Comparative Proteomic Analysis of Rhizoctonia solani Isolates Identifies the Differentially Expressed Proteins with Roles in Virulence
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Sheath Blight Pathogen
2.2. Pathogenicity and Disease Assessment
2.3. Virulence Test on Other Rice Genotypes
2.4. Molecular Characterization
2.5. Protein Extraction
2.6. In Solution Protein Digestion
2.7. LC-MS/MS Analysis
2.8. Peptide Identification and Data Analysis
2.9. Quantitative Real-Time PCR Analysis
2.10. Statistical Analysis
3. Results
3.1. Isolation, Pathogenicity and Molecular Characterization
3.2. Determination of Virulence on Other Rice Genotypes
3.3. Proteomic Analysis
3.4. Pathway Analysis
3.5. Validation of Differentially Expressed Proteins by qRT–PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolfors, F.; Holmquist, L.; Dixelius, C.; Tzelepis, G. A LysM effector protein from the basidiomycete Rhizoctonia solani contributes to virulence through suppression of chitin triggered immunity. Mol. Genet. Genom. 2019, 294, 1211–1218. [Google Scholar] [CrossRef]
- Bernardes-De-Assis, J.; Storari, M.; Zala, M.; Wang, W.; Jiang, D.; Shidong, L.; Jin, M.; McDonald, B.A.; Ceresini, P.C. Genetic structure of populations of the rice-infecting pathogen Rhizoctonia solani AG 1 IA from china. Phytopathology 2009, 99, 1090–1099. [Google Scholar] [CrossRef]
- Kumar, K.V.K.; Raju, S.K.; Reddy, M.S.; Kloepper, J.W.; Lawrence, K.S.; Groth, D.E.; Miller, M.E.; Sudini, H.; Du, B.H. Evaluation of commercially available PGPR for control of rice sheath blight caused by Rhizoctonia solani. J. Pure Appl. Microbiol. 2009, 3, 485–488. [Google Scholar]
- Ghosh, S.; Gupta, S.K.; Jha, G. Identification and functional analysis of AG1-IA specific genes of Rhizoctonia solani. Curr. Genet. 2014, 60, 327–341. [Google Scholar]
- Taheri, P.H.M. Riboflavin-induced resistance against rice sheath blight functions through the potentiation of lignin formation and jasmonic acid signalling pathway. Commun. Agric. Appl. Biol. Sci. 2007, 72, 309–313. [Google Scholar]
- Ou, S.H. Rice Diseases; Common Wealth Mycology Institute: Richmond, Surrey, UK, 1985; pp. 3–10. [Google Scholar]
- Rauf, C.; Ahmad, I.; Ashraf, M. Anastomosis groups of Rhizoctonia solani Kuhn isolates from potato in Pakistan. Pak. J. Bot. 2007, 39, 1335–1340. [Google Scholar]
- Sharon, M.; Sneh, B.; Kuninaga, S.; Hyakumachi, M.; Naito, S. Classification of Rhizoctonia spp. using rDNA-ITS sequence analysis supports the genetic basis of the classical anastomosis grouping. Mycoscience 2008, 49, 93–114. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, N.; Liu, J.; Liu, W.; Wang, G.L. The role of effectors and host immunity in plant-necrotrophic fungal interactions. Virulence 2014, 5, 722–732. [Google Scholar] [CrossRef]
- Zheng, A.; Lin, R.; Zhang, D.; Qin, P.; Xu, L.; Ai, P.; Ding, L.; Wang, Y.; Chen, Y.; Liu, Y.; et al. The evolution and pathogenic mechanisms of the rice sheath blight pathogen. Nat. Commun. 2013, 4, 1424. [Google Scholar] [CrossRef]
- Nagarajkumar, M.; Jayaraj, J.; Muthukrishnan, S.; Bhaskaran, R.; Velazhahan, R. Detoxification of oxalic acid by Pseudomonas fluorescens strain PfMDU2: Implications for the biological control of rice sheath blight caused by Rhizoctonia solani. Microbiol. Res. 2005, 160, 291–298. [Google Scholar]
- Rao, T.B.; Chopperla, R.; Methre, R.; Punniakotti, E.; Venkatesh, V.; Sailaja, B.; Sundaram, R.M. Pectin induced transcriptome of a Rhizoctonia solani strain causing sheath blight disease in rice reveals insights on key genes and RNAi machinery for development of pathogen derived resistance. Plant Mol. Biol. 2019, 100, 59–71. [Google Scholar]
