Abstract
We have previously identified Candida albicans GPH1 (orf19.7021) whose protein product was associated with C. albicans Cdc4. The GPH1 gene is a putative glycogen phosphorylase because its Saccharomyces cerevisiae homolog participates in glycogen catabolism, which involves the synthesis of β-glucan of the fungal cell wall. We made a strain whose CaCDC4 expression is repressed, and GPH1 is constitutively expressed. We established a GPH1 null mutant strain and used it to conduct the in vitro virulence assays that detect cell wall function. The in vitro virulence assay is centered on biofilm formation in which analytic procedures are implemented to evaluate cell surface hydrophobicity; competence, either in stress resistance, germ tube formation, or fibronection association; and the XTT-based adhesion and biofilm formation. We showed that the constitutively expressed GPH1 partially suppresses filamentation when the CaCDC4 expression is repressed. The C. albicans Gph1 protein is reduced in the presence of CaCdc4 in comparison with the absence of CaCdc4. Compared with the wild-type strain, the gph1Δ/gph1Δ mutant displayed a reduction in the capability to form germ tubes and the cell surface hydrophobicity but an increase in binding with fibronectin. Compared with the wild-type strain, the gph1Δ/gph1Δ mutant showed a rise in adhesion, the initial stage of biofilm formation, but displayed a similar capacity to form a mature biofilm. There was no major impact on the gph1Δ/gph1Δ mutant regarding the conditions of cell wall damaging and TOR pathway-associated nutrient depletion. We conclude that GPH1, adversely regulated by the filament suppressor CDC4, contributes to cell wall function in C. albicans.
1. Introduction
The opportunistic human fungal pathogen Candida albicans [1] is a member of the normal microflora on mucosal surfaces in healthy persons [2] but can cause vulvovaginal candidiasis in women [3,4] and oral [5,6] and systemic candidiasis in debilitated and immunocompromised patients [7,8,9,10]. C. albicans can grow in a wide variety of morphological forms, from the ellipsoid blastospore to various filamentous types [11,12,13,14]. A great effort has been made to reveal the underlying mechanism of C. albicans morphogenesis because it is proven to be coupled with virulence and pathogenesis [15,16,17,18]. However, research advancement has been hampered due to C. albicans being a natural diploid with a noncanonical sexual cycle [19,20,21,22]. Still, several positive and negative signaling pathways that control morphological transition have been discovered in C. albicans [23,24,25]. Additionally, cyclin-dependent kinases and their associated cyclins with their regulators have been found to control morphological plasticity in C. albicans [26,27]. Curiously, we and others have recently found that some key cell cycle genes conserved throughout evolution play an essential role in the cell cycle but influence morphogenesis in C. albicans [28,29,30,31,32,33], including the couple cell cycle and morphogenesis [34,35,36,37].
We and others have uncovered that CaCDC4 suppresses filamentation in C. albicans [28,33]. The CaCdc4 has the WD40–repeat and F-box domains, whose homologs participate in binding with Skp1, one of the components of the Skp1-Cdc53/Cul1-F-box (SCF) complex, and the substrate [38], respectively. The CaCDC4 encodes a conventional F-box protein of SCF ubiquitin ligase [39], designated SCFCaCdc4. Notably, we revealed that the domains of F-box and WD40-repeat in the CaCdc4 are critical for filamentous development and repress flocculation [40]. Other than filamentation [41,42,43], flocculation is closely connected to biofilm formation [44,45,46]. Indeed, we found that CaCDC4 negatively regulates biofilm formation in C. albicans [47]. By affinity purification, we identified several novel CaCdc4-interactors [48], among which are Gph1 and Thr1. In the budding yeast Saccharomyces cerevisiae, while the THR1 gene encodes a homoserine kinase that participates in the threonine biosynthesis pathway of [49,50]. Intriguingly, we found that C. albicans THR1 links GCN4 and CaCDC4 to control morphogenesis with the stress response and nutrient sensing [51], indicating that the morphogenesis is intertwined with environmental factors. In S. cerevisiae, GPH1 encodes a glycogen phosphorylase essential for the breakdown of glycogen polysaccharide to glucose-1-phosphate and glucose [52], which feed into glycolysis.
Glucose-1-phosphate can be converted into UDP-glucose in the presence of UTP by Upg1 [53]. UDP-glucose can be directed to either the storage carbohydrate glycogen by Glg1 and Glg 2 [54] or glucose by the trehalose synthase complex [54,55]. Importantly, UDP-glucose can be used as a substrate to become the structural carbohydrate β-glucan, one of the major components of the cell wall [56,57]. Hence, GPH1 appears to be involved in the synthesis of β-glucan and energy conversion in S. cerevisiae and may be shared in fungi including C. albicans. Several lines of evidence point to the association of GPH1 function with the cell wall in C. albicans. Gph1 was found to be non-covalently linked to the cell wall that is enriched in hyphal cells of C. albicans [58]. C. albicans cells treated with fluconazole exhibited a 3.5-fold up-regulated expression of GPH1 [59]. GPH1 was found to be under the Ndt80-dependent transcriptional control for biofilm formation in C. albicans [60]. GPH1 was found to be a common output of Cph2, which is required for the optimal expression of some hypoxia-responsive genes in glycolysis and the citric acid cycle and the regulatory circuit for gastrointestinal (GI) colonization [61,62,63]. In this report, we found that the level of Gph1 protein was decreased in conditions when the expression of CaCDC4 was de-repressed, and the filaments caused by the repressed CaCDC4 expression could be overcame by the constitutive expression of GPH1 in C. albicans. To investigate the role of GPH1 in C. albicans, we generated a gph1 null mutant. Cells of the gph1 null mutant were maintained as the yeast form without growth defect, but they were aggregated after prolonged culture. We made a strain where the expression of GPH1 is under the Tet-on control. In the induced condition, cells of the strain exhibited no morphological changes and peculiarly accumulated glycogen compared with those of the gph1 null mutant and wild-type. The gph1 null mutant did not appear to show growth impairment in a variety of cell wall damaging agents, carbon sources, and amino acid depleted conditions. However, the gph1 null mutant showed a decrease in its cell surface hydrophobicity and its ability to form a germ tube in the hypha-induced condition. Conversely, the gph1 null mutant exhibited an increase in its ability in either binding with fibronectin or adhesion but made no impact on biofilm formation. Hence, GPH1 negatively modulated by the filament suppressor CDC4 is involved in cell wall function in C. albicans.
2. Materials and Methods
2.1. General Manipulation, Media, and Growth Conditions
The E. coli strain DH5α was used for regular manipulation of the plasmids. All C. albicans strains (Table 1) were derived from either the clinically isolated wild-type strain SC5314 [64] or the auxotrophic strain BWP17 (arg4/arg4 his1/his1 ura3/ura3) [65]. The routine usage of media and growth conditions for the strains of E. coli and C. albicans were performed as described previously [66]. The pH of the SD medium with or without agar was adjusted to 7 by 100 mM HEPES after autoclaving because the default SD medium is acidic, which can suppress the filamentation of C. albicans. The E. coli strain DH5α was transformed with plasmid DNA by CaCl2 as described [67] or by electroporation [68]. C. albicans strains were transformed using the LiAc/PEG/ssDNA method [69] or electroporation [70].
