Fusarium oxysporum Casein Kinase 1, a Negative Regulator of the Plasma Membrane H+-ATPase Pma1, Is Required for Development and Pathogenicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolates and Culture Conditions
2.2. Generation of Gene Deletion Mutants and Complemented Strains
2.3. Colony Growth Assays
2.4. Quantification of Microconidia Production and Germination
2.5. Measurement of Pma1 H+-ATPase Activity
2.6. Cytosolic pH Measurements Using the Ratiometric Fluorescent Probe pHluorin
2.7. Measurement of Fungal Survival after Acid Treatment
2.8. Measurement of Colony pH
2.9. Quantification of Invasive Hyphal Growth and Chemotropism
2.10. Western Blot Analysis of MAPK Phosphorylation
2.11. Plant Infection Assays
3. Results
3.1. Generation of ck1 Deletion Mutants
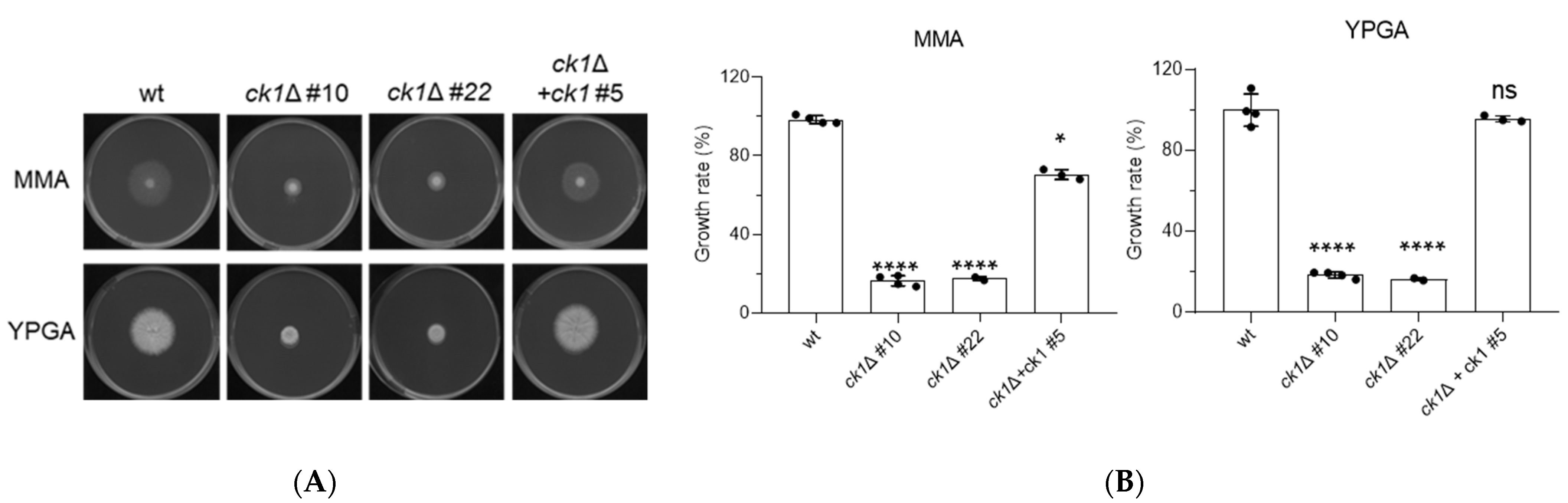
3.2. Ck1 Controls Vegetative Growth and Conidiation of F. oxysporum
3.3. Ck1 Is Required for Tolerance to Membrane and Hyperosmotic Stress
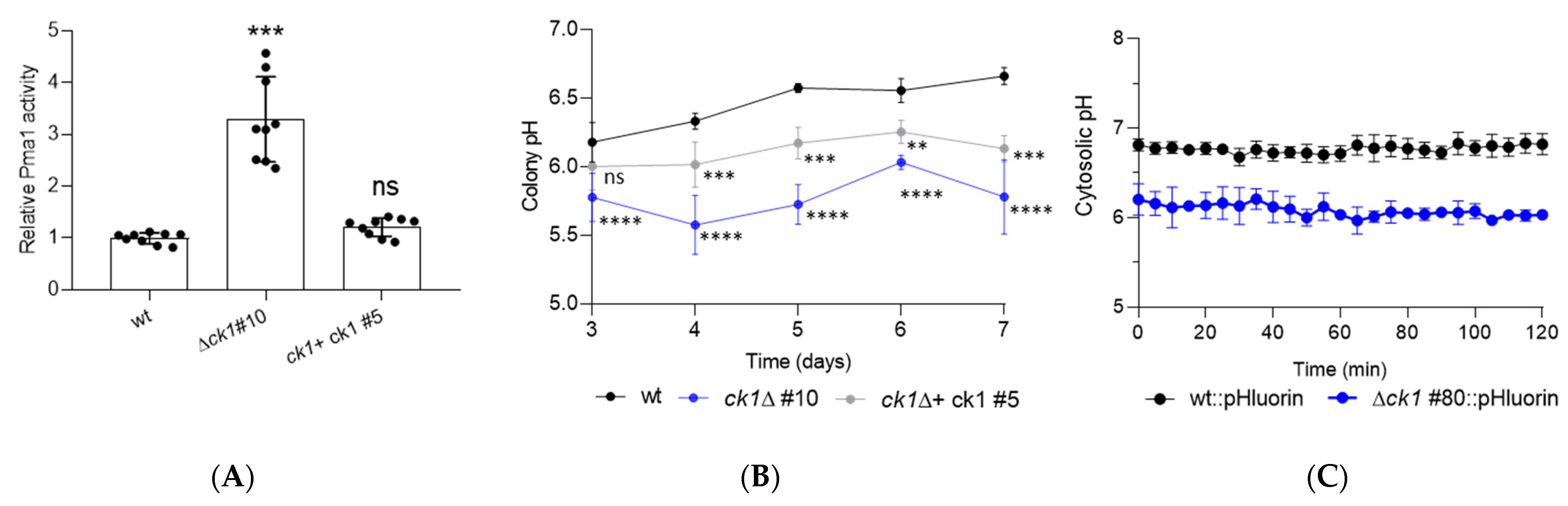
3.4. Ck1 Acts as a Negative Regulator of Pma1 Activity and Controls Both Extra- and Intracellular pH Homeostasis
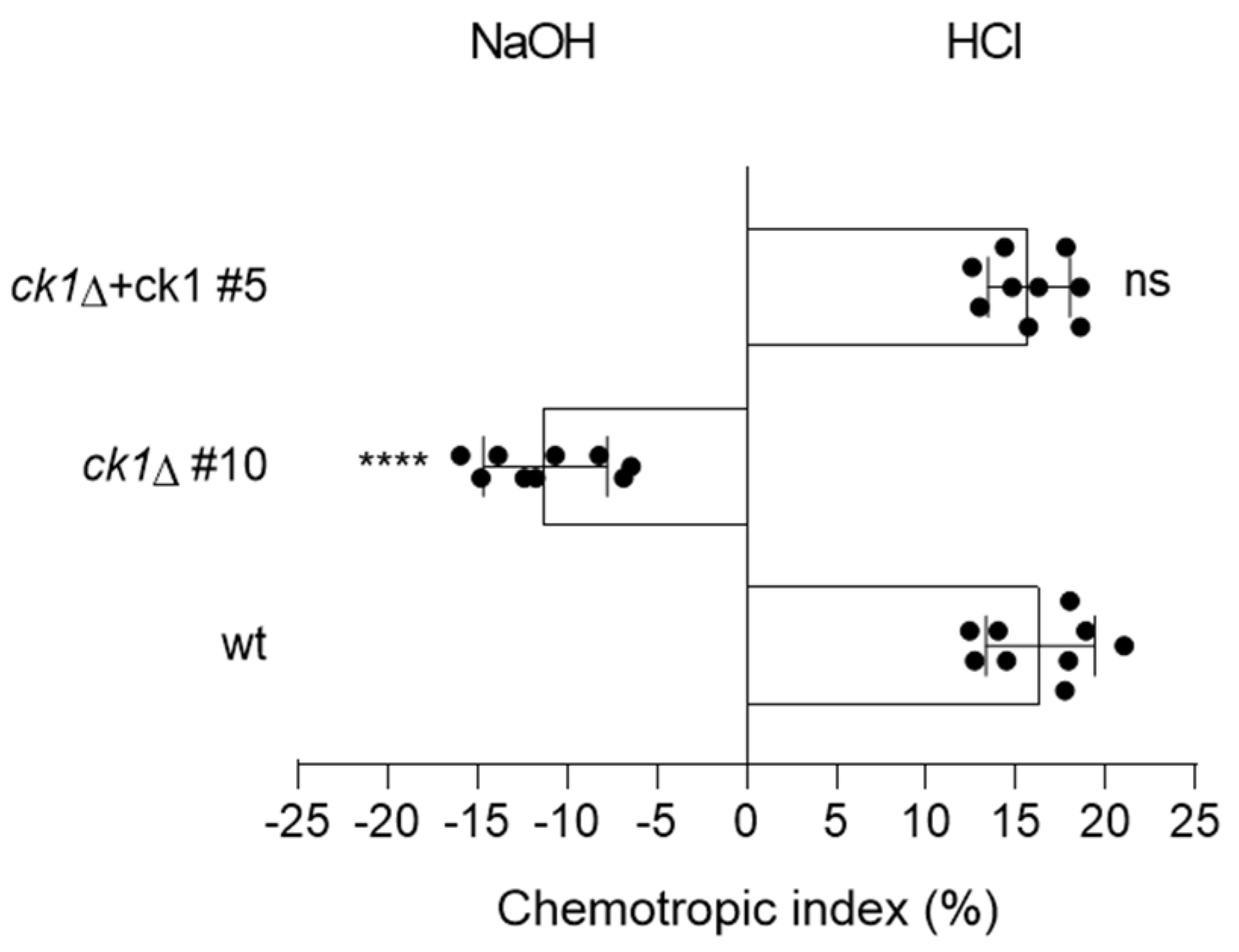
3.5. Ck1 Controls Hyphal Chemotropism towards Acid pH
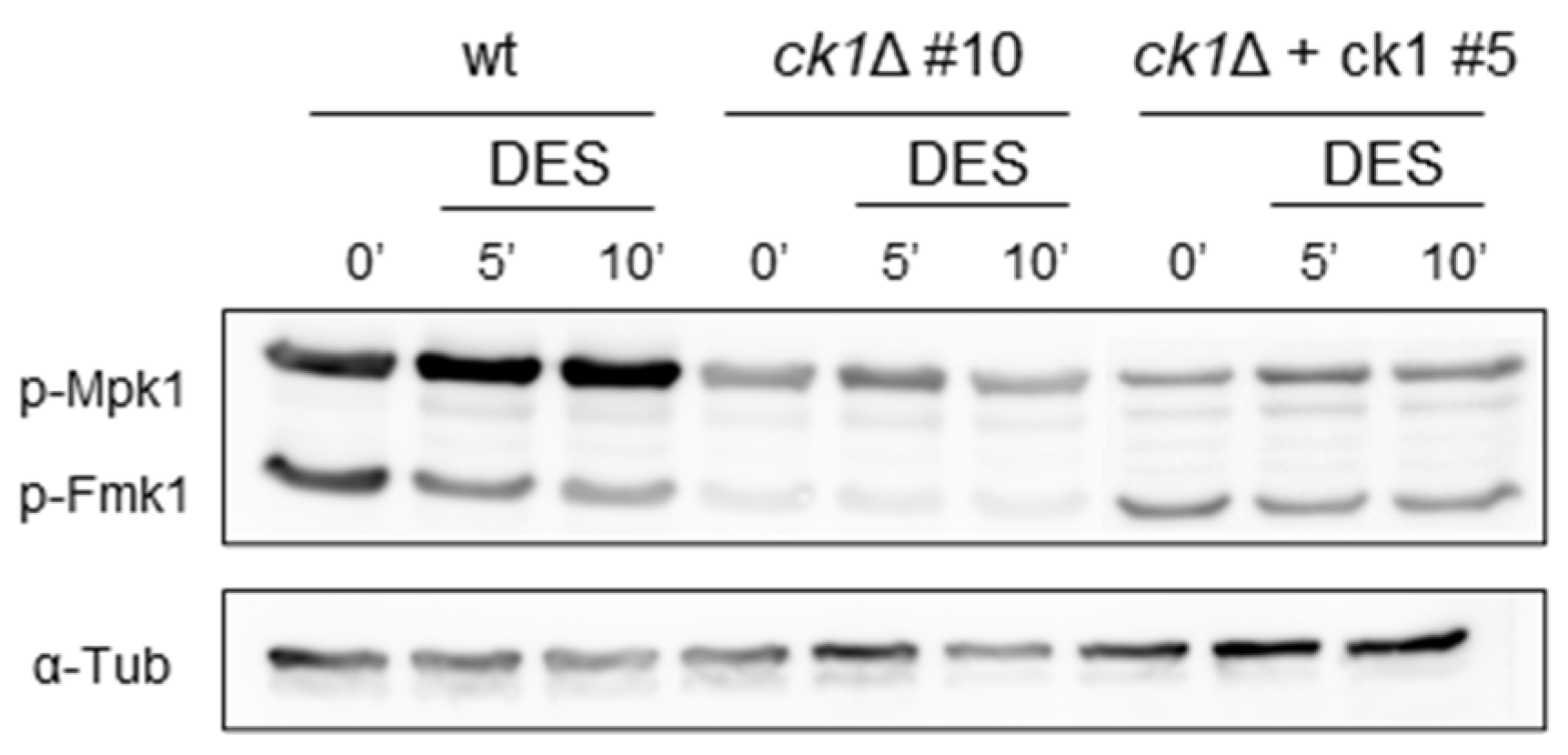
3.6. Ck1 Positively Regulates the MAPK Fmk1
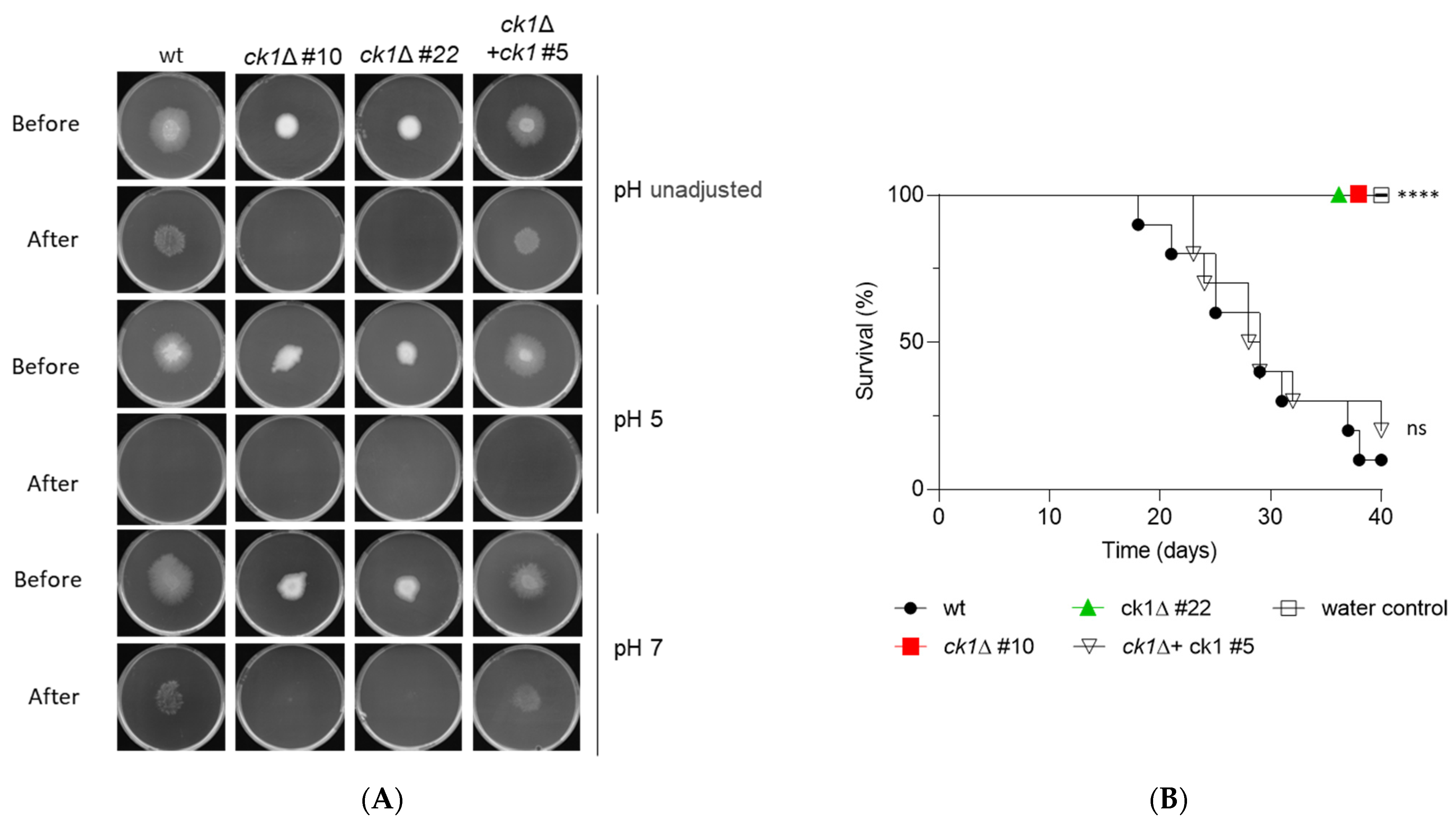
3.7. Ck1 Is Essential for Invasive Hyphal Growth and Pathogenicity of F. oxysporum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandes, T.R.; Segorbe, D.; Prusky, D.; Di Pietro, A. How alkalinization drives fungal pathogenicity. PLoS Pathog. 2017, 13, e1006621. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Kamura, T. The Rim101 pathway mediates adaptation to external alkalization and altered lipid asymmetry: Hypothesis describing the detection of distinct stresses by the Rim21 sensor protein. Curr. Genet. 