- Lore, J.S.; Jain, J.; Hunjan, M.S.; Gargas, G.; Mangat, G.S.; Sandhu, J.S. Virulence spectrum and genetic structure of Rhizoctonia isolates associated with rice sheath blight in the northern region of India. Eur. J. Plant Pathol. 2015, 143, 847–860. [Google Scholar]
- Kim, B.S.; Hwang, B.K. Microbial fungicides in the control of plant diseases. J. Phytopathol. 2007, 155, 641–653. [Google Scholar]
- Yang, Y.; Zhang, H.; Li, G.; Li, W.; Wang, X.; Song, F. Ectopic expression of MgSM1, a Cerato-platanin family protein from Magnaporthe grisea, confers broad-spectrum disease resistance in Arabidopsis. Plant Biotechnol. J. 2009, 7, 763–777. [Google Scholar]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar]
- Komatsu, S.; Tanaka, N. Rice proteome analysis: A step toward functional analysis of the rice genome. Proteomics 2005, 5, 938–949. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Park, Z.Y.; Kim, S.G.; Rakwal, R.; Agrawal, G.K. Comparative secretome investigation of Magnaporthe oryzae proteins responsive to nitrogen starvation. J. Proteome Res. 2011, 10, 3136–3148. [Google Scholar] [CrossRef]
- Prabhukarthikeyan, S.R.; Manikandan, R.; Durgadevi, D.; Keerthana, U.; Harish, S.; Karthikeyan, G.; Raguchander, T. Bio-suppression of turmeric rhizome rot disease and understanding the molecular basis of tripartite interaction among Curcuma longa, Pythium aphanidermatum and Pseudomonas fluorescens. Biol. Control 2017, 111, 23–31. [Google Scholar] [CrossRef]
- Manikandan, R.; Harish, S.; Karthikeyan, G.; Raguchander, T. Comparative proteomic analysis of different isolates of Fusarium oxysporum f.sp. lycopersici to exploit the differentially expressed proteins responsible for virulence on tomato plants. Front. Microbiol. 2018, 9, 420. [Google Scholar] [CrossRef]
- Prabhukarthikeyan, S.R.; Yadav, M.K.; Anandan, A.; Aravindan, S.; Keerthana, U.; Raghu, S.; Baite, M.S.; Parameswaran, M.S.; Panneerselvam, M.S.; Rath, P.C. Bio-protection of brown spot disease of rice and insight into the molecular basis of interaction between Oryza sativa, Bipolaris oryzae and Bacillus amyloliquefaciens. Biol. Control 2019, 137, 104018. [Google Scholar] [CrossRef]
- Xu, S.; Chen, J.; Liu, L.; Wang, X. Proteomics associated with virulence differentiation of Curvularia lunata in Maize in China. J. Integr. Plant Biol. 2007, 49, 487–496. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Kim, S.G.; Chung, W.S.; Bae, H.; Jeong, S.W.; Shin, S.C.; Jeong, M.J.; Park, S.C.; Kwak, Y.S.; Bae, D.W.; et al. Proteomic analysis of Rhizoctonia solani AG-1 sclerotia maturation. Fungal Biol. 2014, 118, 433–443. [Google Scholar]
- Anderson, J.P.; Sperschneider, J.; Win, J.; Kidd, B.; Yoshida, K.; Hane, J.; Diane, G.O.; Saunders, D.G.O.; Singh, K.B. Comparative secretome analysis of Rhizoctonia solani isolates with different host ranges reveals unique secretomes and cell death inducing effectors. Sci. Rep. 2017, 7, 10410. [Google Scholar]
- Lakshman, D.; Roberts, D.P.; Garrett, W.M.; Natarajan, S.S.; Omar, D.; Alkharouf, N.; Pain, A.; Khan, F.; Jambhulkar, P.P.; Mitra, A. Proteomic Investigation of Rhizoctonia solani AG 4 Identifies Secretome and Mycelial Proteins with roles in Plant Cell Wall Degradation and Virulence. J. Agric. Food Chem. 2016, 64, 3101–3110. [Google Scholar] [CrossRef]
- Anderson, J.P.; Hane, J.K.; Stoll, T.; Pain, N.; Hastie, M.L.; Kaur, P.; Hoogland, C.; Gorman, J.J.; Singh, K.B. Proteomic Analysis of Rhizoctonia solani Identifies Infection-specific, Redox Associated Proteins and Insight into Adaptation to Different Plant Hosts. Mol. Cell. Proteom. 2016, 15, 1188–1203. [Google Scholar] [CrossRef]
