In Vitro and In Vivo Activity of Luliconazole (NND-502) against Planktonic Cells and Biofilms of Azole Resistant Aspergillus fumigatus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolates
2.2. Preparation of Fungal Suspensions
2.3. Preparation of LLCZ
2.4. In Vitro Experiments
2.4.1. Broth Microdilution
2.4.2. Growth Kinetics
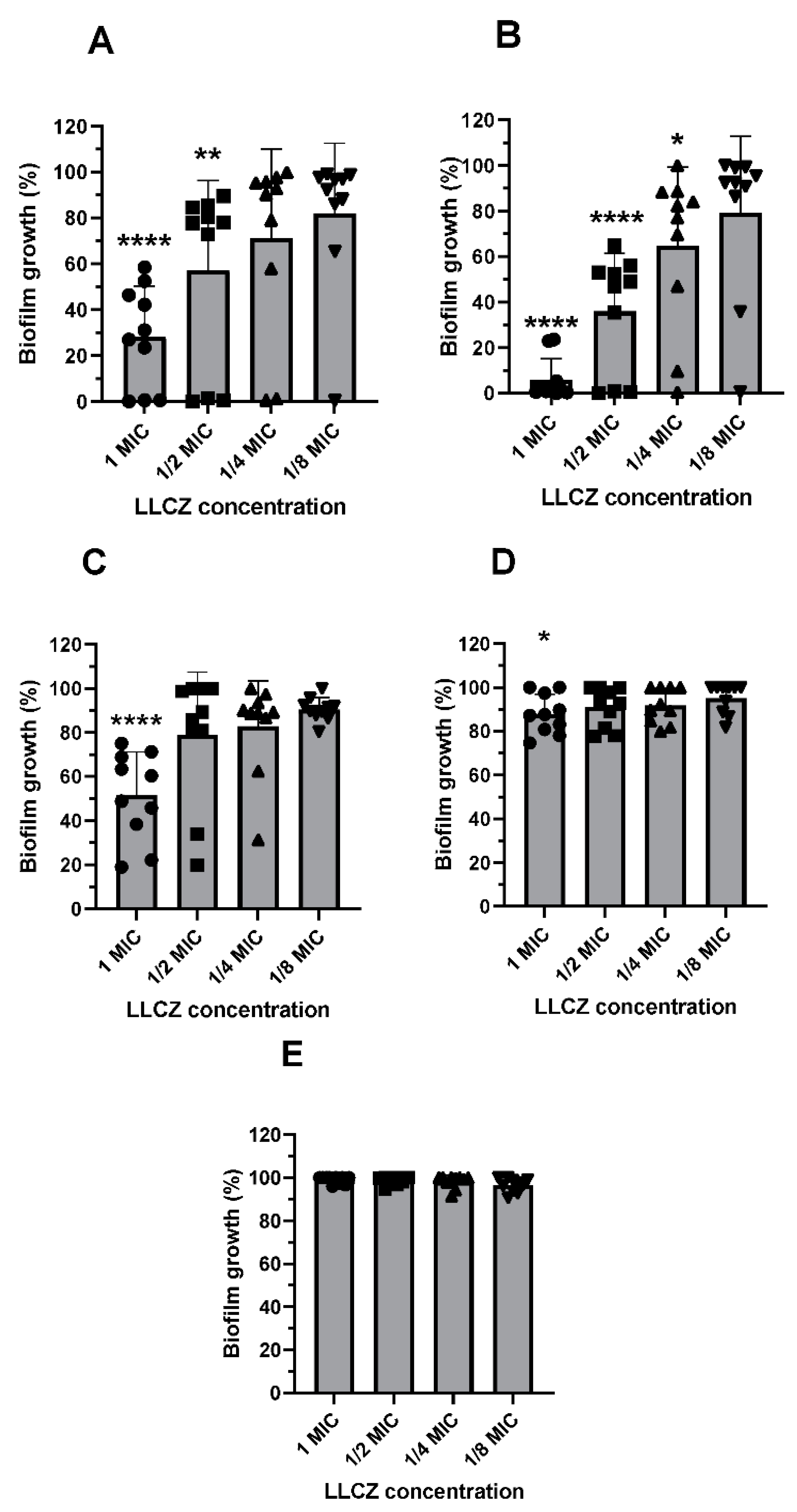
2.4.3. Biofilm Formation & Biofilm Susceptibility Assays
2.4.4. Confocal Laser Scanning Microscopy (CLSM)
2.5. In Vivo Experiments
2.5.1. Handling of Galleria Mellonella Larvae
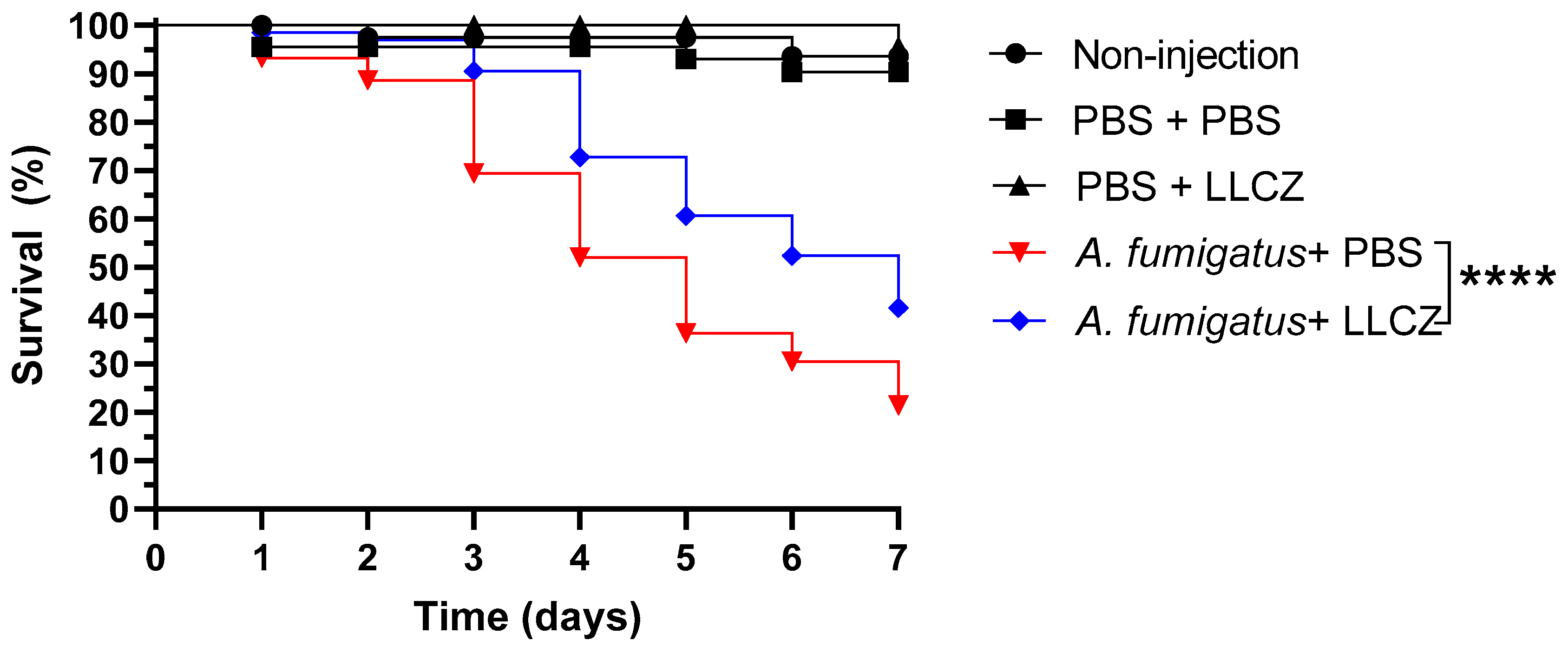
2.5.2. Treatment Assay
2.5.3. Re-Cultivation of A. fumigatus
2.6. Statistical Analysis
2.6.1. In Vitro
2.6.2. In Vivo
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, W.; Latgé, J.P. Microbe Profile: Aspergillus fumigatus: A saprotrophic and opportunistic fungal pathogen. Microbiology 2018, 164, 1009–1011. [Google Scholar] [CrossRef]
- Bowyer, P.; Moore, C.B.; Rautemaa, R.; Denning, D.W.; Richardson, M.D. Azole antifungal resistance today: Focus on Aspergillus. Curr. Infect. Dis. Rep. 2011, 13, 485. [Google Scholar] [CrossRef] [PubMed]
- Lestrade, P.P.A.; Meis, J.F.; Melchers, W.J.G.; Verweij, P.E. Triazole resistance in Aspergillus fumigatus: Recent insights and challenges for patient management. Clin. Microbiol. Infect. 2019, 25, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Lelièvre, L.; Groh, M.; Angebault, C.; Maherault, A.C.; Didier, E.; Bougnoux, M.E. Azole resistant Aspergillus fumigatus: An emerging problem. Med. Mal. Infect. 2013, 43, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Rath, P.-M.; Buchheidt, D.; Spiess, B.; Arfanis, E.; Buer, J.; Steinmann, J. First reported case of azole-resistant Aspergillus fumigatus due to the TR/L98H mutation in Germany. Antimicrob. Agents Chemother. 2012, 56, 6060–6061. [Google Scholar] [CrossRef] [Green Version]
