Cuticular Lipids as a First Barrier Defending Ixodid Ticks against Fungal Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolates, Cultivation and Preparation of Fungal Suspensions
2.2. Collection of Ticks
2.3. Cuticular Hydrocarbons Identification
2.4. Activity of Lipid Extracts to Mycelial Growth and Conidial Viability
2.5. Cytotoxicity of Cuticle Extracts
2.6. Germination of Conidia on Tick Cuticle
2.7. Neutral Lipids from Tick Cuticle
2.8. Statistical Analysis
2.9. Ethics Statement
3. Results
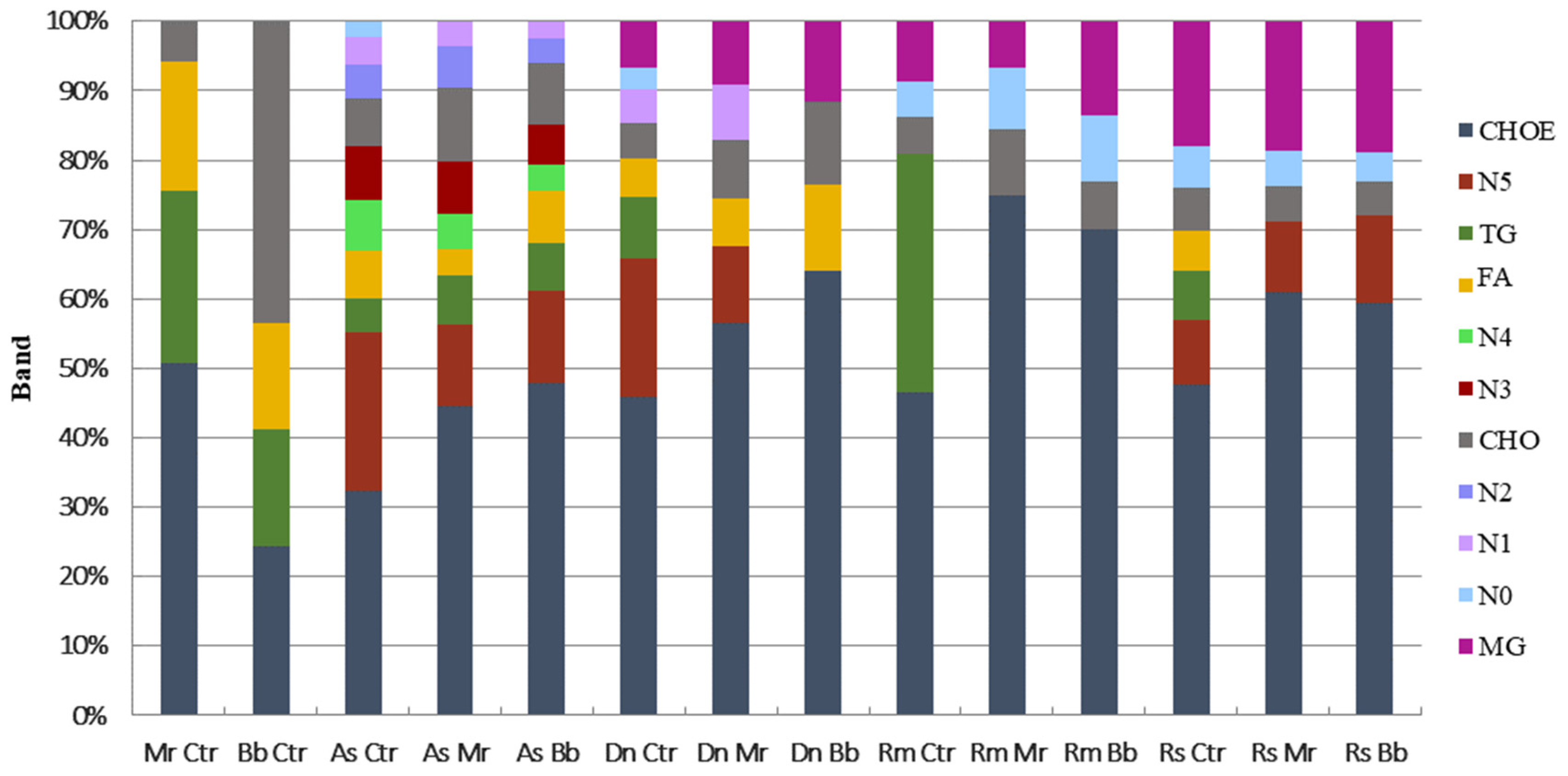
3.1. Cuticular Hydrocarbons Identification
3.2. Activity of Lipid Extracts to Mycelial Growth and Conidial Viability
3.3. Cytotoxicity of Cuticle Extracts
3.4. Germination of Conidia on Tick Cuticle
3.5. Neutral Lipids from Tick Cuticle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parola, P.; Raoult, D. Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Clin. Infect. Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F. The natural history of ticks. Med. Clin. N. Am. 2002, 86, 205–218. [Google Scholar] [CrossRef]
- Sonenshine, D.E.; Roe, M. Biology of Ticks; Oxford University Press: New York, NY, USA, 2014; p. 491. [Google Scholar]
- Ali, A.; Mulenga, A.; Vaz, I.S. Editorial: Tick and Tick-Borne Pathogens: Molecular and Immune Targets for Control Strategies. Front. Physiol. 2020, 11, 1–4. [Google Scholar] [CrossRef]
- Brito, L.G.; Silva Neto, F.G.; Oliveira, M.C.S.; Barbieri, F.S. Bio-ecologia, Importância Médico-Veterinária e Controle de Carrapatos, Com ênfase no Carrapato dos Bovinos; Documentos/Embrapa Rondonia; Embrapa Rondonia: Rodovia, Brazil, 2006; 21p, ISSN 0677-8618. 104. [Google Scholar]
- Gerardi, M.; Ramírez-Hernández, A.; Binder, L.C.; Krawczak, F.S.; Gregori, F.; Labruna, M.B. Comparative susceptibility of different populations of Amblyomma sculptum to Rickettsia rickettsii. Front. Physiol. 2019, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Grisi, L.; Leite, R.C.; de Martins, J.R.S.; de Barros, A.T.M.; Andreotti, R.; Cançado, P.H.D.; Léon, A.A.P.; Villela, H.S. Reassessment of the potential economic impact of cattle parasites in Brazil. Braz. J. Vet. Parasitol. 2014, 23, 150–156. [Google Scholar] [CrossRef]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, 3–14. [Google Scholar] [CrossRef] [PubMed]
- De Damasceno, I.A.M.; Guerra, R.C. Coxiella burnetii and Q fever in brazil: A public health issue. Ciência Saúde Coletiva 2018, 23, 4231–4239. [Google Scholar] [PubMed]
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasites Vectors 2010, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.L.T.; Luo, T.; Zhang, X.; Muraro, L.S.; Pereira, N.A.; Cabezas-Cruz, A.; Dantas-Torres, F.; McBride, J.W.; de Aguiar, D.M. Serological evidence of Ehrlichia minasensis infection in Brazilian dogs. Acta Trop. 2021, 219, 105931. [Google Scholar] [CrossRef] [PubMed]
- Butt, T.M.; Jackson, C.; Magan, N. Fungi as biocontrol agents: Progress, problems and potential. Educ. Psychol. Meas. 2002, 51, 518–521. [Google Scholar]
