Molecular Profiling Reveals Characteristic and Decisive Signatures in Patients after Allogeneic Stem Cell Transplantation Suffering from Invasive Pulmonary Aspergillosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Recruitment
2.2. Blood Sample Collection and Processing
2.3. Whole-Blood Transcriptome Profiling
2.3.1. RNA-seq Data Processing
2.3.2. qPCR-Based Validation
2.3.3. In Silico Investigation of the Gene Expression of Characteristic Immune-Relevant Candidates in Other Studies
2.4. Protein Quantification in Serum
2.5. Statistical Analysis
3. Results
3.1. LGALS2 and MMP1 Are Indicative for IPA in alloSCT Patients within the First Five Weeks after Disease Onset
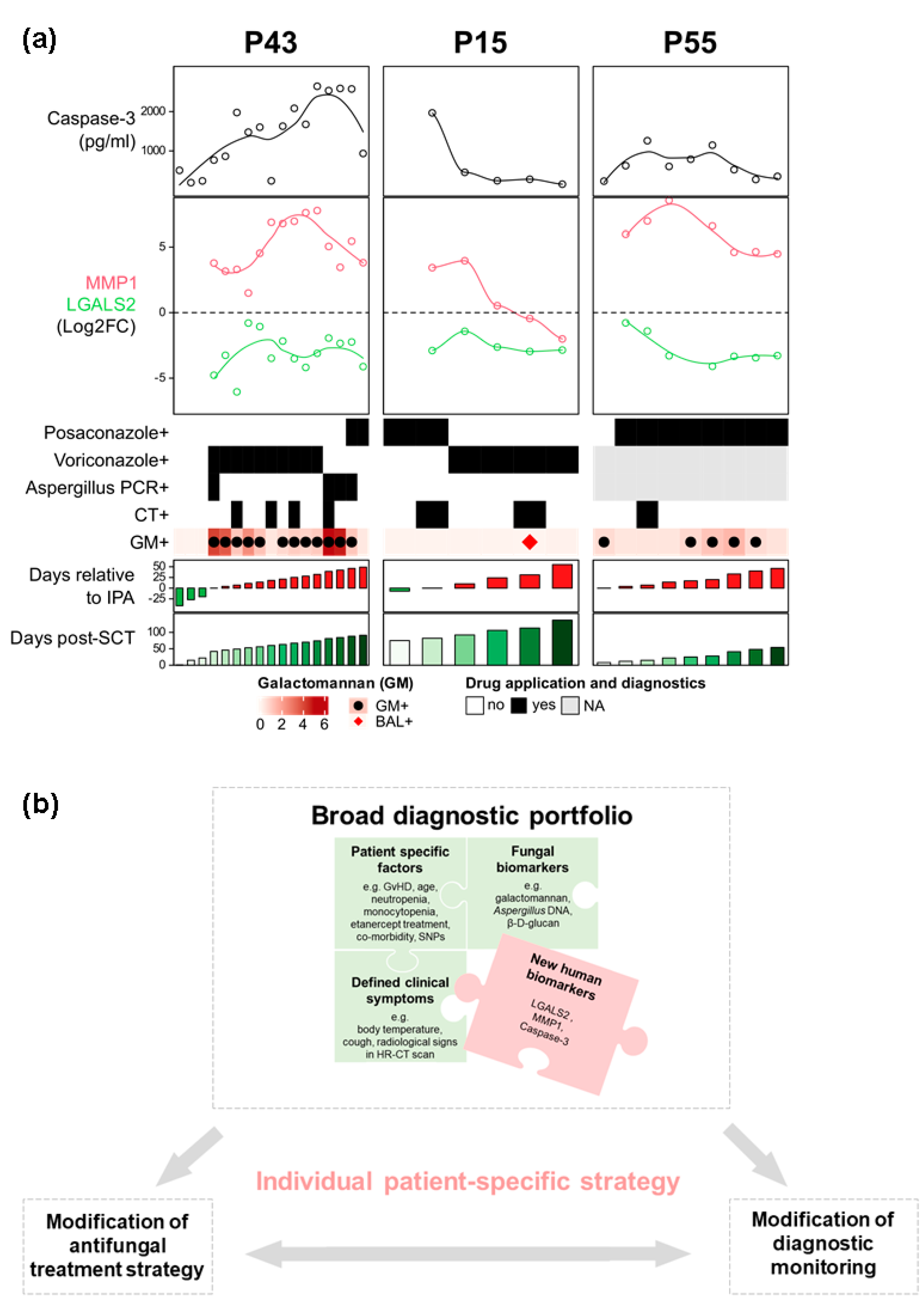
3.2. MMP1, IL-8, and Caspase-3 Serum Protein Levels Show Distinctive Patterns in Probable IPA Cases
3.3. Possible IPA Cases Revealed Higher IL-8 and Caspase-3 Protein Levels Compared with Control Patients
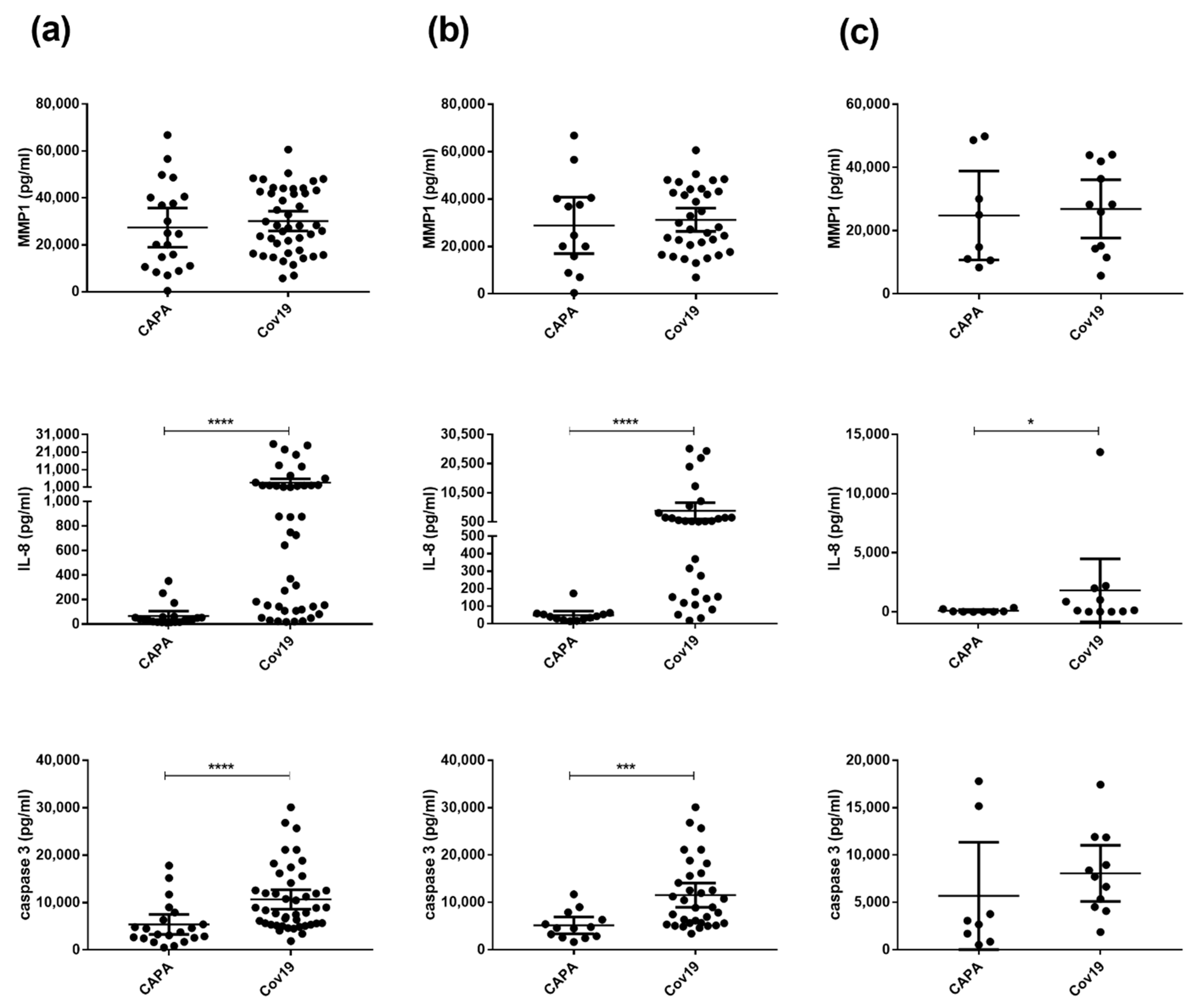
3.4. Patients with CAPA Have Significantly Lower IL-8 and Caspase-3 Serum Levels Compared with COVID-19 Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Ruhnke, M.; Behre, G.; Buchheidt, D.; Christopeit, M.; Hamprecht, A.; Heinz, W.; Heussel, C.P.; Horger, M.; Kurzai, O.; Karthaus, M.; et al. Diagnosis of invasive fungal diseases in haematology and oncology: 2018 update of the recommendations of the infectious diseases working party of the German society for hematology and medical oncology (AGIHO). Mycoses 2018, 61, 796–813. [Google Scholar] [CrossRef] [PubMed]