2021, 67, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Selvig, K.; Alspaugh, J.A. PH response pathways in fungi: Adapting to host-derived and environmental signals. Mycobiology 2011, 39, 249–256. [Google Scholar] [CrossRef]
- Alkan, N.; Espeso, E.A.; Prusky, D. Virulence regulation of phytopathogenic fungi by pH. Antioxid. Redox Signal. 2013, 19, 1012–1025. [Google Scholar] [CrossRef]
- Prusky, D.; McEvoy, J.L.; Leverentz, B.; Conway, W.S. Local modulation of host pH by Colletotrichum species as a mechanism to increase virulence. Mol. Plant Microbe Interact. 2001, 14, 1105–1113. [Google Scholar] [CrossRef]
- Masachis, S.; Segorbe, D.; Turrà, D.; Leon-Ruiz, M.; Fürst, U.; El Ghalid, M.; Leonard, G.; López-Berges, M.S.; Richards, T.A.; Felix, G.; et al. A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat. Microbiol. 2016, 1, 16043. [Google Scholar] [CrossRef]
- Haruta, M.; Sabat, G.; Stecker, K.; Minkoff, B.B.; Sussman, M.R. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 2014, 343, 408–411. [Google Scholar] [CrossRef]
- Kane, P.M. Proton transport and pH control in fungi. Adv. Exp. Med. Biol. 2016, 892, 33–68. [Google Scholar] [CrossRef]
- Ambesi, A.; Miranda, M.; Petrov, V.V.; Slayman, C.W. Biogenesis and function of the yeast plasma-membrane H(+)-ATPase. J. Exp. Biol. 2000, 203, 155–160. [Google Scholar] [CrossRef]
- Palmgren, M.; Morsomme, P. The plasma membrane H(+)-ATPase, a simple polypeptide with a long history. Yeast 2019, 36, 201–210. [Google Scholar] [CrossRef]