- Chandler, D.; Davidson, G.; Pell, J.K.; Ball, B.V.; Shaw, K.; Sunderland, K.D. Fungal biocontrol of Acari. Biocontrol Sci. Technol. 2000, 10, 357–384. [Google Scholar] [CrossRef]
- Faria, M.R.D.; Wraight, S.P. Mycoinsecticides and Mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Lopes, R.B.; Delalibera, Í.; Fernandes, É.K.K.; Luz, C.; Faria, M. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. J. Invertebr. Pathol. 2019, 165, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Angelo, I.C.; Fernandes, É.K.K.; Bahiense, T.C.; Perinotto, W.M.S.; Moraes, A.P.R.; Terra, A.L.M.; Bittencourt, V.R.E.P. Efficiency of Lecanicillium lecanii to control the tick Rhipicephalus microplus. Vet. Parasitol. 2010, 172, 317–322. [Google Scholar] [CrossRef]
- Bittencourt, V.R.E.P.; Peralva, S.L.E.S.; Viegas, E.C.; Alves, B. Avaliaçao dos efeitos do contato de Beauveria bassiana (Bals.) Vuill. com ovos e larvas de Boophilus microplus (Canestrini, 1887) (Acari: Ixodidae). Rev. Bras. De Parasitol. Veterinária 1996, 5, 81–84. [Google Scholar]
- Camargo, M.G.; Nogueira, M.R.S.; Marciano, A.F.; Perinotto, W.M.S.; Coutinho-Rodrigues, C.J.B.; Scott, F.B.; Angelo, I.C.; Prata, M.C.; Bittencourt, V.R.E.P. Metarhizium anisopliae for controlling Rhipicephalus microplus ticks under field conditions. Vet. Parasitol. 2016, 223, 38–42. [Google Scholar] [CrossRef]
- Bernardo, C.C.; Barreto, L.P.; Ribeiro-Silva, C.S.; Luz, C.; Arruda, W.; Fernandes, É.K.K. Conidia and blastospores of Metarhizium spp. and Beauveria bassiana s.l.: Their development during the infection process and virulence against the tick Rhipicephalus Microplus. Ticks Tick-Borne Dis. 2018, 9, 1334–1342. [Google Scholar]
- Mesquita, E.; Marciano, A.F.; Corval, A.R.C.; Fiorotti, J.; Corrêa, T.A.; Quinelato, S.; Gôlo, P.S. Efficacy of a native isolate of the entomopathogenic fungus Metarhizium anisopliae against larval tick outbreaks under semifield conditions. BioControl 2020, 65, 353–362. [Google Scholar] [CrossRef]
- Santi, L.; Beys da Silva, W.O.; Berger, M.; Guimarães, J.A.; Schrank, A.; Vainstein, M.H. Conidial surface proteins of Metarhizium anisopliae: Source of activities related with toxic effects, host penetration and pathogenesis. Toxicon 2010, 55, 874–880. [Google Scholar] [CrossRef]
- Fernandes, É.K.K.; Bittencourt, V.R.E.P. Entomopathogenic fungi against South American tick species. Exp. Appl. Acarol. 2008, 46, 71–93. [Google Scholar] [CrossRef]
- Gindin, G.; Samish, M.; Zangi, G.; Mishoutchenko, A.; Glazer, I. The susceptibility of different species and stages of ticks to entomopathogenic fungi. Exp. Appl. Acarol. 2002, 28, 283–288. [Google Scholar] [CrossRef]
- Butt, T.M.; Wood, M.; Taylor, J.W.D.; Bakirci, S.; Hazir, C.; Ulug, D.; Hazir, S. Differential susceptibility of Hyalomma excavatum adults and nymphs to the entomopathogens Metarhizium anisopliae ARSEF 4556 and Steinernema carpocapsae. Int. J. Pest Manag. 2016, 62, 261–266. [Google Scholar] [CrossRef]
