Antifungal Activity of Human Cathelicidin LL-37, a Membrane Disrupting Peptide, by Triggering Oxidative Stress and Cell Cycle Arrest in Candida auris
Abstract
1. Introduction
2. Materials and Methods
2.1. Candida Strains and Growth Conditions
2.2. Antifungal Susceptibility Profiling
2.3. Combination Studies
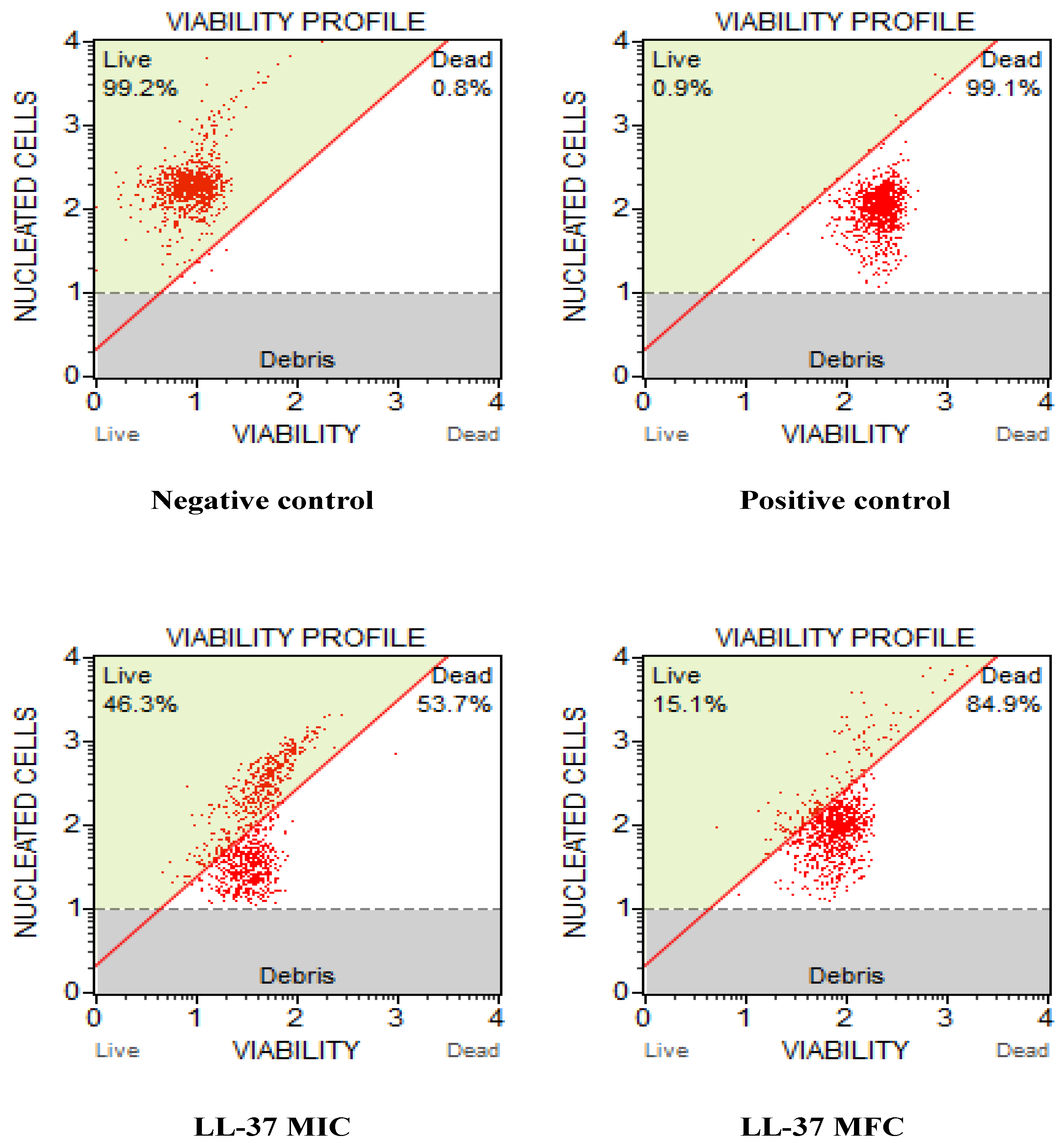
2.4. Cell Viability and Cell Count Assay
2.5. Time-Kill Kinetics
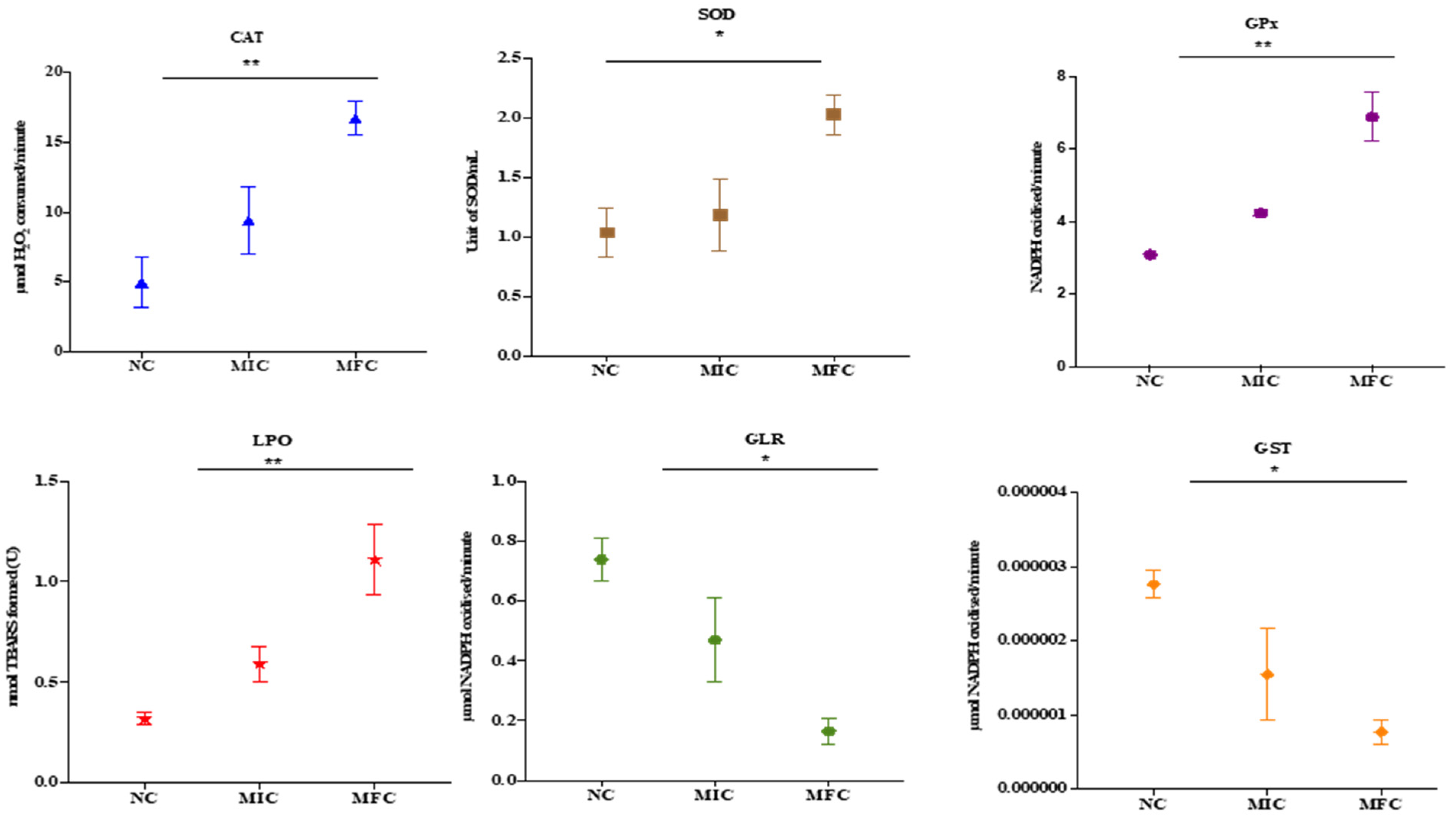
2.6. Effect of Cathelicidin LL-37 on Antioxidant Enzymes
2.7. Antioxidant Assays
2.8. Effect of Cathelicidin LL-37 on C. auris Cell Cycle
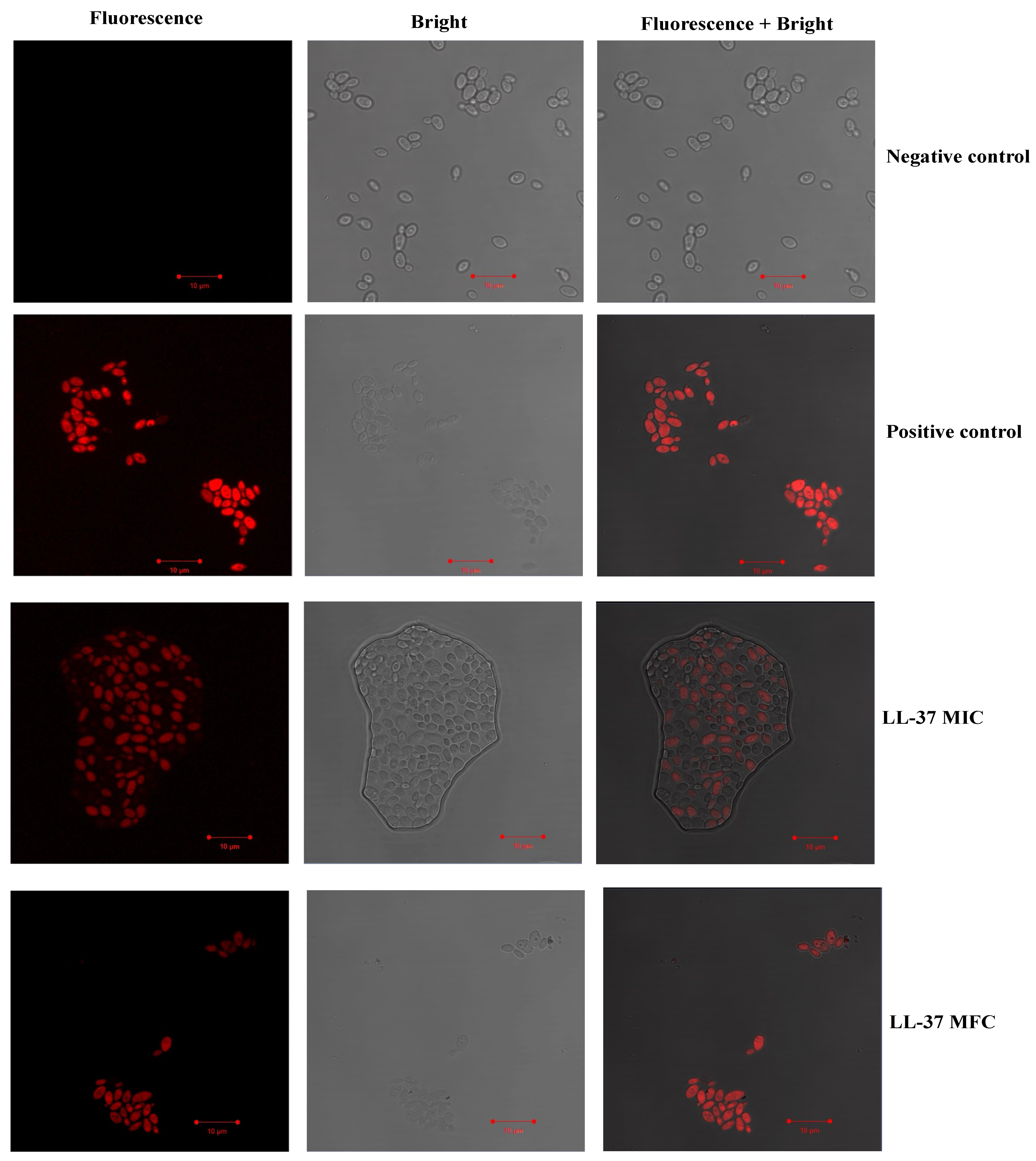
2.9. Effect of Cathelicidin LL-37 on C. auris Membrane Integrity
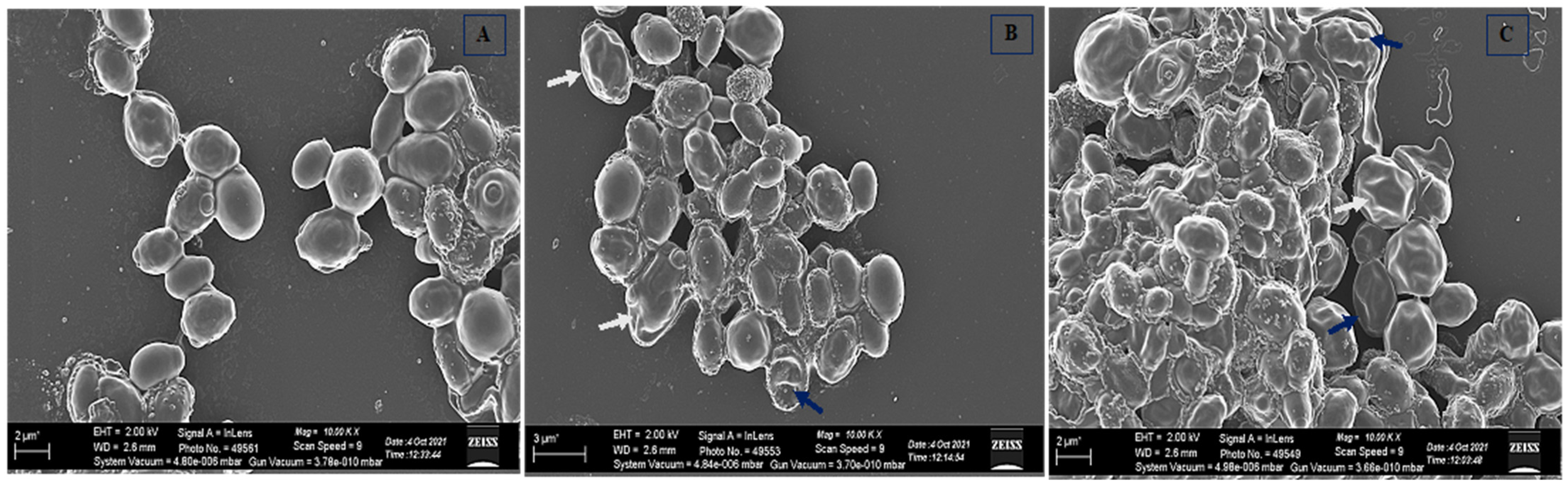
2.10. Scanning Electron Microscopy
2.11. Statistics
3. Results
3.1. Antifungal Potential of Cathelicidin LL-37 against C. auris Isolates
3.2. Antifungal Activity of Cathelicidin LL-37 in Combination with Standard Antifungal Drugs
3.3. Cathelicidin LL-37 Impedes the Growth and Viability of C. auris
3.4. Time-Kill Kinetics of Cathelicidin LL-37 in C. auris Cells
3.5. Cathelicidin LL-37 Modulates the Activity of Antioxidant Enzymes in C. auris
3.6. Cathelicidin LL-37 Arrest Cell Cycle in S Phase in C. auris
3.7. Effect of Cathelicidin LL-37 on C. auris Membrance Integrity
3.8. Effect of Cathelicidin LL-37 Cell Morphology of C. auris MRL6057
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spivak, E.S.; Hanson, K.E. Candida auris: An Emerging Fungal Pathogen. J. Clin. Microbiol. 2018, 56, e01588-17. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.; Quinn, M.; Tsay, S.; Poirot, E.; Chaturvedi, S.; Southwick, K.; Greenko, J.; Fernandez, R.; Kallen, A.; Vallabhaneni, S.; et al. Candida auris in Healthcare Facilities, New York, USA, 2013–2017. Emerg. Infect. Dis. 2018, 24, 1816. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Sood, P.; Rudramurthy, S.M.; Chen, S.; Kaur, H.; Capoor, M.; Chhina, D.; Rao, R.; Eshwara, V.K.; Xess, I.; et al. Incidence, Characteristics, and Outcome of ICU-Acquired Candidemia in India. Intensive Care Med. 2015, 41, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Calvo, B.; Melo, A.S.A.; Perozo-Mena, A.; Hernandez, M.; Francisco, E.C.; Hagen, F.; Meis, J.F.; Colombo, A.L. First report of Candida auris in America: Clinical and Microbiological Aspects of 18 Episodes of Candidemia. J. Infect. 2016, 73, 369–374. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Khan, I.; Shukla, S.; Kumar, P.; Rather, I.A.; Park, Y.-H.; Huh, Y.S.; Han, Y.K. Invasive Fungal Infections and Their Epidemiology: Measures in the Clinical Scenario. Biotechnol. Bioprocess Eng. 2019, 24, 436–444. [Google Scholar] [CrossRef]
- Campione, E.; Cosio, T.; Lanna, C.; Mazzilli, S.; Ventura, A.; Dika, E.; Gaziano, R.; Dattola, A.; Candi, E.; Bianchi, L. Predictive role of vitamin A serum concentration in psoriatic patients treated with IL-17 inhibitors to prevent skin and systemic fungal infections. J. Pharmacol. Sci. 2020, 144, 52–56. [Google Scholar] [CrossRef]
- Cosio, T.; Gaziano, R.; Zuccari, G.; Costanza, G.; Grelli, S.; Di Francesco, P.; Bianchi, L.; Campione, E. Retinoids in fungal infections: From bench to bedside. Pharmaceuticals 2021, 14, 962. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Perlman, D. Antimicrobial Agents and Chemotherapy. Nature 1964, 201, 456–457. [Google Scholar] [CrossRef][Green Version]