- Lecchi, S.; Nelson, C.J.; Allen, K.E.; Swaney, D.L.; Thompson, K.L.; Coon, J.J.; Sussman, M.R.; Slayman, C.W. Tandem phosphorylation of Ser-911 and Thr-912 at the C terminus of yeast plasma membrane H+-ATPase leads to glucose-dependent activation. J. Biol. Chem. 2007, 282, 35471–35481. [Google Scholar] [CrossRef]
- Portillo, F. Regulation of plasma membrane H(+)-ATPase in fungi and plants. Biochim. Biophys. Acta 2000, 1469, 31–42. [Google Scholar] [CrossRef]
- Lee, Y.; Nasution, O.; Choi, E.; Choi, I.G.; Kim, W.; Choi, W. Transcriptome analysis of acetic-acid-treated yeast cells identifies a large set of genes whose overexpression or deletion enhances acetic acid tolerance. Appl. Microbiol. Biotechnol. 2015, 99, 6391–6403. [Google Scholar] [CrossRef]
- Eraso, P.; Mazón, M.J.; Portillo, F. Yeast protein kinase Ptk2 localizes at the plasma membrane and phosphorylates in vitro the C-terminal peptide of the H+-ATPase. Biochim. Biophys. Acta 2006, 1758, 164–170. [Google Scholar] [CrossRef]
- Goossens, A.; De La Fuente, N.; Forment, J.; Serrano, R.; Portillo, F. Regulation of yeast H(+)-ATPase by protein kinases belonging to a family dedicated to activation of plasma membrane transporters. Mol. Cell. Biol. 2000, 20, 7654–7661. [Google Scholar] [CrossRef]
- Estrada, E.; Agostinis, P.; Vandenheede, J.R.; Goris, J.; Merlevede, W.; François, J.; Goffeau, A.; Ghislain, M. Phosphorylation of yeast plasma membrane H+-ATPase by casein kinase I. J. Biol. Chem. 1996, 271, 32064–32072. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Chen, J.S.; Culotta, V.C. Cu/Zn superoxide dismutase and the proton ATPase Pma1p of Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2015, 462, 251–256. [Google Scholar] [CrossRef][Green Version]
- Devare, M.N.; Kim, Y.H.; Jung, J.; Kang, W.K.; Kwon, K.S.; Kim, J.Y. TORC1 signaling regulates cytoplasmic pH through Sir2 in yeast. Aging Cell 2020, 19, e13151. [Google Scholar] [CrossRef]