- Reis, R.C.S.; Melo, D.R.; Bittencourt, V.R.E.P. Efeitos de Beauveria bassiana (Bals) Vuill e Metarhizium anisopliae (Metsc) Sorok sobre fêmeas ingurgitadas de Amblyomma cajennense (Fabricius, 1787) em condições de laboratório. Arq. Bras. Med. Veterinária Zootec. 2004, 56, 788–791. [Google Scholar] [CrossRef]
- Monteiro, S.G.; Carneiro, M.E.; Bittencourt, V.R.E.P.; Daemon, E. Effect of isolate 986 of the fungi Beauveria bassiana (Bals) Vuill on engorged females of Anocentor nitens Neumann, 1897 (Acari: Ixodidae). Arq. Bras. Med. Veterinária Zootec. 1998, 50, 673–676. [Google Scholar]
- Fernandes, É.K.K.; Angelo, I.C.; Rangel, D.E.N.; Bahiense, T.C.; Moraes, Á.M.L.; Roberts, D.W.; Bittencourt, V.R.E.P. An intensive search for promising fungal biological control agents of ticks, particularly Rhipicephalus microplus. Vet. Parasitol. 2011, 182, 307–318. [Google Scholar] [CrossRef]
- Perinotto, W.M.S.; Camargo, M.G.; Golo, P.S.; Angelo, I.C.; Quinelato, S.; Monteiro, C.M.O.; Sá, F.A.; Coutinho-Rodrigues, C.J.B.; Marciano, A.F.; Fiorotti, J.P.; et al. Controle de Dermacentor nitens utilizando uma formulação comercial a base de Metarhizium anisopliae. Rev. Bras. Med. Vet. 2013, 35, 35–42. [Google Scholar]
- Butt, T.M.; Coates, C.J.; Dubovskiy, I.M.; Ratcliffe, N.A. Entomopathogenic Fungi: New insights into host-pathogen interactions. Adv. Genet. 2016, 94, 307–364. [Google Scholar]
- Hartelt, K.; Wurst, E.; Collatz, J.; Zimmermann, G.; Kleespies, R.G.; Oehme, R.M.; Kimmig, P.; Steidle, J.L.M.; Mackenstedt, U. Biological control of the tick Ixodes ricinus with entomopathogenic fungi and nematodes: Preliminary results from laboratory experiments. Int. J. Med. Microbiol. 2008, 298, 314–320. [Google Scholar] [CrossRef]
- Sonenshine, D.E. Biology of Ticks 1; Oxford University Press: New York, NY, USA, 1991. [Google Scholar]
- Cherry, L.M. The production of cuticle wax by engorged females of the cattle tick, Boophilus microplus (Canestrini). J. Exp. Biol. 1969, 50, 705–709. [Google Scholar] [CrossRef]
- Hamilton, J.G.C.; Sonenshine, D.E.; Lusby, W.R. Cholesteryl oleate: Mounting sex pheromone of the hard ticks Dermacentor variabilis (Say) (Acari: Ixodidae). J. Insect Physiol. 1989, 35, 873–879. [Google Scholar] [CrossRef]
- Ment, D.; Gindin, G.; Rot, A.; Soroker, V.; Glazer, I.; Barel, S.; Samish, M. Novel technique for quantifying adhesion of Metarhizium anisopliae conidia, to the tick cuticle. Appl. Environ. Microbiol. 2010, 76, 3521–3528. [Google Scholar] [CrossRef] [PubMed]
- Ment, D.; Gindin, G.; Rot, A.; Eshel, D.; Teper-Bamnolker, P.; Ben-Ze’ev, I.; Glazer, I.; Samish, M. Role of cuticular lipids and water-soluble compounds in tick susceptibility to Metarhizium infection. Biocontrol Sci. Technol. 2013, 23, 956–967. [Google Scholar] [CrossRef]
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monzón, A.; Ownley, B.H.; et al. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef]
