Growth and Yield of Purple Kculli Corn Plants under Different Fertilization Schemes
Abstract
1. Introduction
2. Materials and Methods
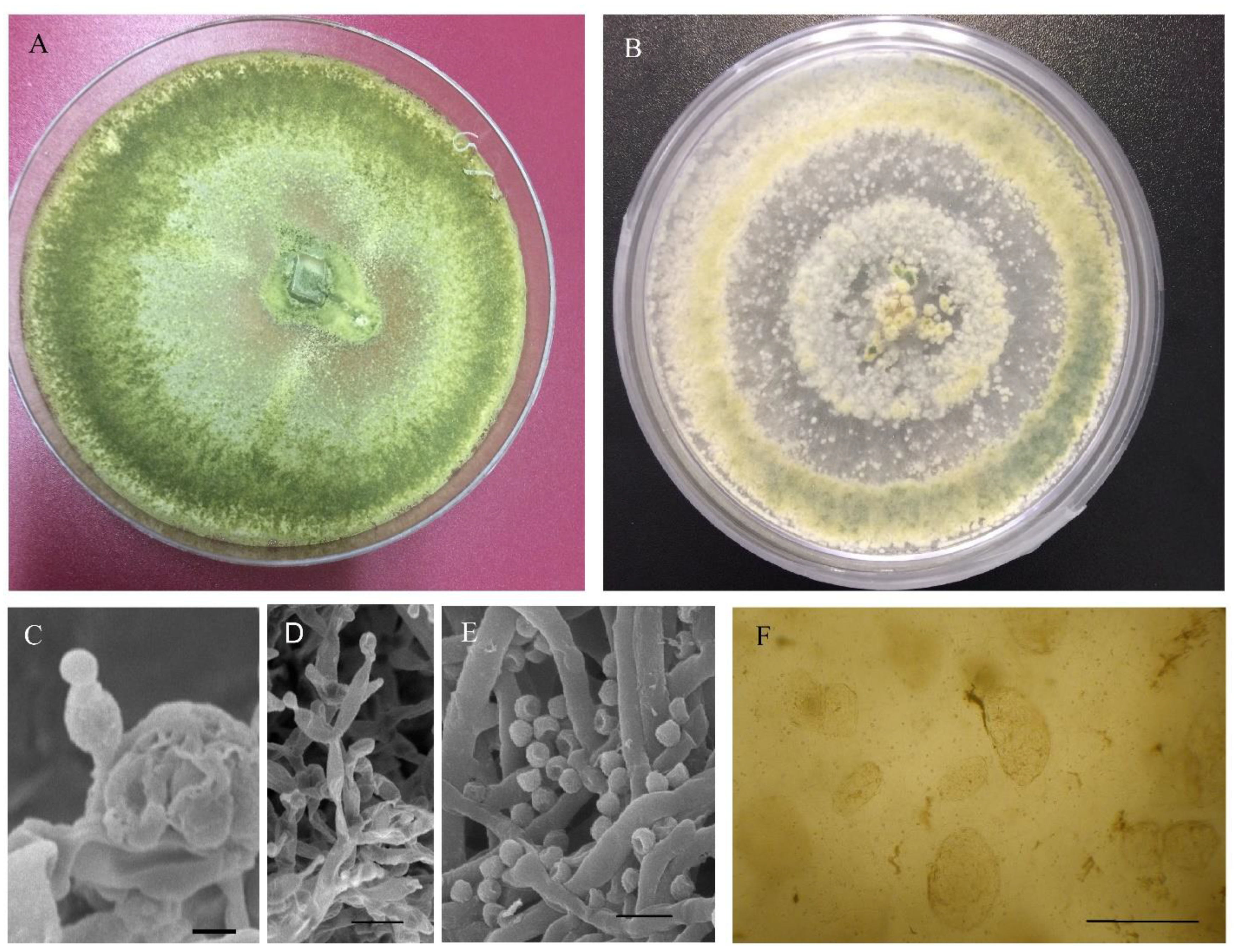
2.1. Fungal Cultures
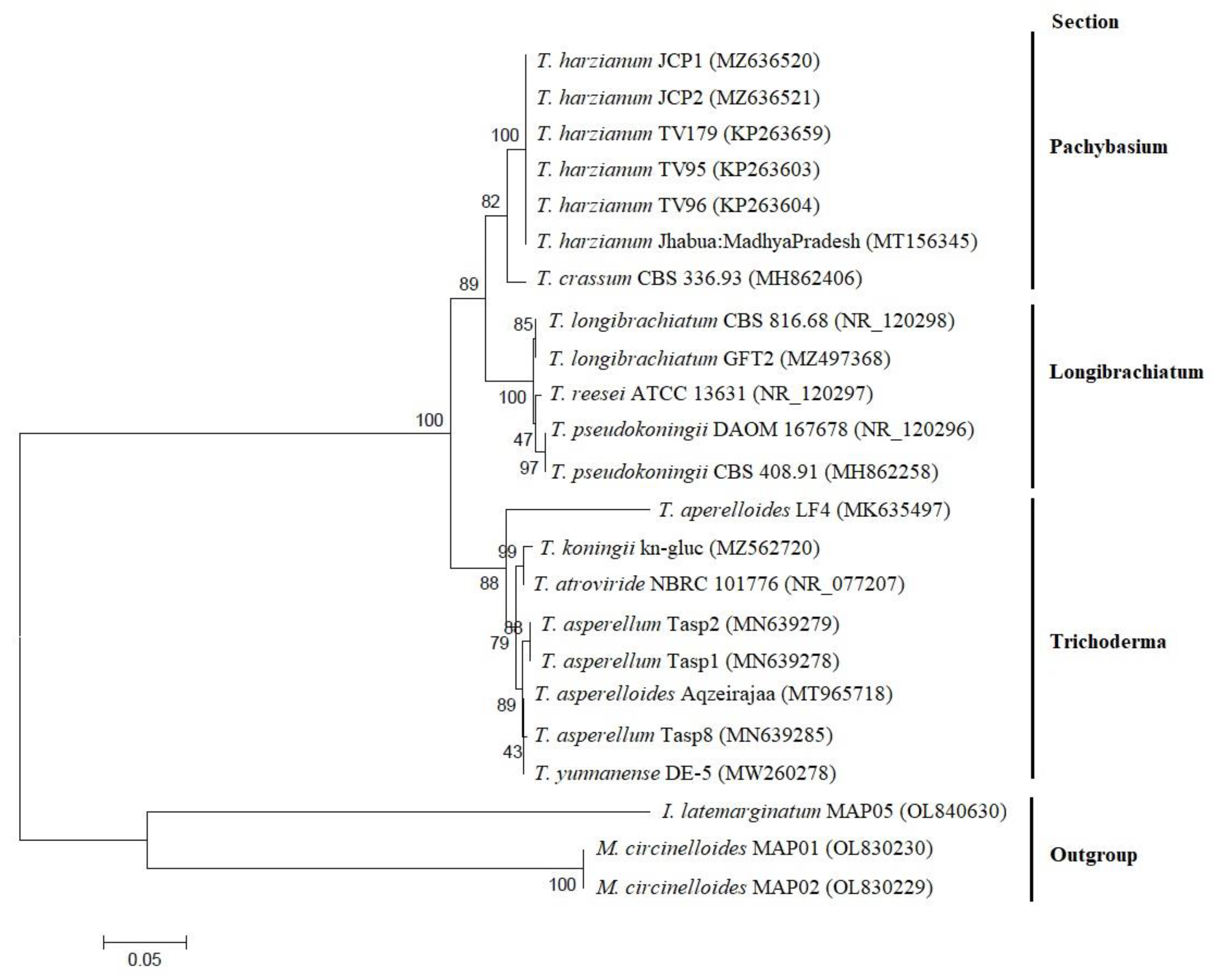
2.2. Molecular and Morphological Characterization
2.3. Corn Accessions
2.4. Treatments and Experimental Design
2.5. Variable Definition
2.5.1. Independent Variables
2.5.2. Growth Dependent Variables
2.5.3. Yield Dependent Variables
2.6. Corn Starch Isolation
2.7. Proximal Analysis of Starches
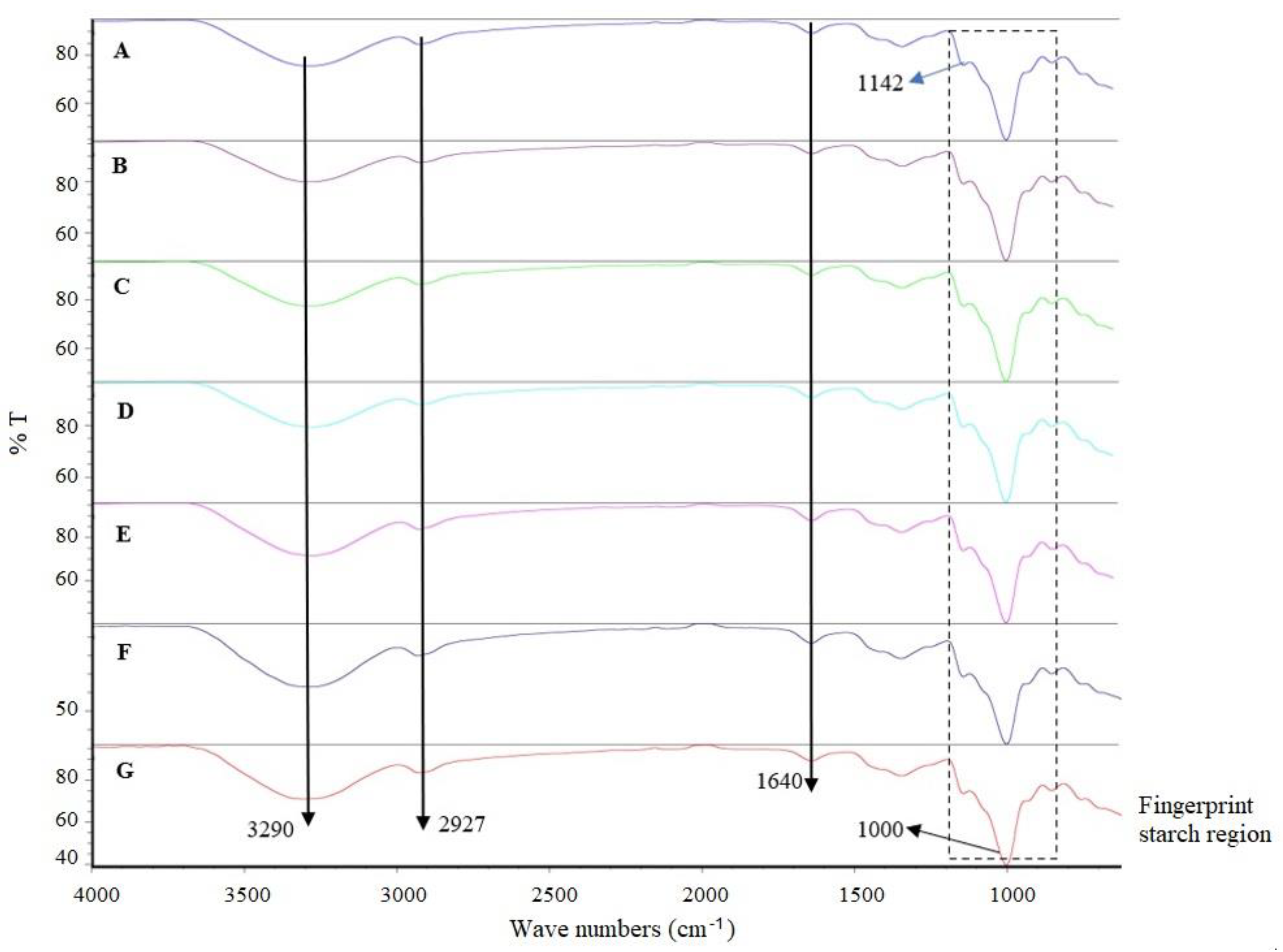
2.8. Fourier Transform Infrared Measurement
2.9. Tristimulus Color
2.10. Statistic Analysis
3. Results and Discussion
3.1. Morphological Characterization
3.2. Molecular Characterization
3.3. Growth Dependent Variables
3.3.1. Plant Height
3.3.2. Stem Diameter
3.3.3. Days to Male and Female Flowering
3.3.4. Floral Asynchrony and Physiological Maturity
3.4. Yield Dependent Variables
3.4.1. Number of Ears per Plant
3.4.2. Ear Weight
3.4.3. Grain Yield
3.4.4. Ear Length
3.4.5. Ear Diameter
3.4.6. Number of Grain Rows
3.4.7. Number of Grains Per Row
3.4.8. One Hundred Grain Weight
3.4.9. Grain Length and Width
3.5. Proximal Chemical Analysis of Native Corn Starches
3.6. Fourier Transform Infrared Spectroscopy
3.7. Tristimulus Color
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Servicio de Información Agroalimentaria y Pesquera (SIAP). Producción Agrícola. 9 January 2017. Available online: https://www.gob.mx/siap/acciones-y-programas/produccion-agricola-33119 (accessed on 22 June 2021).
- Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA). Agenda Técnica Agrícola Hidalgo, 2nd ed.; SAGARPA: Mexico City, Mexico, 2015; pp. 11–92. [Google Scholar]
- Nurhayati, A.Y.; Hariadi, Y.C.; Hasanah, W. Endeavoring to food sustainability by promoting corn cob and rice husk briquetting to fuel energy for small scale industries and household communities. Agric. Agric. Sci. Procedia 2016, 9, 386–395. [Google Scholar] [CrossRef]
- Hernández, A.A.M.; Juárez, L.G.; Fucikovsky, Z.L. Impacto del almacenamiento en la brotación de bulbos de ajo y especies patogénicas de Penicillium y Erwinia asociadas. Rev. Fitotec. Mex. 2006, 29, 283–290. [Google Scholar] [CrossRef]
- Burke, W.J.; Frossard, E.; Kabwe, S.; Jayne, T.S. Understanding fertilizer adoption and effectiveness on maize in Zambia. Food Policy 2019, 86, 101721. [Google Scholar] [CrossRef] [PubMed]
- Ávalos, C.A.M.; Figueroa, V.U.; García, H.J.L.; Vázquez, V.C.; Gallegos, R.M.A.; Orona, C.I. Bioinoculantes y abonos orgánicos en la producción de maíz forrajero. Nova Sci. 2018, 10, 170–189. [Google Scholar] [CrossRef]
- Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). Agenda Técnica Agrícola Hidalgo, 5th ed.; INIFAP: Mexico City, Mexico, 2017; pp. 55–79. [Google Scholar]
- Lira-Saldivar, R.H. Uso de Biofertilizantes en la Agricultura Ecológica, 1st ed.; Serie Agricultura Orgánica Núm. 14; Artículos Técnicos de INTAGRI: Mexico City, Mexico, 2017; pp. 1–9. [Google Scholar]
- Okur, N. A review: Bio-fertilizers- power of beneficial microorganisms in soils. Biomed. J. Sci. Tech. Res. 2018, 4, 4028–4029. [Google Scholar] [CrossRef]