- Krel, M.; Petraitis, V.; Petraitiene, R.; Jain, M.R.; Zhao, Y.; Li, H.; Walsh, T.J.; Perlin, D.S. Host biomarkers of invasive pulmonary aspergillosis to monitor therapeutic response. Antimicrob. Agents Chemother. 2014, 58, 3373–3378. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Upton, A.; Kirby, K.A.; Marr, K.A. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: Biological risk factors for infection according to time after transplantation. Clin. Infect. Dis. 2008, 47, 1041–1050. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- White, P.L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Armstrong-James, D.; Youngs, J.; Bicanic, T.; Abdolrasouli, A.; Denning, D.W.; Johnson, E.; Mehra, V.; Pagliuca, T.; Patel, B.; Rhodes, J.; et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis. Eur. Respir. J. 2020, 56, 2002554. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Florl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Seelbinder, B.; Wolf, T.; Priebe, S.; McNamara, S.; Gerber, S.; Guthke, R.; Linde, J. GEO2RNAseq: An easy-to-use R pipeline for complete pre-processing of RNA-seq data. bioRxiv 2019, 771063. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Cortez, K.J.; Lyman, C.A.; Kottilil, S.; Kim, H.S.; Roilides, E.; Yang, J.; Fullmer, B.; Lempicki, R.; Walsh, T.J. Functional genomics of innate host defense molecules in normal human monocytes in response to Aspergillus fumigatus. Infect. Immun. 2006, 74, 2353–2365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets--update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Netea, M.G.; Teerenstra, S.; Earnest, A.; Vonk, A.G.; Schlamm, H.T.; Herbrecht, R.; Troke, P.F.; Kullberg, B.J. Early proinflammatory cytokines and C-reactive protein trends as predictors of outcome in invasive aspergillosis. J. Infect. Dis. 2010, 202, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, S.M.; Lagrou, K.; Rodrigues, C.S.; Campos, C.F.; Bernal-Martinez, L.; Rodrigues, F.; Silvestre, R.; Alcazar-Fuoli, L.; Maertens, J.A.; Cunha, C.; et al. Evaluation of bronchoalveolar lavage fluid cytokines as biomarkers for invasive pulmonary aspergillosis in at-risk patients. Front. Microbiol. 2017, 8, 2362. [Google Scholar] [CrossRef]
