRef]
- Theodoro, R.C.; de Melo Teixeira, M.; Felipe, M.S.S.; dos Santos Paduan, K.; Ribolla, P.M.; San-Blas, G.; Bagagli, E. Genus Paracoccidioides: Species recognition and biogeographic aspects. PLoS ONE 2012, 7, e37694. [Google Scholar] [CrossRef]
- Turissini, D.A.; Gomez, O.M.; Teixeira, M.M.; McEwen, J.G.; Matute, D.R. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet. Biol. 2017, 106, 9–25. [Google Scholar] [CrossRef]
- Matute, D.R.; McEwen, J.G.; Puccia, R.; Montes, B.A.; San-Blas, G.; Bagagli, E.; Rauscher, J.T.; Restrepo, A.; Morais, F.; Niño-Vega, G.; et al. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol. Biol. Evol. 2006, 23, 65–73. [Google Scholar] [CrossRef]
- Mattos, K.; Cocio, T.A.; Chaves, E.G.A.; Borges, C.L.; Venturini, J.; de Carvalho, L.R.; Mendes, R.P.; Paniago, A.M.M.; Weber, S.S. An Update on the occurrence of Paracoccidioides species in the midwest region, Brazil: Molecular epidemiology, clinical aspects and serological profile of patients from Mato Grosso Do Sul state. PLoS Negl. Trop. Dis. 2021, 15, e0009317. [Google Scholar] [CrossRef]
- Salazar, M.E.; Restrepo, A.; Stevens, D.A. Inhibition by Estrogens of conidium-to-yeast conversion in the fungus Paracoccidioides brasiliensis. Infect. Immun. 1988, 56, 711–713. [Google Scholar] [CrossRef]
- Restrepo, A.; Salazar, M.E.; Cano, L.E.; Stover, E.P.; Feldman, D.; Stevens, D.A. Estrogens inhibit mycelium-to-yeast transformation in the fungus Paracoccidioides brasiliensis: Implications for resistance of females to Paracoccidioidomycosis. Infect. Immun. 1984, 46, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Aristizábal, B.H.; Clemons, K.V.; Cock, A.M.; Restrepo, A.; Stevens, D.A. Experimental paracoccidioides brasiliensis Infection in mice: Influence of the hormonal status of the host on tissue responses. Med. Mycol. 2002, 40, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Giannini, M.J.; Ricci, L.C.; Uemura, M.A.; Toscano, E.; Arns, C.W. Infection and apparent invasion of vero cells by Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 1994, 32, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, A.P.; Gesztesi, J.L.; Franco, M.F.; de Souza, W.; de Moraes, J.Z.; Travassos, L.R.; Lopes, J.D. Binding of Paracoccidioides brasiliensis to laminin through surface glycoprotein gp43 leads to enhancement of fungal pathogenesis. Infect. Immun. 1994, 62, 1465–1469. [Google Scholar] [CrossRef]
- da Silva Neto, B.R.; de Fátima da Silva, J.; Mendes-Giannini, M.J.S.; Lenzi, H.L.; de Almeida Soares, C.M.; Pereira, M. The Malate Synthase of Paracoccidioides brasiliensisis a linked surface protein that behaves as an anchorless adhesin. BMC Microbiol. 2009, 9, 1–12. [Google Scholar] [CrossRef]
- Andreotti, P.F.; Monteiro da Silva, J.L.; Bailão, A.M.; de Almeida Soares, C.M.; Benard, G.; Soares, C.P.; Mendes-Giannini, M.J.S. Isolation and partial characterization of a 30 KDa adhesin from Paracoccidioides brasiliensis. Microbes Infect. 2005, 7, 875–881. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Báo, S.N.; Andreotti, P.F.; de Faria, F.P.; Felipe, M.S.S.; dos Santos Feitosa, L.; Mendes-Giannini, M.J.S.; de Almeida Soares, C.M. Glyceraldehyde-3-phosphate dehydrogenase of Paracoccidioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect. Immun. 2006, 74, 382–389. [Google Scholar] [CrossRef]