- Tavera-Zavala, D.D.; Hernández-Escareño, J.J.; Ulibarri, G.; Sánchez-Yáñez, J.M. Inoculación de Trichoderma harzianum en Zea mays y su efecto a la adición del fertilizante nitrogenado al 50%. J. Selva Andina Res. Soc. 2017, 8, 115–123. [Google Scholar] [CrossRef]
- Ramírez-Cariño, H.F.; Guadarrama-Mendoza, P.C.; Sánchez-López, V.; Cuervo-Parra, J.A.; Ramírez-Reyes, T.I.; Valadez-Blanco, R. Biocontrol of Alternaria alternata and Fusarium oxysporum by Trichoderma asperelloides and Bacillus licheniformis in tomato plants. Antonie Van Leeuwenhoek 2020, 113, 1247–1261. [Google Scholar] [CrossRef]
- Al-Zubade, A.; Phillips, T.; Williams, A.M.; Jacobsen, K.; Van Sanford, D. Impact of nitrogen rate in conventional and organic production systems on yield and bread baking quality of soft red winter wheat. Agronomy 2021, 11, 1683. [Google Scholar] [CrossRef]
- Bernard, G. Is a Haber-Bosch world sustainable? Population, nutrition, cereals, nitrogen and environment. J. Soc. Political Econ. Stud. 2014, 39, 166. [Google Scholar]
- Chinnamuthu, C.R.; Boopathi, P.M. Nanotechnology and agroecosystem. Madras Agric. J. 2009, 96, 17–31. [Google Scholar]
- Ngosong, C.; Bongkisheri Tanyi, C.B.; Nanganoa, L.T.; Tening, A.S. Optimizing nitrogen fertilization regimes for sustainable maize (Zea mays L.) production on the volcanic soils of Buea Cameroon. Adv. Agric. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, C.; Guo, Q.; Zhang, J.; Ruiz-Menjivar, J. The impact of agricultural chemical inputs on environment: Global evidence from informetrics analysis and visualization. Int. J. Low-Carbon Technol. 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Baioni, R.J.N.; Piña, G.V. Desarrollo de Nuevas Formulaciones de Bioinoculantes Agrícolas Mediante Tecnologías de Inmovilización. Bachelor’s Thesis, Facultad de Ingeniería, Universidad ORT Uruguay, Montevideo, Uruguay, August 2018. [Google Scholar]
- Samaniego-Fernández, L.M.; Harouna, M.; Corbea, O.; Rondón-Castillo, J.R.; Placeres-Espinosa, I. Aislamiento, identificación y evaluación de cepas autóctonas de Trichoderma spp. antagonistas de patógenos del suelo. Rev. Prot. Veg. 2018, 33, 1–11. [Google Scholar]
- Salinas-Moreno, Y.; Esquivel-Esquivel, G.; Ramírez-Díaz, J.L.; Alemán, T.I.; Bautista-Ramírez, E.; Santillán-Fernández, A. Selección de germoplasma de maíz morado (Zea mays L.) con potencial para extracción de pigmentos. Rev. Fitotec. Mex. 2021, 44, 309–321. [Google Scholar] [CrossRef]
- Piña, D.P.C. Comparación de Rendimiento y Contenido de Antocianinas de 6 Variedades de Maíz Morado (Zea mays L.) en el Distrito de Ichocán, Provincia de San Marcos, Región Cajamarca. Bachelor’s Thesis, Facultad de Ciencias Agrarias, Universidad Nacional de Cajamarca, Cajamarca, Peru, December 2018. [Google Scholar]
- Guillén-Sánchez, J.; Mori-Arismendi, S.; Paucar-Mecacho, L.M. Características y propiedades funcionales del maíz morado (Zea mays L.) var. subnigroviolaceo. Sci. Agropecu. 2014, 5, 211–217. [Google Scholar]
- Boñón, C.D.M.; Paredes, C.E.R. Caracterización de Antocianinas de la Coronta de Zea mays L. “Maíz Morado” Para la Elaboración de un Protector Solar. Bachelor’s Thesis, Facultad de Ciencias de la Salud, Universidad Privada Antonio Guillermo Urrelo, Cajamarca, Peru, January 2020. [Google Scholar]