- Heldt, S.; Prattes, J.; Eigl, S.; Spiess, B.; Flick, H.; Rabensteiner, J.; Johnson, G.; Pruller, F.; Wolfler, A.; Niedrist, T.; et al. Diagnosis of invasive aspergillosis in hematological malignancy patients: Performance of cytokines, Asp LFD, and Aspergillus PCR in same day blood and bronchoalveolar lavage samples. J. Infect. 2018, 77, 235–241. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Lewis, R.E.; Leventakos, K.; Kontoyiannis, D.P. Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood 2009, 114, 5393–5399. [Google Scholar] [CrossRef]
- Gibson, P.G.; Wark, P.A.; Simpson, J.L.; Meldrum, C.; Meldrum, S.; Saltos, N.; Boyle, M. Induced sputum IL-8 gene expression, neutrophil influx and MMP-9 in allergic bronchopulmonary aspergillosis. Eur. Respir. J. 2003, 21, 582–588. [Google Scholar] [CrossRef]
- Stanzani, M.; Orciuolo, E.; Lewis, R.; Kontoyiannis, D.P.; Martins, S.L.; St John, L.S.; Komanduri, K.V. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood 2005, 105, 2258–2265. [Google Scholar] [CrossRef]
- Weiss, L.A.; Lester, L.A.; Gern, J.E.; Wolf, R.L.; Parry, R.; Lemanske, R.F.; Solway, J.; Ober, C. Variation in ITGB3 is associated with asthma and sensitization to mold allergen in four populations. Am. J. Respir. Crit. Care Med. 2005, 172, 67–73. [Google Scholar] [CrossRef]
- Jenks, J.D.; Hoenigl, M. Treatment of Aspergillosis. J. Fungi 2018, 4, 98. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Peghin, M.; Cervera, C.; Gudiol, C.; Ruiz-Camps, I.; Moreno, A.; Royo-Cebrecos, C.; Rosello, E.; de la Bellacasa, J.P.; Ayats, J.; et al. Causes of death in a contemporary cohort of patients with invasive aspergillosis. PLoS ONE 2015, 10, e0120370. [Google Scholar] [CrossRef] [PubMed]