- Yu, G.; Baeder, D.Y.; Regoes, R.R.; Rolff, J. Predicting Drug Resistance Evolution: Insights from Antimicrobial Peptides and Antibiotics. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172687. [Google Scholar] [CrossRef] [PubMed]
- Gharehbolagh, S.A.; Izadi, A.; Talebi, M.; Sadeghi, F.; Zarrinnia, A.; Zarei, F.; Darmiani, K.; Borman, A.M.; Mahmoudi, S. New weapons to fight a new enemy: A systematic review of drug combinations against the drug-resistant fungus Candida auris. Mycoses 2021, 64, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Agerberth, B.; Gunne, H.; Odeberg, J.; Kogner, P.; Boman, H.G.; Gudmundsson, G.H. FALL-39, a Putative Human Peptide Antibiotic, Is Cysteine-Free and Expressed in Bone Marrow and Testis. Proc. Natl. Acad. Sci. USA 1995, 92, 195–199. [Google Scholar] [CrossRef]
- Johansson, J.; Gudmundsson, G.H.; Rottenberg, M.E.; Berndt, K.D.; Agerberth, B. Conformation-Dependent Antibacterial Activity of the Naturally Occurring Human Peptide LL-37. J. Biol. Chem. 1998, 273, 3718–3724. [Google Scholar] [CrossRef] [PubMed]
- de Yang, B.; Chen, Q.; Schmidt, A.P.; Anderson, G.M.; Wang, J.M.; Wooters, J.; Oppenheim, J.J.; Chertov, O. LL-37, the Neutrophil Granule- and Epithelial Cell-Derived Cathelicidin, Utilizes Formyl Peptide Receptor-like 1 (FPRL1) as a Receptor to Chemoattract Human Peripheral Blood Neutrophils, Monocytes, and T Cells. J. Exp. Med. 2000, 192, 1069–1074. [Google Scholar] [CrossRef]
