Keratinocyte Response to Infection with Sporothrix schenckii
Abstract
1. Introduction
2. Materials and Methods
2.1. HaCaT Keratinocyte Culture
2.2. Sporothrix schenckii Culture
2.3. Cell Infection
2.4. Cytotoxicity Analysis
2.5. Analysis of Changes in the Cytoskeleton of Infected Keratinocytes
2.6. Opsonization of Conidia and Yeast Cells of S. schenckii
2.7. Analysis of Surface Receptor Expression in Infected Keratinocytes
2.8. Cytokine Analysis in Infected Keratinocytes
2.9. Statistical Analysis
3. Results
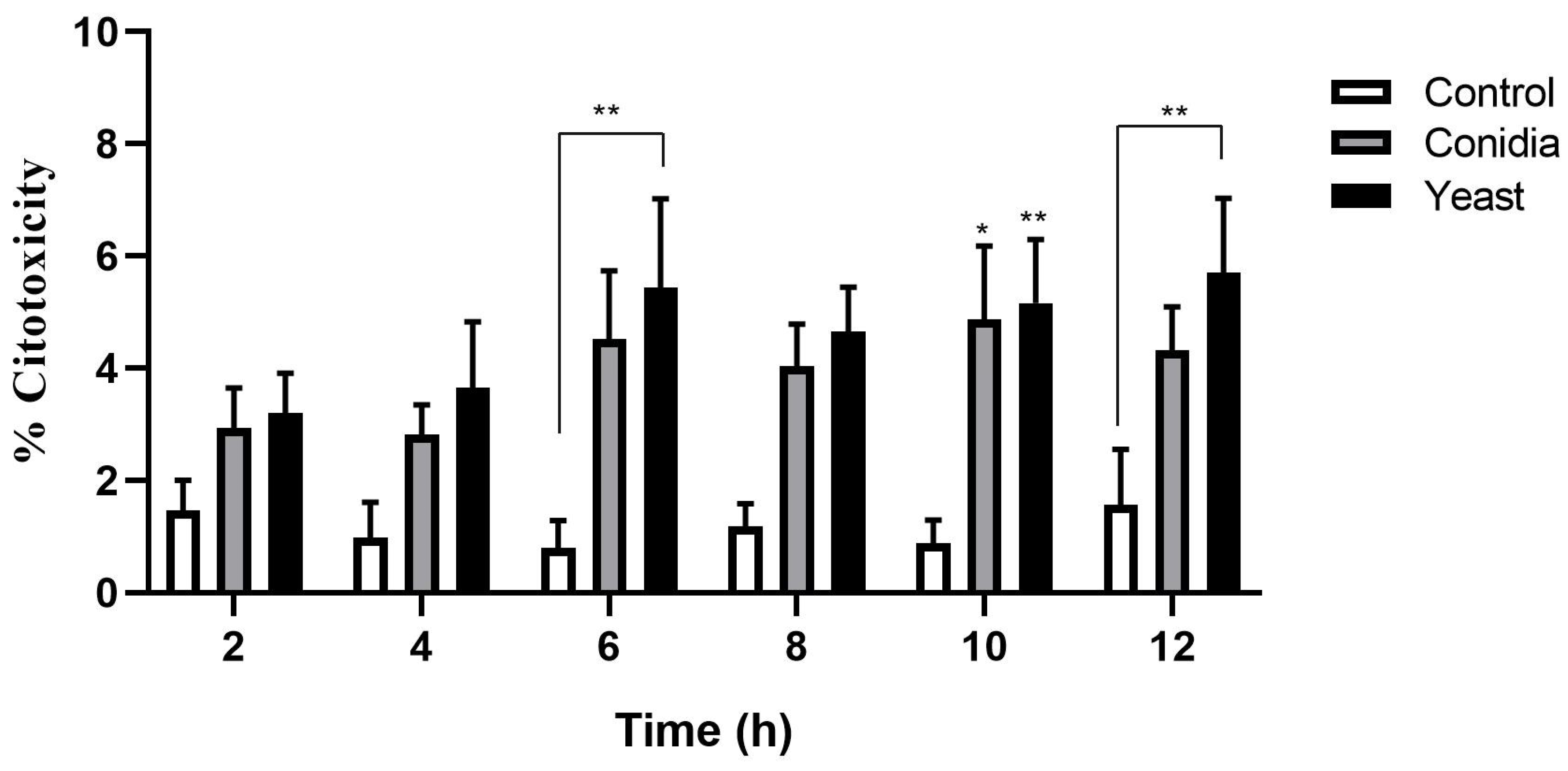
3.1. S. schenckii Yeast Cells Induce a Cytotoxic Effect
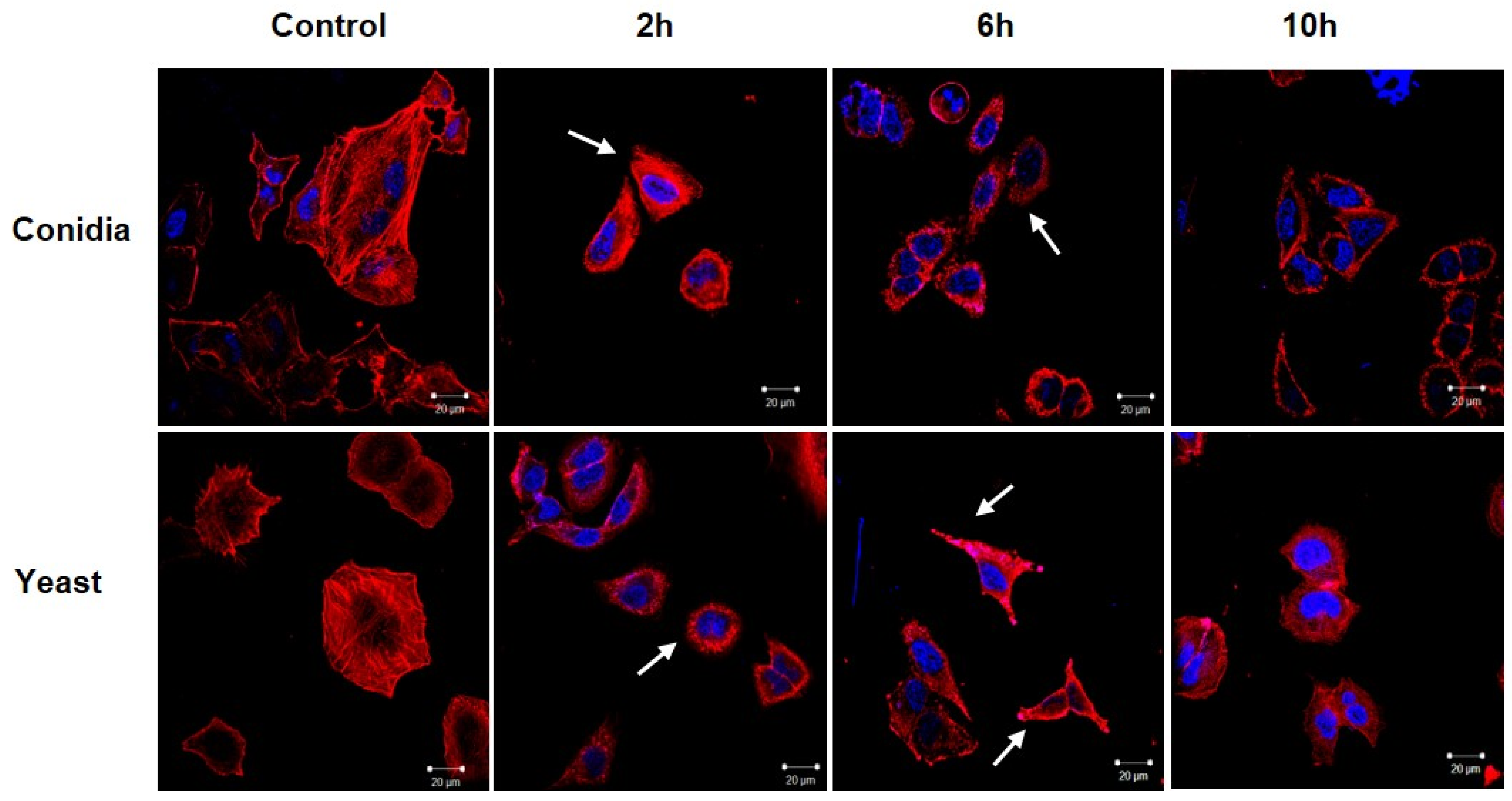
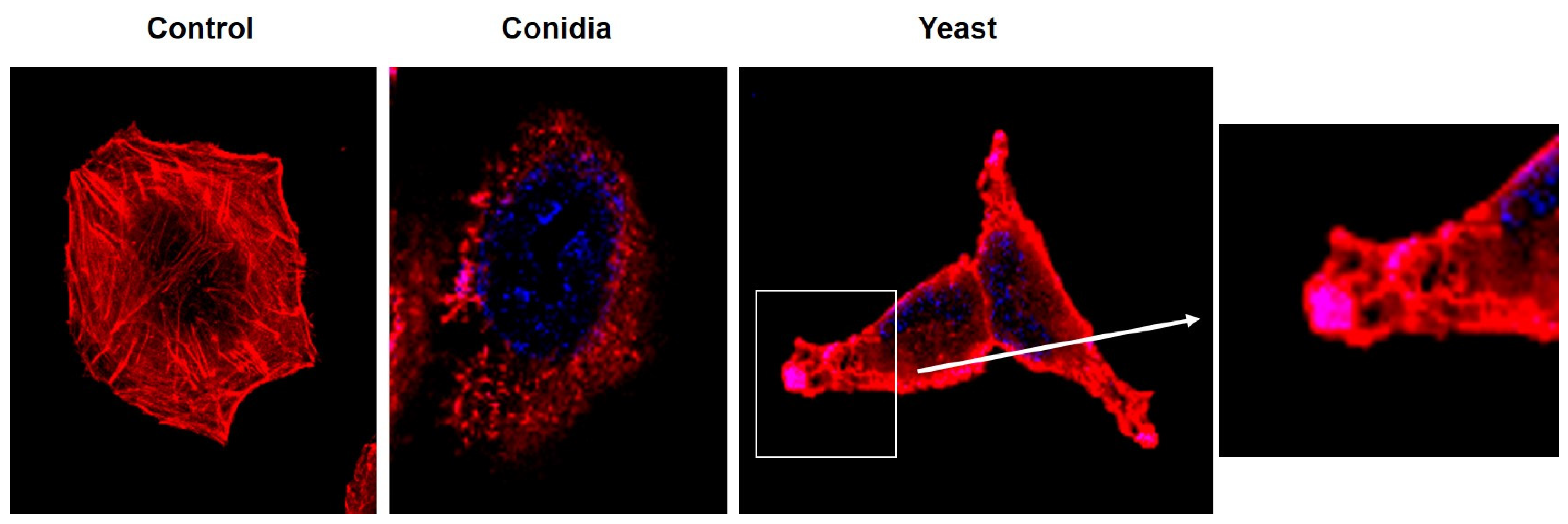
3.2. Sporothrix schenckii Induces Changes in the Actin Cytoskeleton in Human Keratinocytes
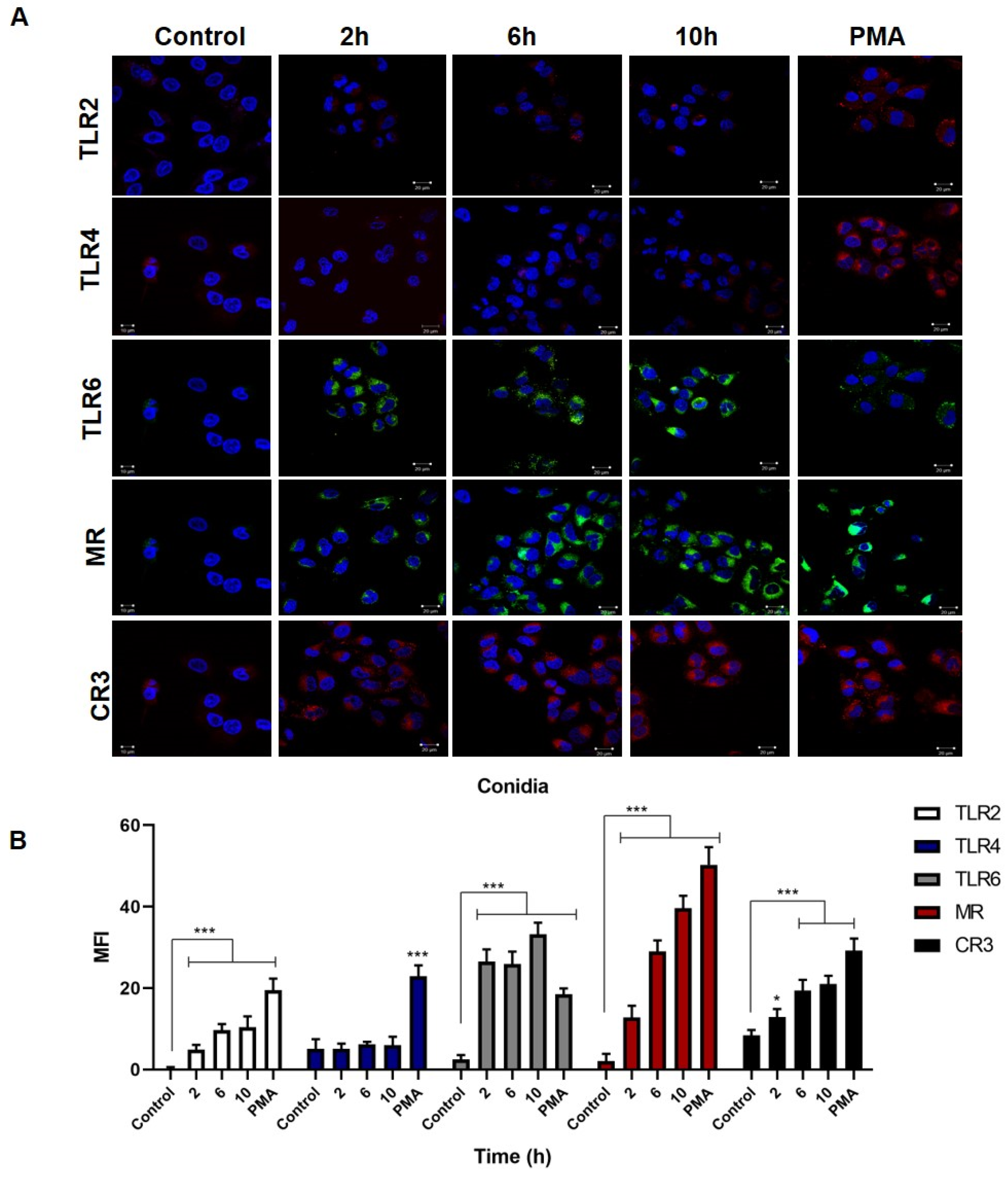
3.3. Overexpression of TLR2, TLR6, MR and CR3 Receptors by Keratinocytes Infected with Conidia and Yeast Cells of Sporothrix schenckii
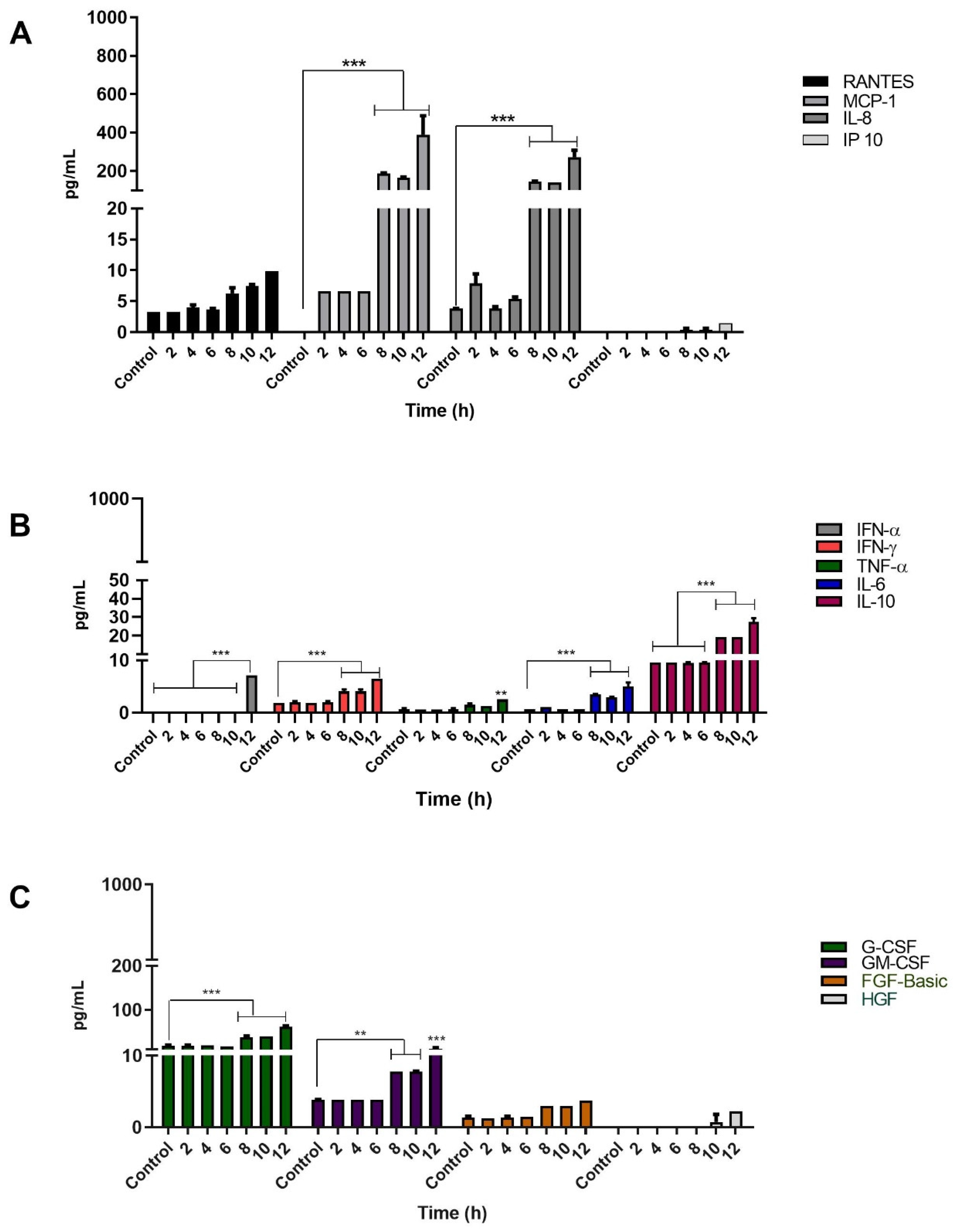
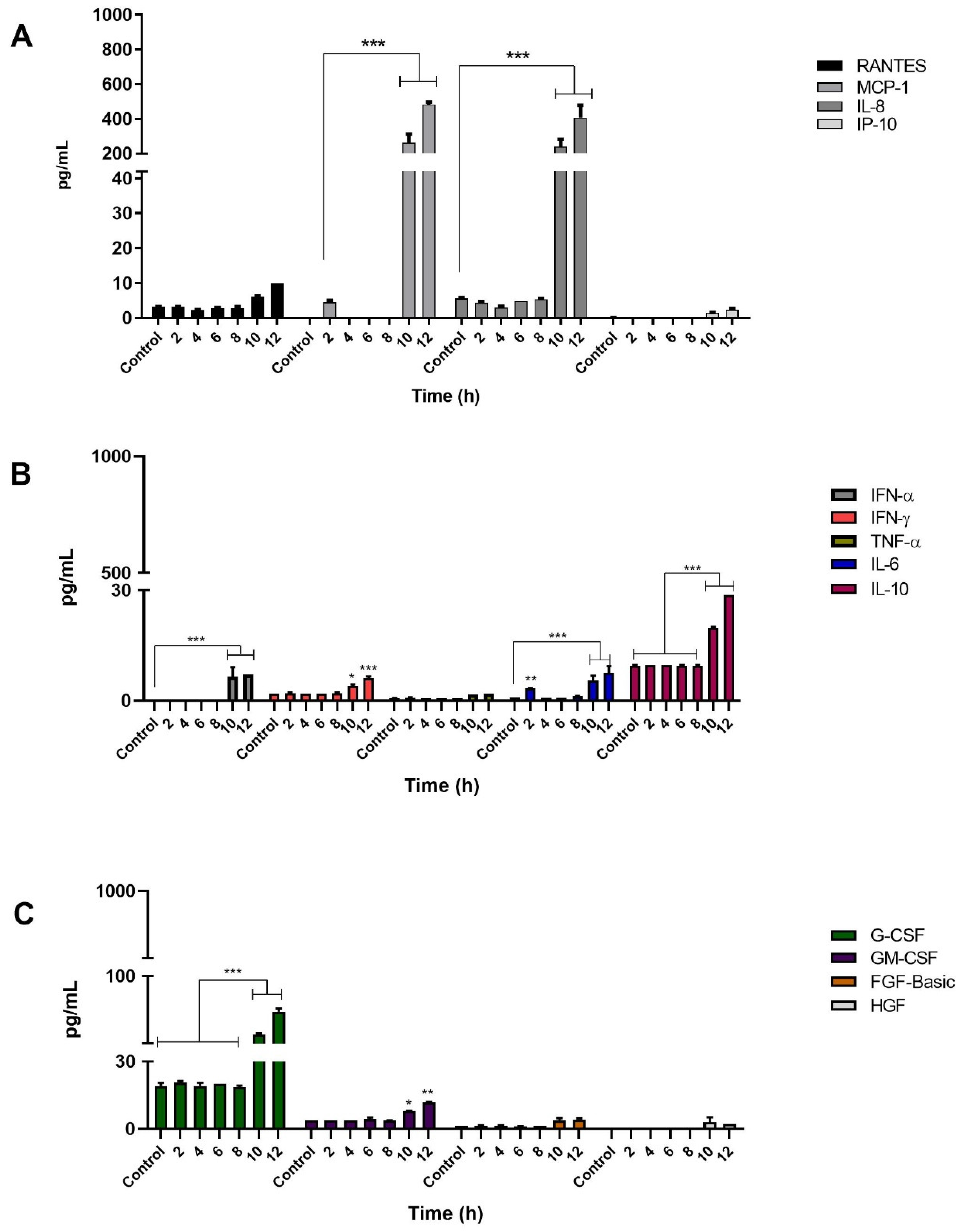
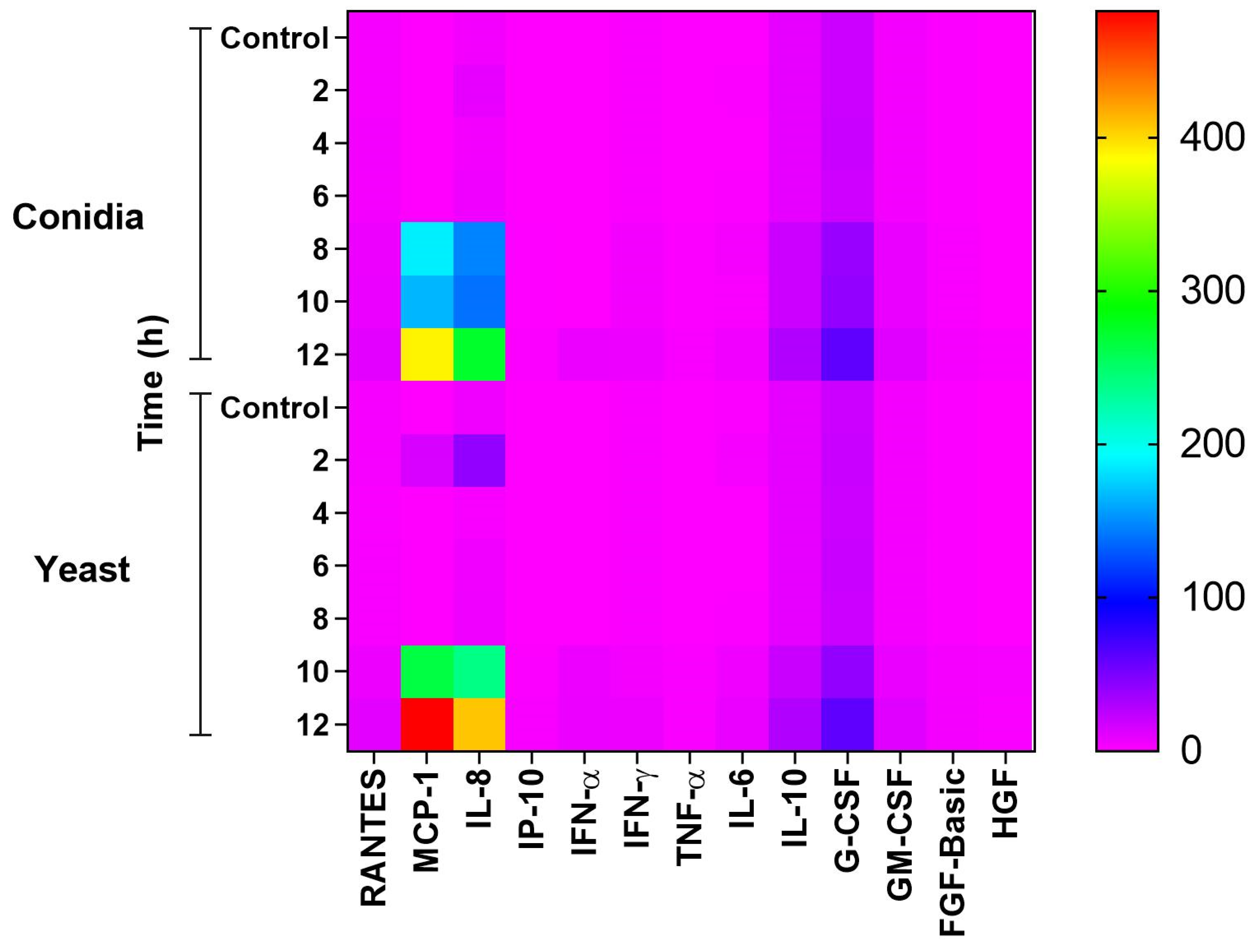
3.4. Infection of Keratinocytes with Sporothrix schenckii Induces the Production of Proinflammatory Cytokines, Chemokines and Growth Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marimon, R.; Cano, J.; Gene, J.; Sutton, D.A.; Kawasaki, M.; Guarro, J. Sporothrix brasiliensis, S. globosa and S. mexicana, three new Sporothrix species of clinical interest. J. Clin. Microbiol. 2007, 45, 3198–3206. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Chung, W.H.; Hung, S.I.; Chun, H.H.; Wang, Z.W.; Chen, C.H.; Lu, S.C.; Kuo, T.T.; Hong, H.S. Detection of Sporothrix schenckii in clinical samples by a nested PCR assay. J. Clin. Microbiol. 2003, 41, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Bonifaz, A.; Gutierrez-Galhardo, M.C.; Mochizuki, T.; Li, S. Global epidemiology of sporotrichosis. Med. Mycol. 2014, 53, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Lopez, B.L.M.; Schubach, A.; Costa, R.O. Sporothrix schenckii and sporotrichosis. An. Acad. Bras. Cienc. 2006, 78, 293–308. [Google Scholar] [CrossRef]