- Ma, H.; Sheng, C.; Qiao, C.; Zhao, H.; Niu, D. A comparative proteomic approach to identify defence-related proteins between resistant and susceptible rice cultivars challenged with the fungal pathogen Rhizoctonia solani. Plant Growth Regul. 2019, 90, 73–88. [Google Scholar] [CrossRef]
- Mora, L.; Bramley, P.M.; Fraser, P.D. Development and optimisation of a label-free quantitative proteomic procedure and its application in the assessment of genetically modified tomato fruit. Proteomics 2013, 13, 2016–2030. [Google Scholar] [CrossRef]
- Li, L.; Luo, Z.; Huang, X.; Zhang, L.; Zhao, P.; Ma, H.; Li, X.; Ban, Z.; Liu, X. Label-free quantitative proteomics to investigate strawberry fruit proteome changes under controlled atmosphere and low temperature storage. J. Proteom. 2015, 120, 44–57. [Google Scholar] [CrossRef]
- Sharma, N.R.; Teng, P.S.; Oliver, F.M. Comparisons of assessment methods for rice sheath blight disease. Philipp. Phytopathol. 1990, 26, 20–24. [Google Scholar]
- IRRI. Standard Evaluation System (SES) of Rice (Revised), 5th ed.; International Rice Research Institute: Manila, Philippines, 2013. [Google Scholar]
- Prasad, B.; Eizenga, G.C. Rice sheath blight disease resistance identified in Oryza species accessions. Plant Dis. 2008, 92, 1503–1509. [Google Scholar]
- Wheeler, B.E.J. An Introduction to Plant Diseases; John Wiley and Sons Limited: London, UK, 1969; p. 301. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungalribosomal RNA genes for phylogenetics. In PCR Protocols. A Guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Matsumoto, M. Trials of direct detection and identification of Rhizoctonia solani AG1 and AG 2 subgroups using specifically primed PCR analysis. Mycoscience 2002, 43, 185–189. [Google Scholar]
- Meng, H.; Wang, S.; Yang, W.; Ding, X.; Li, N.; Chu, Z.; Li, X. Identification of virulence associated milRNAs and their bidirectional targets in Rhizoctonia solani and maize during infection. BMC Plant Biol. 2021, 21, 155. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedure for Agricultural Research; John Wiley and Sons: New York, NY, USA, 1984. [Google Scholar]
- Perez-Riverol, Y.; Csordas; Bai, A.; Bernal-Llinares, J.; Hewapathirana, M.; Kundu, S.; Inuganti, D.J.; Griss, A.; Mayer, J.; Eisenacher, G.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, 442–450. [Google Scholar]
- Deutsch, E.W.; Bandeira, N.; Sharma, V.; Perez-Riverol, Y.; Carver, J.J.; Kundu, D.J.; Garcia-Seisdedos, D.; Jarnuczak, A.F.; Hewapathirana, S.; Pullman, B.S.; et al. The ProteomeXchange consortium in 2020: Enabling ‘big data’ approaches in proteomics. Nucleic Acids Res. 2020, 48, 1145–1152. [Google Scholar]
- Adhipathi, P.; Singh, V.; Meena, S.C. Virulence diversity of Rhizoctonia solani causing sheath blight disease in rice and its host pathogen interaction. Bioscan 2013, 8, 949–952. [Google Scholar]
- Singh, R.; Murti, S.; Mehilalm, T.A.; Prasad, D. Virulence Diversity in Rhizoctonia solani Causing Sheath Blight in Rice. J. Plant Pathol. Microb. 2015, 6, 296. [Google Scholar] [CrossRef]
- Pavani, S.L.; Singh, V. Assessment of virulence diversity of Rhizoctonia solani causing sheath blight disease in rice from Eastern Up. Curr. J. Appl. Sci. Technol. 2018, 26, 1–10. [Google Scholar]
- El-Shafey, R.A.S.; Elamawi, R.M.; Saleh, M.M.; Tahoon, A.M.; Emeran, A.A. Morphological, pathological and molecular characterisation of rice sheath blight disease causal organism Rhizoctonia solani AG-1 IA in Egypt. Arch. Phytopathol. Plant Prot. 2019, 52, 507–529. [Google Scholar]