- Bader, O.; Tünnermann, J.; Dudakova, A.; Tangwattanachuleeporn, M.; Weig, M.; Groß, U. Environmental isolates of azole-resistant Aspergillus fumigatus in Germany. Antimicrob. Agents Chemother. 2015, 59, 4356–4359. [Google Scholar] [CrossRef] [Green Version]
- Blankenship, J.R.; Mitchell, A.P. How to build a biofilm: A fungal perspective. Curr. Opin. Microbiol. 2006, 9, 588–594. [Google Scholar] [CrossRef]
- Ramage, G.; Williams, C. Chapter Two—The clinical importance of fungal biofilms. Adv. Appl. Microbiol. 2013, 84, 27–83. [Google Scholar]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2017, 24, e1–e38. [Google Scholar] [CrossRef] [Green Version]
- Riat, A.; Neofytos, D.; Coste, A.; Harbarth, S.; Bizzini, A.; Grandbastien, B.; Pugin, J.; Lamoth, F. First case of Candida auris in Switzerland: Discussion about preventive strategies. Swiss. Med. Wkly. 2018, 148, w14622. [Google Scholar]
- Khanna, D.; Bharti, S. Luliconazole for the treatment of fungal infections: An evidence-based review. Core Evid. 2014, 9, 113–124. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food and Drug Administration. LUZU (Luliconazole) Cream, 1% for Topical Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204153s000lbl.pdf (accessed on 7 September 2021).
- U.S. Food and Drug Administration. Tertiary Pharmacology Review, Application Number: 204153Orig1s000. 2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204153Orig1s000PharmR.pdf (accessed on 7 September 2021).