- Fallahi, M.; Saremi, H.; Javan-Nikkhah, M.; Somma, S.; Haidukowski, M.; Logrieco, A.F.; Moretti, A. Isolation, molecular identification and mycotoxin profile of Fusarium species isolated from maize kernels in Iran. Toxins 2019, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Parra, J.A.; Sánchez-López, V.; Romero-Cortes, T.; Ramírez-Lepe, M. Hypocrea viridescens ITV43 potential biological control agent for Moniliophthora roreri Cif & Par, Phytophthora megasperma and P. capsici. Afr. J. Microbiol. Res. 2014, 8, 1704–1712. [Google Scholar] [CrossRef][Green Version]
- Juárez-Hernández, J.; Castillo-Hernández, D.; Pérez-Parada, C.; Nava-Galicia, S.; Cuervo-Parra, J.A.; Surian-Cruz, E.; Díaz-Godínez, G.; Sánchez-Hernández, C.; Bibbins-Martínez, M. Isolation of fungi from a textile industry effluent and the screening of their potential to degrade industrial dyes. J. Fungi 2021, 7, 805. [Google Scholar] [CrossRef]
- Romero-Cortes, T.; López-Pérez, P.A.; Pérez, E.V.H.; Medina-Toledo, A.K.; Aparicio-Burgos, J.E.; Cuervo-Parra, J.A. Confrontation of Trichoderma asperellum VSL80 against Aspergillus niger via the effect of enzymatic production. Chil. J. Agric. Anim. Sci. 2019, 35, 68–80. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- García-Núñez, H.G.; Martínez-Campos, A.R.; Hermosa-Prieto, M.R.; Monte-Vázquez, E.; Aguilar-Ortigoza, C.J.; González-Esquivel, C.E. Morphological and molecular characterization of native isolates of Trichoderma and its potential biocontrol against Phytophthora infestans. Rev. Mex. Fitopatol. 2017, 35, 58–79. [Google Scholar] [CrossRef]
- Acurio, V.R.D.; España, I.C.K. Aislamiento, caracterización y evaluación de Trichoderma spp. como promotor de crecimiento vegetal en pasturas de Raygrass (Lolium perenne) y trébol blanco (Trifolium repens). LA Granja 2017, 25, 53–61. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Miranda, M.D.; Moreno, M.L.F.; Páramo, A.L.A. Identificación morfológica y molecular de especies autóctonas Trichoderma spp., aisladas de suelos de importancia agrícola. El Higo Rev. Cienc. y Tecnol. 2021, 11, 26–42. [Google Scholar] [CrossRef]
- Medina, H.T.; Cañedo, T.D.; Aguirre, A.C.; Tello, F.H. Línea de Base de la Diversidad Genética del Maíz Peruano con Fines de Bioseguridad, 1st ed.; Ministerio del Ambiente, Grupo Raso: Lima, Perú, 2018; pp. 25–143. [Google Scholar]
- Centro Internacional de Mejoramiento del Maíz y el Trigo (CIMMYT). CIMMYTMA 12580, Zea mays L. subsp. mays, PERU 760. 2 July 2020. Available online: http://mgb.cimmyt.org/gringlobal/accessiondetail?id=12573 (accessed on 11 February 2022).
- Centro Internacional de Mejoramiento del Maíz y el Trigo (CIMMYT). CIMMYTMA 12473, Zea mays L. subsp. mays, PERU 389. 2 July 2020. Available online: http://mgb.cimmyt.org/gringlobal/accessiondetail?id=12466 (accessed on 11 February 2022).
- Centro Internacional de Mejoramiento del Maíz y el Trigo (CIMMYT). GRIN-Global, CIMMYT- Maize Germplasm Bank. Passport Data. Search Accessions GRID-Global Software, Version 2.0.4.0. 2 July 2020. Available online: http://mgb.cimmyt.org/gringlobal/search (accessed on 11 February 2022).