- Yu, Y.; Chun-Hao, J.; Chao, W.; Liu-Jun, C.; Li, H.; Xu, Q.; Jian-Hua, G. An improved strategy for stable biocontrol agents selecting to control rice sheath blight caused by Rhizoctonia solani. Microbiol. Res. 2017, 203, 1–9. [Google Scholar]
- Shu, C.; Zou, C.; Chen, J.L.; Fang, T.; Run, H.Y.; Er-Xun, Z. Genetic diversity and population structure of Rhizoctonia solani AG-1 IA, the causal agent of rice sheath blight, in South China. Can. J. Plant Pathol. 2014, 36, 179–186. [Google Scholar] [CrossRef]
- Moni, Z.R.; Ali, M.A.; Alam, M.S.; Rahman, M.A.; Bhuiyan, M.R.; Mian, M.S.; Iftekharuddaula, K.M.; Latif, M.A.; Khan, M.A.I. Morphological and Genetical Variability among Rhizoctonia solani isolates causing sheath blight disease of rice. Rice Sci. 2016, 23, 42–50. [Google Scholar]
- Nagaraj, B.T.; Sunkad, G.; Pramesh, D.; Manjunath, K.N.; Patil, M.B.; Yadav, M.K.; Patil, N.B. Morphological, genetic and virulence diversity of Rhizoctonia solani isolates from different rice growing regions of Southern India. Res. J. Biotechnol. 2019, 14, 16–23. [Google Scholar]
- Deng, Y.Z.; Qu, Z.; He, Y.; Naqvi, N.I. Sorting nexinSnx41 is essential for conidiation and mediates glutathione-based antioxidant defense during invasive growth in Magnaporthe oryzae. Autophagy 2012, 8, 1058–1070. [Google Scholar] [CrossRef]
- Zheng, H.; Guo, Z.; Xi, Y.; Yuan, M.; Lin, Y.; Wu, C.; Abubakar, Y.S.; Dou, X.; Li, G.; Wang, Z.; et al. Sorting nexin (MoVps17) is required for fungal development and plant infection by regulating endosome dynamics in the rice blast fungus. Environ. Microbiol. 2017, 19, 4301–4317. [Google Scholar] [CrossRef]
- Gacek-Matthews, A.; Berger, H.; Sasaki, T.; Wittstein, T.; Gruber, C.; Lewis, Z.A.; Strauss, J. KdmB, a Jumonji Histone H3 Demethylase, Regulates Genome-Wide H3K4 Trimethylation and Is Required for Normal Induction of Secondary Metabolism in Aspergillus nidulans. PLoS Genet. 2016, 12, e1006222. [Google Scholar] [CrossRef]
- Bachleitner, S.; Sorensen, J.L.; Gacek-Matthews, A.; Sulyok, M.; Studt, L.; Strauss, J. Evidence of a Demethylase-Independent Role for the H3K4-Specific Histone Demethylases in Aspergillus nidulans and Fusarium graminearum Secondary Metabolism. Front. Microbiol. 2019, 10, 1759. [Google Scholar] [CrossRef]
- Huh, A.; Dubey, A.; Kim, S.; Jeon, J.; Lee, Y. MoJMJ1, encoding a histone demethylase containing JmjC domain, is required for pathogenic development of the rice blast fungus, Magnaporthe oryzae. Plant Pathol. J. 2017, 33, 193–205. [Google Scholar]
- Li, Z.; Srivastava, P. Heat-shock proteins. Curr. Protoc. Immunol. 2004, 1, Appendix 1T. [Google Scholar] [CrossRef]
- Chen, L.; Geng, X.; Ma, Y.; Zhao, J.; Che, W.; Xing, X.; Shi, Y.; Sun, B.; Li, H. The ER Lumenal Hsp70 Protein FpLhs1 Is Important for Conidiation and Plant Infection in Fusarium pseudograminearum. Front. Microbiol. 2019, 10, 1401. [Google Scholar] [CrossRef]
- Presti, L.L.; LopezDiaz, C.; Turra, D.; di Pietro, A.; Hampel, M.; Heimel, K.; Kahmann, R. A conserved co-chaperone is required for virulence in fungal plant pathogens. New Phytol. 2016, 209, 1135–1148. [Google Scholar]
- Yang, J.; Liu, M.; Liu, X.; Yin, Z.; Sun, Y.; Zhang, H.; Zheng, X.; Wang, P.; Zhang, Z. Heat shock proteins MoSsb1, MoSsz1, and MoZuo1 attenuate MoMkk1-mediated CWI signaling and are important for growth and pathogenicity of Magnaporthe oryzae. Mol. Plant-Microbe Interact. 2018, 31, 1211–1221. [Google Scholar] [CrossRef]