- Lopes, J.D.; Moura-Campos, M.C.; Vicentini, A.P.; Gesztesi, J.L.; de-Souza, W.; Camargo, Z.P. Characterization of glycoprotein Gp43, the major laminin-binding protein of Paracoccidioides brasiliensis. Braz. J. Med. Biol. Res. 1994, 27, 2309–2313. [Google Scholar]
- Pereira, L.A.; Báo, S.N.; Barbosa, M.S.; da Silva, J.L.M.; Felipe, M.S.S.; de Santana, J.M.; Mendes-Giannini, M.J.S.; de Almeida Soares, C.M. Analysis of the Paracoccidioides brasiliensis triosephosphate isomerase suggests the potential for adhesin function. FEMS Yeast Res. 2007, 7, 1381–1388. [Google Scholar] [CrossRef]
- Mendes-Giannini, M.J.S.; Andreotti, P.F.; Vincenzi, L.R.; da Silva, J.L.M.; Lenzi, H.L.; Benard, G.; Zancopé-Oliveira, R.; de Matos Guedes, H.L.; Soares, C.P. Binding of extracellular matrix proteins to Paracoccidioides brasiliensis. Microbes Infect. 2006, 8, 1550–1559. [Google Scholar] [CrossRef]
- Gesztesi, J.L.; Puccia, R.; Travassos, L.R.; Vicentini, A.P.; de Moraes, J.Z.; Franco, M.F.; Lopes, J.D. Monoclonal antibodies against the 43,000 Da glycoprotein from Paracoccidioides brasiliensis modulate laminin-mediated fungal adhesion to epithelial cells and pathogenesis. Hybridoma 1996, 15, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, A.P.; Moraes, J.Z.; Gesztesi, J.L.; Franco, M.F.; de Souza, W.; Lopes, J.D. Laminin-binding epitope on Gp43 from Paracoccidioides brasiliensis is recognized by a monoclonal antibody raised against Staphylococcus aureus laminin receptor. J. Med. Vet. Mycol. 1997, 35, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ywazaki, C.Y.; Maza, P.K.; Suzuki, E.; Takahashi, H.K.; Straus, A.H. Role of host glycosphingolipids on Paracoccidioides brasiliensis adhesion. Mycopathologia 2011, 171, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Gomez, B.L.; Restrepo, A.; Hamilton, A.J.; Cano, L.E. Recognition of extracellular matrix proteins by Paracoccidioides brasiliensis yeast cells. Med. Mycol. 2005, 43, 637–645. [Google Scholar] [CrossRef] [PubMed][Green Version]
- da Silva, J.D.F.; de Oliveira, H.C.; Marcos, C.M.; da Silva, R.A.M.; da Costa, T.A.; Calich, V.L.G.; Almeida, A.M.F.; Mendes-Giannini, M.J.S. Paracoccidoides brasiliensis 30 KDa adhesin: Identification as a 14-3-3 protein, cloning and subcellular localization in infection models. PLoS ONE 2013, 8, e62533. [Google Scholar] [CrossRef]
- de Oliveira, H.C.; da Silva, J.D.F.; Scorzoni, L.; Marcos, C.M.; Rossi, S.A.; de Paula E Silva, A.C.A.; Assato, P.A.; da Silva, R.A.M.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Importance of adhesins in virulence of Paracoccidioides spp. Front. Microbiol. 2015, 6, 303. [Google Scholar] [CrossRef]
- Almeida, B.R.; Barros, B.C.S.; Araújo, A.C.L.; Alcantara, C.; Suzuki, E. Paracoccidioides Species present distinct fungal adherence to epithelial lung cells and promote different IL-8 secretion levels. Med. Microbiol Immunol. 2020, 209, 59–67. [Google Scholar] [CrossRef]
- Maza, P.K.; Straus, A.H.; Toledo, M.S.; Takahashi, H.K.; Suzuki, E. Interaction of epithelial cell membrane rafts with Paracoccidioides brasiliensis leads to fungal adhesion and Src-family kinase activation. Microbes Infect. 2008, 10, 540–547. [Google Scholar] [CrossRef]