- Castro, A.R.; Morejón, R.R.; Díaz, S.S.H.; Álvarez, E.G. Efecto de borde y la validez de los muestreos en el cultivo del arroz. Cultiv. Trop. 2013, 34, 70–75. [Google Scholar]
- Chura, J.; Mendoza-Cortez, J.W.; Carlos, C.J. Dosis y fraccionamiento de nitrógeno en dos densidades de siembra del maíz amarillo duro. Sci. Agropecu. 2019, 10, 241–248. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística y Geografía (INEGI). Prontuario de Información Geográfica Municipal de los Estados Unidos Mexicanos. Apan, Hidalgo, Clave Geoestadística 13008. 9 December 2009. Available online: https://web.archive.org/web/20170327170244/http://www3.inegi.org.mx/sistemas/mexicocifras/datos-geograficos/13/13008.pdf (accessed on 5 September 2021).
- Servició Meteorológico Nacional (SMN). Estación: 00013002 APAN (DGE) Normales Climatológicas Periodo 1951–2010. 1 December 2010. Available online: https://smn.conagua.gob.mx/es/informacion-climatologica-por-estado?estado=hgo (accessed on 5 August 2021).
- Xiu, K.A. Evaluación del Crecimiento del maíz VS-536 Inoculado con Microorganismos (Micorrizas y Azospirillum), y con la Adición de Fertilizantes Químicos en un Suelo Luvisol, Technical Report of Professional Residence, Instituto Tecnológico de la Zona Maya, Ingeniería en Agronomía, Juan Sarabia, Quintana Roo, México. 5 December 2014. Available online: http://www.itzonamaya.edu.mx/web_biblio/archivos/res_prof/agro/agro-2014-5.pdf (accessed on 22 March 2022).
- Sandoval, C.F.J. Intervalo Seda Antesis y su Efecto en el Rendimiento de Siete Híbridos de Maíz Usados en Chile. Bachelor’s Thesis, Universidad de Chile, Facultad de Ciencias Agronómicas, Santiago, Chile, November 2015. [Google Scholar]
- Verhulst, N.; Sayre, K.; Govaerts, B. Manual de Determinación de Rendimiento, 1st ed.; SAGARPA, CIMMYT: Mexico City, Mexico, 2012; pp. 27–34. [Google Scholar]
- Zamudio-González, B.; Espinosa-Calderón, A.; Tadeo-Robledo, M.; Escastín-Dionicio, J.J.; Martínez, R.N.; Felix-Reyes, A.; Cardenas, M.A.L.; Turrent, F.A. Maize hybrids and varieties for seed production in planting double rows. Rev. Mex. Cienc. Agric. 2015, 6, 1491–1505. [Google Scholar]
- Sandhu, K.S.; Singh, N.; Kaur, M. Characteristics of the different corn types and their grain fractions: Physicochemical, thermal, morphological, and rheological properties of starches. J. Food Eng. 2004, 64, 119–127. [Google Scholar] [CrossRef]
- Bustillos-Rodríguez, J.C.; Tirado-Gallegos, J.M.; Ordóñez-García, M.; Zamudio-Flores, P.B.; Ornelas-Paz, J.J.; Acosta-Muñiz, C.H.; Gallegos-Morales, G.; Páramo-Calderón, D.E.; Ríos-Velasco, C. Physicochemical, thermal and rheological properties of three native corn starches. Food Sci. Technol. 2019, 39, 149–157. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 21st ed.; Latimer, G.W., Ed.; Association of Official Analytical Chemists (AOAC): Washington, DC, USA, 2019; pp. 777–801. [Google Scholar]
- Zamudio-Flores, P.B.; Vargas-Torres, A.; Gutiérrez-Meraz, F.; Bello-Pérez, L.A. Caracterización fisicoquímica de almidones doblemente modificados de plátano. Agrociencia 2010, 44, 283–295. [Google Scholar]
- Sánchez, G.J.J.; Goodman, M.M.; Rawlings, J.O. Appropriate characters for racial classification in maize. Econ. Bot. 1993, 47, 44–59. [Google Scholar] [CrossRef]
- Analytical Software. Statistix 10, 1st ed.; Analytical Software: Tallahassee, FL, USA, 2017; pp. 7–445. [Google Scholar]
- Romero-Arenas, O.; Huerta, L.M.; Huato, D.M.A.; Domínguez, H.F.; Arellano, V.D.A. Características de Trichoderma harzianum, como agente limitante en el cultivo de hongos comestibles. Rev. Colomb. Biotecnol. 2009, 11, 143–151. [Google Scholar]
- Pavone, M.D.; Dorta, B. Diversidad del hongo Trichoderma spp. en plantaciones de maíz de Venezuela. Interciencia 2015, 40, 23–31. [Google Scholar]
- Manjunatha, S.E.; Yadahalli, K.B.; Uday, G.; Kalappnavar, I.K.; Kachapur, R.M.; Priyanka, K. Trichoderma harzianum GenBank Direct Submission. 6 March 2020. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MT156345 (accessed on 11 August 2021).
- Morgulis, A.; Coulouris, G.; Raytselis, Y.; Madden, T.L.; Agarwala, R.; Schäffer, A.A. Database indexing for production MegaBLAST searches. Bioinformatics 2008, 24, 1757–1764. [Google Scholar] [CrossRef]
- Umesha, S.; Srikantaiah, M.; Prasanna, K.S.; Sreeramulu, K.R.; Divya, M.; Lakshmipathi, R.N. Comparative effect of organics and biofertilizers on growth and yield of maize (Zea mays L). Curr. Agric. Res. J. 2014, 2, 55–62. [Google Scholar] [CrossRef]
- Montejo-Martínez, D.; Casanova-Lugo, F.; García-Gómez, M.; Oros-Ortega, I.; Díaz- Echeverría, V.; Morales-Maldonado, E.R. Respuesta foliar y radical del maíz a la fertilización biológica-química en un suelo luvisol. Agron. Mesoam. 2018, 29, 325–341. [Google Scholar] [CrossRef]
- Ahmed, O.S.; Elsadig, I.A.; Mohamed, A.B.E.A. Effect of bio-fertilizer on growth and yield of two maize (Zea mays L.) cultivars at Shambat, Sudan. Sch. J. Agric. Vet. Sci. 2016, 3, 313–317. [Google Scholar] [CrossRef]
- Salinas, T.R.R. Mejoramiento Poblacional de un Compuesto de Maíz Morado (Zea mays L.) Canaán a 2735 Msnm—Ayacucho. Bachelor’s Thesis, Facultad de Ciencias Agrarias, Universidad Nacional de San Cristóbal de Huamanga, Ayacucho, Peru, April 2015. [Google Scholar]
- Pérez de Luque, A.; Tille, S.; Jhonson, I.; Pascual-Pardo, D.; Ton, J.; Cameron, D.D. The interactive effects of arbuscular mycorrhiza and plant growth-promoting rhizobacteria synergistically enhance host plant defenses against pathogens. Sci. Rep. 2017, 7, 16409. [Google Scholar] [CrossRef]
- Del Abarza, V.S.; Altamirano, F.E.; Zankar, G.C.; Boccardo, R.J.; Britos, J. Fertilización biológica con Trichoderma spp. y Azospirillum spp. en maíz. Agrotecnia 2017, 25, 31. [Google Scholar] [CrossRef]
- Quintal, V.Y.Y. Bacterias Rizósfericas Asociadas al Cultivo de Maíz (Zea mays L.) con Potencial Biofertilizante en el Estado de Campeche. Bachelor’s Thesis, Instituto Tecnológico de Chiná, Chiná, Campeche, Mexico, November 2020. [Google Scholar]
- Maidana, E.; Melgarejo, A.M.; Amarilla, D.; Ocampos, L.V.; Colman, P.; Mendoza, M.; Bogado, M.; Franco, R.; Silvero, O. Características agronómicas del maíz inoculado con diferentes dosis de Azospirillum brasiliense. Rev. Soc. Cient. Parag. 2020, 25, 49–57. [Google Scholar] [CrossRef]