- Bui, D.; Lee, Y.; Lim, J.Y.; Fu, M.; Kim, J.; Choi, G.J.; Son, H.; Lee, Y.W. Heat shock protein 90 is required for sexual and asexual development, virulence, and heat shock response in Fusarium graminearum. Sci. Rep. 2016, 6, 28154. [Google Scholar] [CrossRef]
- Wang, J.C. Cellular roles of DNA topoisomerases: A molecular perspective. Nat. Rev. Mol. Cell Biol. 2002, 3, 430–440. [Google Scholar]
- Baldwin, T.K.; Urban, M.; Brown, N.; Hammond-Kosack, K.E. A role for topoisomerase in Fusarium graminearum and F. culmorum pathogenesis and sporulation. Mol. Plant Microbe Interact. 2010, 23, 566–577. [Google Scholar]
- Gerhold, D.; Thiyagarajan, M.; Kmiec, E.B. The topoisomerase I gene from Ustilago maydis: Sequence, disruption and mutant phenotype. Nucleic Acids Res. 1994, 22, 3773–3778. [Google Scholar] [CrossRef][Green Version]
- Klages-Mundt, N.L.; Kumar, A.; Zhang, Y.; Kapoor, P.; Shen, X. The nature of actin family proteins in chromatin-modifying complexes. Front. Genet. 2018, 9, 398. [Google Scholar] [CrossRef]
- Kitamura, H.; Matsumori, H.; Kalendova, A.; Hozak, P.; Goldberg, I.G.; Nakao, M.; Saitoh, N.; Harata, M. The actin family protein ARP6 contributes to the structure and the function of the nucleolus. Biochem. Biophys. Res. Commun. 2015, 464, 554–560. [Google Scholar] [CrossRef]
- Garaiova, M.; Zambojova, V.; Simova, Z.; Griac, P.; Hapala, I. Squalene epoxidase as a target for manipulation of squalene levels in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2013, 14, 310–323. [Google Scholar] [CrossRef]
- Karst, F.; Lacroute, F. Ergosterol biosynthesis in Saccharomyces cerevisiae: Mutants deficient in the early steps of the pathway. Mol. Gen. Genet. 1977, 154, 269–277. [Google Scholar]
- Zhang, Y.; Yang, H.; Turra, D.; Zhou, S.; HazalAyhan, D.; DeIulio, G.A.; Guo, L.; Broz, K.; Wiederhold, N.; Coleman, J.J.; et al. The genome of opportunistic fungal pathogen Fusarium oxysporum carries a unique set of lineage-specific chromosomes. Commun. Biol. 2020, 3, 50. [Google Scholar] [CrossRef]
- Ma, Y.M.; Li, Y.; Liu, J.Y.; Song, Y.C.; Tan, R.X. Anti-Helicobacter pylori metabolites from Rhizoctonia sp. Cy064, an endophytic fungus in Cynodondactylon. Fitoterapia 2004, 75, 451–456. [Google Scholar]
- Aliferis, K.A.; Jabaji, S. 1H NMR and GC-MS metabolic fingerprinting of developmental stages of Rhizoctonia solani sclerotia. Metabolomics 2010, 6, 96–108. [Google Scholar]
- Liang, X.; Wang, X.-H.; Luo, R.-Y.; Lu, S.-Q.; Guo, Z.-J.; Wang, M.-A.; Liu, Y.; Zhou, L.-G. Secondary metabolites of rice sheath blight pathogen Rhizoctonia solani Kuhn and their biological activities. J. Integr. Agric. 2015, 14, 80–87. [Google Scholar]
- Elleuche, S.; Poggeler, S.A. Cyanase is transcriptionally regulated by arginine and involved in cyanate decomposition in Sordaria macrospora. Fungal Genet. Biol. 2008, 45, 1458–1469. [Google Scholar]
- Marsalova, L.; Vitamvas, P.; Hynek, R.; Prasil, I.T.; Kosova, K. Proteomic Response of Hordeum vulgare cv. Tadmor and Hordeum marinum to Salinity Stress: Similarities and Differences between a Glycophyte and a Halophyte. Front. Plant Sci. 2016, 7, 1154. [Google Scholar] [CrossRef]
- Linder, T. Cyanase-independent utilization of cyanate as a nitrogen source in ascomycete yeasts. World J. Microb. Biot. 2019, 35, 3. [Google Scholar] [CrossRef]
- Mchunu, N.P.; Permaul, K.; Abdul Rahman, A.Y.; Saito, J.A.; Singh, S.; Alam, M. Xylanase Super producer: Genome sequence of a compost-loving thermophilic fungus, Thermomyces lanuginosus Strain SSBP. Genome Announc. 2013, 1, 4–5. [Google Scholar]
- Winger, A.M.; Heazlewood, J.L.; Chan, L.J.G.; Petzold, C.J.; Permaul, K.; Singh, S. Secretome analysis of the thermophilic xylanase hyper-producer Thermomyces lanuginosus SSBP cultivated on corn cobs. J. Ind. Microbiol. Biotechnol. 2014, 41, 1687–1696. [Google Scholar]