- Hivary, S.; Fatahinia, M.; Halvaeezadeh, M.; Mahmoudabadi, A.Z. The potency of luliconazole against clinical and environmental Aspergillus nigri complex. Iran J. Microbiol. 2019, 11, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Abastabar, M.; Rahimi, N.; Meis, J.F.; Aslani, N.; Khodavaisy, S.; Nabili, M.; Rezaei-Matehkolaei, A.; Makimura, K.; Badali, H. Potent activities of novel imidazoles lanoconazole and luliconazole against a collection of azole-resistant and -susceptible Aspergillus fumigatus strains. Antimicrob. Agents Chemother. 2016, 60, 6916–6919. [Google Scholar] [CrossRef] [Green Version]
- Gharaghani, M.; Taghipour, S.; Zarei, M. A molecular identification, biofilm formation and antifungal susceptibility of Rhodotorula spp. Mol. Biol. Rep. 2020, 47, 8903–8909. [Google Scholar] [CrossRef] [PubMed]
- Niwano, Y.; Kuzuhara, N.; Goto, Y.; Munechika, Y.; Kodama, H.; Kanai, K.; Yoshida, M.; Miyazaki, T.; Yamaguchi, H. Efficacy of NND-502, a novel imidazole antimycotic agent, in experimental models of Candida albicans and Aspergillus fumigatus infections. Int. J. Antimicrob. Agents 1999, 12, 221–228. [Google Scholar] [CrossRef]
- Seufert, R.; Sedlacek, L.; Kahl, B.; Hogardt, M.; Hamprecht, A.; Haase, G.; MacKenzie, C.R.; Essig, A.; Stehling, A.; Sutharsan, S.; et al. Prevalence and characterization of azole-resistant Aspergillus fumigatus in patients with cystic fibrosis: A prospective multicentre study in Germany. J. Antimicrob. Chemother. 2018, 73, 2047–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J.; The Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). EUCAST Definitive Document E.DEF 9.3.2. Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Formingmoulds. 2020, pp. 1–23. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_9.3.2_Mould_testing_definitive_revised_2020.pdf (accessed on 7 September 2021).