- Kang, W.K.; Kim, Y.H.; Kang, H.A.; Kwon, K.S.; Kim, J.Y. Sir2 phosphorylation through cAMP-PKA and CK2 signaling inhibits the lifespan extension activity of Sir2 in yeast. eLife 2015, 4, e09709. [Google Scholar] [CrossRef]
- Herrador, A.; Livas, D.; Soletto, L.; Becuwe, M.; Léon, S.; Vincent, O. Casein kinase 1 controls the activation threshold of an α-arrestin by multisite phosphorylation of the interdomain hinge. Mol. Biol. Cell 2015, 26, 2128–2138. [Google Scholar] [CrossRef]
- Robinson, L.C.; Bradley, C.; Bryan, J.D.; Jerome, A.; Kweon, Y.; Panek, H.R. The Yck2 yeast casein kinase 1 isoform shows cell cycle-specific localization to sites of polarized growth and is required for proper septin organization. Mol. Biol. Cell 1999, 10, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.C.; Menold, M.M.; Garrett, S.; Culbertson, M.R. Casein kinase I-like protein kinases encoded by YCK1 and YCK2 are required for yeast morphogenesis. Mol. Cell. Biol. 1993, 13, 2870–2881. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.F.; Papanayotou, I.; Davis, N.G. The yeast kinase Yck2 has a tripartite palmitoylation signal. Mol. Biol. Cell 2011, 22, 2702–2715. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.I.; Rodriguez, N.; Irrizary, J.; Liboro, K.; Bogarin, T.; Macias, M.; Eivers, E.; Porter, E.; Filler, S.G.; Park, H. Yeast casein kinase 2 governs morphology, biofilm formation, cell wall integrity, and host cell damage of Candida albicans. PLoS ONE 2017, 12, e0187721. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Liu, Y.; Solis, N.; Spotkov, J.; Hamaker, J.; Blankenship, J.R.; Yeaman, M.R.; Mitchell, A.P.; Liu, H.; Filler, S.G. Transcriptional responses of Candida albicans to epithelial and endothelial cells. Eukaryot. Cell 2009, 8, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, T.B.; Patel, S.; Jiang, L.; Xue, C. The casein kinase I protein Cck1 regulates multiple signaling pathways and is essential for cell integrity and fungal virulence in Cryptococcus neoformans. Eukaryot. Cell 2011, 10, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.B.; Chen, N.; Zhu, X.M.; Su, Z.Z.; Wang, J.Y.; Lu, J.P.; Liu, X.H.; Lin, F.C. The casein kinase MoYck1 regulates development, autophagy, and virulence in the rice blast fungus. Virulence 2019, 10, 719–733. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, D.; Liu, Q.; Hou, R.; Xu, J.R.; Wang, G. Stage-specific genetic interaction between FgYCK1 and FgBNI4 during vegetative growth and conidiation in Fusarium graminearum. Int. J. Mol. Sci. 2022, 23, 9106. [Google Scholar] [CrossRef]
- Di Pietro, A.; García-MacEira, F.I.; Méglecz, E.; Roncero, M.I. A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 2001, 39, 1140–1152. [Google Scholar] [CrossRef]
- Turrà, D.; El Ghalid, M.; Rossi, F.; Di Pietro, A. Fungal pathogen uses sex pheromone receptor for chemotropic sensing of host plant signals. Nature 2015, 527, 521–524. [Google Scholar] [CrossRef]
- Di Pietro, A.; Roncero, M.I. Cloning, expression, and role in pathogenicity of pg1 encoding the major extracellular endopolygalacturonase of the vascular wilt pathogen Fusarium oxysporum. Mol. Plant Microbe Interact. 1998, 11, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Catlett, N.L.; Lee, B.-N.; Yoder, O.C.; Turgeon, B.G. Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet. Rep. 2003, 50, 9–11. [Google Scholar] [CrossRef]
- Punt, P.J.; Van den Hondel, C.A. Transformation of filamentous fungi based on hygromycin b and phleomycin resistance markers. Methods Enzymol. 1992, 216, 447–457. [Google Scholar] [CrossRef] [PubMed]