- Ranjan, B.; Pillai, S.; Permaul, K.; Singh, S. Expression of a novel recombinant cyanate hydratase (rTl-Cyn) in Pichia pastoris, characteristics and applicability in the detoxification of cyanate. Bioresour. Technol. 2017, 238, 582–588. [Google Scholar]
- Ranjan, B.; Pillai, S.; Permaul, K.; Singh, S. A novel strategy for the efficient removal of toxic cyanate by the combinatorial use of recombinant enzymes immobilized on aminosilane modified magnetic nanoparticles. Bioresour. Technol. 2018, 253, 105–111. [Google Scholar]
- Ranjan, B.; Pillai, S.; Singh, K.P.S. Simultaneous removal of heavy metals and cyanate in a wastewater sample using immobilized cyanate hydratase on magnetic-multiwall carbon nanotubes. J. Hazard. Mater. 2019, 363, 73–80. [Google Scholar]
- Sutzl, L.; Laurent, C.V.F.P.; Abrera, A.T.; Schutz, G.; Ludwig, R.; Haltrich, D. Multiplicity of enzymatic functions in the CAZy AA3 family. Appl. Microbiol. Biotechnol. 2018, 102, 2477–2492. [Google Scholar]
- Daniel, G.; Volc, J.; Kubatova, E.; Nilsson, T. Ultrastructural and immunocytochemical studies on the H2O2-producing enzyme pyranose oxidase in Phanerochaete chrysosporium grown under liquid culture conditions. Appl. Environ. Microbiol. 1992, 58, 3667–3676. [Google Scholar]
- Abrera, A.T.; Sutzl, L.; Haltrich, D. Pyranose oxidase: A versatile sugar oxidoreductase for bioelectrochemical applications. Bioelectrochemistry 2020, 132, 107409. [Google Scholar] [CrossRef]
- Takakura, Y. Tricholoma matsutake fruit bodies secrete hydrogen peroxide as a potent inhibitor of fungal growth. Can. J. Microbiol. 2015, 61, 6. [Google Scholar] [CrossRef]
- Marzluf, G.A. Molecular genetics of sulfur assimilation in filamentous fungi and yeast. Annu. Rev. Microbiol. 1997, 51, 73–96. [Google Scholar] [CrossRef]
- Lima, J.O.; Pereira, J.F.; Rincones, J.; Barau, J.G.; Araujo, E.F.; Pereira, G.A.G.; Queiroz, M.V. The glyceraldehyde-3-phosphate dehydrogenase gene of Moniliophthora perniciosa, the causal agent of witches’ broom disease of The obroma cacao Genet. Mol. Biol. 2009, 32, 362–366. [Google Scholar] [CrossRef]
- Miki, T.S.; Grobhans, H. The multifunctional RNase XRN2. Biochem. Soc. Trans. 2013, 41, 825–830. [Google Scholar]
- Mateyak, M.K.; Pupek, J.K.; Garino, A.E.; Knapp, M.C.; Colmer, S.F.; Kinzy, T.G.; Dunaway, S. Demonstration of translation elongation factor 3 activity from a non-fungal species, Phytophthora infestans. PLoS ONE 2018, 13, e0190524. [Google Scholar] [CrossRef]
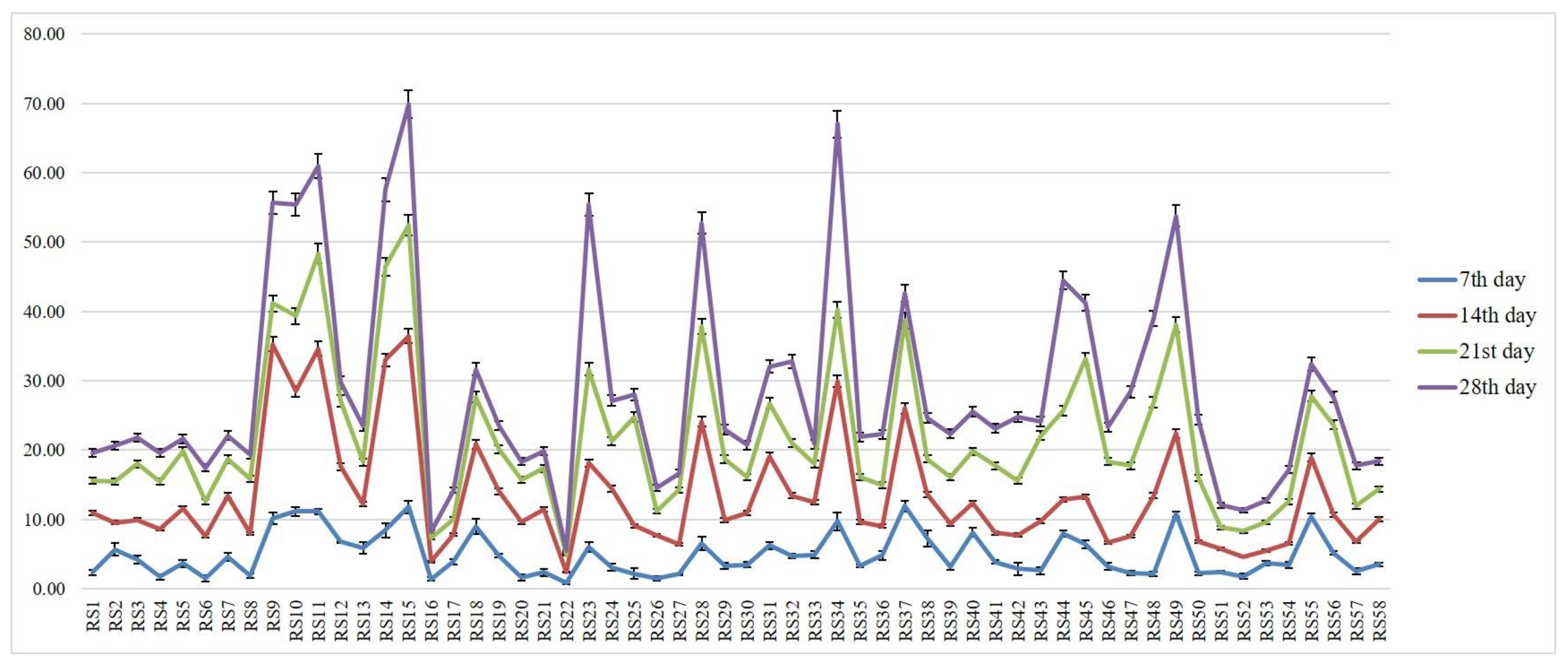
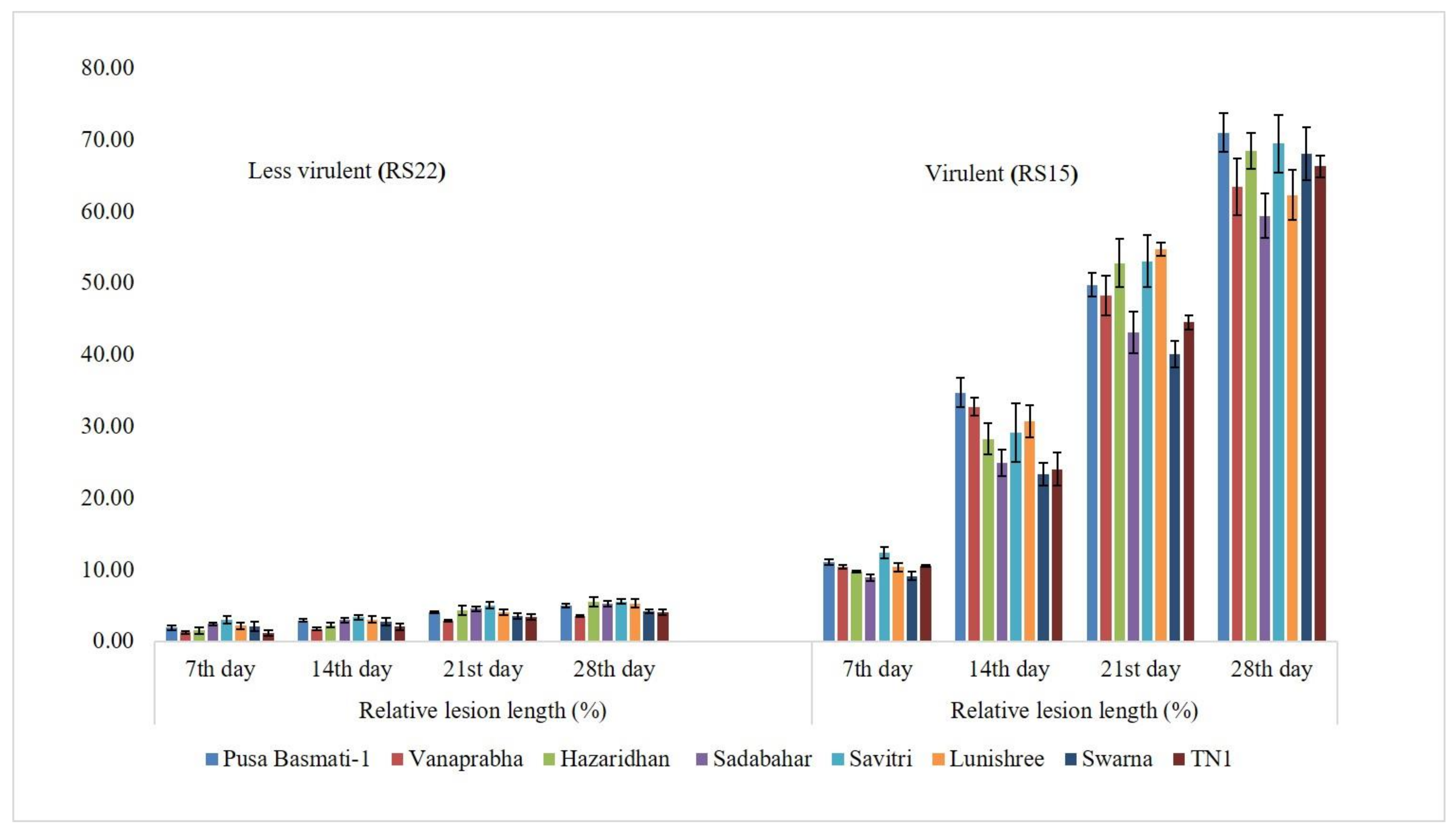
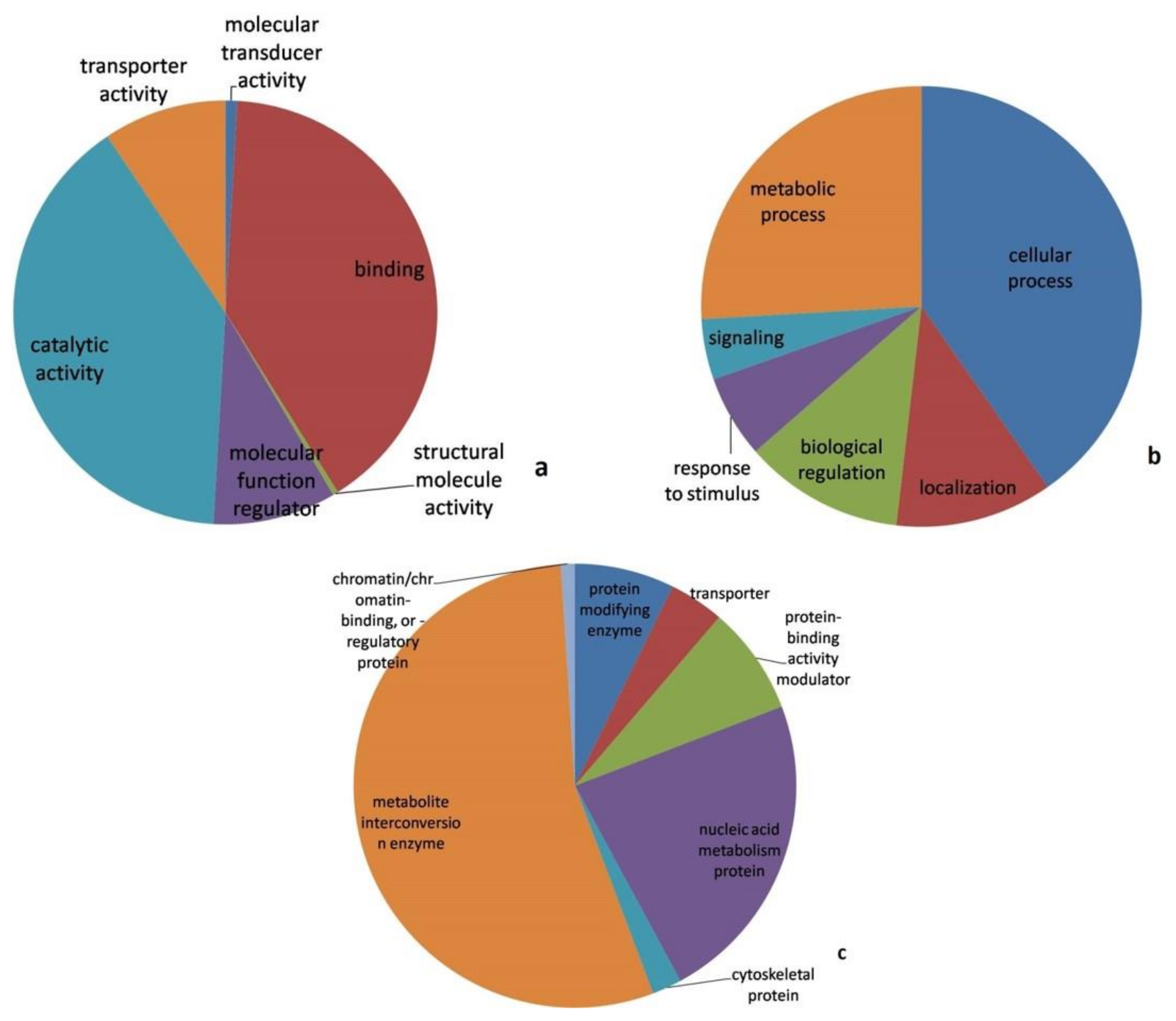
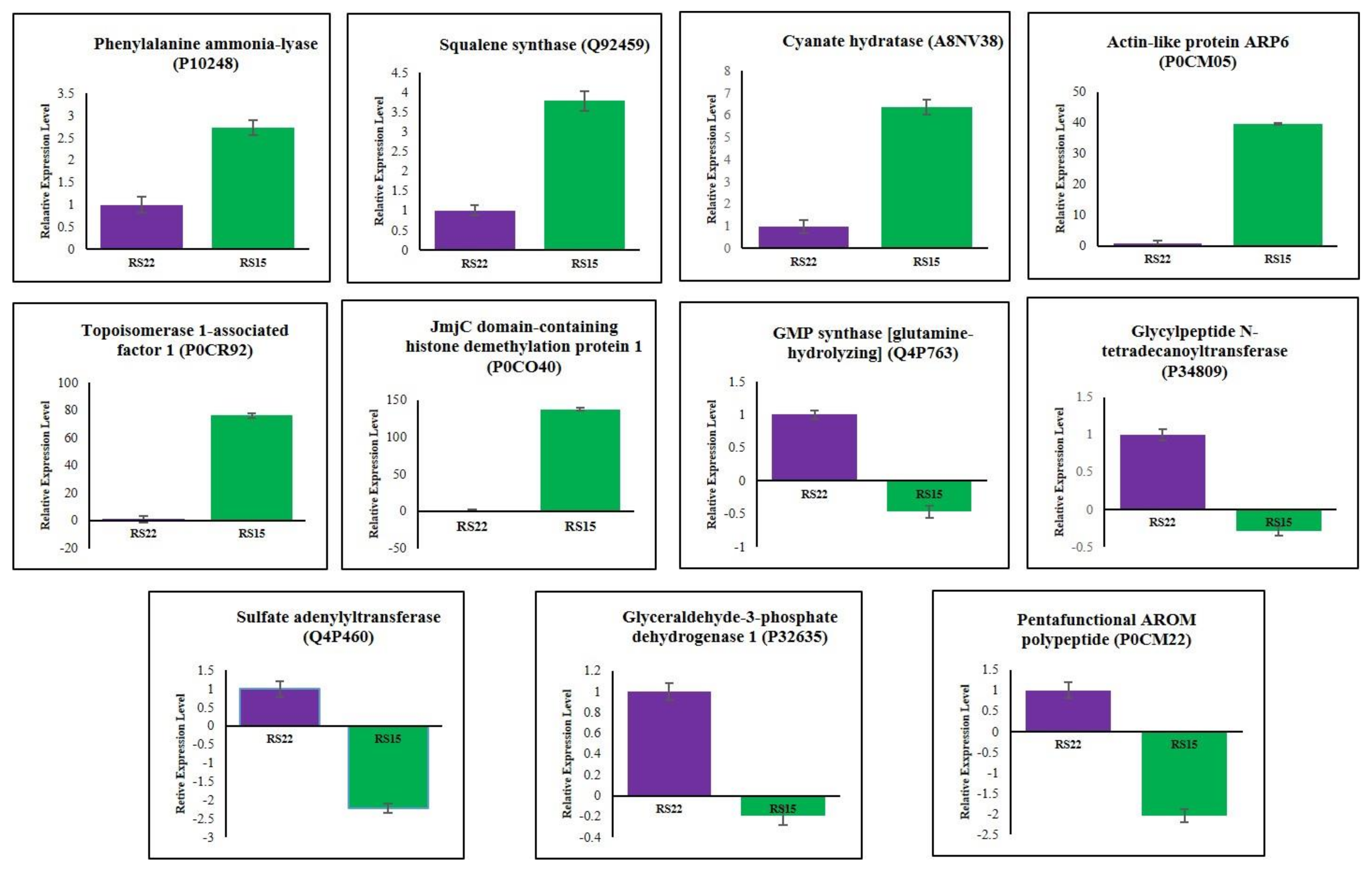
| Isolates | PDI * | Average Virulent Index | Disease Reaction |
|---|---|---|---|
| RS1 | 25.93 (30.61) k | 2.33 | Less virulent |
| RS2 | 25.93 (30.61) k | 2.33 | Less virulent |
| RS3 | 33.33 (35.26) j | 3.00 | Less virulent |
| RS4 | 25.93 (30.61) k | 2.33 | Less virulent |
| RS5 | 33.33 (35.26) j | 3.00 | Less virulent |
| RS6 | 18.52 (25.49) l | 1.67 | Less virulent |
| RS7 | 25.93 (30.61) k | 2.33 | Less virulent |
| RS8 | 18.52 (25.49) l | 1.67 | Less virulent |
| RS9 | 77.78 (61.93) d | 7.00 | Moderately virulent |
| RS10 | 77.78 (61.93) d | 7.00 | Moderately virulent |
| RS11 | 85.19 (67.51) c | 7.67 | Moderately virulent |
| RS12 | 48.15 (43.94) h | 4.33 | Moderately virulent |
| RS13 | 33.33 (35.26) j | 3.00 | Less virulent |
| RS14 | 77.78 (61.93) d | 7.00 | Moderately virulent |
| RS15 | 100.00 (88.19) a | 9.00 | Highly virulent |