- Lass-Florl, C. Current challenges in the diagnosis of fungal infections. Methods Mol. Biol. 2017, 1508, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Meis, J.F.; Chowdhary, A.; Rhodes, J.L.; Fisher, M.C.; Verweij, P.E. Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Philos. Trans. R Soc. Lond. B Biol. Sci. 2016, 371, 20150460. [Google Scholar] [CrossRef] [PubMed]
- Nedel, W.L.; Kontoyiannis, D.P.; Pasqualotto, A.C. aspergillosis in patients treated with monoclonal antibodies. Rev. Iberoam. Micol. 2009, 26, 175–183. [Google Scholar] [CrossRef]
- Zoran, T.; Weber, M.; Springer, J.; White, P.L.; Bauer, J.; Schober, A.; Loffler, C.; Seelbinder, B.; Hunniger, K.; Kurzai, O.; et al. Treatment with etanercept and low monocyte concentration contribute to the risk of invasive aspergillosis in patients post allogeneic stem cell transplantation. Sci. Rep. 2019, 9, 17231. [Google Scholar] [CrossRef] [PubMed]
- Alkharabsheh, O.; Alsayed, A.; Morlote, D.M.; Mehta, A. Cerebral invasive aspergillosis in a case of chronic lymphocytic leukemia with Bruton tyrosine kinase inhibitor. Curr. Oncol. 2021, 28, 81. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, A.; Joosse, M.E.; de Boer, F.; Eefting, M.; Rijnders, B.J.A. Invasive fungal infections in patients treated with Bruton’s tyrosine kinase inhibitors. Neth. J. Med. 2020, 78, 294–296. [Google Scholar] [PubMed]
- Styczyński, J. Infections following CAR-T cells therapy: Current state-of-the-art review and recommendations. Acta Haematologica Polonica 2020, 51, 11–16. [Google Scholar] [CrossRef][Green Version]