- Bittencourt, V.R.E.P.; Mascarenhas, A.G.; Faccini, J.L.H. Mecanismo de infecção do fungo Metarhizium anisopliae no carrapato Boophilus microplus em condições experimentais. Ciência Rural 1999, 29, 351–354. [Google Scholar] [CrossRef]
- Kirkland, B.H.; Westwood, G.S.; Keyhani, N.O. Pathogenicity of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae to ixodidae tick species Dermacentor variabilis, Rhipicephalus sanguineus, and Ixodes scapularis. J. Med. Entomol. 2004, 41, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.F.N.; Inglis, P.W.; Humber, R.A.; Kipnis, A.; Luz, C. Occurrence of Metarhizium spp. in Central Brazilian soils. J. Basic Microbiol. 2013, 53, 251–259. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, W.B.; Humber, R.A.; Luz, C. Occurrence of pathogenic fungi to Amblyomma cajennense in a rural area of Central Brazil and their activities against vectors of Rocky Mountain spotted fever. Vet. Parasitol. 2012, 188, 156–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Braga, G.U.L.; Flint, S.D.; Miller, C.D.; Anderson, A.J.; Roberts, D.W. Variability in response to UV-B among species and strains of Metarhizium isolated from sites at latitudes from 61° N to 54° S. J. Invertebr. Pathol. 2001, 78, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Barreto, L.P.; Luz, C.; Mascarin, G.M.; Roberts, D.W.; Arruda, W.; Fernandes, É.K.K. Effect of heat stress and oil formulation on conidial germination of Metarhizium anisopliae s. s. on tick cuticle and artificial medium. J. Invertebr. Pathol. 2016, 138, 94–103. [Google Scholar] [CrossRef]
- Bligh, G.E.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.I.; Ochoa, B. Quantification in the subnanomolar range of phospholipids and neutral lipids by monodimensional thin-layer chromatography and image analysis. J. Lipid Res. 1997, 38, 1482–1489. [Google Scholar] [CrossRef]
- Arnhold, E. Package in the R environment for analysis of variance and complementary analyses. Braz. J. Vet. Res. Anim. Sci. 2014, 50, 488–492. [Google Scholar] [CrossRef]
- Lin, L.; Fang, W.; Liao, X.; Wang, F.; Wei, D.; Leger, R.J.S. The MrCYP52 cytochrome P450 monoxygenase gene of Metarhizium robertsii is important for utilizing insect epicuticular hydrocarbons. PLoS ONE. 2011, 6, e28984. [Google Scholar] [CrossRef] [PubMed]
- Keyhani, N.O. Lipid biology in fungal stress and virulence: Entomopathogenic fungi. Fungal Biol. 2018, 122, 420–429. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Bagnères, A.-G. (Eds.) Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Cambridge University Press: Cambridge, UK, 2010; pp. 19–34. [Google Scholar]
- Gibbs, A.G. Lipid melting and cuticular permeability: New insights into an old problem. J. Insect Physiol. 2002, 48, 391–400. [Google Scholar] [CrossRef]
- Ment, D.; Gindin, G.; Soroker, V.; Glazer, I.; Rot, A.; Samish, M. Metarhizium anisopliae conidial responses to lipids from tick cuticle and tick mammalian host surface. J. Invertebr. Pathol. 2010, 103, 132–139. [Google Scholar] [CrossRef]