- López-Berges, M.S.; Rispail, N.; Prados-Rosales, R.C.; Di Pietro, A. A nitrogen response pathway regulates virulence functions in Fusarium oxysporum via the protein kinase TOR and the bZIP protein MeaB. Plant Cell 2010, 22, 2459–2475. [Google Scholar] [CrossRef]
- Kahm, M.; Navarrete, C.; Llopis-Torregrosa, V.; Herrera, R.; Barreto, L.; Yenush, L.; Ariño, J.; Ramos, J.; Kschischo, M. Potassium starvation in yeast: Mechanisms of homeostasis revealed by mathematical modeling. PLoS Comput. Biol. 2012, 8, e1002548. [Google Scholar] [CrossRef]
- Fernandes, T.R.; Serrano, A.; Di Pietro, A. In vivo monitoring of cytosolic pH using the ratiometric pH sensor pHluorin. Methods Mol. Biol. 2022, 2391, 99–107. [Google Scholar] [CrossRef]
- Shaw, S.; Knüsel, S.; Abbühl, D.; Naguleswaran, A.; Etzensperger, R.; Benninger, M.; Roditi, I. Cyclic AMP signalling and glucose metabolism mediate pH taxis by African trypanosomes. Nat. Commun. 2022, 13, 603. [Google Scholar] [CrossRef]
- Méchin, V.; Damerval, C.; Zivy, M. Total protein extraction with TCA-acetone. Methods Mol. Biol. 2007, 355, 1–8. [Google Scholar] [CrossRef]
- Nordzieke, D.E.; Fernandes, T.R.; El Ghalid, M.; Turrà, D.; Di Pietro, A. NADPH oxidase regulates chemotropic growth of the fungal pathogen Fusarium oxysporum towards the host plant. New Phytol. 2019, 224, 1600–1612. [Google Scholar] [CrossRef]
- Agostinis, P.; Pinna, L.A.; Meggio, F.; Marin, O.; Goris, J.; Vandenheede, J.R.; Merlevede, W. A synthetic peptide substrate specific for casein kinase I. FEBS Lett. 1989, 259, 75–78. [Google Scholar] [CrossRef]