- Wudhikarn, K.; Palomba, M.L.; Pennisi, M.; Garcia-Recio, M.; Flynn, J.R.; Devlin, S.M.; Afuye, A.; Silverberg, M.L.; Maloy, M.A.; Shah, G.L.; et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J. 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.O.; Leao-Cordeiro, J.A.B.; Paula, H.; Ataides, F.S.; Saddi, V.A.; Vilanova-Costa, C.; Silva, A. Association between polymorphisms in the genes encoding toll-like receptors and dectin-1 and susceptibility to invasive aspergillosis: A systematic review. Rev. Soc. Bras. Med. Trop. 2018, 51, 725–730. [Google Scholar] [CrossRef]
- Lupianez, C.B.; Villaescusa, M.T.; Carvalho, A.; Springer, J.; Lackner, M.; Sanchez-Maldonado, J.M.; Canet, L.M.; Cunha, C.; Segura-Catena, J.; Alcazar-Fuoli, L.; et al. Common genetic polymorphisms within NFkappaB-related genes and the risk of developing invasive aspergillosis. Front. Microbiol. 2016, 7, 1243. [Google Scholar] [CrossRef]
- Zhao, Y.; Nagasaki, Y.; Paderu, P.; Sugrue, M.W.; Leather, H.L.; Wingard, J.R.; Perlin, D.S. Applying host disease status biomarkers to therapeutic response monitoring in invasive aspergillosis patients. Med. Mycol. 2019, 57, 38–44. [Google Scholar] [CrossRef]
- Lu, M.; Zhan, X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018, 9, 77–102. [Google Scholar] [CrossRef]
- Ashkenazi-Hoffnung, L.; Oved, K.; Navon, R.; Friedman, T.; Boico, O.; Paz, M.; Kronenfeld, G.; Etshtein, L.; Cohen, A.; Gottlieb, T.M.; et al. A host-protein signature is superior to other biomarkers for differentiating between bacterial and viral disease in patients with respiratory infection and fever without source: A prospective observational study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1361–1371. [Google Scholar] [CrossRef]
- Lydon, E.C.; Henao, R.; Burke, T.W.; Aydin, M.; Nicholson, B.P.; Glickman, S.W.; Fowler, V.G.; Quackenbush, E.B.; Cairns, C.B.; Kingsmore, S.F.; et al. Validation of a host response test to distinguish bacterial and viral respiratory infection. EBioMedicine 2019, 48, 453–461. [Google Scholar] [CrossRef]