2.2. Strain Usage and Construction
To enable constitutive expression of GPH1 in C. albicans carrying the expression-repressible CaCDC4, the coding sequence of the GPH1 gene was PCR-amplified from genomic DNA of the C. albicans wild-type strain SC5314 [64])with a pair of primers, CaGPH1-XhoI-F and CaGph1-XhoI-R (Table 2) and cloned into the plasmid vector p6HF-ACT1p [71] to generate p6HF-ACT1p-GPH1 capable of constitutively expressing GPH1. The CaCDC4 expression repressible strain CaCDC4 M3/− [40] (Table 1), whose CaCDC4 expression is repressed with 2.5 mM methionine and cysteine (Met/Cys) [72], was used to introduce the NcoI-linearized plasmid p6HF-ACT1p-GPH1, along with the empty plasmid p6HF-ACT1p and p6HF-ACT1p-CaCDC4 (51) targeting and integrating at the RPS1 locus to generate CaCDC4 M3/−|GPH1, CaCDC4 M3/−|p6HF-ACT1p, and CaCDC4 M3/−|CaCDC4, respectively (Table 1). Moreover, GPH1 was deleted in the C. albicans wild-type strain SC5314 with the CaSAT1-flipper method [73]. Briefly, both the upstream and downstream regions of GPH1 were amplified with primer pairs CaGPH1-U-F_KpnI/CaGPH1-U-R_XhoI and CaGPH1-D-F_SacII/CaGPH1-D-R_SacI, respectively (Table 2), and with template DNA of the genomic DNA extracted from SC5314. These were consecutively cloned into plasmid pSFS2A with a CaSAT1-flipper cassette at KpnI/XhoI and SacII/SacI sites to make plasmid pSF2A-gph1Δ. A cassette freed from pSF2A-gph1Δ using KpnI/SacII was introduced into SC5314 and was selected for nourseothricin positive (Nou+) GPH1/gph1ΔSF, following the CaSAT1-popped out by induction in YP–maltose (the glucose is replaced with maltose in YPD) to make gph1 heterozygous null mutant, GPH1/gph1Δ. The cassette pSF2A-gph1Δ was introduced into GPH1/gph1Δ and selected for Nou+ (gph1ΔSF/gph1Δ), following the CaSAT1-popped out by induction in YP–maltose for FLP/FRT recombination to generate gph1 homozygous null mutant, gph1Δ/gph1Δ. To make a GPH1 reintegrated strain, the DNA cassettes were PCR-amplilied with primer pairs CaGPH1-U-F_KpnI/GPH1-D-XhoI-R and CaGPH1-D-F_SacII/CaGPH1-D-R_SacI, respectively (Table 2), and with template DNA of the SC5314 genomic DNA. These were subsequently cloned into plasmid pSFS2A to become pSF2A-gph1, which contains a CaSAT1-flipper cassette flanked with the GPH1 upstream region plus the GPH1 ORF and GPH1 downstream region. A cassette freed from pSF2A-gph1 using KpnI/SacII was introduced into gph1Δ/gph1Δ and selected for nourseothricin positive (Nou+) gph1Δ/gph11Δ+GPH1-SAT1-FLIP, following the CaSAT1-popped out by induction in YP–maltose to make the GPH1 complement strain gph1Δ/gph1Δ+GPH1. The strain gcn4Δ/gcn4Δ was created as previously described [74].

Table 1.
Candida albicans strains used in this study.
Table 1.
Candida albicans strains used in this study.
| Name of the Strain | Parental Strain | Genotype | Source |
|---|---|---|---|
| SC5314 | Wild-type strain | [64] | |
| BWP17 | ura3::imm434/ura3::imm434 iro1/iro1::imm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | [65] | |
| CaCDC4 M3/− | BWP17 | Cacdc4Δ::dpl200/Cacdc4::pFA-HIS1-MET3p-CaCDC4 | [40] |
| CaCDC4 M3/−|p6HF-ACT1p | CaCDC4 M3/− | Cacdc4Δ::dpl200/Cacdc4::pFA-HIS1-MET3p-CaCDC4 RPS1/rps1::p6HF- ACT1p | This study |
| CaCDC4 M3/−|CaCDC4 | CaCDC4 M3/− | Cacdc4Δ::dpl200/Cacdc4::pFA-HIS1-MET3p-CaCDC4 RPS1/rps1Δ::p6HF-ACT1p-CaCDC4 | This study |
| CaCDC4 M3/−|GPH1 | CaCDC4 M3/− | Cacdc4Δ::dpl200/Cacdc4::pFA-HIS1-MET3p-CaCDC4 RPS1/rps1Δ::p6HF-ACT1p-GPH1 | This study |
| gcn4Δ/gcn4Δ | SC5314 | gcn4::FRT/gcn4::FRT | [74] |
| GPH1/gph1ΔSF | SC5314 | GPH1/gph1Δ::SAT1-FLIP | This study |
| GPH1/gph1Δ | GPH1/gph1ΔSF | GPH1/gph1Δ::FRT | This study |
| gph1ΔSF/gph1Δ | GPH1/gph1Δ | gph1Δ::SAT1-FLIP/gph1Δ::FRT | This study |
| gph1Δ/gph11Δ | gph1ΔSF/gph1Δ | gph1Δ::FRT/gph1Δ::FRT | This study |
| gph1Δ/gph11Δ+GPH1-SAT1-FLIP | gph1Δ/gph1Δ | gph1Δ::FRT/gph1Δ::FRT::GPH1-SAT1-FLIP | This study |
| gph1Δ/gph11Δ+GPH | gph1Δ/gph11Δ+GPH1-SAT1-FLIP | gph1Δ::FRT/gph1Δ::FRT::GPH1 | This study |
| Tet-on-GPH1 | SC5314 | ADH1/adh1::PTET-GPH1-SAT1 | This study |

Table 2.
Synthetic oligonucleotide primers used in this study.
Table 2.
Synthetic oligonucleotide primers used in this study.
| Name | Sequence (5′→3′) 1 |
|---|---|
| CaGPH1-U-F_KpnI | CGGGGTACCCCACCTAACTAATAACTATTGC |
| CaGPH1-U-R_XhoI | CCGCTCGAGGGGTAAGATAATCCATTGGC |
| CaGPH1-D-F_SacII | TCCCCGCGGGAAAGTAAGACAACGAGCGA |
| CaGPH1-D-R_SacI | CTAGGAGCTCCTTAGCTGAGTTAGGATCTG |
| GPH1-D-XhoI-R | GGGCTCGAGTCTTTCTCTCCCTTCATTGC |
| CaGPH1-XhoI-F (p6HF-ACT1p) | CCGCTCGAGATGCCAATGGATTATCTTACC |
| CaGph1-XhoI-R (p6HF-ACT1p) | CCGCTCGAGCTAAACATTGGATGGTTCAAC |
| GPH1-probe-F | CTGATTTAGATCAAGTGGCTGA |
| GPH1-probe-R | GACGAATGTAATGGCAGAGTT |
| front of GPH1-F_SpeI | GGACTAGTATGCCAATGGATTATCTTACC |
| front of GPH1-R_SpeI | GGACTAGTAACCCGTAACCCCAACCAC |
| CaACT1-F | ACGGTGAAGTTGCTGCTTTA |
| CaACT1-R | GCATTTCTTGTTCGAAATCC |
1 Restriction enzyme sites are shaded in grey.
2.3. Nucleic Acid Extraction and PCR Analysis
Candida albicans cells were grown to the mid-log phase, and genomic DNA was isolated using a MasterPure™ Yeast DNA Purification Kit (Epicentre, Madison, WI, USA), following the manufacturer’s instructions, as described previously [35]. The total RNA derived from cells cultured to the mid-log phase was extracted using a MasterPureTM Yeast RNA Purification Kit (Epicentre, Madison, WI, USA), following the manufacturer’s instructions, as described previously [51]. Subsequently, 5 μg of total RNA was used to generate cDNA using a SuperScript III Reverse Transcriptase Kit (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s instructions. The cDNA was then subjected to PCR with a pair of GPH1-specific primers, the front of GPH1-F_SpeI, and the front of GPH1-R_SpeI (Table 2), annealing the downstream of the GPH1 coding sequence with a predictive product of 623 bp. The primers CaACT1-F and CaACT1-R were used to generate a C. albicans ACT1-specific product that was used as a loading control. To confirm the correctness of the GPH1 deletion strain, Southern blotting analyses with DIG-labelled probe amplified by a pair of primers, GPH1-probe-F and GPH1-probe-R (Table 2), were performed as previously described [74].
2.4. Protein Extraction and Western Blotting
The total protein was extracted from the C. albicans cells, as described previously (74). The protein was partially purified from cells containing the p6HF-ACT1p plasmid with the open reading frame of the gene integrated at RPS1 capable of generating a tagged (6×His and FLAG) protein using Ni2+-NTA-agarose beads (Qiagen, Germantown, MD, USA), as previously described [75]. Precipitated proteins were resolved using 10% SDS-PAGE and transferred electrophoretically to PVDF membranes (Pall Corporation, Port Washington, NY, USA). They were then probed with a polyclonal antibody to FLAG (Sigma) in a 1:2000 dilution and visualized using a SuperSignal West Pico Chemiluminescent Substrate Kit (Pierce). The proteins detected were recorded with a Luminescent Image Analyzer (FUJIFILM LAS-1000) and analyzed by ImageGauge 3.46 and L Process v 1.96 (FUJIFILM). ImageJ (National Institutes of Health, Bethesda, MD, USA) was used to quantify the levels of proteins.