| RS16 | 11.11 (19.47) m | 1.00 | Less virulent |
| RS17 | 11.11 (19.47) m | 1.00 | Less virulent |
| RS18 | 55.56 (48.20) g | 5.00 | Moderately virulent |
| RS19 | 33.33 (35.26) j | 3.00 | Less virulent |
| RS20 | 18.52 (25.49) l | 1.67 | Less virulent |
| RS21 | 18.52 (25.49) l | 1.67 | Less virulent |
| RS22 | 11.11 (19.47) m | 1.00 | Less virulent |
| RS23 | 77.78 (61.93) d | 7.00 | Moderately virulent |
| RS24 | 40.74 (39.66) i | 3.67 | Less virulent |
| RS25 | 40.74 (39.66) i | 3.67 | Less virulent |
| RS26 | 11.11 (19.47) m | 1.00 | Less virulent |
| RS27 | 11.11 (19.47) m | 1.00 | Less virulent |
| RS28 | 77.78 (61.93) d | 7.00 | Moderately virulent |
| RS29 | 25.93 (30.61) k | 2.33 | Less virulent |
| RS30 | 25.93 (30.61) k | 2.33 | Less virulent |
| RS31 | 55.56 (48.20) g | 5.00 | Moderately virulent |
| RS32 | 48.15 (43.94) h | 4.33 | Moderately virulent |
| RS33 | 18.52 (25.49) l | 1.67 | Less virulent |
| RS34 | 92.59 (74.77) b | 8.33 | Highly virulent |
| RS35 | 25.93 (30.61) k | 2.33 | Less virulent |
| RS36 | 25.93 (30.61) k | 2.33 | Less virulent |
| RS37 | 70.37 (57.05) e | 6.33 | Moderately virulent |
| RS38 | 33.33 (35.26) j | 3.00 | Less virulent |
| RS39 | 33.33 (35.26) j | 3.00 | Less virulent |
| RS40 | 33.33 (35.26) j | 3.00 | Less virulent |
| RS41 | 33.33 (35.26) j | 3.00 | Less virulent |
| RS42 | 33.33 (35.26) j | 3.00 | Less virulent |
| RS43 | 33.33 (35.26) j | 3.00 | Less virulent |
| RS44 | 62.96 (52.52) f | 5.67 | Moderately virulent |
| RS45 | 62.96 (52.52) f | 5.67 | Moderately virulent |
| RS46 | 33.33 (35.26) j | 3.00 | Less virulent |
| RS47 | 40.74 (39.66) i | 3.67 | Less virulent |
| RS48 | 55.56 (48.20) g | 5.00 | Moderately virulent |
| RS49 | 77.78 (61.93) d | 7.00 | Moderately virulent |
| RS50 | 33.33 (35.26) j | 3.00 | Less virulent |
| RS51 | 11.11 (19.47) m | 1.00 | Less virulent |
| RS52 | 11.11 (19.47) m | 1.00 | Less virulent |
| RS53 | 11.11 (19.47) m | 1.00 | Less virulent |
| RS54 | 18.52 (25.49) l | 1.67 | Less virulent |
| RS55 | 48.15 (43.94) h | 4.33 | Moderately virulent |
| RS56 | 40.74 (39.66) i | 3.67 | Less virulent |
| RS57 | 11.11 (19.47) m | 1.00 | Less virulent |
| RS58 | 11.11 (19.47) m | 1.00 | Less virulent |
| Accessions | Description | Fold Change ** | Category |
|---|---|---|---|
| P0CN36 | Protein EFR3 | 2.034 | Up-regulated |
| Q4P3U5 | Protein EFR3 | 2.034 | Up-regulated |
| * P10248 | Phenylalanine ammonia-lyase | 2.054 | Up-regulated |
| Q4PB37 | Pre-mRNA-splicing factor | 2.181 | Up-regulated |
| Q4P0P3 | Mediator of RNA polymerase II transcription subunit 14 | 2.270 | Up-regulated |
| P0CQ16 | Lon protease homolog, mitochondrial | 2.293 | Up-regulated |
| Q6QWR1 | Pyranose 2-oxidase | 2.411 | Up-regulated |
| * Q92459 | Squalene synthase | 2.664 | Up-regulated |
| Q4P652 | Pre-mRNA-splicing factor CEF1 | 2.691 | Up-regulated |
| * A8NV38 | Cyanate hydratase | 2.718 | Up-regulated |
| P0CM56 | Probable O-acetyltransferase CAS1 | 2.773 | Up-regulated |
| Q4P9P3 | ATP-dependent RNA helicase DRS1 | 2.829 | Up-regulated |
| P32186 | Elongation factor 1-alpha | 3.004 | Up-regulated |
| P0CR93 | Topoisomerase 1-associated factor 1 | 3.034 | Up-regulated |
| P0CM04 | Actin-like protein ARP6 | 3.096 | Up-regulated |
| * P0CM05 | Actin-like protein ARP6 | 3.706 | Up-regulated |
| Q4P3H6 | Actin cytoskeleton-regulatory complex protein SLA1 | 4.904 | Up-regulated |
| * P0CR92 | Topoisomerase 1-associated factor 1 | 5.207 | Up-regulated |
| A8Q513 | Exportin-T | 8.935 | Up-regulated |
| Q4P525 | Glutamyl-tRNA (Gln) amidotransferase subunit B, mitochondrial | 8.935 | Up-regulated |