- Rivas-Santiago, B.; Hernandez-Pando, R.; Carranza, C.; Juarez, E.; Contreras, J.L.; Aguilar-Leon, D.; Torres, M.; Sada, E. Expression of Cathelicidin LL-37 during Mycobacterium Tuberculosis Infection in Human Alveolar Macrophages, Monocytes, Neutrophils, and Epithelial Cells. Infect. Immun. 2008, 76, 935–941. [Google Scholar] [CrossRef]
- Doss, M.; White, M.R.; Tecle, T.; Hartshorn, K.L. Human Defensins and LL-37 in Mucosal Immunity. J. Leukoc. Biol. 2010, 87, 79–92. [Google Scholar] [CrossRef]
- Bucki, R.; Leszczyńska, K.; Namiot, A.; Sokołowski, W. Cathelicidin LL-37: A Multitask Antimicrobial Peptide. Arch. Immunol. Ther. Exp. 2010, 58, 15–25. [Google Scholar] [CrossRef]
- Moncla, B.J.; Pryke, K.; Rohan, L.C.; Graebing, P.W. Degradation of Naturally Occurring and Engineered Antimicrobial Peptides by Proteases. Adv. Biosci. Biotechnol. 2011, 2, 404. [Google Scholar] [CrossRef]
- Aoki, W.; Ueda, M. Characterization of Antimicrobial Peptides toward the Development of Novel Antibiotics. Pharmaceuticals 2013, 6, 1055–1081. [Google Scholar] [CrossRef]
- Ahmad, A.; Wani, M.Y.; Khan, A.; Manzoor, N.; Molepo, J. Synergistic Interactions of Eugenol-Tosylate and Its Congeners with Fluconazole against Candida albicans. PLoS ONE 2015, 10, e0145053. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiao, P.; Li, Y.; Gong, Y.; Chen, X.; Sun, S. The Synergistic Antifungal Effect and Potential Mechanism of D-Penicillamine Combined With Fluconazole Against Candida albicans. Front. Microbiol. 2019, 10, 2853. [Google Scholar] [CrossRef] [PubMed]
- Klepser, M.E.; Ernst, E.J.; Lewis, R.E.; Ernst, M.E.; Pfaller, M.A. Influence of Test Conditions on Antifungal Time-Kill Curve Results: Proposal for Standardized Methods. Antimicrob. Agents Chemother. 