- Puma, V.J.M. Dos Fuentes de Material Orgánica y el Rendimiento del Maíz Morado (Zea mays L.) cv. “Canteño” en Zonas Áridas. Bachelor’s Thesis, Universidad Nacional de San Agustín de Arequipa, Arequipa, Peru, October 1998. [Google Scholar]
- Ebel, R.; Pozas, C.J.G.; Soria, M.F.; Cruz, G.J. Manejo orgánico de la milpa: Rendimientos de maíz, frijol y calabaza en monocultivo y policultivo. Terra Latinoam. 2017, 35, 149–160. [Google Scholar] [CrossRef]
- Rabanal-Atalaya, M.; Medina-Hoyos, A. Evaluación del rendimiento, características morfológicas y químicas de variedades del maíz morado (Zea mays L.) en la región Cajamarca-Perú. Terra Latinoam. 2021, 39, e829. [Google Scholar] [CrossRef]
- Pepó, P.; Karancsi, G.L. Effect of fertilization on the NPK uptake of different maize (Zea mays L.) genotypes. Cereal Res. Commun. 2017, 45, 699–710. [Google Scholar] [CrossRef]
- Alemayehu, M.; Jemberie, M.; Yeshiwas, T.; Aklile, M. Integrated application of compound NPS fertilizer and farmyard manure for economical production of irrigated potato (Solanum tuberosum L.) in highlands of Ethiopia. Cogent Food Agric. 2020, 6, 1724385. [Google Scholar] [CrossRef]
- Zulueta-Rodríguez, R.; Gómez-Merino, F.C.; Alemán-Chávez, I.; Núñez-Camargo, M.C.; Lara-Capistrán, L. Respuesta del cultivo de maíz a la bioinoculación y fertilización química reducida en campo. Terra Latinoam. 2020, 38, 597–612. [Google Scholar] [CrossRef]
- Arango, O.M.J. Abonos Orgánicos Como Alternativa Para la Conservación y Mejoramiento de los Suelos. Bachelor’s Thesis, Corporación Universitaria Lasallista, Caldas, Antioquia, Colombia, June 2017. [Google Scholar]
- Jjagwe, J.; Chelimo, K.; Karungi, J.; Komakech, A.J.; Lederer, J. Comparative performance of organic fertilizers in maize (Zea mays L.) growth, yield, and economic results. Agronomy 2020, 10, 69. [Google Scholar] [CrossRef]
- Tamayo-Aguilar, Y.; Martín-Alonso, G.; Herrera-Altuve, J.A.; Cesar-Gainza, A.; Abad-Michael, M.; Nápoles-García, M.C.; Rivera-Espinosa, R.; Juárez-López, P. Biofertilizantes en la sucesión Canavalia ensiformis-Solanum lycopersicum: Rendimiento y calidad en frutos de tomate. Rev. Fitotec. Mex. 2021, 44, 341–347. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; Pérez-Tapia, V.; Bedmar, E.J.; Santillana, N. Purple corn-associated rhizobacteria with potential for plant growth promotion. J. Appl. Microbiol. 2018, 124, 1254–1264. [Google Scholar] [CrossRef]
- Roldán, A.; Salinas, G.J.; Alguacil, M.M.; Caravaca, F. Soil sustainability indicators following conservation tillage practices under subtropical maize and bean crops. Soil Tillage Res. 2006, 93, 273–282. [Google Scholar] [CrossRef]
- Yépez, E. Caracterización Morfológica y Evaluación Fenológica de Sesenta y Cinco Entradas de Maíz (Zea mays L.) del Banco de Germoplasma del CICA-Kayra, Cusco. Bachelor’s Thesis, Universidad Nacional San Antonio Abad del Cusco, Facultad de Agronomía y Zootecnia, Cusco, Peru, August 2011. [Google Scholar]
- Salhuana, W. Diversidad y descripción de las razas de maíz en el Perú. In Cincuenta Años del Programa Cooperativo de Investigación en Maíz (PCIM) 1953–2003, 1st ed.; Salhuana, W.M., Valdez, M.A., Scheuch, H.F., Davelouis, M.J., Eds.; UNALM: Lima, Peru, 2004; pp. 1–35. [Google Scholar]
- Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA). INIA 606 “Choclero Prolífico”. Estación Experimental Agraria Santa Ana, Huancayo, Peru. 6 April 2004. Plegable No. 3. Available online: http://pgc-snia.inia.gob.pe:8080/jspui/bitstream/inia/685/3/Trip-Choclo-INIA606.pdf (accessed on 24 September 2021).
- Sharma, L.K.; Zaeen, A.A.; Bali, S.K. Improving nitrogen and phosphorus efficiency for optimal plant growth and yield. In New Visions in Plant Science, 1st ed.; Celik, O., Ed.; IntechOpen: London, UK, 2018; pp. 13–140. [Google Scholar] [CrossRef]
- Okoth, A.S.; Otadoh, A.J.; Ochanda, O.J. Improved seedling emergence and growth of maize and beans by Trichoderma harzianum. Trop. Subtrop. Agroecosyst. 2011, 13, 65–71. [Google Scholar]
- Justiniano, A.E. Fenología e Intensidad de Color en Corontas del Maíz Morado (Zea mays L.) en sus Diferentes Estados de Desarrollo en la Localidad de La Molina. Masters’s Thesis, Universidad Nacional Agraria La Molina, Escuela de Postgrado, Lima, Peru, September 2010. [Google Scholar]
- Pedraza, G.M.; Idrogo, V.G.; Pedraza, G.S. Densidad de siembra y comportamiento agronómico de tres variedades de maíz morado (Zea mays L.). Rev. ECIPerú 2017, 14, 20–40. [Google Scholar] [CrossRef]
- Medina-Hoyos, A.; Narro-León, L.A.; Chávez-Cabrera, A. Cultivo de maíz morado (Zea mays L.) en zona altoandina de Perú: Adaptación e identificación de cultivares de alto rendimiento y contenido de antocianinas. Sci. Agropecu. 2020, 11, 291–299. [Google Scholar] [CrossRef]
- Uribe, V.G.; Petit, J.; Dzib, E.R. Respuesta del cultivo de maíz a la aplicación de biofertilizantes en el sistema roza, tumba y quema en suelo alfisol (chac-lu’um, nomenclatura maya), en Yucatán, México. Agric. Andin. 2007, 13, 3–18. [Google Scholar]
- Manrique, C.A. Maíz Peruano Morado (Zea mays L. amilaceae St.), Folleto R. I. N° 04-00, 1st ed.; Instituto Nacional de Investigación Agraria: Lima, Peru, 2000; pp. 1–21. [Google Scholar]
- Oscanoa, C.; Sevilla, R. Razas de Maíz en la Sierra Central del Perú Junín, Huancavelica y Ayacucho, 1st ed.; Instituto Nacional de Innovación Agraria: La Molina, Peru, 2008; pp. 122–135. [Google Scholar]
- Castillo, A.R. Selección por Intensidad de color en Corontas del Maíz Morado (Zea mays L.) Variedad INIA 601 en el Distrito de Monsefú—Lambayeque-2019. Bachelor’s Thesis, Facultad de Agronomía, Universidad Nacional Pedro Ruiz Gallo, Lambayeque, Peru, February 2019. [Google Scholar]
- Borrero, C.A.; Silva, H.M.R. Efecto de Trichoderma (in vitro) en los microorganismos no patógenos descomponedores de la materia orgánica de un suelo oxisol clase IV del piedemonte llanero. Orinoquia 2005, 9, 6–14. [Google Scholar]
- Endicott, S.; Brueland, B.; Keith, R.; Schon, R.; Bremer, C.; Farmham, D.; DeBruin, J.; Clausen, C.; Strachan, S.; Carter, P. Maíz, Crecimiento y Desarrollo. DuPont, Pioneer, Iowa, EE. UU. 23 March 2015. Available online: https://www.pioneer.com/CMRoot/International/Latin_America_Central/Chile/Servicios/Informacion_tecnica/Corn_Growth_and_Development_Spanish_Version.pdf (accessed on 28 September 2021).
- Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA). Proyecto PNIA 022_PI, Estudio del Contenido de Antocianinas en Diferentes Variedades de Maíz Morado y Pisos Altitudinales Para el Mejoramiento del Agrocomercio en la Zona Altoandina. Ministerio de Agricultura y Riego, Instituto Nacional de Innovación Agraria: La Molina, Lima, Peru, 30 October 2019. Available online: https://cdn.www.gob.pe/uploads/document/file/572078/pub_cuadrifolio-022-ma%C3%ADz-morado.pdf (accessed on 10 October 2021).
- Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA). Maíz INIA 615—Negro Canaán, Nueva Variedad de Maíz Morado Para la Sierra Peruana. Dirección de Investigación Agraria, Estación Experimental Agraria Canaán—Ayacucho: Lima, Perú, 17 November 2007. Plegable No. 17. Volume 11. Available online: https://www.inia.gob.pe/wp-content/uploads/investigacion/programa/sistProductivo/variedad/maiz-amilaceo/INIA_615.pdf (accessed on 10 September 2021).
- MacRobert, J.F.; Setimela, P.; Gethi, J.; Worku, R.M. Manual de Producción de Semilla de Maíz Híbrido, 1st ed.; Centro Internacional de Mejoramiento de Maíz y Trigo: Mexico City, Mexico, 2015; pp. 1–26. [Google Scholar]
- Begazo, T.J.J. Marco de Siembra en el Rendimiento de Maíz Morado (Zea mays L.) “Ecotipo Arequipeño” en la Irrigación Majes 2012–2013. Bachelor’s Thesis, Universidad Nacional de San Agustín, Arequipa, Peru, May 2013. [Google Scholar]
- Lazo, C.R.N. Fertilización Potásica y Fosfórica en el Rendimiento de Maíz Morado (Zea mays L.) P.M. 581, El Cural—Arequipa. Bachelor’s Thesis, Universidad Nacional de San Agustín de Arequipa, Arequipa, Peru, February 1999. [Google Scholar]
- Quispe, J.F.; Arroyo, C.K.; Gorriti, G.A. Características morfológicas y químicas de 3 cultivares de maíz morado (Zea mays L.) en Arequipa—Perú. Rev. Soc. Quím. Perú 2011, 77, 205–217. [Google Scholar]
- Golik, S.; Schierenbeck, M.; Dietz, J.I.; Fleitas, M.C. Maíz: Crecimiento y desarrollo del cultivo de maíz. In Cereales de Verano, 1st ed.; Simón, M.R., Golik, S.I., Eds.; Editorial de la Universidad de la Palta, La Palta: Buenos Aires, Argentina, 2018; pp. 26–40. [Google Scholar]
- Pozo, H.M.R. Efecto del Guano de Islas y Trébol (Medicago hispida G.) en el Rendimiento del Cultivo de Maíz Morado (Zea mays L.) en Condiciones de Azángaro, Huanta, Ayacucho. Bachelor’s Thesis, Universidad Nacional de Huancavelica, Acobamba, Perú, January 2015. [Google Scholar]
- Pinedo, T.R.E. Niveles de Fertilización en dos Variedades de Maíz Morado (Zea mays L.) en la Localidad de Canaán—Ayacucho. Master’s Thesis, Universidad Nacional Agraria la Molina, Lima, Peru, May 2015. [Google Scholar]
- Pinedo, R.; Rodríguez, G.; Valverde, N. Niveles de fertilización en dos variedades de maíz morado (Zea mays L.) en la localidad de Canaán-Ayacucho. Aporte St. 2017, 10, 39–50. [Google Scholar]
- Parihar, C.M.; Jat, S.L.; Singh, A.K.; Kumar, B.; Singh, Y.; Pradhan, S.; Pooniya, V.; Dhauja, A.; Chaudhary, V.; Jat, M.L.; et al. Conservation agricultural in irrigated intensive maize-based systems of north-western India: Effects on crops yields, water productivity and economic profitability. Field Crop. Res. 2016, 193, 104–116. [Google Scholar] [CrossRef]
- Galindo, F.S.; Teixeira, F.M.C.M.; Buzetti, S.; Oagliari, P.H.; Santini, J.M.K.; Alves, C.J.; Megda, M.M.; Nogueira, T.A.R.; Andreotti, M.; Arf, O. Maize yield response to nitrogen rates and sources associated with Azospirillum brasilense. Agron. J. 2019, 111, 1985–1997. [Google Scholar] [CrossRef]
- Brotodjojo, R.R.R.; Arbiwati, D. Growth and yield of hybrid corn under different fertilizer applications. J. Adv. Agric. Technol. 2018, 5, 149–152. [Google Scholar] [CrossRef]
- García, A.J.; Fischer, G.; Riaño, H.N. Effect of fertilization level on water use and production of corn (Zea mays L.) in a cereal producing area in Colombia—A modeling exercise using AquaCrop-FAO. Agron. Colomb. 2017, 35, 68–74. [Google Scholar] [CrossRef]
- Chong, E.A. Fertilización de maíces nativos con aplicadora manual para pequeñas parcelas. Acta Fitogenét. 2019, 6, 1. [Google Scholar]
- Sierra-Macías, M.; Andrés-Meza, P.; Palafox-Caballero, A.; Meneses-Márquez, I.; Francisco-Nicolás, N.; Zamnada-Martínez, A.; Rodríguez-Montalvo, F.; Espinosa-Calderón, A.; Tadeo-Roblero, M. Variación morfológica de maíces nativos (Zea mays L.) en el estado de Veracruz, México. Agro Product. 2014, 7, 58–65. [Google Scholar]
- Carrera-Valtierra, J.A.; Ron, P.J.; Sánchez, G.J.J.; Jiménez, C.A.A.; Márquez, S.F.; Sahagún, C.L.; Sesmas, G.J.J.; Sitt, M.M. Razas de Maíz de Michoacán de Ocampo, su Origen, Relaciones Fitogeográficas y Filogenéticas, 1st ed.; Gobierno del Estado de Michoácan, COECyT, UACH: Morelia, Michoacán, Mexico, 2011; pp. 33–71. [Google Scholar]
- Secretaria de Comercio y Fomento Industrial (SCFI). Norma Oficial Mexicana NOM 247 SSA1 2008 Cereales. Alimentos-Almidón o Fécula de maíz:1-6. 27 July 2009. Available online: http://intranet.dif.df.gob.mx/transparencia/new/art_15/10/_anexos/NORMA%20Oficial%20Mexicana%20NOM%20247%20SSA1%202008%20Cereales.pdf (accessed on 22 March 2022).