- Öz, Y.; Özdemir, H.G.; Gökbolat, E.; Kiraz, N.; Ilkit, M.; Seyedmousavi, S. Time-kill kinetics and in vitro antifungal susceptibility of non-fumigatus Aspergillus species isolated from patients with ocular mycoses. Mycopathologia 2016, 181, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Kirchhoff, L.; Dittmer, S.; Furnica, D.-T.; Buer, J.; Steinmann, E.; Rath, P.-M.; Steinmann, J. Inhibition of azole-resistant Aspergillus fumigatus biofilm at various formation stages by antifungal drugs, including olorofim. J. Antimicrob. Chemother. 2022, dkac062. [Google Scholar] [CrossRef]
- Olsowski, M.; Hoffmann, F.; Hain, A.; Kirchhoff, L.; Theegarten, D.; Todt, D.; Steinmann, E.; Buer, J.; Rath, P.-M.; Steinmann, J. Exophiala dermatitidis isolates from various sources: Using alternative invertebrate host organisms (Caenorhabditis elegans and Galleria mellonella) to determine virulence. Sci. Rep. 2018, 8, 12747. [Google Scholar] [CrossRef]
- Slater, J.L.; Gregson, L.; Denning, D.W.; Warn, P.A. Pathogenicity of Aspergillus fumigatus mutants assessed in Galleria mellonella matches that in mice. Med. Mycol. 2011, 49, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Campbell, C.K. Fine structure and physiology of conidial germination in Aspergillus fumigatus. Trans. Brit. Mycol. Soc. 1971, 57, 393–395. [Google Scholar] [CrossRef]
- Bastos, R.W.; Rossato, L.; Valero, C.; Lagrou, K.; Colombo, A.L.; Goldman, G.H. Potential of gallium as an antifungal agent. Front. Cell. Infect. Microbiol. 2019, 9, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, 383–392. [Google Scholar] [CrossRef]
- Tits, J.; Cammue, B.P.A.; Thevissen, K. Combination therapy to treat fungal biofilm-based infections. Int. J. Mol. Sci. 2020, 21, 8873. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, H.; Zeng, R.; Liang, G.; Zheng, N.; Liu, W. Effects of itraconazole and micafungin on Aspergillus fumigatus Biofilms. Mycopathologia 2021, 186, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Desoubeaux, G.; Cray, C. Rodent models of invasive aspergillosis due to Aspergillus fumigatus: Still a long path toward standardization. Front. Microbiol. 2017, 8, 841. [Google Scholar] [CrossRef]