1998, 42, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, S.; Ahmad, A.; Khan, A.; Manzoor, N.; Khan, L.A. Effect of Diallyldisulphide on an Antioxidant Enzyme System in Candida Species. Can. J. Microbiol. 2010, 56, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Maras, B.; Angiolella, L.; Mignogna, G.; Vavala, E.; Macone, A.; Colone, M.; Pitari, G.; Stringaro, A.; Dupré, S.; Palamara, A.T. Glutathione Metabolism in Candida albicans Resistant Strains to Fluconazole and Micafungin. PLoS ONE 2014, 9, e98387. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Alam, M.B.; Quan, K.T.; Kwon, K.-R.; Ju, M.-K.; Choi, H.-J.; Lee, J.S.; Yoon, J.-I.; Majumder, R.; Rather, I.A.; et al. Antioxidant Efficacy and the Upregulation of Nrf2-Mediated HO-1 Expression by (+)-Lariciresinol, a Lignan Isolated from Rubia Philippinensis, through the Activation of P38. Sci. Rep. 2017, 7, 46035. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Rather, I.A.; Park, Y.-H. Partially Purified Exo-Polysaccharide from Lactobacillus Sakei Probio 65 with Antioxidant, α-Glucosidase and Tyrosinase Inhibitory Potential. J. Food Biochem. 2016, 40, 264–274. [Google Scholar] [CrossRef]
- Zhang, N.; Fan, Y.; Li, C.; Wang, Q.; Leksawasdi, N.; Li, F.; Wang, S. Cell Permeability and Nuclear DNA Staining by Propidium Iodide in Basidiomycetous Yeasts. Appl. Microbiol. Biotechnol. 2018, 102, 4183–4191. [Google Scholar] [CrossRef]
- Suchodolski, J.; Feder-Kubis, J.; Krasowska, A. Antifungal Activity of Ionic Liquids Based on (−)-Menthol: A Mechanism Study. Microbiol. Res. 2017, 197, 56–64. [Google Scholar] [CrossRef]
- CDC. Candida auris: Information for Laboratorians and Health Professionals; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019.