- Blanche, S.; Sun, X. Physical characterization of starch extrudates as a function of melting transitions and extrusion conditions. Adv. Polym. Technol. 2004, 23, 277–290. [Google Scholar] [CrossRef]
- Tovar-Benítez, T. Caracterización Morfológica y Térmica del Almidón de Maíz (Zea mays L.) Obtenido por Diferentes Métodos de Aislamiento. Bachelor’s Thesis, Universidad Autónoma del Estado de Hidalgo, Hidalgo, Mexico, June 2008. [Google Scholar]
- Tirado-Gallegos, J.M.; Zamudio-Flores, P.B.; Ornelas-Paz, J.J.; Ríos Velasco, C.; Acosta-Muñiz, C.H.; Gutiérrez-Meraz, F.; Islas-Hernández, J.J.; René, S.D. Efecto del método de aislamiento y el estado de madurez en las propiedades fisicoquímicas, estructurales y reológicas de almidón de manzana. Rev. Mex. Ing. Quim. 2016, 15, 391–408. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Yousif, E.; Gadallah, M.; Sorour, A.M. Physico-chemical and rheological properties of modified corn starches and its effect on noodle quality. Ann. Agric. Sci. 2012, 57, 19–27. [Google Scholar] [CrossRef]
- Acton, Q. Advances in Intracellular Space Research and Application, 1st ed.; Scholarly Brief: Atlanta, GA, USA, 2013; pp. 10–254. [Google Scholar]
- Cauvain, S.P.; Young, L.S. The ICC Handbook of Cereals, Flour, Dough & Product Testing: Methods and Applications, 1st ed.; DEStech Publications, Inc.: Lancaster, UK, 2009; pp. 23–456. [Google Scholar]
- Eckhoff, S.; Watson, S. Corn and sorghum starches: Production. In Starch: Chemistry and Technology, 3rd ed.; BeMiller, J.N., Whistler, R., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 373–439. [Google Scholar] [CrossRef]
- Yu, H.; Cheng, L.; Yin, J.; Yan, S.; Liu, K.; Zhang, F.; Xu, B.; Li, L. Structure and physicochemical properties of starches in lotus (Nelumbo nucifera Gaertn.) rhizome. Food Sci. Nutr. 2013, 1, 273–283. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Andrade-Mahecha, M.M.; Sobral, P.J.A.; Menegalli, F.C. Isolation and characterization of the flour and starch of plantain bananas (Musa paradisiaca). Stärke 2012, 64, 382–391. [Google Scholar] [CrossRef]
- Fang, J.M.; Fowler, P.A.; Sayers, C.; Williams, P.A. The chemical modification of a range of starches under aqueous reaction conditions. Carbohydr. Polym. 2004, 55, 283–289. [Google Scholar] [CrossRef]
- Almeida, D.V.; da Silva Nornberg, B.F.; Geracitano, L.A.; Barros, D.M.; Monserrat, J.M.; Marins, L.F. Induction of phase II enzymes and hsp70 genes by copper sulfate through the electrophile-responsive element (EpRE): Insights obtained from a transgenic zebrafish model carrying an orthologous EpRE sequence of mammalian origin. Fish Physiol. Biochem. 2010, 36, 347–353. [Google Scholar] [CrossRef]
- Liu, T.Y.; Ma, Y.; Yu, S.F.; Shi, J.; Xue, S. The effect of ball milling treatment on structure and porosity of maize starch granule. Innov. Food Sci. Emerg. Technol. 2011, 12, 586–593. [Google Scholar] [CrossRef]
- Fang, J.; Fowler, P.; Tomkinson, J.; Hill, C. The preparation and characterization of a series of chemically modified potato starches. Carbohydr. Polym. 2002, 47, 245–252. [Google Scholar] [CrossRef]
- Agama-Acevedo, E.; de la Rosa, A.P.B.; Méndez-Montealvo, G.; Bello-Pérez, L.A. Physicochemical and biochemical characterization of starch granules isolated of pigmented maize hybrids. Stärke 2008, 60, 433–441. [Google Scholar] [CrossRef]
- Xie, X.; Seib, P. Laboratory procedure to wet-mill 100 g of grain sorghum into six fractions 1. Cereal Chem. 2000, 77, 392–395. [Google Scholar] [CrossRef]
- Lee, Y.T.; Puligundla, P. Characteristics of reduced-fat muffins and cookies with native and modified rice starches. Emir. J. Food Agric. 2016, 28, 311–316. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, Y.; Eckhoff, S.R.; Feng, H. Sonication enhanced cornstarch separation. Stärke 2005, 57, 240–245. [Google Scholar] [CrossRef]
- Sánchez-Rivera, M.M.; García-Suárez, F.J.L.; Velázquez del Valle, M.; Gutiérrez-Meraz, F.; Bello-Pérez, L. Partial characterization of banana starches oxidized by different levels of sodium hypochlorite. Carbohydr. Polym. 2005, 62, 50–56. [Google Scholar] [CrossRef]
- Ferron, L.; Colombo, R.; Mannucci, B.; Papetti, A. A new Italian purple corn variety (Moradyn) by product extract: Antiglycative and hypoglycemic in vitro activities and preliminary bioaccessibility studies. Molecules 2020, 25, 1958. [Google Scholar] [CrossRef]
- Mansilla, P.S.; Nazar, M.C.; Pérez, G.T. Flour functional properties of purple maize (Zea mays L.) from Argentina. Influence of environmental growing conditions. Int. J. Biol. Macromol. 2020, 146, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gonzales, M.E.; Luna-Vidal, D.; Tao, T.; Chandrasekaran, S.; Chatham, L.; Juvik, J.; Singh, V.; Kumar, D. Relationship of phenolic composition of selected purple maize (Zea mays L.) genotypes with their anti-inflammatory, anti-adipogenic and anti-diabetic potential. Food Chem. 2019, 289, 739–750. [Google Scholar] [CrossRef]
- Chichizola, J.B.; López, T.E.; Navarro, R.J.M.; Salinas, A.F. Acopio, Procesamiento y Exportación de Maíz Morado. Plan de Negocios, Trabajo Aplicativo Final. Universidad Peruana de Ciencias Aplicadas, Escuela de Posgrado: Arequipa, Peru, 30 July 2009. Available online: http://hdl.handle.net/10757/273917 (accessed on 10 September 2021).
- Garnica, V.A.; Esparza, R.S. Trichoderma: Un hongo biofertilizante. Saber Más Rev. De Divulg. 2017, 31, 34–37. [Google Scholar]



| Treatments | Key |
|---|---|
| Control | T1 |
| Chemical fertilizer 1 | T2 |