- Arenas, R.; Sánchez-Cárdenas, C.D.; Ramirez-Hobak, L.; Ruíz Arriaga, L.F.; Vega Memije, M.E. Sporotrichosis: From KOH to molecular biology. J. Fungi. 2018, 4, 62. [Google Scholar] [CrossRef]
- Mayorga-Rodríguez, J.; Mayorga-Garibaldi, J.L.; Muñoz-Estrada, V.F.; De León Ramírez, R.M. Esporotricosis: Serie de 1134 casos en una zona endémica de México. Med. Cutan. Ibero. Lat. Am. 2019, 47, 24–28. [Google Scholar]
- Toriello, C.; Brunner-Mendoza, C.; Ruiz-Baca, E.; Duarte-Escalante, E.; Pérez-Mejía, A.; Del Rocío Reyes-Montes, M. Sporotrichosis in Mexico. Braz. J. Microbiol. 2021, 52, 49–62. [Google Scholar] [CrossRef]
- Carlos, I.Z. Sporotrichosis: New Developments and Future Prospects; Springer: Cham, Switzerland, 2015; pp. 1–185. ISBN 9783319119113. [Google Scholar]
- Kajiwara, H.; Saito, M.; Ohga, S.; Uenotsuchi, T.; Yoshida, S.-I. Impaired host defense against Sporothrix schenckii in mice with chronic granulomatous disease. Infect. Immun. 2004, 72, 5073–5079. [Google Scholar] [CrossRef]
- Scott, E.N.; Muchmore, H.G.; Fine, D.P. Activation of the alternative complement pathway by Sporothrix schenckii. Infect. Immun. 1986, 51, 6–9. [Google Scholar] [CrossRef]
- Guzman-Beltran, S.; Perez-Torres, A.; Coronel-Cruz, C.; Torres-Guerrero, H. Phagocytic receptors on macrophages distinguish between different Sporothrix schenckii morphotypes. Microbes Infect. 2012, 14, 1093–1101. [Google Scholar] [CrossRef]
- Martínez-Álvarez, J.A.; Pérez-García, L.A.; Mellado-Mojica, E.; López, M.G.; Martínez-Duncker, I.; Lopes-Bezerra, L.M.; Mora-Montes, H.M. Sporothrix schenckii sensu stricto and Sporothrix brasiliensis are differentially recognized by human peripheral blood mononuclear cells. Front. Microbiol. 2017, 8, 843. [Google Scholar] [CrossRef] [PubMed]
- Piipponen, M.; Li, D.; Landén, N.X. The immune functions of keratinocytes in skin wound healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef] [PubMed]