- Gomez, P.; Hackett, T.L.; Moore, M.M.; Knight, D.A.; Tebbutt, S.J. Functional genomics of human bronchial epithelial cells directly interacting with conidia of aspergillus fumigatus. BMC Genom. 2010, 11, 358. [Google Scholar] [CrossRef]
- Hohl, T.M. Immune responses to invasive aspergillosis: New understanding and therapeutic opportunities. Curr. Opin. Infect. Dis. 2017, 30, 364–371. [Google Scholar] [CrossRef]
- Brinchmann, M.F.; Patel, D.M.; Iversen, M.H. The role of galectins as modulators of metabolism and inflammation. Mediators Inflamm. 2018, 2018, 9186940. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Inoue, K.; Sato, H.; Iida, A.; Ohnishi, Y.; Sekine, A.; Sato, H.; Odashiro, K.; Nobuyoshi, M.; Hori, M.; et al. Functional variation in LGALS2 confers risk of myocardial infarction and regulates lymphotoxin-alpha secretion in vitro. Nature 2004, 429, 72–75. [Google Scholar] [CrossRef]
- Tomalka, J.; Hise, A.G. Inflammasomes in aspergillosis--it takes two to tango. Cell Host Microbe 2015, 17, 290–292. [Google Scholar] [CrossRef]
- Femenia, F.; Huet, D.; Lair-Fulleringer, S.; Wagner, M.C.; Sarfati, J.; Shingarova, L.; Guillot, J.; Boireau, P.; Chermette, R.; Berkova, N. Effects of conidia of various aspergillus species on apoptosis of human pneumocytes and bronchial epithelial cells. Mycopathologia 2009, 167, 249–262. [Google Scholar] [CrossRef]
- Gayathri, L.; Akbarsha, M.A.; Ruckmani, K. In vitro study on aspects of molecular mechanisms underlying invasive aspergillosis caused by gliotoxin and fumagillin, alone and in combination. Sci. Rep. 2020, 10, 14473. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, S.; Xia, H.; Shi, D.; Chen, Y.; Zheng, S.; Chen, Y.; Gao, H.; Guo, F.; Ji, Z.; et al. Cytokine signature associated with disease severity in COVID-19. Front. Immunol. 2021, 12, 3276. [Google Scholar] [CrossRef]