| Chemical fertilizer + Bioinoculants | T3 |
| Bioinoculants 2 | T4 |
| Organic fertilizer | T5 |
| Organic fertilizer + Bioinoculants | T6 |
| Chemical fertilizer + Bioinoculants + Organic fertilizer | T7 |
| Key | Plant Height at Stage VT 1 (cm) ± SE 2 | Stem Diameter (cm) ± SE |
|---|---|---|
| T1 | 185.63 ± 0.60 a | 1.30 ± 0.42 a |
| T2 | 253.30 ± 0.07 b | 2.02 ± 0.27 b |
| T3 | 263.33 ± 0.05 c | 1.94 ± 0.33 ab |
| T4 | 283.46 ± 0.08 d | 1.89 ± 0.42 ab |
| T5 | 262.70 ± 0.06 c | 1.87 ± 0.30 ab |
| T6 | 275.43 ± 0.04 d | 1.83 ± 0.30 ab |
| T7 | 315.34 ± 0.03 e | 2.06 ± 0.21 b |
| Stages R0 and R1 (Beginning of Pollen Shedding and Emergence of Silks on Ears) | Stage R6 (Physiological Maturity) | |||
|---|---|---|---|---|
| Key | MF 1 ± SE 5 | FF 2 ± SE | FA 3 ± SE | Time to Harvest 4 (Days) ± SE |
| T1 | 118.67 ± 0.9 d | 128.67 ± 1.0 d | 10.00 ± 0.00 e | 180.67 ± 1.53 e |
| T2 | 113.67 ± 0.5 c | 122.00 ± 0.80 c | 8.33 ± 0.50 d | 169.00 ± 1.00 d |
| T3 | 100.67 ± 0.3 a | 106.33 ± 0.40 a | 5.67 ± 0.40 b | 149.33 ± 1.53 a |
| T4 | 115.33 ± 0.2 c | 123.67 ± 0.30 c | 8.33 ± 0.50 d | 166.67 ± 0.58 c |
| T5 | 111.00 ± 0.6 b | 118.33 ± 0.30 b | 7.33 ± 0.30 c | 166.33 ± 1.15 c |
| T6 | 105.33 ± 0.4 b | 112.67 ± 0.50 b | 7.33 ± 0.30 c | 154.67 ± 1.05 b |
| T7 | 98.67 ± 0.1 a | 103.33 ± 0.20 a | 4.67 ± 0.10 a | 147.67 ± 0.23 a |
| Key | Total Number of Plants ± SE 1 | Number of Ears ± SE | Number of Ears per Plant | Ear Weight (g) ± SE | Grain Yield (t ha−1) ± SE |
|---|---|---|---|---|---|
| T1 | 81.00 ± 1.00 a | 57.00 ± 1.00 a | 0.70 | 25.15 ± 0.02 a | 1.02 ± 0.01 a |
| T2 | 96.00 ± 0.00 b | 111.60 ± 0.50 b | 1.16 | 85.18 ± 0.08 b | 4.35 ± 0.10 b |
| T3 | 96.00 ± 0.00 b | 112.60 ± 0.50 b | 1.17 | 71.63 ± 0.06 c | 3.88 ± 0.07 c |
| T4 | 96.00 ± 0.00 b | 92.00 ± 1.00 c | 0.96 | 61.44 ± 0.04 d | 3.03 ± 0.02 d |
| T5 | 91.30 ± 0.50 c | 77.30 ± 0.50 d | 0.85 | 72.91 ± 0.06 c | 3.25 ± 0.06 c |
| T6 | 93.30 ± 0.50 c | 84.60 ± 0.50 e | 0.90 | 74.19 ± 0.05 c | 3.46 ± 0.03 c |
| T7 | 96.00 ± 0.00 b | 114.30 ± 0.50 b | 1.19 | 96.62 ± 0.07 e | 6.19 ± 0.07 e |
| Key | Ear Diameter (cm) ± SE 1 | Ear Length (cm) ± SE | Number of Rows per Ear ± SE | Number of Grains per Row ± SE | Weight of 100 Grains ± SE |
|---|---|---|---|---|---|
| T1 | 2.90 ± 0.58 c | 8.43 ± 0.78 d | 7.68 ± 2.58 d | 9.17 ± 0.61 e | 23.80 ± 0.15 a |
| T2 | 3.52 ± 0.75 b | 17.20 ± 0.59 b | 8.39 ± 2.13 c | 16.16 ± 0.54 c | 45.20 ± 0.60 bc |
| T3 | 3.38 ± 0.81 b | 16.30 ± 0.10 bc | 8.12 ± 2.39 c | 13.74 ± 0.93 d | 45.00 ± 0.12 bc |
| T4 | 3.46 ± 0.56 b | 14.56 ± 0.92 c | 9.01 ± 1.46 a | 15.47 ± 0.04 c | 44.10 ± 0.08 b |
| T5 | 3.70 ± 0.62 a | 16.52 ± 0.91 bc | 9.09 ± 1.62 a | 16.00 ± 0.51 c | 44.00 ± 0.11 b |
| T6 | 3.62 ± 0.58 ab | 17.25 ± 0.23 b | 9.29 ± 1.61 a | 19.82 ± 0.66 b | 44.40 ± 0.12 b |
| T7 | 3.79 ± 0.56 a | 18.95 ± 0.77 a | 8.99 ± 1.48 b | 20.37 ± 0.18 a | 45.60 ± 0.05 bc |
| Key | Grain Length (cm) ± SE 1 | Grain Width (cm) ± SE |
|---|---|---|
| T1 | 0.81 ± 0.02 a | 0.54 ± 0.07 a |
| T2 | 1.07 ± 0.01 c | 1.35 ± 0.04 b |
| T3 | 1.02 ± 0.01 bc | 1.29 ± 0.01 b |
| T4 | 1.03 ± 0.01 bc | 1.15 ± 0.01 c |
| T5 | 1.05 ± 0.04 bc | 1.13 ± 0.01 c |
| T6 | 0.95 ± 0.07 b | 1.14 ± 0.02 c |
| T7 | 1.10 ± 0.01 c | 1.71 ± 0.03 d |
| Moisture % ± SE 1 | Ash % ± SE | Fat % ± SE | Protein % ± SE | |
|---|---|---|---|---|
| T1 | 7.94 ± 0.06 c | 0.35 ± 0.03 a | 0.27 ± 0.05 a | 0.37 ± 0.03 ab |
| T2 | 7.93 ± 0.27 c | 0.43 ± 0.09 a | 0.31 ± 0.11 a | 0.39 ± 0.02 ab |
| T3 | 7.97 ± 0.12 c | 0.46 ± 0.07 a | 0.35 ± 0.07 a | 0.35 ± 0.01 b |
| T4 | 10.24 ± 0.09 a | 0.35 ± 0.04 a | 0.36 ± 0.07 a | 0.39 ± 0.00 ab |
| T5 | 8.96 ± 0.16 b | 0.41 ± 0.05 a | 0.37 ± 0.02 a | 0.39 ± 0.02 ab |
| T6 | 6.92 ± 0.05 d | 0.41 ± 0.04 a | 0.34 ± 0.03 a | 0.39 ± 0.00 ab |
| T7 | 6.80 ± 0.65 d | 0.35 ± 0.03 a | 0.36 ± 0.03 a | 0.41 ± 0.00 a |
| L ± SE 1 | a ± SE | b ± SE | |
|---|---|---|---|
| T1 | 91.76 ± 0.45 bc | 0.90 ± 0.02 a | 2.40 ± 0.05 b |
| T2 | 90.76 ± 0.28 c | 0.93 ± 0.02 a | 2.75 ± 0.05 a |
| T3 | 92.69 ± 0.41 b | 0.15 ± 0.02 e | 2.42 ± 0.06 b |
| T4 | 91.98 ± 1.11 bc | 0.15 ± 0.02 e | 2.31 ± 0.14 bc |
| T5 | 92.44 ± 0.57 b | 0.20 ± 0.02 d | 2.35 ± 0.07 b |
| T6 | 90.77 ± 1.08 c | 0.25 ± 0.01 c | 2.15 ± 0.14 c |
| T7 | 96.60 ± 0.70 a | 0.41 ± 0.02 b | 1.60 ± 0.06 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Cortes, T.; Tamayo-Rivera, L.; Morales-Ovando, M.A.; Aparicio Burgos, J.E.; Pérez España, V.H.; Peralta-Gil, M.; Cuervo-Parra, J.A. Growth and Yield of Purple Kculli Corn Plants under Different Fertilization Schemes. J. Fungi 2022, 8, 433. https://doi.org/10.3390/jof8050433
Romero-Cortes T, Tamayo-Rivera L, Morales-Ovando MA, Aparicio Burgos JE, Pérez España VH, Peralta-Gil M, Cuervo-Parra JA. Growth and Yield of Purple Kculli Corn Plants under Different Fertilization Schemes. Journal of Fungi. 2022; 8(5):433. https://doi.org/10.3390/jof8050433
Chicago/Turabian StyleRomero-Cortes, Teresa, Lis Tamayo-Rivera, Mario A. Morales-Ovando, José E. Aparicio Burgos, Victor H. Pérez España, Martin Peralta-Gil, and Jaime A. Cuervo-Parra. 2022. "Growth and Yield of Purple Kculli Corn Plants under Different Fertilization Schemes" Journal of Fungi 8, no. 5: 433. https://doi.org/10.3390/jof8050433
APA StyleRomero-Cortes, T., Tamayo-Rivera, L., Morales-Ovando, M. A., Aparicio Burgos, J. E., Pérez España, V. H., Peralta-Gil, M., & Cuervo-Parra, J. A. (2022). Growth and Yield of Purple Kculli Corn Plants under Different Fertilization Schemes. Journal of Fungi, 8(5), 433. https://doi.org/10.3390/jof8050433