- Roupé, K.M.; Nybo, M.; Sjöbring, U.; Alberius, P.; Schmidtchen, A.; Sørensen, O.E. Injury is a major inducer of epidermal innate immune responses during wound healing. J. Investig. Dermatol. 2010, 130, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Köllisch, G.; Kalali, B.N.; Voelcker, V.; Wallich, R.; Behrendt, H.; Ring, J. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology 2005, 114, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Lebre, M.C.; Van der Aar, A.M.G.; Van Baarsen, L.; Van Capel, T.M.M.; Schuitemaker, J.H.N.; Kapsenberg, M.L.; de Jong, E.C. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J. Investig. Dermatol. 2007, 127, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Fukui, A.; Shigeishi, H.; Ishida, Y.; Nishi, H.; Tobiume, K.; Takechi, M.; Kamata, N. Expression and function of RIG-I in oral keratinocytes and fibroblasts. Cell. Physiol. Biochem. 2014, 34, 1556–1565. [Google Scholar] [CrossRef]
- Kimura, K.; Matsuzaki, Y.; Nishikawa, Y.; Kitamura, H.; Akasaka, E.; Rokunohe, D.; Sawamura, D. Characterization of retinoic acid-inducible gene-I (RIG-I) expression corresponding to viral infection and UVB in human keratinocytes. J. Dermatol. Sci. 2012, 66, 64–70. [Google Scholar] [CrossRef]
- Van den Berg, L.M.; Zijlstra-Willems, E.M.; Richters, C.D.; Ulrich, M.M.W.; Geijtenbeek, T.B.H. Dectin-1 activation induces proliferation and migration of human keratinocytes enhancing wound re-epithelialization. Cell. Immunol. 2014, 289, 49–54. [Google Scholar] [CrossRef]
- Harder, J.; Núñez, G. Functional expression of the intracellular pattern recognition receptor NOD1 in human keratinocytes. J. Investig. Dermatol. 2009, 129, 1299–1302. [Google Scholar] [CrossRef][Green Version]
- Wang, J.N.; Li, M. The Immune function of keratinocytes in anti-pathogen infection in the skin. Int. J. Dermatol. Venereol. 2020, 3, 231–238. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016, 12, e1005638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Xu, X.G.; Zhang, M.; Jiang, P.; Zhou, X.Y.; Li, Z.Z.; Zhang, M.F. Sporotrichosis: Clinical and histopathological manifestations. Am. J. Dermatopathol. 