2.5. Germ Tube Formation Assay
The morphological plasticity of C. albicans plays a vital role in biofilm maturation, as previously discussed. Germ tube formation is a prerequisite of the development of hyphal and pseudohyphal forms, and hence the length of the germ tube under hyphal induction condition is used for the assessment of yeast-to-hypha transition. To promote germ tube formation, 1 × 106 C. albicans cells/mL are transferred into the cell culture medium RPMI 1640 supplemented with 10% (v/v) fetal calf serum (FCS), 2 mM L-glutamine, penicillin (100 U/mL), and streptomycin (100 μg/mL) and seeded into a 24-well plate. After 1 h incubation at 37 °C, cells were visualized and recorded, and the germ tube length was determined with the Photoshop 6 software.
2.6. Cell Surface Hydrophobicity Assay
Cell surface hydrophobicity (CSH) was measured using the microbial adhesion assay to hydrocarbons (MATH) [76]. The assay was conducted as previously described [77]. Briefly, C. albicans cells grown to the mid-log phase at 30 °C were collected and washed twice with PBS. The cell suspension with an OD600 between 0.4 and 0.5 was set up in PBS (A0); 3 mL of the cell suspension was overlaid with 0.4 mL of the hydropho-bichydrocarbon, n-hexadecane (SIGMA, H6703). Following robust vortexing, the phases were left to separate for 10 min at 30 °C, and the OD600 of the aqueous phase was quantified (A1). The percentage of hydrophobicity is calculated as follows: hydrophobicity (%) = [1 − (A1/A0)] × 100.
2.7. Fibronectin (FN)-C. albicans Association Assay
To specifically assess the binding of fibronectin with C. albicans cells, C. albicans cells (1 × 106) from the mid-log phase were sub-cultured in 2 mL RPMI 1640 medium supplemented with 0.0001% human fibronectin (Sigma-Aldrich, St. Louis, MO, USA) for 1 h at 30 °C at 200 rpm. Subsequently, cells were examined microscopically or washed three times with 2 mL PBS before harvesting. Cells were resuspended in 80 μL PBS, with the addition of 20 μL 5× sample loading buffer (with β-ME), boiled for 10 min, and rested on ice for 10 min. The cells were spun down for 10 min, and 90 µL of the supernatant was transferred to a fresh tube prior to immunoblot analysis with specific FN-specific antibody [78].
2.8. Adhesion Assay
The adhesion assay was conducted as previously described [77]. In brief, C. albicans cells were grown overnight in YPD at 37 °C with 180 rpm agitation. Cells were collected by centrifugation for 5 min at 10,000× g, washed with PBS, and standardized to 5 × 107 C. albicans cells/mL in RPMI-1640 medium supplemented with L-glutamine and buffered with MOPS acid. Next, 100 μL (5 × 106 cells) aliquots of the cell suspension were placed in each well of a nonpyrogenic polystyrene flat-bottom 96-well microtiter plate and incubated for 1 h at 37 °C. The wells were washed three times with 10 mM PBS before being quantified by the XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5- carboxanilide) reduction assay [79]. Briefly, the adherent cells were incubated with XTT (0.5 mg/mL + 1 μM menadione in Ringer’ solution) in the dark. The absorbance of reduced XTT was measured in a microtiter plate reader at 490 nm, as described previously [47].
2.9. Biofilm Formation Assay
To assess the ability of C. albicans cells to form biofilm, cells of the strains were prepared as described in the adhesion assay, except that the cells were standardized to 1 × 106 C. albicans cells/mL after washing. Then, 200 μL aliquots (2 × 105 cells) of the C. albicans cell suspension was placed in the wells of a 96-well microtiter plate and incubated for 48 h at 37 °C before XTT reduction assay.
2.10. Spotting Assay
The spotting assays were carried out as previously described [74]. Concisely, cells of the C. albicans strains were grown in YPD to the mid-log phase. The cultured strains were diluted to an optical density of 1.0 at OD600 (approximately 2 × 107 cells/mL) and then serially diluted from 107 to 102 cells/mL. The diluted cultures were spotted on agar plates at a volume of 5 μL and left to grow.
2.11. Cellular Image Observation and Recording
The images of the cultured cells were recorded with a Nikon 50i microscope at 400× magnification. Colonies were photographed with a MEIJI stereoscopic microscope EMZ5 at 40× magnification. The monographs were digitized and processed using Adobe Photoshop software.
2.12. Statistical Analysis
Unless stated otherwise, three independent assays were conducted, and each sample was assayed in triplicate. Statistical analyses were performed using GraphPad Prism software, v.8.0 (GraphPad Software, Inc., La Jolla, CA, USA), by one-way analysis of variance (ANOVA), followed by Tukey’s post hoc analysis. The results are expressed as the mean ± standard deviation (SD). The p < 0.05 indicate a statistically significant difference. The asterisks used to indicate statistically significant difference are as follows: * p < 0.05; ** p < 0.01; *** p < 0.005; **** p < 0.001.
3. Results
3.1. The Filamentous Growth Caused by the Repressed CaCDC4 Expression Could Be Partially Suppressed by the Constitutive GHP1 Expression in C. albicans
We previously identified the Gph1 protein as a C. albicans Cdc4-interactor [48]. To understand the functional link between CaCDC4 and GPH1, a C. albicans strain capable of repressing CaCDC4 expression with methionine and cysteine (Met/Cys) and constitutively expressing GPH1, together with those expressing CaCDC4 and none, were created (Figure 1A). To evaluate the outcome of GPH1 expression on the filamentous growth of cells with CaCDC4 expression being repressed, the cells of the above strains, together with their parental strain, were plated onto YPD rich media (Figure 1B) or were grown in the minimum media (Figure 1C) with or without 2.5 mM Met/Cys. After obtaining the constructed strains, we unexpectedly found that the expression of CaCDC4 of CaCDC4 M3/− on plates of YPD-rich media appeared to be repressed; hence, they grew as filamentous forms but could mostly be suppressed when constitutively expressing CaCDC4 from the p6HF-based plasmid (Figure 1B). As expected, the constitutive expression of CaCDC4 but not the empty plasmid completely suppressed the filamentous mode of growth when the CaCDC4 expression was repressed (Figure 1C). It appeared that the filaments as a result of the repression of CaCDC4 expression were mixed with the hyphal and pseudohyphal cells, similar to previous observations by us [40] and others [28]. Significantly, the constitutive GPH1 expression could somewhat suppress the filamentous development when the CaCDC4 expression was repressed (Figure 1C). Curiously, the suppression of filamentous growth by GPH1 on SD plates appeared to be indistinct (Figure 1D). Overall, these results suggest that GPH1 is functionally related to CaCDC4 regarding the control morphogenesis and that GPH1 negatively modulates hyphal formation.
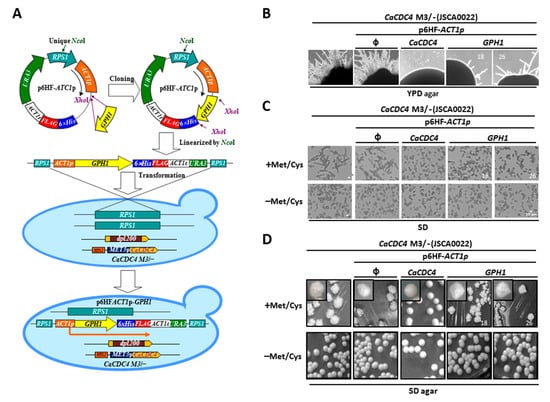
Figure 1.
The constitutive expression of GPH1 suppresses the filamentous mode of growth when the expression of CaCDC4 is repressed in C. albicans. (A) The diagram illustrates the strains used. The cells were (B) plated on YPD plate or were grown in the SD media (C) or plate (D) with or without 2.5 mM Met/Cys. The “ϕ” represents empty plasmid p6HF-ACT1p. Bars represent 10 μm. “18” and “26” represent different isolates of strains with p6HF-ACT1p-GPH1.