- Marin, O.; Meggio, F.; Sarno, S.; Andretta, M.; Pinna, L.A. Phosphorylation of synthetic fragments of inhibitor-2 of protein phosphatase-1 by casein kinase-1 and -2. Evidence that phosphorylated residues are not strictly required for efficient targeting by casein kinase-1. Eur. J. Biochem. 1994, 223, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Arneborg, N.; Jespersen, L.; Jakobsen, M. Individual cells of Saccharomyces cerevisiae and Zygosaccharomyces bailii exhibit different short-term intracellular pH responses to acetic acid. Arch. Microbiol. 2000, 174, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.R.; Mariscal, M.; Serrano, A.; Segorbe, D.; Fernández-Acero, T.; Martín, H.; Turrà, D.; Di Pietro, A. Cytosolic pH controls fungal MAPK signaling and pathogenicity. bioRxiv 2022. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Z.; Wang, C.; Li, Y.; Xu, J.R. Germination and infectivity of microconidia in the rice blast fungus Magnaporthe oryzae. Nat. Commun. 2014, 5, 4518. [Google Scholar] [CrossRef]
- Lee, Y.; Nasution, O.; Lee, Y.M.; Kim, E.; Choi, W.; Kim, W. Overexpression of PMA1 enhances tolerance to various types of stress and constitutively activates the SAPK pathways in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 229–239. [Google Scholar] [CrossRef]
- Guaragnella, N.; Antonacci, L.; Passarella, S.; Marra, E.; Giannattasio, S. Achievements and perspectives in yeast acetic acid-induced programmed cell death pathways. Biochem. Soc. Trans. 2011, 39, 1538–1543. [Google Scholar] [CrossRef]
- Martínez-Muñoz, G.A.; Kane, P. Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. J. Biol. Chem. 2017, 292, 7743. [Google Scholar] [CrossRef]
- Yenush, L.; Mulet, J.M.; Ariño, J.; Serrano, R. The Ppz protein phosphatases are key regulators of K+ and pH homeostasis: Implications for salt tolerance, cell wall integrity and cell cycle progression. EMBO J. 2002, 21, 920–929. [Google Scholar] [CrossRef]