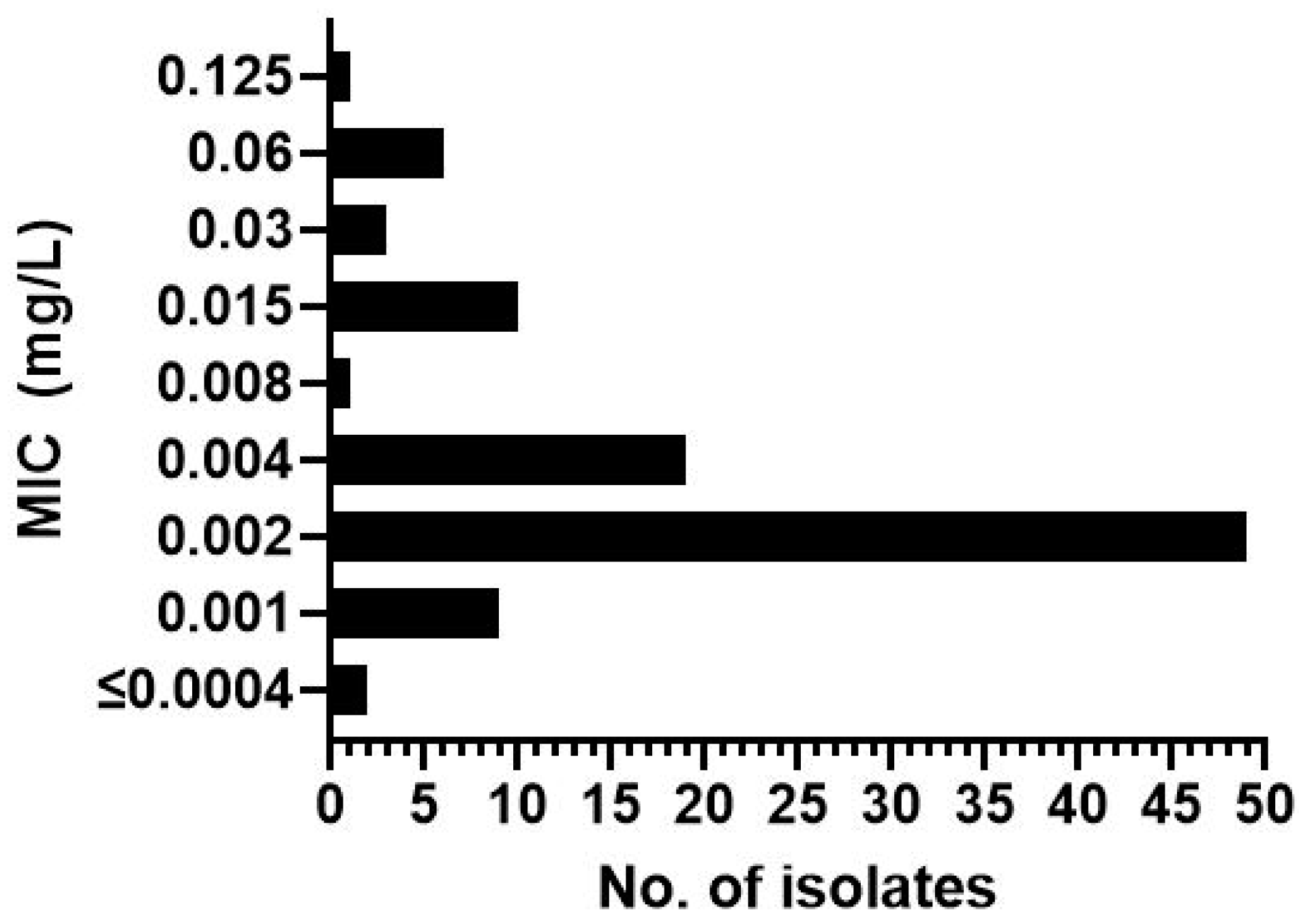


| Isolate | MIC (mg/L) | Mutation | Isolate | MIC (mg/L) | Mutation | Isolate | MIC (mg/L) | Mutation | Isolate | MIC (mg/L) | Mutation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1954 * | 0.002 | TR34/L98H | 1962 | 0.002 | TR34 | 2120 | 0.06 | TR34 | 2320 | 0.015 | Wild type |
| 2107 * | 0.06 | TR46 | 1964 | 0.001 | TR34 | 2122 | 0.002 | TR34 | 2321 | 0.015 | Wild type |
| 2514 * | 0.002 | TR34/L98H | 1966 | 0.002 | TR34 | 2123 | 0.004 | TR34 | 2325 | 0.03 | Wild type |
| 2607 * | 0.002 | TR34/L98H | 1970 | 0.001 | TR34 | 2130 | 0.002 | TR34 | 2328 | 0.002 | Wild type |
| 2608 * | 0.002 | TR34/L98H | 1972 | 0.002 | TR34 | 2142 | 0.002 | TR34 | 2331 | 0.015 | TR34/L98H |
| 2135 * | 0.03 | TR34/L98H | 1974 | 0.001 | TR34 | 2147 | 0.002 | Wild type | 2332 | 0.004 | TR34/L98H |
| 2515 * | 0.002 | TR34/L98H | 1975 | ≤0.0004 | Wild type | 2166 | 0.002 | TR34/L98H | 2333 | 0.015 | TR34/L98H |
| 2119 * | 0.002 | TR34/L98H | 1976 | 0.004 | TR34 | 2173 | 0.002 | TR34 | 2334 | 0.015 | Wild type |
| m1215 * | 0.002 | Wild-type | 1978 | 0.002 | TR34 | 2199 | 0.002 | TR34 | 2344 | 0.015 | Wild type |
| ATCC 204305 * | 0.002 | Wild-type | 1979 | 0.004 | Wild type | 2204 | 0.002 | TR34 | 2346 | 0.06 | TR34 |
| 1944 | 0.004 | TR34/L98H | 1984 | 0.004 | TR34/L98H | 2246 | 0.002 | TR34 | 2347 | 0.015 | TR34/L98H |
| 1965 | 0.004 | TR34 | 1986 | 0.002 | TR34 | 2249 | 0.002 | TR34 | 2348 | 0.06 | TR34/L98H |
| 1968 | 0.004 | TR34 | 2010 | 0.004 | TR34 | 2250 | 0.002 | TR34 | 2688 | 0.002 | TR34 |
| 1971 | 0.002 | TR34 | 2026 | 0.002 | TR34 | 2258 | 0.002 | TR34 | 2689 | 0.002 | TR34 |
| 2102 | 0.004 | TR34 | 2035 | 0.002 | Wild type | 2259 | 0.002 | TR34 | 2690 | 0.03 | TR34 |
| 2133 | 0.002 | TR34 | 2036 | 0.001 | Wild type | 2300 | 0.004 | TR34/L98H | 2691 | 0.008 | TR34 |
| 2136 | 0.004 | TR34 | 2039 | 0.001 | TR34 | 2310 | 0.004 | TR34/L98H | 2692 | 0.001 | Wild type |