3.2. C. albicans Gph1 Protein Being Reduced in the Presence of CaCdc4 May Be the Result of Polyubiquitin-Proteasome-Dependent Degradation
Because GPH1 negatively modulates hyphal development (Figure 1B,C), we presumed that Gph1, like Sol1 [28] and our recently characterized Thr1 [51], is the target of CaCdc4 and is governed by ubiquitination for degradation. To assess the possible regulation of CaCdc4 and Gph1, cells of the same strains as in Figure 1 were grown in the minimum media with or without Met/Cys, and the proteins were extracted and subjected to Western blotting analysis. The protein level of Gph1, with or without expression of CaCDC4, showed no apparent difference (Figure 2A). However, the de-repressed CaCDC4 expression led to a reduction of the level of Gph1 protein with translation inhibitor cycloheximide (Figure 2B,C), suggesting that Gph1 protein is targeted for degradation by CaCdc4. The results indicate that the CaCdc4 negatively regulates the level of Gph1 protein and that the Gph1 adversely controls filamentation.
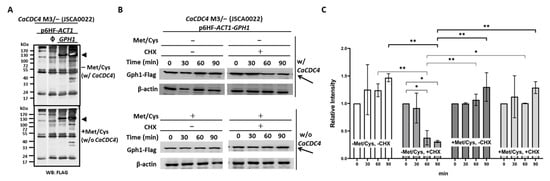
Figure 2.
Gph1 protein level decreases in the presence of CaCdc4 in C. albicans. (A) Cells of strains, as indicated, were grown in SD with or without 2.5 mM Met/Cys. Triangles indicate the migrated position of Gph1 protein. The “ϕ” represents empty plasmid p6HF-ACT1p. (B) Cells of strain CaCDC4 M3/– carrying p6HF-ACT1-GPH1 were grown in SD with or without 2.5 mM Met/Cys and in the presence or absence of cycloheximide (CHX) for the indicated time. Arrows indicate the Gph1 protein. Cells of all strains were collected and subjected to Western blotting analysis after growing in the indicated condition. SD donates synthetic defined medium. The anti-FLAG antibodies used as the Gph1 are tagged with FLAG. The β-actin used as a loading control was detected by the anti-β-actin antibody. (C) To quantify the protein levels, two independent experiments, including the one in (B), were used. The Gph1 levels were normalized to those of β-actin and expressed as the relative intensity. Statistical analyses were performed by one way ANOVA, with * p < 0.05 and ** p < 0.01 as indicated. Representative data from one of the three independent experiments are shown.
3.3. Cells Overexpressing or Lacking GPH1 Bear No Morphological Changes but Those without GPH1were Apt to Aggregate for a More Extended Period in Normal Growth Condition
To evaluate the role of GPH1 in morphogenesis, we made a strain Tet-on-GPH1 (Supplementary Figure S1A), where the expression of GPH1 is induced by doxycycline (Dox). The massive induction of GPH1 expression, both transcriptionally (Supplementary Figure S1B) and translationally (Supplementary Figure S1C), was confirmed. However, no morphological alteration could be observed, suggesting that cells overexpressing GPH1 were unable to interfere with cellular morphology in C. albicans (Supplementary Figure S1D). To further determine whether GPH1 has a role in morphogenesis, the CaSAT1-flipper method (73) was used to create the GPH1 homozygous null mutant (gph1Δ/gph1Δ). Southern blotting analyses were used to validate the mutants. As shown in Supplementary Figure S2B, the PvuII-digested genomic DNAs extracted from each of the strains could be detected with a probe specific to the GPH1 locus flanked with PvuII sites generating the expected sizes (Supplementary Figure S2A). Therefore, we proved that the created mutants were correct. By RT-PCR analyses, as expected, the GPH1 expression was only observed in the wild-type SC5314 (GPH1/GPH1), the GPH1 heterozygous null mutant (GPH1/gph1Δ), and the GPH1 complementation strain gph1Δ/gph1Δ+GPH1, but not in the homozygous null mutant (gph1Δ/gph1Δ) (Supplementary Figure S2C). The expression level of GPH1 was approximately two-fold less in GPH1/gph1Δ) and gph1Δ/gph1Δ+GPH1 than that of SC5314 (Supplementary Figure S2C). No apparent morphological alteration between the GPH1 null mutant and the wild-type in the normal growth condition could be found (Supplementary Figure S3A). However, after prolonged incubation, compared with cells of the wild-type, those of the GPH1 null mutants exhibited an increase in aggregation (Supplementary Figure S3B), suggesting that cells lacking GPH1 may alter the properties of the cell wall, consequently promoting the cell–cell interaction.
3.4. The GPH1 Null Mutant Shows No Growth Defect in Normal Growth Condition and Various Stressful Conditions
To determine if GPH1 can have a general effect on growth, cells of the gph1Δ/gph1Δ, together with the gph1 heterozygous null mutant (GPH1/gph1Δ), the GPH1 complement strain gph1Δ/gph1Δ+GPH1, and the wild-type SC5314 (GPH1/GPH1), were grown in either liquid or semi-solid YPD. Cells lacking GPH1 showed no growth defect both in YPD liquid medium (Supplementary Figure S4A,B) and YPD plate (Supplementary Figure S4C), suggesting that GPH1 bears no role in the maintenance of growth. GPH1 is involved in the synthesis of β-glucan and energy conversion in S. cerevisiae; hence, we presumed that this is common in fungi, including C. albicans. We set up the spotting assays with conditions including various cell wall damaging agents, different carbon sources, and distinct nutrient-depleted states at either 30 or 37 °C. However, with cells of the wild-type, those of the GPH1 null mutant showed no consequence in growth ability (Supplementary Figure S5), suggesting that either GPH1 plays no role or the presence of GPH1 redundant genes in the cell wall structure and energy conversion. As a result, we have sought alternative assays that may reveal the GPH1 function.
3.5. C. albicans Cells Lacking GPH1 Reduce the Ability to Form Germ Tube in Response to the Hypha-Inducing Condition
While the GPH1 homozygous null mutant of C. albicans did not directly contribute to the yeast-to-hypha transition, we sought to determine the influence of the hyphal induction condition on the mutant. Cells of the GPH1 homozygous null mutant (gph1Δ/gph1Δ), together with the GPH1 heterozygous null mutant (GPH1/gph1Δ), the GPH1 complement strain gph1Δ/gph1Δ+GPH1, and the wild-type SC5314 (GPH1/GPH1), were grown exponentially in YPD and transferred to RPMI 1640 supplemented with 10% fetal calf serum at 37 °C and were subjected to analysis of the length of the germ tube. The ability of the GPH1 homozygous null mutant to form a germ tube was reduced compared to that of the GCN4 homozygous null mutant, which is known to be impaired in filamentation under the hyphal induction condition [80] (Figure 3), suggesting that C. albicans GPH1 is indirectly involved in the yeast-to-hypha transition.
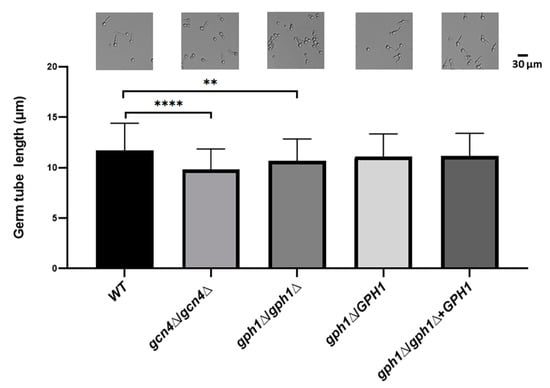
Figure 3.
GPH1 acts positively in germ tube formation in C. albicans. The exponentially cultured strains were subjected to 10% fetal calf serum for 1 h to induce germ tube formation. The length of the germ tube was determined from 20 randomly picked germ tube-cells. The representative monographs were from the differential interference contrast (DIC) (also known as Nomarski) microscopy. The GCN4 null mutant gcn4Δ/gcn4Δ, known to reduce germ tube formation under the hyphal induction condition, was used as a control. Statistical analyses were performed by one-way ANOVA, with ** p < 0.01 and **** p < 0.001 as indicated. Representative data from one of the three independent experiments are shown.
3.6. C. albicans Cells Lacking GPH1 Reduce Their Cell Surface Hydrophobicity (CSH)
C. albicans is capable of forming biofilms on abiotic or biotic surfaces. Catheters, dentures, prosthesis (abiotic), and mucosal cell surfaces (biotic) are the most common substrates [81]. Notably, hydrophobic attachment to abiotic and biotic surfaces is also critical in the initial step of biofilm formation, demonstrated by the fact that the adhesion of C. albicans to polymeric materials correlates with the cell surface hydrophobicity (CSH) phenotype [82]. Cells of the strains were grown exponentially in YPD and subjected to incubation with hydrophobic molecule n-hexadecane; the percentage of hydrophobicity (CSH) of the cells was determined as the percentage of cells not bound with the n-hexadecane. It appeared that the GPH1 homozygous null mutant decreased further in CSH compared to that of the GCN4 homozygous null mutant (Figure 4), which is known to decrease in biofilm formation [83], suggesting that C. albicans GPH1 is required to maintain CSH in C. alibicans.