- Ment, D.; Churchill, A.C.L.; Gindin, G.; Belausov, E.; Glazer, I.; Rehner, S.A.; Rot, A.; Donzelli, B.G.G.; Samish, M. Resistant ticks inhibit Metarhizium infection prior to haemocoel invasion by reducing fungal viability on the cuticle surface. Environ. Microbiol. 2012, 14, 1570–1583. [Google Scholar] [CrossRef]
- Yoder, J.A.; Domingus, J.L. Identification of hydrocarbons that protect ticks (Acari: Ixodidae) against fire ants (Hymenoptera: Formicidae), but not lizards (Squamata: Polychrotidae), in an allomonal defense secretion. Int. J. Acarol. 2003, 29, 87–91. [Google Scholar] [CrossRef]
- Arruda, W.; Lübeck, I.; Schrank, A.; Vainstein, M.H. Morphological alterations of Metarhizium anisopliae during penetration of Boophilus microplus ticks. Exp. Appl. Acarol. 2005, 37, 231–244. [Google Scholar] [CrossRef]
- Pedrini, N.; Zhang, S.; Juárez, M.P.; Keyhani, N.O. Molecular characterization and expression analysis of a suite of cytochrome P450 enzymes implicated in insect hydrocarbon degradation in the entomopathogenic fungus Beauveria bassiana. Microbiology 2010, 156, 2549–2557. [Google Scholar] [CrossRef]
- Leger, R.J.S.; Cooper, R.M.; Charnley, A.K. Utilization of alkanes by entomopathogenic fungi. J. Invertebr. Pathol. 1988, 52, 356–359. [Google Scholar] [CrossRef]
- Sosa-Gomez, D.R.; Boucias, D.G.; Nation, J.L. Attachment of Metarhizium anisopliae to the southern green stink bug Nezara viridula cuticle and fungistatic effect of cuticular lipids and aldehydes. J. Invertebr. Pathol. 1997, 69, 31–39. [Google Scholar] [CrossRef]
- Borges, M.; Aldrich, J.R. Instar-specific defensive secretions of stink bugs (Heteroptera: Pentatomidae). Experientia 1992, 48, 893–896. [Google Scholar] [CrossRef]
- Silva, R.A.; Quintela, E.D.; Mascarin, G.M.; Pedrini, N.; Lião, L.M.; Ferri, P.H. Unveiling chemical defense in the rice stalk stink bug against the entomopathogenic fungus Metarhizium anisopliae. J. Invertebr. Pathol. 2015, 127, 93–100. [Google Scholar] [CrossRef]
- Dietemann, V.; Peeters, C.; Liebig, J.; Thivet, V.; Hölldobler, B. Cuticular hydrocarbons mediate discrimination of reproductives and nonreproductives in the ant Myrmecia Gulosa. Proc. Natl. Acad. Sci. USA 2003, 100, 10341–10346. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Guglielmone, A.A.; Mangold, A.J.; Castellá, J. Patterns of cuticular hydrocarbon variation and genetic similarity between natural populations of Amblyomma cajennense (Acari: Ixodidae). Acta Trop. 1993, 55, 61–78. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Estrada-Peña, R.; Peiró, J.M. Differentiation of Rhipicephalus ticks (Acari: Ixodidae) by gas chromatography of cuticular hydrocarbons. J. Parasitol. 1992, 78, 982–993. [Google Scholar] [CrossRef]
- Maya-Monteiro, C.M.; Daffre, S.; Logullo, C.; Lara, F.A.; Alves, E.W.; Capurro, M.L.; Zingali, R.; Almeida, I.C.; Oliveira, P.L. HeLp, a Heme Lipoprotein from the Hemolymph of the Cattle Tick, Boophilus microplus. J. Biol. Chem. 2000, 275, 36584–36589. [Google Scholar] [CrossRef]