| A8N142 | rRNA biogenesis protein RRP36 | 9.116 | Up-regulated |
| P18694 | Heat shock 70 kDa protein 2 | 15.800 | Up-regulated |
| * P0CO40 | JmjC domain-containing histone demethylation protein 1 | 17.385 | Up-regulated |
| A8PTG4 | tRNA-dihydrouridine(47) synthase [NAD(P)(+)] | 3.265 | Up-regulated |
| P0CO41 | JmjC domain-containing histone demethylation protein 1 | 14.604 | Up-regulated |
| A8PWG8 | mRNA cleavage and polyadenylation factor CLP1 | 2.836 | Up-regulated |
| P0CR62 | Sorting nexin-4 | 17.915 | Up-regulated |
| * Q4P763 | GMP synthase [glutamine-hydrolyzing] | 0.018 | Down-regulated |
| Q4P257 | Elongation factor G, mitochondrial | 0.024 | Down-regulated |
| * P34809 | Glycylpeptide N-tetradecanoyltransferase | 0.072 | Down-regulated |
| * Q4P460 | Sulfate adenylyltransferase | 0.124 | Down-regulated |
| Q4P149 | 5′-3′ exoribonuclease 2 | 0.157 | Down-regulated |
| * P32635 | Glyceraldehyde-3-phosphate dehydrogenase 1 | 0.160 | Down-regulated |
| P0CO63 | Protein phosphatase methylesterase 1 | 0.247 | Down-regulated |
| P0CM63 | Protein CFT1 | 0.259 | Down-regulated |
| P0CM89 | Endoribonuclease | 0.267 | Down-regulated |
| P0CM88 | Endoribonuclease | 0.292 | Down-regulated |
| P0CM62 | Protein CFT1 | 0.292 | Down-regulated |
| P0CO62 | Protein phosphatase methylesterase 1 | 0.298 | Down-regulated |
| Q4P4C1 | Inositol-pentakisphosphate 2-kinase | 0.307 | Down-regulated |
| P0CO60 | Putative lipase ATG15 | 0.333 | Down-regulated |
| P0CM23 | Pentafunctional AROM polypeptide | 0.343 | Down-regulated |
| P0CO61 | Putative lipase ATG15 | 0.353 | Down-regulated |
| P0CP58 | Pescadillo homolog | 0.427 | Down-regulated |
| Q4P0P0 | Eukaryotic translation initiation factor 3 subunit C | 0.440 | Down-regulated |
| I3ZNU9 | Orsellinic acid synthase ArmB | 0.458 | Down-regulated |
| A0A1B1ZGB5 | Adenylate-forming reductase Nps10 | 0.458 | Down-regulated |
| * P0CM22 | Pentafunctional AROM polypeptide | 0.487 | Down-regulated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabhukarthikeyan, S.R.; Parameswaran, C.; Sawant, S.B.; Naveenkumar, R.; Mahanty, A.; Keerthana, U.; Yadav, M.K.; Anandan, A.; Panneerselvam, P.; Bag, M.K.; et al. Comparative Proteomic Analysis of Rhizoctonia solani Isolates Identifies the Differentially Expressed Proteins with Roles in Virulence. J. Fungi 2022, 8, 370. https://doi.org/10.3390/jof8040370
Prabhukarthikeyan SR, Parameswaran C, Sawant SB, Naveenkumar R, Mahanty A, Keerthana U, Yadav MK, Anandan A, Panneerselvam P, Bag MK, et al. Comparative Proteomic Analysis of Rhizoctonia solani Isolates Identifies the Differentially Expressed Proteins with Roles in Virulence. Journal of Fungi. 2022; 8(4):370. https://doi.org/10.3390/jof8040370
Chicago/Turabian StylePrabhukarthikeyan, Seenichamy Rathinam, Chidambaranathan Parameswaran, Shraddha Bhaskar Sawant, Ramasamy Naveenkumar, Arabinda Mahanty, Umapathy Keerthana, Manoj Kumar Yadav, Annamalai Anandan, Periyasamy Panneerselvam, Manas Kumar Bag, and et al. 2022. "Comparative Proteomic Analysis of Rhizoctonia solani Isolates Identifies the Differentially Expressed Proteins with Roles in Virulence" Journal of Fungi 8, no. 4: 370. https://doi.org/10.3390/jof8040370
APA StylePrabhukarthikeyan, S. R., Parameswaran, C., Sawant, S. B., Naveenkumar, R., Mahanty, A., Keerthana, U., Yadav, M. K., Anandan, A., Panneerselvam, P., Bag, M. K., & Rath, P. C. (2022). Comparative Proteomic Analysis of Rhizoctonia solani Isolates Identifies the Differentially Expressed Proteins with Roles in Virulence. Journal of Fungi, 8(4), 370. https://doi.org/10.3390/jof8040370