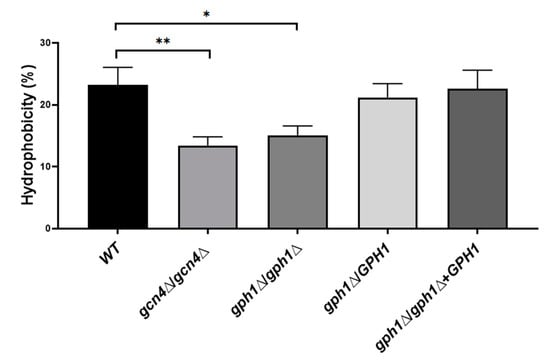
Figure 4.
GPH1 has a role in positively regulating cell surface hydrophobicity in C. albicans. The hydrophobicity was measured according to a microbial adhesion to hydrocarbons (MATH) test, as described in the Materials and Methods. All assays are representative of at least three independent experiments performed in triplicate. The original cells read as an absorbance value: A0; the cells left after subjecting to n-hexadecane read as an absorbance value: A1; the hydrophobicity (%) = [1 − (A1/A0)] × 100. The GCN4 null mutant gcn4Δ/gcn4Δ, known to reduce biofilm formation, was included in the assay. Statistical analyses were performed by one-way ANOVA, with * p < 0.05 and ** p < 0.01 as indicated.
3.7. C. albicans Cells Lacking GPH1 Increase Their Ability to Bind Fibronectin
Adhesion in C. albicans refers to the adherence of candida cells to host tissues. It is a phenomenon employing several adhesion proteins (called adhesins, flocculins, or agglutinins) expressed on morphologically changing cell surfaces. Adhesins are agglutinin-like sequences (ALS) that are members of a family of glycosylphosphatidylinositol (GPI)-linked cell surface glycoproteins, capable of binding several extracellular matrix proteins (ECM) of mammalian cells, such as fibronectin (FN), lamin, fibrinogen, and collagen type I and IV [84,85,86,87,88]. To assess the ability of C. albicans cells associating with FN, cells of the strains were incubated with FN for 1 h, before washing, harvesting, boiling in the presence of loading dye, and subjecting to Western blotting analysis with a specific anti-FN antibody. It appeared that cells lacking GPH1 increase the ability to bind FN compared with the wild-type strain, suggesting that C. albicans GPH1 suppresses the binding with FN (Figure 5).
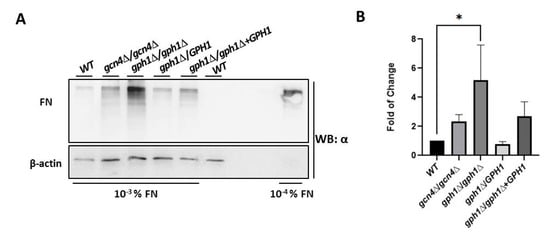
Figure 5.
GPH1 negatively regulates binding with fibronectin (FN) in C. albicans. Western blots for FN after pre-treatment of C. albicans cells with 10−3% fibronectin for 1 h, as described in the Materials and Methods. The GCN4 null mutant gcn4Δ/gcn4Δ, known to reduce biofilm formation, was included in the assay. The fibronectin was detected by the antibody specific to fibronectin. The 10−4% fibronectin was used as a control. The β-actin was used as a loading control and was detected by an anti-β-actin antibody. (A) The represented Western blot. (B) The fold of change in association with fibronectin in comparison with the wild-type strain SC5314 (WT). Statistical analyses were performed by one-way ANOVA, with * p < 0.05 as indicated.
3.8. C. albicans Cells Lacking GPH1 Improve Adhesion Ability but Remain Unchanged in Biofilm Formation
Because CaCDC4 negatively regulates biofilm formation, we attempted to ascertain whether GPH1 plays a similar role. Cells (5 × 106 cells in 100 μL) of the strains were subjected to biofilm induction condition for 1 h at 37 °C in each well of the nonpyrogenic polystyrene flat-bottom 96-well microtiter plate to determine the ability in adhesion assessed by the XTT reduction assay. Cells (5 × 106 cells in 100 μL) of the strains were subjected to biofilm induction condition for 1 h at 37 °C in each well of the nonpyrogenic polystyrene flat-bottom 96-well microtiter plate to assess the ability in adhesion before the XTT reduction assay. As shown in Figure 6A, cells the GPH1 mull mutants, both the homozygote and heterozygote, and the GPH1 complement strain increased in adhesion to polystyrene as those of the GCN4 homozygous null mutant, compared with the wild-type, suggesting that GPH1 has a role in inhibiting adhesion to the polystyrene. Cells (2 × 105 cells in 200 μL) of the strains were subjected to biofilm induction condition for 48 h at 37 °C in each well of the nonpyrogenic polystyrene flat-bottom 96-well microtiter plate to assess the ability in biofilm formation before the XTT reduction assay. Unlike cells of the GCN4 homozygous null mutant exhibiting increased ability in biofilm formation, those of all GPH1-related strains showed similar ability in biofilm formation (Figure 6B), as compared with those of wild-type, suggesting that GPH1 does not affect biofilm formation.
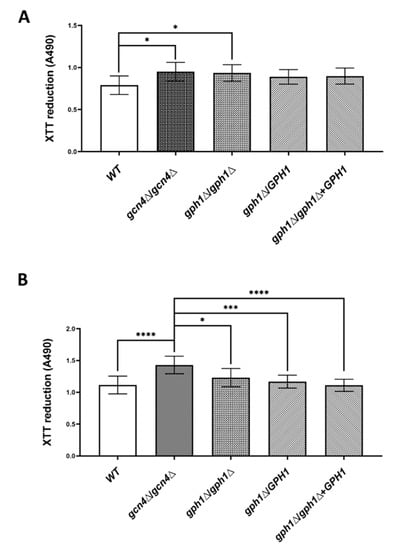
Figure 6.
GPH1 has a role in adhesion but not in biofilm formation in C. albicans. Cells of the strains were induced for adhesion in RPMI-1640 medium supplemented with L-glutamine and buffered with MOPS acid as described in the Materials and Methods and were subjected to in vitro XTT reduction assay for adhesion assay (A) or biofilm formation assay (B). Statistical analyses were performed by one-way ANOVA, with * p < 0.05, *** p < 0.005, **** p < 0.001 as indicated. The GCN4 null mutant gcn4Δ/gcn4Δ, known to reduce biofilm formation, was included in the assay.
4. Discussion
In this study, we characterized a CaCdc4-associated protein Gph1 that had been identified previously [48]. The functional interaction between the Gph1-encoded gene GPH1 and CaCDC4 was assessed. Because the F-box and WD40-repeat domains are present in CaCdc4, we presumed that CaCDC4 encodes a standard F-box protein of SCF ubiquitin ligase [39] named as SCFCaCdc4. We verified that those domains are indispensable for filamentation [40] to control its targets via SCFCaCdc4 ubiquitin ligase-dependent degradation. We revealed that the filamentous development due to the repressed CaCDC4 expression in C. albicans was moderately suppressed by the constitutive expression ofGPH1 (Figure 1B,C), which is a positive regulator of filamentous growth. The reason for this can be justified by the hindrance of Gph1 being entirely degraded by the SCFCaCdc4 ubiquitin ligase, resembling the degradation of Sol1 blocked in the CaCdc4-depleted C. albicans cells [28]. Indeed, we were able to observe the decreased level of Gph1 when the CaCDC4 was de-repressed with the translation inhibitor cycloheximide in C. albicans (Figure 2B,C). Hence, Gph1 denotes a typical SCFCaCdc4 target, which is negatively regulated by CaCdc4 in a ubiquitin-proteasome-dependent manner.