- Gutierrez, A.C.; Gołębiowski, M.; Pennisi, M.; Peterson, G.; García, J.J.; Manfrino, R.G.; López Lastra, C.C. Cuticle fatty acid composition and differential susceptibility of three species of cockroaches to the entomopathogenic fungi Metarhizium anisopliae (Ascomycota, Hypocreales). J. Econ. Entomol. 2015, 108, 752–760. [Google Scholar] [CrossRef] [PubMed]




| Hydrocarbons | RT * | A. sculptum | D. nitens | R. microplus | R. sanguineus |
|---|---|---|---|---|---|
| Heneicosane | 24.179 | ■ | □ | □ | □ |
| Octasiloxane | 26.000 | ■ | □ | □ | □ |
| Heptadecane | 28.133 | ■ | □ | ■ | □ |
| Tetradecen | 30.125 | ■ | ■ | ■ | □ |
| Tetratetracontane | 28.283 | ■ | □ | □ | □ |
| Octacpsyl | 28.392 | ■ | □ | □ | □ |
| Octatriacontril | 29.783 | ■ | □ | □ | □ |
| Cyclononasiloxane | 38.042 | ■ | □ | □ | □ |
| 3,7-dimetilnonano | 13.183 | □ | ■ | □ | □ |
| Pentadecane | 17.758 | □ | ■ | □ | □ |
| 2,3,5-trimetil Decane | 17.925 | □ | ■ | □ | □ |
| Heneicosanol | 26.142 | □ | ■ | □ | □ |
| 9 (E)-eicoseno | 22.142 | □ | ■ | □ | □ |
| 1,2 Propanediol | 34.067 | □ | ■ | □ | □ |
| Squalen | 39.675 | □ | ■ | ■ | □ |
| Cholesta 3,5 diene | 40.642 | □ | ■ | ■ | □ |
| Tridecan | 13.000 | □ | □ | ■ | □ |
| Eicoisene | 17.767 | □ | □ | ■ | □ |
| Heneicosasnol | 26.142 | □ | □ | ■ | □ |
| Benzene | 34.067 | □ | □ | ■ | □ |
| Pyran | 39.933 | □ | □ | ■ | □ |
| 9,12-Octadecadienoyl chloride | 33.783 | □ | □ | □ | □ |
| 1,19 Eicosadiene | 34.650 | □ | □ | □ | ■ |
| 13-Octadecenal | 41.417 | □ | □ | □ | ■ |
| Nonadecatriene-5,14-diol | 55.273 | □ | □ | □ | ■ |
| Orixane | 56.008 | □ | □ | □ | ■ |
| 12-Tricosanone | 56.700 | □ | □ | □ | ■ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro-Silva, C.S.; Muniz, E.R.; Lima, V.H.; Bernardo, C.C.; Arruda, W.; Castro, R.N.; Gôlo, P.S.; Angelo, I.C.; Fernandes, É.K.K. Cuticular Lipids as a First Barrier Defending Ixodid Ticks against Fungal Infection. J. Fungi 2022, 8, 1177. https://doi.org/10.3390/jof8111177
Ribeiro-Silva CS, Muniz ER, Lima VH, Bernardo CC, Arruda W, Castro RN, Gôlo PS, Angelo IC, Fernandes ÉKK. Cuticular Lipids as a First Barrier Defending Ixodid Ticks against Fungal Infection. Journal of Fungi. 2022; 8(11):1177. https://doi.org/10.3390/jof8111177
Chicago/Turabian StyleRibeiro-Silva, Cárita S., Elen R. Muniz, Valesca H. Lima, Cíntia C. Bernardo, Walquíria Arruda, Rosane N. Castro, Patrícia S. Gôlo, Isabele C. Angelo, and Éverton K. K. Fernandes. 2022. "Cuticular Lipids as a First Barrier Defending Ixodid Ticks against Fungal Infection" Journal of Fungi 8, no. 11: 1177. https://doi.org/10.3390/jof8111177
APA StyleRibeiro-Silva, C. S., Muniz, E. R., Lima, V. H., Bernardo, C. C., Arruda, W., Castro, R. N., Gôlo, P. S., Angelo, I. C., & Fernandes, É. K. K. (2022). Cuticular Lipids as a First Barrier Defending Ixodid Ticks against Fungal Infection. Journal of Fungi, 8(11), 1177. https://doi.org/10.3390/jof8111177