| 2198 | 0.009 | TR34 | 2040 | 0.001 | TR34 | 2311 | 0.002 | TR34/L98H | 2693 | 0.002 | TR34 |
| 2237 | 0.004 | TR34 | 2044 | 0.004 | TR34 | 2312 | 0.004 | TR34/L98H | 2694 | 0.06 | TR34 |
| 2513 | 0.001 | TR34/L98H | 2047 | 0.002 | TR34 | 2313 | 0.002 | TR34/L98H | 2702 | 0.002 | TR34 |
| 2453 | 0.125 | TR46 | 2087 | 0.002 | TR34 | 2314 | 0.001 | TR34/L98H | 2707 | 0.002 | TR34 |
| 2487 | 0.06 | TR46 | 2089 | 0.002 | TR34 | 2315 | 0.004 | TR34/L98H | 2708 | 0.004 | TR34/L98H |
| 1950 | 0.002 | TR34 | 2091 | 0.002 | TR34 | 2316 | 0.015 | TR34/L98H | 2711 | 0.002 | TR34 |
| 1958 | ≤0.0004 | Wild type | 2101 | 0.004 | TR34 | 2317 | 0.015 | TR34/L98H | 2712 | 0.002 | TR34 |
| 1959 | 0.002 | TR34 | 2116 | 0.002 | TR34 | 2319 | 0.015 | Wild type | 2713 | 0.002 | Wild type |
| 2714 | 0.002 | TR34 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furnica, D.-T.; Dittmer, S.; Sanders, M.I.; Steinmann, J.; Rath, P.-M.; Kirchhoff, L. In Vitro and In Vivo Activity of Luliconazole (NND-502) against Planktonic Cells and Biofilms of Azole Resistant Aspergillus fumigatus. J. Fungi 2022, 8, 350. https://doi.org/10.3390/jof8040350
Furnica D-T, Dittmer S, Sanders MI, Steinmann J, Rath P-M, Kirchhoff L. In Vitro and In Vivo Activity of Luliconazole (NND-502) against Planktonic Cells and Biofilms of Azole Resistant Aspergillus fumigatus. Journal of Fungi. 2022; 8(4):350. https://doi.org/10.3390/jof8040350
Chicago/Turabian StyleFurnica, Dan-Tiberiu, Silke Dittmer, Maike Isabell Sanders, Joerg Steinmann, Peter-Michael Rath, and Lisa Kirchhoff. 2022. "In Vitro and In Vivo Activity of Luliconazole (NND-502) against Planktonic Cells and Biofilms of Azole Resistant Aspergillus fumigatus" Journal of Fungi 8, no. 4: 350. https://doi.org/10.3390/jof8040350
APA StyleFurnica, D.-T., Dittmer, S., Sanders, M. I., Steinmann, J., Rath, P.-M., & Kirchhoff, L. (2022). In Vitro and In Vivo Activity of Luliconazole (NND-502) against Planktonic Cells and Biofilms of Azole Resistant Aspergillus fumigatus. Journal of Fungi, 8(4), 350. https://doi.org/10.3390/jof8040350