- Plassmeyer, M.; Alpan, O.; Corley, M.J.; Premeaux, T.A.; Lillard, K.; Coatney, P.; Vaziri, T.; Michalsky, S.; Pang, A.P.S.; Bukhari, Z.; et al. Caspases and therapeutic potential of caspase inhibitors in moderate-severe SARS CoV2 infection and long COVID. Allergy 2021, 77, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Volling, K.; Brakhage, A.A.; Saluz, H.P. Apoptosis inhibition of alveolar macrophages upon interaction with conidia of Aspergillus fumigatus. FEMS Microbiol. Lett. 2007, 275, 250–254. [Google Scholar] [CrossRef]
- Garlanda, C.; Hirsch, E.; Bozza, S.; Salustri, A.; De Acetis, M.; Nota, R.; Maccagno, A.; Riva, F.; Bottazzi, B.; Peri, G.; et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 2002, 420, 182–186. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Zhou, W.; Rui, Y.; He, B.; Shi, Y.; Su, X. Pentraxin 3 in bronchoalveolar lavage fluid and plasma in non-neutropenic patients with pulmonary aspergillosis. Clin. Microbiol. Infect. 2019, 25, 504–510. [Google Scholar] [CrossRef] [PubMed]

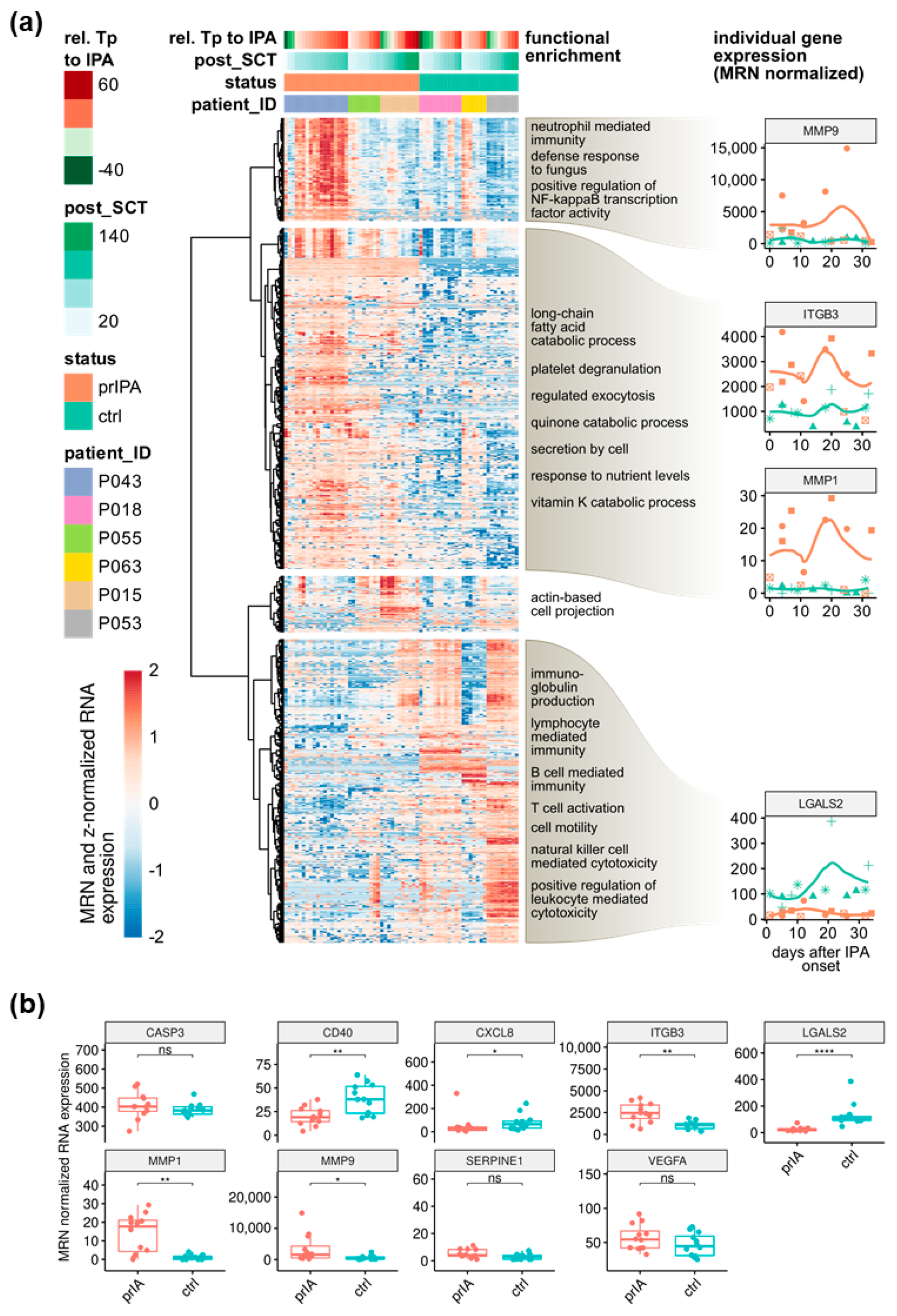
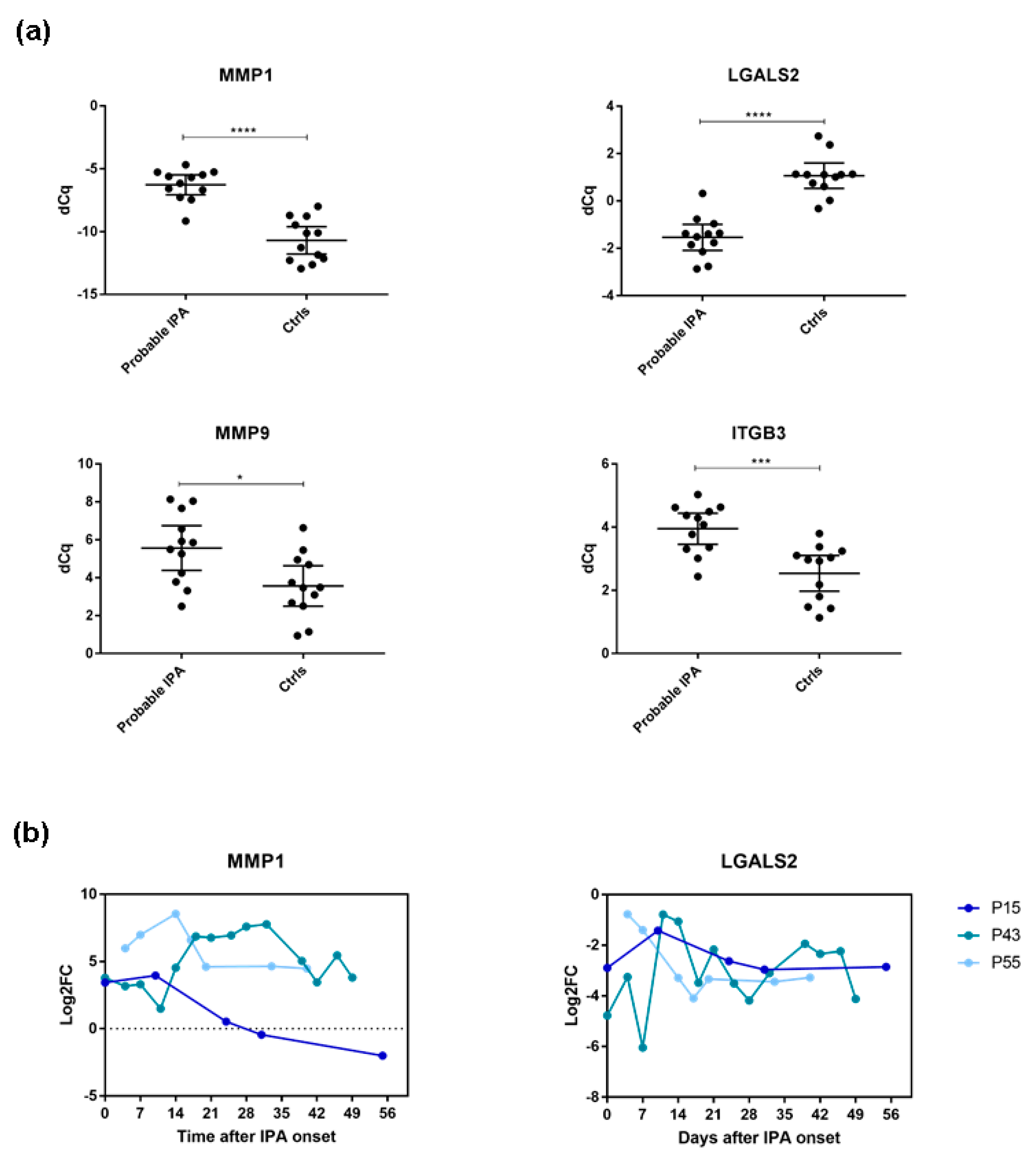
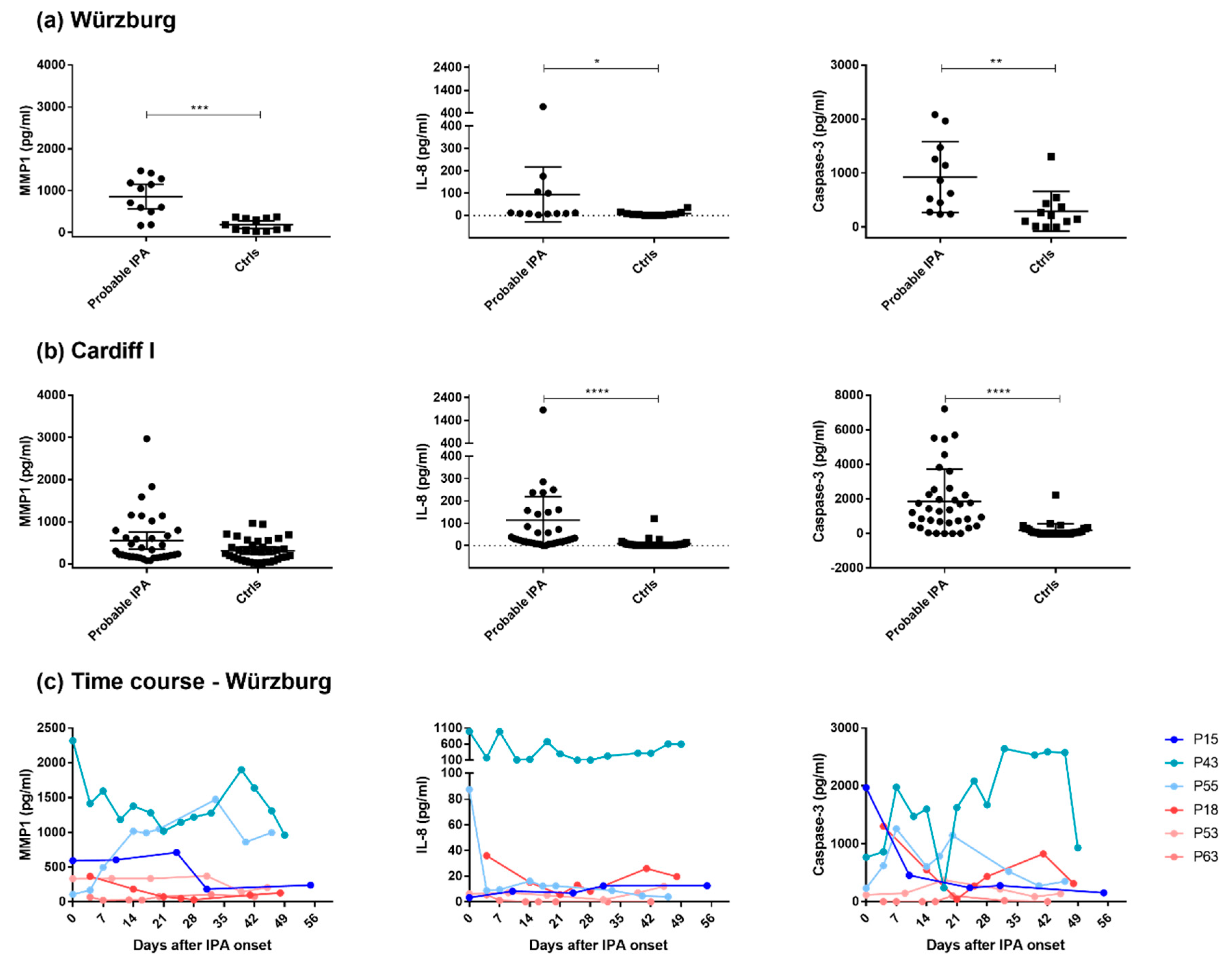
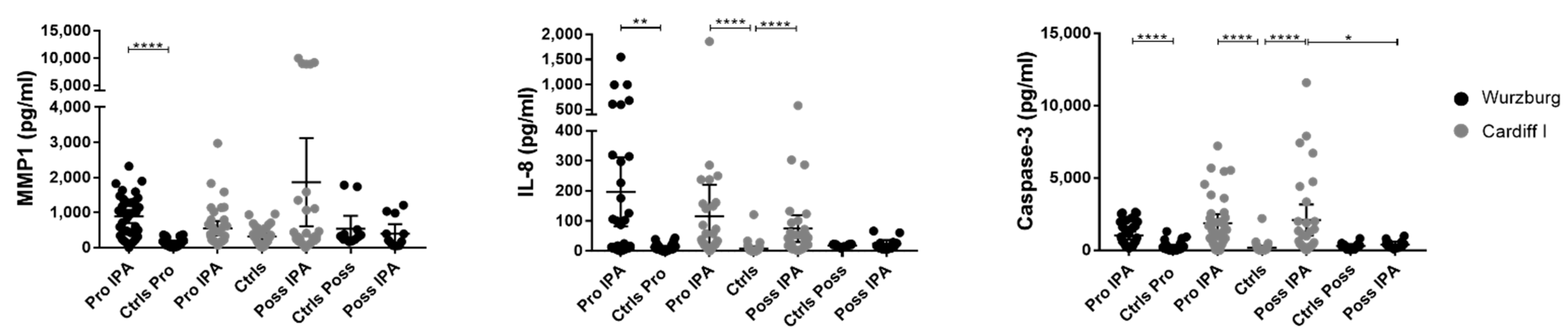
| Patient | Status | Age | Sex | Antifungal Treatment | Prophylaxis | GvHD (Grade) | No. of Samples |
|---|---|---|---|---|---|---|---|
| P15 | case | 63 | M | VRC | PSC, FLC | pulmonary (III) | 11 |
| P53 | control | 59 | M | NT | FLC | no GvHD | 9 |
| P43 | case | 60 | F | VRC, PSC | FLC | skin (III), intestinal (IV) | 18 |
| P18 | control | 51 | F | NT | PSC | no GvHD | 12 |