- Wang, G.; Narayana, J.L.; Mishra, B.; Zhang, Y.; Wang, F.; Wang, C.; Zarena, D.; Lushnikova, T.; Wang, X. Design of antimicrobial peptides: Progress made with human cathelicidin LL-37. Adv. Exp. Med. Biol. 2019, 1117, 215–240. [Google Scholar] [CrossRef]
- Gronberg, A.; Mahlapuu, M.; Ståhle, M.; Whately-Smith, C.; Rollman, O. Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: A randomized, placebo-controlled clinical trial. Wound Repair Regen. 2014, 22, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Merelli, M.; Righi, E.; Diaz-Martin, A.; Rosello, E.M.; Luzzati, R.; Parra, A.; Trecarichi, E.M.; Sanguinetti, M.; Posteraro, B.; et al. Epidemiology, Species Distribution, Antifungal Susceptibility, and Outcome of Candidemia across Five Sites in Italy and Spain. J. Clin. Microbiol. 2013, 51, 4167–4172. [Google Scholar] [CrossRef] [PubMed]
- Leszczyńska, K.; Namiot, A.; Cruz, K.; Byfield, F.J.; Won, E.; Mendez, G.; Sokołowski, W.; Savage, P.B.; Bucki, R.; Janmey, P.A. Potential of Ceragenin CSA-13 and Its Mixture with Pluronic F-127 as Treatment of Topical Bacterial Infections. J. Appl. Microbiol. 2011, 110, 229–238. [Google Scholar] [CrossRef] [PubMed]
- García-Quintanilla, M.; Pulido, M.R.; Moreno-Martínez, P.; Martín-Peña, R.; López-Rojas, R.; Pachón, J.; McConnell, M.J. Activity of Host Antimicrobials against Multidrug-Resistant Acinetobacter Baumannii Acquiring Colistin Resistance through Loss of Lipopolysaccharide. Antimicrob. Agents Chemother. 2014, 58, 2972–2975. [Google Scholar] [CrossRef]
- Haisma, E.M.; de Breij, A.; Chan, H.; van Dissel, J.T.; Drijfhout, J.W.; Hiemstra, P.S.; el Ghalbzouri, A.; Nibbering, P.H. LL-37-Derived Peptides Eradicate Multidrug-Resistant Staphylococcus Aureus from Thermally Wounded Human Skin Equivalents. Antimicrob. Agents Chemother. 2014, 58, 4411–4419. [Google Scholar] [CrossRef]
- den Hertog, A.L.; van Marle, J.; van Veen, H.A.; Van’t Hof, W.; Bolscher, J.G.M.; Veerman, E.C.I.; Nieuw Amerongen, A.V. Candidacidal Effects of Two Antimicrobial Peptides: Histatin 5 Causes Small Membrane Defects, but LL-37 Causes Massive Disruption of the Cell Membrane. Biochem. J. 2005, 388, 689–695. [Google Scholar] [CrossRef]
- Tsai, P.W.; Cheng, Y.L.; Hsieh, W.P.; Lan, C.Y. Responses of Candida albicans to the Human Antimicrobial Peptide LL-37. J. Microbiol. 2014, 52, 581–589. [Google Scholar] [CrossRef]
- Scarsini, M.; Tomasinsig, L.; Arzese, A.; D’Este, F.; Oro, D.; Skerlavaj, B. Antifungal Activity of Cathelicidin Peptides against Planktonic and Biofilm Cultures of Candida Species Isolated from Vaginal Infections. Peptides 2015, 71, 211–221. [Google Scholar] [CrossRef]
- Spitzer, M.; Robbins, N.; Wright, G.D. Combinatorial Strategies for Combating Invasive Fungal Infections. Virulence 2017, 8. [Google Scholar] [CrossRef]
- Fakhim, H.; Chowdhary, A.; Prakash, A.; Vaezi, A.; Dannaoui, E.; Meis, J.F.; Badali, H. In Vitro Interactions of Echinocandins with Triazoles against Multidrug-Resistant Candida auris. Antimicrob. Agents Chemother. 2017, 61, e01056-17. [Google Scholar] [CrossRef]
- Jaggavarapu, S.; Burd, E.M.; Weiss, D.S. Micafungin and Amphotericin B Synergy against Candida auris. Lancet Microbe 2020, 1, e314–e315. [Google Scholar] [CrossRef]