However, the fact that the GPH1 expression-induced strain showed no enhancement of filamentous development (Supplementary Figure S1) and the GPH1 homozygous null mutant could still form filaments under the hypha-induced conditions but with the reduced ability in germ tube formation (Figure 3), suggests that GPH1 serves no direct role to control yeast-to-hypha transition. The likely reason is that the property of the cell wall of the GPH1 null mutant has altered, which is evidenced by the improved ability to aggregate in cells lacking GPH1 (Supplementary Figure S3B); as a consequence, the ability in hyphal formation is affected. The change in cell wall property may alter the structural organization and cell wall layers, which affects the ability in flocculation. We tested if GPH1 has a role in calcium-dependent self-recognition mediated by adhesins or flocculins [45,89], which has been demonstrated to be required by the C. albicans CaCDC4 [40]. Cells lacking GPH1 did not appear to affect the ability in flocculation (Supplementary Figure S6). Hence, the altered cell wall property mediated by GPH1 has no role in the function of flocculins, including expression and activity, specifically those of Ca2+-dependency. The change in cell wall property in C. albicans cells lacking GPH1 was revealed in their decreased cell surface hydrophobicity (CSH) (Figure 4) and increased ability to bind fibronectin (Figure 5), both of which are related to cell adhesion. Of note, while increased CSH [90] and binding ability to fibronectin [91] of C. albicans is known to accompany enhanced biofilm formation, C. albicans lacking GPH1 appeared to affect biofilm formation oppositely with regards to CSH and binding to fibronection. Nevertheless, our data indeed demonstrated that the homozygous gph1 null mutant improves cell adhesion on polystyrene surfaces (Figure 6A). Interestingly, no improvement of biofilm formation was found in the homozygous gph1 null mutant (Figure 6B). Biofilms form in a sequential process involving adherence of yeast cells to the substrate, proliferation of the yeast cells, development of hyphal or pseudohyphal cells in the upper part of the biofilm, encircled in an accumulated extracellular polymer matrix consisting of proteins and polysaccharides that form a three-dimensional structure with water channels, and finally, dispersion of yeast cells from the biofilm to seed new sites [92,93]. The loss of GPH1 in C. albicans may improve only the initial step of adherence but not the final stage of maturation in biofilm formation.
How the loss of GPH1 in C. albicans cells influences the cell wall property and related functions may be far more complicated than predicted. Of note, the homozygous gph1 null mutant did not show accumulation of glycogen in C. albicans cells (unpublished data), which is inconsistent with the homozygous gph1 null mutant affecting the no growth defect by the cell wall damaging agents, diverse carbon sources, or the nutrient-depleted conditions (Supplementary Figure S5). We presume that the loss of GPH1 in C. albicans results in the compensation of GPH1 function related to the character of the cell wall architecture in which the function of many genes in a diverse aspect has interfered. As a consequence, the cell wall is reorganized such that CSH, the ability to bind fibronectin, and adhering to the abiotic surface are altered.
5. Conclusions
Our findings indicates that C. albicans CaCdc4 controls the polyubiquitin- proteasome-dependent degradation of Gph1. While GPH1 is a positive regulator of filamentation, GPH1 is negatively controlled by the CaCDC4, which suppresses filamentation. C. albicans cells lacking GPH1 affect several features associated with the cell wall structure. Hence, the alterations of these features impact on the adhesion of the early stage of biofilm formation and other related virulent attributes, but not on mature biofilm formation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8030233/s1, Figures S1–S6: Candida albicans GPH1 JoF supplmentary.
Author Contributions
W.-C.L. and J.-C.S. conceived and designed the study and supervised the project; H.-C.H. and C.-W.C. designed and materialized the study; H.-C.H., W.C.L. and P.-S.H. created the strains and conducted several phenotypic analyses; H.-C.H. established important phenotypic analyses and reagents; T.-L.T. engaged in the creation of the initial strains and early analyses; S.-H.W. provided critical materials; C.-W.C., S.-H.W. and T.-H.L. provided critical interpretation and consultation of data. All authors analyzed the data, discussed the results, and commented on the manuscript. J.-C.S. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the support for this research provided by grants from the Ministry of Science and Technology of Taiwan, the Republic of China to J.C.S. (NSC 101-2629-B-040-001-MY3; MOST 105-2320-B-040-027-MY3; MOST 108-2320-B-040-009; MOST 110-2320-B-040-024-MY3), and Chung Shan Medical University Research Grant to J.C.S. (CSMU-INT-109-10).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank A.J.P. Brown (University of Aberdeen, UK) for C. albicans strain SC5314, A. Mitchell (University of Georgia) for C. albicans strain BWP17, and M. Niimi (National Institute of Infectious Diseases, Tokyo, Japan) for p6HF-ACT1p.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Pande, K.; Chen, C.; Noble, S.M. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat. Genet. 2013, 45, 1088–1091. [Google Scholar] [CrossRef]
- Ruhnke, M. Epidemiology of Candida albicans infections and role of non-Candida-albicans yeasts. Curr. Drug. Targets 2006, 7, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Fidel, P.L., Jr. History and update on host defense against vaginal candidiasis. Am. J. Reprod. Immunol. 2007, 57, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A. Vulvovaginal Candida albicans infections: Pathogenesis, immunity and vaccine prospects. BJOG 2015, 122, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Front. Microbiol. 2015, 6, 1391. [Google Scholar] [CrossRef]
- Garcia-Cuesta, C.; Sarrion-Perez, M.G.; Bagan, J.V. Current treatment of oral candidiasis: A literature review. J. Clin. Exp. Dent. 2014, 6, e576–e582. [Google Scholar] [CrossRef]
- Fortun, J.; Martin-Davila, P.; Gomez-Garcia de la Pedrosa, E.; Pintado, V.; Cobo, J.; Fresco, G.; Meije, Y.; Ros, L.; Alvarez, M.E.; Luengo, J.; et al. Emerging trends in candidemia: A higher incidence but a similar outcome. J. Infect. 2012, 65, 64–70. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Benjamin, D.K., Jr.; Calandra, T.F.; Edwards, J.E., Jr.; Filler, S.G.; Fisher, J.F.; Kullberg, B.J.; Ostrosky-Zeichner, L.; et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 503–535. [Google Scholar] [CrossRef]
- Teoh, F.; Pavelka, N. How Chemotherapy Increases the Risk of Systemic Candidiasis in Cancer Patients: Current Paradigm and Future Directions. Pathogens 2016, 5, 6. [Google Scholar] [CrossRef]
- Sudbery, P. Morphogenesis of a human fungal pathogen requires septin phosphorylation. Dev. Cell 2007, 13, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Sudbery, P.; Gow, N.; Berman, J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004, 12, 317–324. [Google Scholar] [CrossRef]
- Whiteway, M.; Bachewich, C. Morphogenesis in Candida albicans. Annu Rev. Microbiol. 2007, 61, 529–553. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, C.; Liu, H. Candida albicans hyphal initiation and elongation. Trends Microbiol. 2014, 22, 707–714. [Google Scholar] [CrossRef]