2011, 33, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Kupper, T.S. Inflammatory skin diseases, T cells, and immune surveillance. N. Engl. J. Med. 1999, 341, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tsoi, L.C.; Billi, A.C.; Ward, N.L.; Harms, P.W.; Zeng, C.; Maverakis, E.; Kahlenberg, J.M.; Gudjonsson, J.E. Cytokinocytes: The diverse contribution of keratinocytes to immune responses in skin. JCI Insight 2020, 5, e142067. [Google Scholar] [CrossRef]
- Santiago-Téllez, A.; Castrillón-Rivera, L.E.; Palma-Ramos, A.; Bello-López, J.M.; Sainz-Espuñes, T.; Contreras-Paredes, A.; Luna-Herrera, J.; Castañeda-Sánchez, J.I. Keratinocyte infection by Actinomadura madurae triggers an inflammatory response. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 392–398. [Google Scholar] [CrossRef]
- Crack, L.R.; Jones, L.; Malavige, G.N.; Patel, V.; Ogg, G.S. Human antimicrobial peptides LL-37 and human β-defensin-2 reduce viral replication in keratinocytes infected with varicella zoster virus. Clin. Exp. Dermatol. 2012, 37, 534–543. [Google Scholar] [CrossRef]
- Achterman, R.R.; Moyes, D.L.; Thavaraj, S.; Smith, A.R.; Blair, K.M.; White, T.C.; Naglik, J.R. Dermatophytes activate skin keratinocytes via mitogen-activated protein kinase signaling and induce immune responses. Infect. Immun. 2015, 83, 1705–1714. [Google Scholar] [CrossRef]
- Mario, D.A.N.; Santos, R.C.V.; Denardi, L.B.; de Almeida Vaucher, R.; Santurio, J.M.; Alves, S.H. Interference of melanin in the susceptibility profile of Sporothrix species to amphotericin B. Revista Iberoamericana de Micología 2016, 33, 21–25. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; Figueiredo-Carvalho, M.H.; Brito-Santos, F.; Almeida-Silva, F.; Oliveira, M.M.; Zancopé-Oliveira, R.M. Melanins protect Sporothrix brasiliensis and Sporothrix schenckii from the antifungal effects of terbinafine. PLoS ONE 2016, 11, e0152796. [Google Scholar] [CrossRef]
- Arrillaga-Moncrieff, I.; Capilla, J.; Mayayo, E.; Marimon, R.; Mariné, M.; Gené, J.; Cano, J.; Guarro, J. Different virulence levels of the species of Sporothrix in a murine model. Clin. Microbiol. Infect. 2009, 15, 651–655. [Google Scholar] [CrossRef]