- Zimmermann, G.R.; Lehár, J.; Keith, C.T. Multi-Target Therapeutics: When the Whole Is Greater than the Sum of the Parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Bondaryk, M.; Staniszewska, M.; Zielińska, P.; Urbańczyk-Lipkowska, Z. Natural Antimicrobial Peptides as Inspiration for Design of a New Generation Antifungal Compounds. J. Fungi 2017, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, D.M.; Desbois, A.P.; Coote, P.J. Enhanced Efficacy of Synergistic Combinations of Antimicrobial Peptides with Caspofungin versus Candida albicans in Insect and Murine Models of Systemic Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1055–1062. [Google Scholar] [CrossRef]
- Mwangi, J.; Hao, X.; Lai, R.; Zhang, Z. Antimicrobial peptides: New hope in the war against multidrug resistance. Zool. Res. 2019, 40, 488. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chi, J.; Yan, Y.; Luo, R.; Feng, Z.; Zheng, Y.; Xian, D.; Li, X.; Quan, G.; Liu, D.; et al. Membrane-disruptive peptides/peptidomimetics-based therapeutics: Promising systems to combat bacteria and cancer in the drug-resistant era. Acta Pharm. Sin. B 2021, 11, 2609–2644. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Induction of oxidative stress as a possible mechanism of the antifungal action of three phenylpropanoids. FEMS Yeast Res. 2011, 11, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, A.; Khan, L.A.; Padoa, C.J.; van Vuuren, S.; Manzoor, N. Effect of two monoterpene phenols on antioxidant defence system in Candida albicans. Microb. Pathog. 2015, 20, 50–56. [Google Scholar] [CrossRef]
- Kaloriti, D.; Jacobsen, M.; Yin, Z.; Patterson, M.; Tillmann, A.; Smith, D.A.; Cook, E.; You, T.; Grimm, M.J.; Bohovych, I.; et al. Mechanisms underlying the exquisite sensitivity of Candida albicans to combinatorial cationic and oxidative stress that enhances the potent fungicidal activity of phagocytes. Mbio 2014, 5, e01334-14. [Google Scholar] [CrossRef]
- Peterson, R.L.; Galaleldeen, A.; Villarreal, J.; Taylor, A.B.; Cabelli, D.E.; Hart, P.J.; Culotta, V.C. The phylogeny and active site design of eukaryotic Cu-only superoxide dismutases. J. Biol. Chem. 2016, 291, 20911–20923. [Google Scholar] [CrossRef]
- Dantas, A.D.S.; Day, A.; Ikeh, M.; Kos, I.; Achan, B.; Quinn, J. Oxidative stress responses in the human fungal pathogen, Candida albicans. Biomolecules 2015, 5, 142–165. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Biomarkers of lipid peroxidation in clinical material. Biochim. Biophys. Acta 2014, 1840, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Setiawati, S.; Nuryastuti, T.; Ngatidjan, N.; Mustofa, M.; Jumina, J.; Fitriastuti, D. In vitro antifungal activity of (1)-N-2-Methoxybenzyl-1,10-Phenanthrolinium bromide against Candida albicans and its effects on membrane Integrity. Mycobiology 2017, 45, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Oren, Z.; Shai, Y. Mode of action of linear amphipathic α-Helical antimicrobial peptides. Biopolymers 1998. [Google Scholar] [CrossRef]
- Epand, R.M.; Vogel, H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta-Biomembr. 1999, 1462, 11–28. [Google Scholar] [CrossRef]


| Study ID | Clinical ID |
|---|---|
| CAU-01 | MRL 3499 |
| CAU-02 | MRL3785 |
| CAU-03 | MRL4000 |
| CAU-04 | MRL2921 |
| CAU-05 | MRL5762 |
| CAU-06 | MRL5765 |
| CAU-07 | MRL6277 |
| CAU-08 | MRL6065 |
| CAU-09 | MRL6057 |
| CAU-10 | MRL6173 |
| C. auris | MIC/MFC (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cathelicidin LL-37 | Amphotericin B | Caspofungin | Fluconazole | |||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| CAU-01 | 50 | 100 | 0.5 (S) | 1.0 | 0.25 (S) | 0.5 | 16.0 (S) | FS |
| CAU-02 | 25 | 50 | 0.12 (S) | 0.5 | 0.25 (S) | 0.5 | 16.0 (S) | FS |