- Brand, A. Hyphal growth in human fungal pathogens and its role in virulence. Int. J. Microbiol. 2012, 2012, 517529. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Garcia, F.; Sanchez, M.; Nombela, C.; Pla, J. Virulence genes in the pathogenic yeast Candida albicans. FEMS Microbiol. Rev. 2001, 25, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.; van de Veerdonk, F.L.; Brown, A.J.; Netea, M.G. Candida albicans morphogenesis and host defence: Discriminating invasion from colonization. Nat. Rev. Microbiol. 2012, 10, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Rizzetto, L.; Weil, T.; Cavalieri, D. Systems Level Dissection of Candida Recognition by Dectins: A Matter of Fungal Morphology and Site of Infection. Pathogens 2015, 4, 639–661. [Google Scholar] [CrossRef]
- Ene, I.V.; Bennett, R.J. The cryptic sexual strategies of human fungal pathogens. Nat. Rev. Microbiol. 2014, 12, 239–251. [Google Scholar] [CrossRef]
- Zhang, N.; Magee, B.B.; Magee, P.T.; Holland, B.R.; Rodrigues, E.; Holmes, A.R.; Cannon, R.D.; Schmid, J. Selective Advantages of a Parasexual Cycle for the Yeast Candida albicans. Genetics 2015, 200, 1117–1132. [Google Scholar] [CrossRef]
- Hickman, M.A.; Zeng, G.; Forche, A.; Hirakawa, M.P.; Abbey, D.; Harrison, B.D.; Wang, Y.M.; Su, C.H.; Bennett, R.J.; Wang, Y.; et al. The ‘obligate diploid’ Candida albicans forms mating-competent haploids. Nature 2013, 494, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.J. The parasexual lifestyle of Candida albicans. Curr. Opin. Microbiol. 2015, 28, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.; Sudbery, P.E. Candida albicans: A molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 2002, 3, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Van Dijck, P.; Datta, A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol. Mol. Biol. Rev. 2007, 71, 348–376. [Google Scholar] [CrossRef] [PubMed]
- Martchenko, M.; Levitin, A.; Whiteway, M. Transcriptional activation domains of the Candida albicans Gcn4p and Gal4p homologs. Eukaryot. Cell 2007, 6, 291–301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berman, J. Morphogenesis and cell cycle progression in Candida albicans. Curr. Opin. Microbiol. 2006, 9, 595–601. [Google Scholar] [CrossRef]
- Perez-Martin, J.; Bardetti, P.; Castanheira, S.; de la Torre, A.; Tenorio-Gomez, M. Virulence-specific cell cycle and morphogenesis connections in pathogenic fungi. Semin. Cell Dev. Biol. 2016, 57, 93–99. [Google Scholar] [CrossRef]
- Atir-Lande, A.; Gildor, T.; Kornitzer, D. Role for the SCFCDC4 Ubiquitin Ligase in Candida albicans Morphogenesis. Mol. Biol. Cell 2005, 16, 2772–2785. [Google Scholar] [CrossRef]
- Bensen, E.S.; Clemente-Blanco, A.; Finley, K.R.; Correa-Bordes, J.; Berman, J. The Mitotic Cyclins Clb2p and Clb4p Affect Morphogenesis in Candida albicans. Mol. Biol. Cell 2005, 16, 3387–3400. [Google Scholar] [CrossRef]
- Bensen, E.S.; Filler, S.G.; Berman, J. A forkhead transcription factor is important for true hyphal as well as yeast morphogenesis in Candida albicans. Eukaryot. Cell 2002, 1, 787–798. [Google Scholar] [CrossRef]
- Butler, D.K.; All, O.; Goffena, J.; Loveless, T.; Wilson, T.; Toenjes, K.A. The GRR1 gene of Candida albicans is involved in the negative control of pseudohyphal morphogenesis. Fungal Genet. Biol. 2006, 43, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Wang, Y.M.; Zheng, X.D.; Shi, Q.M.; Zhang, T.T.; Bai, C.; Li, D.; Sang, J.L.; Wang, Y. The F-box protein Grr1 regulates the stability of Ccn1, Cln3 and Hof1 and cell morphogenesis in Candida albicans. Mol. Microbiol. 2006, 62, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.C.; White, A.; Cheng, Y.C.; Rosamond, J. Identification and functional characterization of Candida albicans CDC4. J. Biomed. Sci. 2005, 12, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Bates, S. Candida albicans Cdc15 is essential for mitotic exit and cytokinesis. Sci. Rep. 2018, 8, 8899. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.; Tseng, T.L.; Wang, J.Y.; Shen, Y.T.; Lin, T.H.; Shieh, J.C. Candida albicans DBF4 gene inducibly duplicated by the mini-Ura-blaster is involved in hypha-suppression. Mutat. Res. 2015, 779, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.C.; Chang, T.W.; Wu, C.H.; Yang, S.Y.; Lee, T.L.; Li, W.C.; Chien, T.; Cheng, Y.C.; Shieh, J.C. Candida albicans Dbf4-dependent Cdc7 kinase plays a novel role in the inhibition of hyphal development. Sci. Rep. 2016, 6, 33716. [Google Scholar] [CrossRef]
- Umeyama, T.; Kaneko, A.; Niimi, M.; Uehara, Y. Repression of CDC28 reduces the expression of the morphology-related transcription factors, Efg1p, Nrg1p, Rbf1p, Rim101p, Fkh2p and Tec1p and induces cell elongation in Candida albicans. Yeast 2006, 23, 537–552. [Google Scholar] [CrossRef]
- Agam, G.; Shamir, A.; Shaltiel, G.; Greenberg, M.L. Myo-inositol-1-phosphate (MIP) synthase: A possible new target for antibipolar drugs. Bipolar Disord. 2002, 4 (Suppl. 1), 15–20. [Google Scholar] [CrossRef]
- Hochstrasser, M. Protein degradation or regulation: Ub the judge. Cell 1996, 84, 813–815. [Google Scholar] [CrossRef]
- Chin, C.; Lai, W.C.; Lee, T.L.; Tseng, T.L.; Shieh, J.C. Dissection of the Candida albicans Cdc4 protein reveals the involvement of domains in morphogenesis and cell flocculation. J. Biomed. Sci. 2013, 20, 97. [Google Scholar] [CrossRef]
- Galan-Ladero, M.A.; Blanco-Blanco, M.T.; Hurtado, C.; Perez-Giraldo, C.; Blanco, M.T.; Gomez-Garcia, A.C. Determination of biofilm production by Candida tropicalis isolated from hospitalized patients and its relation to cellular surface hydrophobicity, plastic adherence and filamentation ability. Yeast 2013, 30, 331–339. [Google Scholar] [PubMed]
- Ramage, G.; VandeWalle, K.; Lopez-Ribot, J.L.; Wickes, B.L. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol. Lett. 2002, 214, 95–100. [Google Scholar] [PubMed]
- Ryan, O.; Shapiro, R.S.; Kurat, C.F.; Mayhew, D.; Baryshnikova, A.; Chin, B.; Lin, Z.Y.; Cox, M.J.; Vizeacoumar, F.; Cheung, D.; et al. Global gene deletion analysis exploring yeast filamentous growth. Science 2012, 337, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, N.L.; Zhang, A.Q.; Nobile, C.J.; Johnson, A.D.; Ribbeck, K. Mucins suppress virulence traits of Candida albicans. MBio 2014, 5, e01911-14. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Klis, F.M. Flocculation, adhesion and biofilm formation in yeasts. Mol. Microbiol. 2006, 60, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, K.J.; Reynolds, T.B.; Fink, G.R. Origins of variation in the fungal cell surface. Nat. Rev. Microbiol. 2004, 2, 533–540. [Google Scholar] [CrossRef]
- Tseng, T.L.; Lai, W.C.; Lee, T.L.; Hsu, W.H.; Sun, Y.W.; Li, W.C.; Cheng, C.W.; Shieh, J.C. A role of Candida albicans CDC4 in the negative regulation of biofilm formation. Can. J. Microbiol. 2015, 61, 247–255. [Google Scholar] [CrossRef]
- Tseng, T.L.; Lai, W.C.; Jian, T.; Li, C.; Sun, H.F.; Way, T.D.; Shieh, J.C. Affinity purification of Candida albicans CaCdc4-associated proteins reveals the presence of novel proteins involved in morphogenesis. Biochem. Biophys. Res. Commun. 2010, 395, 152–157. [Google Scholar] [CrossRef]
- Ramos, C.; Calderon, I.L. Biochemical evidence that the Saccharomyces cerevisiae THR4 gene encodes threonine synthetase. FEBS Lett. 1994, 351, 357–359. [Google Scholar] [CrossRef]
- Schultes, N.P.; Ellington, A.D.; Cherry, J.M.; Szostak, J.W. Saccharomyces cerevisiae homoserine kinase is homologous to prokaryotic homoserine kinases. Gene 1990, 96, 177–180. [Google Scholar] [CrossRef]
- Lee, Y.T.; Fang, Y.Y.; Sun, Y.W.; Hsu, H.C.; Weng, S.M.; Tseng, T.L.; Lin, T.H.; Shieh, J.C. THR1 mediates GCN4 and CDC4 to link morphogenesis with nutrient sensing and the stress response in Candida albicans. Int. J. Mol. Med. 2018, 42, 3193–3208. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.K.; Tugendreich, S.; Fletterick, R.J. Molecular analysis of GPH1, the gene encoding glycogen phosphorylase in Saccharomyces cerevisiae. Mol. Cell Biol. 1989, 9, 1659–1666. [Google Scholar] [PubMed]