| P55 | case | 62 | M | Ambisome | PSC | skin (NA) | 9 |
| P63 | control | 55 | M | NT | FLC | skin (III) and intestinal (I) | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoran, T.; Seelbinder, B.; White, P.L.; Price, J.S.; Kraus, S.; Kurzai, O.; Linde, J.; Häder, A.; Loeffler, C.; Grigoleit, G.U.; et al. Molecular Profiling Reveals Characteristic and Decisive Signatures in Patients after Allogeneic Stem Cell Transplantation Suffering from Invasive Pulmonary Aspergillosis. J. Fungi 2022, 8, 171. https://doi.org/10.3390/jof8020171
Zoran T, Seelbinder B, White PL, Price JS, Kraus S, Kurzai O, Linde J, Häder A, Loeffler C, Grigoleit GU, et al. Molecular Profiling Reveals Characteristic and Decisive Signatures in Patients after Allogeneic Stem Cell Transplantation Suffering from Invasive Pulmonary Aspergillosis. Journal of Fungi. 2022; 8(2):171. https://doi.org/10.3390/jof8020171
Chicago/Turabian StyleZoran, Tamara, Bastian Seelbinder, Philip Lewis White, Jessica Sarah Price, Sabrina Kraus, Oliver Kurzai, Joerg Linde, Antje Häder, Claudia Loeffler, Goetz Ulrich Grigoleit, and et al. 2022. "Molecular Profiling Reveals Characteristic and Decisive Signatures in Patients after Allogeneic Stem Cell Transplantation Suffering from Invasive Pulmonary Aspergillosis" Journal of Fungi 8, no. 2: 171. https://doi.org/10.3390/jof8020171
APA StyleZoran, T., Seelbinder, B., White, P. L., Price, J. S., Kraus, S., Kurzai, O., Linde, J., Häder, A., Loeffler, C., Grigoleit, G. U., Einsele, H., Panagiotou, G., Loeffler, J., & Schäuble, S. (2022). Molecular Profiling Reveals Characteristic and Decisive Signatures in Patients after Allogeneic Stem Cell Transplantation Suffering from Invasive Pulmonary Aspergillosis. Journal of Fungi, 8(2), 171. https://doi.org/10.3390/jof8020171