- Ferreira-Fernandes, G.; Oliveira dos Santos, P.; Messias-Rodrigues, A.; Sasaki, A.A.; Burger, E.; Pires de Camargo, Z. Characterization of virulence profile, protein secretion and immunogenicity of different Sporothrix schenckii sensu stricto isolates compared with S. globosa and S. brasiliensis species. Virulence 2013, 4, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Charoenvit, Y.; Taylor, R.L. Experimental sporotrichosis in Syrian hamsters. Infect Immun. 1979, 23, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Brito, M.M.; Conceição-Silva, F.; Morgado, F.N.; Raibolt, P.S.; Schubach, A.; Schubach, T.P.; Schäffer, G.M.; Borba, C.M. Comparison of virulence of different Sporothrix schenckii clinical isolates using experimental murine model. Med. Mycol. 2007, 45, 721–729. [Google Scholar] [CrossRef]
- Mendes-Giannini, M.J.S.; Soares, C.P.; Silva, J.L.M.; Andreotti, P.F. Interaction of pathogenic fungi with host cells: Molecular and cellular approaches. FEMS Immunol Med Microbiol. 2005, 45, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Duarte, A.R.; Castrejón-Jiménez, N.S.; Baltierra-Uribe, S.L.; Pérez-Rangel, S.J.; Carapia-Minero, N.; Castañeda-Sánchez, J.I.; García-Pérez, B.E. Candida glabrata survives and replicates in human osteoblasts. FEMS Pathog. Dis. 2016, 74, ftw030. [Google Scholar] [CrossRef] [PubMed]
- Sabanero, L.M.; Tsutsumi, F.V.; Barbosa, S.G.; López, R.E.; Sandoval, B.G. Interaction of yeasts of Sporothrix schenckii with epithelia. Res. Sci. 2006, 36, 10–14. [Google Scholar]
- Flores, I.; Cruz, T.A.; Nieto, J.L.; González, F.R.; Soto, C.I.; García, C.G. Evaluation of changes in actin filaments of RK13 cells infected with Malassezia pachydermatis. Open J. Vet. Med. 2018, 8, 15–24. [Google Scholar] [CrossRef][Green Version]
- Wasylnka, J.A.; Moore, M.M. Uptake of Aspergillus fumigatus conidia by phagocytic and nonphagocytic cells in vitro: Quantitation using strains expressing green fluorescent protein. Infect. Immun. 2002, 70, 3156–3163. [Google Scholar] [CrossRef]