- Daran, J.M.; Dallies, N.; Thines-Sempoux, D.; Paquet, V.; Francois, J. Genetic and biochemical characterization of the UGP1 gene encoding the UDP-glucose pyrophosphorylase from Saccharomyces cerevisiae. Eur. J. Biochem. 1995, 233, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Francois, J.; Parrou, J.L. Reserve carbohydrates metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2001, 25, 125–145. [Google Scholar]
- Fuhring, J.I.; Cramer, J.T.; Schneider, J.; Baruch, P.; Gerardy-Schahn, R.; Fedorov, R. A quaternary mechanism enables the complex biological functions of octameric human UDP-glucose pyrophosphorylase, a key enzyme in cell metabolism. Sci. Rep. 2015, 5, 9618. [Google Scholar] [CrossRef]
- Daran, J.M.; Bell, W.; Francois, J. Physiological and morphological effects of genetic alterations leading to a reduced synthesis of UDP-glucose in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1997, 153, 89–96. [Google Scholar]
- Aimanianda, V.; Simenel, C.; Garnaud, C.; Clavaud, C.; Tada, R.; Barbin, L.; Mouyna, I.; Heddergott, C.; Popolo, L.; Ohya, Y.; et al. The Dual Activity Responsible for the Elongation and Branching of beta-(1,3)-Glucan in the Fungal Cell Wall. MBio 2017, 8, e00619-17. [Google Scholar] [CrossRef]
- Urban, C.; Sohn, K.; Lottspeich, F.; Brunner, H.; Rupp, S. Identification of cell surface determinants in Candida albicans reveals Tsa1p, a protein differentially localized in the cell. FEBS Lett. 2003, 544, 228–235. [Google Scholar] [CrossRef]
- Copping, V.M.; Barelle, C.J.; Hube, B.; Gow, N.A.; Brown, A.J.; Odds, F.C. Exposure of Candida albicans to antifungal agents affects expression of SAP2 and SAP9 secreted proteinase genes. J. Antimicrob. Chemother. 2005, 55, 645–654. [Google Scholar] [CrossRef]
- Nobile, C.J.; Fox, E.P.; Nett, J.E.; Sorrells, T.R.; Mitrovich, Q.M.; Hernday, A.D.; Tuch, B.B.; Andes, D.R.; Johnson, A.D. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 2012, 148, 126–138. [Google Scholar]
- Perez, J.C.; Kumamoto, C.A.; Johnson, A.D. Candida albicans commensalism and pathogenicity are intertwined traits directed by a tightly knit transcriptional regulatory circuit. PLoS Biol. 2013, 11, e1001510. [Google Scholar] [CrossRef]
- Lane, S.; Di Lena, P.; Tormanen, K.; Baldi, P.; Liu, H. Function and Regulation of Cph2 in Candida albicans. Eukaryot. Cell 2015, 14, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Rosenbach, A.; Dignard, D.; Pierce, J.V.; Whiteway, M.; Kumamoto, C.A. Adaptations of Candida albicans for growth in the mammalian intestinal tract. Eukaryot. Cell 2010, 9, 1075–1086. [Google Scholar] [CrossRef]
- Gillum, A.M.; Tsay, E.Y.; Kirsch, D.R. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 1984, 198, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.B.; Davis, D.; Mitchell, A.P. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 1999, 181, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, X.; Wang, Q.; Zhang, F.; Lou, Z.; Zhang, Q.; Zhou, D.X. Structural basis of a histone H3 lysine 4 demethylase required for stem elongation in rice. PLoS Genet. 2013, 9, e1003239. [Google Scholar] [CrossRef]
- Warren, G.; Sherratt, D. Incompatibility and transforming efficiency of ColE1 and related plasmids. Mol. Gen. Genet. 1978, 161, 39–47. [Google Scholar] [CrossRef]
- Dower, W.J.; Miller, J.F.; Ragsdale, C.W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988, 16, 6127–6145. [Google Scholar] [CrossRef]
- Gietz, R.D. Yeast transformation by the LiAc/SS carrier DNA/PEG method. Methods Mol. Biol. 2014, 1205, 1–12. [Google Scholar]
- Becker, D.M.; Lundblad, V. Introduction of DNA into yeast cells. Curr. Protoc. Mol. Biol. Chapter. 2001, 27, 13.7.1–13.7.10. [Google Scholar] [CrossRef]
- Kaneko, A.; Umeyama, T.; Hanaoka, N.; Monk, B.C.; Uehara, Y.; Niimi, M. Tandem affinity purification of the Candida albicans septin protein complex. Yeast 2004, 21, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Care, R.S.; Trevethick, J.; Binley, K.M.; Sudbery, P.E. The MET3 promoter: A new tool for Candida albicans molecular genetics. Mol. Microbiol. 1999, 34, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Reuss, O.; Vik, A.; Kolter, R.; Morschhauser, J. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 2004, 341, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.C.; Sun, H.F.; Lin, P.H.; Ho Lin, H.L.; Shieh, J.C. A new rapid and efficient system with dominant selection developed to inactivate and conditionally express genes in Candida albicans. Curr. Genet. 2016, 62, 213–235. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.C.; Cheng, Y.C.; Su, M.C.; Moore, M.; Choo, Y.; Klug, A. Tailor-made zinc-finger transcription factors activate FLO11 gene expression with phenotypic consequences in the yeast Saccharomyces cerevisiae. PLoS ONE 2007, 2, e746. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M. Isolation of pigmented and nonpigmented mutants of Serratia marcescens with reduced cell surface hydrophobicity. J. Bacteriol. 1984, 160, 480–482. [Google Scholar] [CrossRef]
- Silva-Dias, A.; Miranda, I.M.; Branco, J.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: Relationship among Candida spp. Front. Microbiol. 2015, 6, 205. [Google Scholar] [CrossRef]
- Vogel, M.; Köberle, M.; Schäffler, H.; Treiber, M.; Autenrieth, I.; Schumacher, U. Rifampicin induced virulence determinants increase Candida albicans biofilm formation [version 1; peer review: 3 approved with reservations]. F1000Research 2013, 2, 106. [Google Scholar] [CrossRef]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L., Jr.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- Gildor, T.; Shemer, R.; Atir-Lande, A.; Kornitzer, D. Coevolution of cyclin Pcl5 and its substrate Gcn4. Eukaryot Cell 2005, 4, 310–318. [Google Scholar] [CrossRef][Green Version]
- Fanning, S.; Mitchell, A.P. Fungal biofilms. PLoS Pathog. 2012, 8, e1002585. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, Z.; Xu, J. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology 2003, 149, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sanchez, S.; Aubert, S.; Iraqui, I.; Janbon, G.; Ghigo, J.M.; d’Enfert, C. Candida albicans biofilms: A developmental state associated with specific and stable gene expression patterns. Eukaryot. Cell 2004, 3, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I. Combating Fungal Infections: Problems and Remedy; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Chaffin, W.L.; Lopez-Ribot, J.L.; Casanova, M.; Gozalbo, D.; Martinez, J.P. Cell wall and secreted proteins of Candida albicans: Identification, function, and expression. Microbiol. Mol. Biol. Rev. 1998, 62, 130–180. [Google Scholar] [CrossRef]
- Hostetter, M.K. Adhesins and ligands involved in the interaction of Candida spp. with epithelial and endothelial surfaces. Clin. Microbiol. Rev. 1994, 7, 29–42. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Klotz, S.A.; Smith, R.L. A fibronectin receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J. Infect. Dis. 1991, 163, 604–610. [Google Scholar] [CrossRef]
- Veelders, M.; Bruckner, S.; Ott, D.; Unverzagt, C.; Mosch, H.U.; Essen, L.O. Structural basis of flocculin-mediated social behavior in yeast. Proc. Natl. Acad. Sci. USA 2010, 107, 22511–22516. [Google Scholar] [CrossRef]
- Masuoka, J.; Hazen, K.C. Cell wall mannan and cell surface hydrophobicity in Candida albicans serotype A and B strains. Infect. Immun. 2004, 72, 6230–6236. [Google Scholar] [CrossRef]
- Nett, J.E.; Cabezas-Olcoz, J.; Marchillo, K.; Mosher, D.F.; Andes, D.R. Targeting Fibronectin To Disrupt In Vivo Candida albicans Biofilms. Antimicrob. Agents Chemother. 2016, 60, 3152–3155. [Google Scholar] [CrossRef]
- Dominic, R.M.; Shenoy, S.; Baliga, S. Candida biofilms in medical devices: Evolving trends. Kathmandu Univ. Med. J. (KUMJ) 2007, 5, 431–436. [Google Scholar]
- Finkel, J.S.; Mitchell, A.P. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 2011, 9, 109–118. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).