- Yenush, L.; Merchan, S.; Holmes, J.; Serrano, R. pH-Responsive, posttranslational regulation of the Trk1 potassium transporter by the type 1-related Ppz1 phosphatase. Mol. Cell. Biol. 2005, 25, 8683–8692. [Google Scholar] [CrossRef]
- Palmieri, D.; Vitale, S.; Lima, G.; Di Pietro, A.; Turrà, D. A bacterial endophyte exploits chemotropism of a fungal pathogen for plant colonization. Nat. Commun. 2020, 11, 5264. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariscal, M.; Miguel-Rojas, C.; Hera, C.; Fernandes, T.R.; Di Pietro, A. Fusarium oxysporum Casein Kinase 1, a Negative Regulator of the Plasma Membrane H+-ATPase Pma1, Is Required for Development and Pathogenicity. J. Fungi 2022, 8, 1300. https://doi.org/10.3390/jof8121300
Mariscal M, Miguel-Rojas C, Hera C, Fernandes TR, Di Pietro A. Fusarium oxysporum Casein Kinase 1, a Negative Regulator of the Plasma Membrane H+-ATPase Pma1, Is Required for Development and Pathogenicity. Journal of Fungi. 2022; 8(12):1300. https://doi.org/10.3390/jof8121300
Chicago/Turabian StyleMariscal, Melani, Cristina Miguel-Rojas, Concepción Hera, Tânia R. Fernandes, and Antonio Di Pietro. 2022. "Fusarium oxysporum Casein Kinase 1, a Negative Regulator of the Plasma Membrane H+-ATPase Pma1, Is Required for Development and Pathogenicity" Journal of Fungi 8, no. 12: 1300. https://doi.org/10.3390/jof8121300
APA StyleMariscal, M., Miguel-Rojas, C., Hera, C., Fernandes, T. R., & Di Pietro, A. (2022). Fusarium oxysporum Casein Kinase 1, a Negative Regulator of the Plasma Membrane H+-ATPase Pma1, Is Required for Development and Pathogenicity. Journal of Fungi, 8(12), 1300. https://doi.org/10.3390/jof8121300