- Mendes-Giannini, M.J.S.; Hanna, S.A.; Monteiro da Silva, J.L.; Andreotti, P.F.; Benard, G.; Lenzi, H.L.; Soares, C.P. Invasion of epithelial mammalian cells by Paracoccidioides brasiliensis leads to cytoskeletal rearrangement and apoptosis of the host cell. Microbes Infect. 2004, 6, 882–891. [Google Scholar] [CrossRef]
- Chen, S.H.; Stins, M.F.; Huang, S.H.; Chen, Y.H.; Kwon-Chung, K.J.; Chang, Y.; Kim, K.S.; Suzuki, K.; Jong, A.Y. Cryptococcus neoformans induces alterations in the cytoskeleton of human brain microvascular endothelial cells. J. Med. Microbiol. 2003, 52, 961–970. [Google Scholar] [CrossRef]
- García-Pérez, B.E.; Mondragón-Flores, R.; Luna-Herrera, J. Internalization of Mycobacterium tuberculosis by macropinocytosis in non-phagocytic cells. Microb. Pathog. 2003, 35, 49–55. [Google Scholar] [CrossRef]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.S.S.; Mathews, H.L.; Bezerra, L.M.L. Differences in virulence of Sporothrix schenckii conidia related to culture conditions and cell-wall components. J. Med. Microbiol. 1999, 48, 195–203. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lloyd, K.O.; Bitoon, M.A. Isolation and purification of a peptido-rhamnomannan from the yeast form of Sporothrix schenckii. Structural and immunochemical studies. J. Immunol. 1971, 107, 663–671. [Google Scholar] [PubMed]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2004, 4, 11–24. [Google Scholar] [CrossRef]
- Gazi, U.; Martinez-Pomares, L. Influence of the mannose receptor in host immune responses. Immunobiology 2009, 214, 554–561. [Google Scholar] [CrossRef]
- Szolnoky, G.; Bata-Csörgö, Z.; Kenderessy, A.S.; Kiss, M.; Pivarcsi, A.; Novák, Z.; Nagy Newman, K.; Michel, G.; Ruzicka, T.; Maródi, L.; et al. A mannose-binding receptor is expressed on human keratinocytes and mediates killing of Candida albicans. J. Investig. Dermatol. 2001, 117, 205–213. [Google Scholar] [CrossRef]
- Jensen, R.K.; Bajic, G.; Sen, M.; Springer, T.A.; Vorup-Jensen, T.; Andersen, G.R. Complement receptor 3 forms a compact high-affinity complex with iC3b. J. Immunol. 2021, 206, 3032–3042. [Google Scholar] [CrossRef]