| CAU-03 | 100 | 200 | 2.0 ® | 4.0 | 0.25 (S) | 0.5 | 250.0 (R) | FS |
| CAU-04 | 50 | 100 | 2.0 (R) | 4.0 | 0.5 (S) | 1.0 | 250.0 (R) | FS |
| CAU-05 | 100 | 200 | 2.0 (R) | 4.0 | 0.25 (S) | 0.5 | 500.0 (R) | FS |
| CAU-06 | 50 | 100 | 2.0 (R) | 4.0 | 0.25 (S) | 0.5 | 500.0 (R) | FS |
| CAU-07 | 25 | 50 | 0.5 (S) | 1.0 | 0.25 (S) | 1.0 | 125.0 (R) | FS |
| CAU-08 | 100 | 200 | 1.0 (S) | 2.0 | 0.25 (S) | 0.5 | 125.0 (R) | FS |
| CAU-09 | 50 | 100 | 4.0 (R) | 8.0 | 2.0 (R) | 4.0 | 125.0 (R) | FS |
| CAU-10 | 50 | 100 | 0.25 (S) | 0.5 | 0.25 (S) | 0.5 | (R) | FS |
| Test Agent | Strains | MIC Alone (µg/mL) | MIC in Combination (µg/mL) | FICI | INT | ||
|---|---|---|---|---|---|---|---|
| MIC-A | LL-37-A | MIC-B | LL-37-B | ||||
| LL-37-FLZ | CAU-01 | 16 | 50 | 16 | 3.125 | 1.06 | IND |
| CAU-02 | 16 | 25 | 16 | 3.125 | 1.13 | IND | |
| CAU-03 | 250 | 100 | 63 | 12.5 | 0.38 | SYN | |
| CAU-04 | 250 | 50 | 63 | 12.5 | 0.50 | SYN | |
| CAU-05 | 500 | 100 | 63 | 12.5 | 0.25 | SYN | |
| CAU-06 | 500 | 50 | 63 | 12.5 | 0.38 | SYN | |
| CAU-07 | 125 | 25 | 63 | 12.5 | 0.63 | ADD | |
| CAU-08 | 125 | 100 | 32 | 6.25 | 0.32 | SYN | |
| CAU-09 | 125 | 50 | 32 | 6.25 | 0.38 | SYN | |
| CAU-10 | 32 | 50 | 8 | 1.56 | 0.27 | SYN | |
| LL-37-AmB | CAU-01 | 0.5 | 50 | 0.125 | 0.78 | 0.27 | SYN |
| CAU-02 | 0.12 | 25 | 0.031 | 0.195 | 0.26 | SYN | |
| CAU-03 | 2 | 100 | 0.25 | 1.56 | 0.14 | SYN | |
| CAU-04 | 2 | 50 | 0.5 | 3.125 | 0.31 | SYN | |
| CAU-05 | 2 | 100 | 0.25 | 1.56 | 0.14 | SYN | |
| CAU-06 | 2 | 50 | 0.5 | 3.125 | 0.31 | SYN | |
| CAU-07 | 0.5 | 25 | 0.062 | 0.39 | 0.14 | SYN | |
| CAU-08 | 1 | 100 | 0.25 | 1.56 | 0.27 | SYN | |
| CAU-09 | 4 | 50 | 0.5 | 3.16 | 0.20 | SYN | |
| CAU-10 | 0.25 | 50 | 0.031 | 0.20 | 0.13 | SYN | |
| LL-37-CAS | CAU-01 | 0.25 | 50 | 0.062 | 0.39 | 0.26 | SYN |
| CAU-02 | 0.25 | 25 | 0.062 | 0.39 | 0.26 | SYN | |
| CAU-03 | 0.25 | 100 | 0.031 | 0.195 | 0.13 | SYN | |
| CAU-04 | 0.5 | 50 | 0.062 | 0.39 | 0.13 | SYN | |
| CAU-05 | 0.25 | 100 | 0.031 | 0.195 | 0.13 | SYN | |
| CAU-06 | 0.25 | 50 | 0.031 | 0.195 | 0.13 | SYN | |
| CAU-07 | 0.25 | 25 | 0.031 | 0.195 | 0.13 | SYN | |
| CAU-08 | 0.25 | 100 | 0.062 | 0.39 | 0.25 | SYN | |
| CAU-09 | 2 | 50 | 0.5 | 3.125 | 0.13 | SYN | |
| CAU-10 | 0.25 | 50 | 0.031 | 0.195 | 0.13 | SYN | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rather, I.A.; Sabir, J.S.M.; Asseri, A.H.; Ali, S. Antifungal Activity of Human Cathelicidin LL-37, a Membrane Disrupting Peptide, by Triggering Oxidative Stress and Cell Cycle Arrest in Candida auris. J. Fungi 2022, 8, 204. https://doi.org/10.3390/jof8020204
Rather IA, Sabir JSM, Asseri AH, Ali S. Antifungal Activity of Human Cathelicidin LL-37, a Membrane Disrupting Peptide, by Triggering Oxidative Stress and Cell Cycle Arrest in Candida auris. Journal of Fungi. 2022; 8(2):204. https://doi.org/10.3390/jof8020204
Chicago/Turabian StyleRather, Irfan A., Jamal S. M. Sabir, Amer H. Asseri, and Sajad Ali. 2022. "Antifungal Activity of Human Cathelicidin LL-37, a Membrane Disrupting Peptide, by Triggering Oxidative Stress and Cell Cycle Arrest in Candida auris" Journal of Fungi 8, no. 2: 204. https://doi.org/10.3390/jof8020204
APA StyleRather, I. A., Sabir, J. S. M., Asseri, A. H., & Ali, S. (2022). Antifungal Activity of Human Cathelicidin LL-37, a Membrane Disrupting Peptide, by Triggering Oxidative Stress and Cell Cycle Arrest in Candida auris. Journal of Fungi, 8(2), 204. https://doi.org/10.3390/jof8020204