- Monteiro da Silva, J.L.; Andreotti, P.F.; Benard, G.; Soares, C.P.; Miranda, E.T.; Mendes-Giannini, M.J.S. Epithelial cells treated with genistein inhibit adhesion and endocytosis of Paracoccidioides brasiliensis. Antonie Van Leeuwenhoek 2007, 92, 129–135. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Silva, J.D.F.D.; Silva, J.L.M.D.; Andreotti, P.F.; Soares, C.P.; Benard, G.; Giannini, M.J.S.M. Induction of apoptosis in A549 pulmonary cells by two Paracoccidioides brasiliensis samples. Mem. Inst. Oswaldo Cruz 2009, 104, 749–754. [Google Scholar] [CrossRef]
- de Barros, B.C.S.C.; Almeida, B.R.; Suzuki, E. Paracoccidioides brasiliensis downmodulates α3 integrin levels in human lung epithelial cells in a TLR2-dependent manner. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maza, P.K.; Oliveira, P.; Toledo, M.S.; Paula, D.M.B.; Takahashi, H.K.; Straus, A.H.; Suzuki, E. Paracoccidioides brasiliensis induces secretion of IL-6 and IL-8 by lung epithelial cells. modulation of host cytokine levels by fungal proteases. Microbes Infect. 2012, 14, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, C.; Maza, P.K.; Barros, B.C.S.C.; Suzuki, E. Role of protein kinase c in cytokine secretion by lung epithelial cells during infection with Paracoccidioides brasiliensis. Pathog. Dis. 2015, 73, ftv045. [Google Scholar] [CrossRef] [PubMed]
- Barros, B.C.S.C.; Maza, P.K.; Alcantara, C.; Suzuki, E. Paracoccidioides brasiliensis Induces recruitment of α3 and α5 integrins into epithelial cell membrane rafts, leading to cytokine secretion. Microbes Infect. 2016, 18, 68–77. [Google Scholar] [CrossRef]
- de Oliveira, P.; Juliano, M.A.; Tanaka, A.S.; Carmona, A.K.; Dos Santos, S.M.B.; de Barros, B.C.S.C.; Maza, P.K.; Puccia, R.; Suzuki, E. Paracoccidioides brasiliensis induces cytokine secretion in epithelial cells in a protease-activated receptor-dependent (PAR) manner. Med. Microbiol. Immunol. 2017, 206, 149–156. [Google Scholar] [CrossRef]
- Barron, S.L.; Saez, J.; Owens, R.M. In vitro models for studying respiratory host-pathogen interactions. Adv. Biol. 2021, 5, e2000624. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, B.C.S.C.; Almeida, B.R.; Barros, D.T.L.; Toledo, M.S.; Suzuki, E. Respiratory Epithelial Cells: More Than Just a Physical Barrier to Fungal Infections. J. Fungi 2022, 8, 548. https://doi.org/10.3390/jof8060548
Barros BCSC, Almeida BR, Barros DTL, Toledo MS, Suzuki E. Respiratory Epithelial Cells: More Than Just a Physical Barrier to Fungal Infections. Journal of Fungi. 2022; 8(6):548. https://doi.org/10.3390/jof8060548
Chicago/Turabian StyleBarros, Bianca C. S. C., Bruna R. Almeida, Debora T. L. Barros, Marcos S. Toledo, and Erika Suzuki. 2022. "Respiratory Epithelial Cells: More Than Just a Physical Barrier to Fungal Infections" Journal of Fungi 8, no. 6: 548. https://doi.org/10.3390/jof8060548
APA StyleBarros, B. C. S. C., Almeida, B. R., Barros, D. T. L., Toledo, M. S., & Suzuki, E. (2022). Respiratory Epithelial Cells: More Than Just a Physical Barrier to Fungal Infections. Journal of Fungi, 8(6), 548. https://doi.org/10.3390/jof8060548