- Long, K.H.; Gomez, F.J.; Morris, R.E.; Newman, S.L. Identification of heat shock protein 60 as the ligand on Histoplasma capsulatum that mediates binding to CD18 receptors on human macrophages. J. Immunol. 2003, 170, 487–494. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, J.; Imrich, A.; Cushion, M.; Kinane, T.B.; Koziel, H. Pneumocystis activates human alveolar macrophage NF-kappa B signaling through mannose receptors. Infect. Immun. 2004, 72, 3147–3160. [Google Scholar] [CrossRef] [PubMed]
- Vukman, K.V.; Ravidà, A.; Aldridge, A.M.; O’Neill, S.M. Mannose receptor and macrophage galactose-type lectin are involved in Bordetella pertussis mast cell interaction. J. Leukoc. Biol. 2013, 94, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, Q.; Sun, J.; Shen, Y.; Liu, W. Inflammatory response of human keratinocytes triggered by Sporothrix schenckii via Toll-like receptor 2 and 4. J. Dermatol. Sci. 2012, 66, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Ozinsky, A.; Underhill, D.M.; Fontenot, J.D.; Hajjar, A.M.; Smith, K.D.; Wilson, C.B.; Schroeder, L.; Aderem, A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 13766–13771. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Saini, V.; Arora, S. MCP-1: Chemoattractant with a role beyond immunity: A review. Clin. Chim. Acta 2010, 411, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, G.; Lin, J.; Li, C.; Jiang, N.; Xu, Q.; Wang, Q.; Zhang, J. Role of the mannose receptor during Aspergillus fumigatus infection and interaction with Dectin-1 in corneal epithelial cells. Cornea 2016, 35, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Fayyazi, A.; Schweyer, S.; Soruri, A.; Duong, L.Q.; Radzun, H.J.; Peters, J.; Parwaresch, R.; Berger, H. T lymphocytes and altered keratinocytes express interferon-gamma and interleukin 6 in lichen planus. Arch. Dermatol. Res. 1999, 291, 485–490. [Google Scholar] [CrossRef]
- Fujisawa, H.; Kondo, S.; Wang, B.; Shivji, G.M.; Sauder, D.N. The expression and modulation of IFN-alpha and IFN-beta in human keratinocytes. J. Interferon Cytokine Res. 1997, 17, 721–725. [Google Scholar] [CrossRef]
- Stanifer, M.L.; Pervolaraki, K.; Boulant, S. Differential regulation of type I and type III interferon signaling. Int. J. Mol. Sci. 2019, 20, 1445. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Oliveira, A.H.; de Oliveira, G.G.; Carnevale, N.F.; Portuondo, D.F.; Batista-Duharte, A.; Carlos, I.Z. Anti-inflammatory activity of Vismia guianensis (Aubl.) Pers. extracts and antifungal activity against Sporothrix schenckii. J. Ethnopharmacol. 2017, 4, 266–274. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W. G-CSF: A key regulator of neutrophil production, but that’s not all! Growth Factors 2005, 23, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, Y.J.; Jegal, J.; Jo, B.G.; Choi, H.S.; Yang, M.H. Haplopine Ameliorates 2,4-Dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice and TNF-α/IFN-γ-induced inflammation in human keratinocyte. Antioxidants 2021, 10, 806. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paredes-Rojas, A.; Palma-Ramos, A.; Castrillón-Rivera, L.E.; Mendoza-Pérez, F.; Navarro-González, M.d.C.; Arenas-Guzmán, R.; Castañeda-Sánchez, J.I.; Luna-Herrera, J. Keratinocyte Response to Infection with Sporothrix schenckii. J. Fungi 2022, 8, 437. https://doi.org/10.3390/jof8050437
Paredes-Rojas A, Palma-Ramos A, Castrillón-Rivera LE, Mendoza-Pérez F, Navarro-González MdC, Arenas-Guzmán R, Castañeda-Sánchez JI, Luna-Herrera J. Keratinocyte Response to Infection with Sporothrix schenckii. Journal of Fungi. 2022; 8(5):437. https://doi.org/10.3390/jof8050437
Chicago/Turabian StyleParedes-Rojas, Araceli, Alejandro Palma-Ramos, Laura Estela Castrillón-Rivera, Felipe Mendoza-Pérez, María del Carmen Navarro-González, Roberto Arenas-Guzmán, Jorge Ismael Castañeda-Sánchez, and Julieta Luna-Herrera. 2022. "Keratinocyte Response to Infection with Sporothrix schenckii" Journal of Fungi 8, no. 5: 437. https://doi.org/10.3390/jof8050437
APA StyleParedes-Rojas, A., Palma-Ramos, A., Castrillón-Rivera, L. E., Mendoza-Pérez, F., Navarro-González, M. d. C., Arenas-Guzmán, R., Castañeda-Sánchez, J. I., & Luna-Herrera, J. (2022). Keratinocyte Response to Infection with Sporothrix schenckii. Journal of Fungi, 8(5), 437. https://doi.org/10.3390/jof8050437






